Andean Flora as a Source of New Repellents against Insect Pests: Behavioral, Morphological and Electrophysiological Studies on Sitophilus zeamais (Coleoptera: Curculionidae)
Abstract
:Highlights
- -
- Sustainable control of stored product insect pests is receiving increasing attention, essential oils could represent an effective, environmentally-safe control method.
- -
- Aromatic plants from megadiverse countries could be a source of new effective repellents or insecticides to be considered in IPM strategies.
- -
- In this study we investigated the repellent effect of EOs from two Lamiaceae species of Ecuador Andean flora belonging to the Clinopdium genus against the maize weevil.
- -
- The EOs where chemically characterized, then used for behavioral assays and EAG experiments. We also carried out a study on the antennal sensory structures of the maize weevil.
- -
- EOs revealed to be perceived trough the antennal olfactory sensilla and showed a clear repellent activity towards the weevil.
1. Introduction
2. Materials and Methods
2.1. Essential Oils Chemical Analyses
2.2. Insect Cultures and Rearing Conditions
2.3. Behavioural Assays
2.4. Electron Microscopy Observation
2.5. Statistical Analyses
3. Results
3.1. Essential Oils Compositions
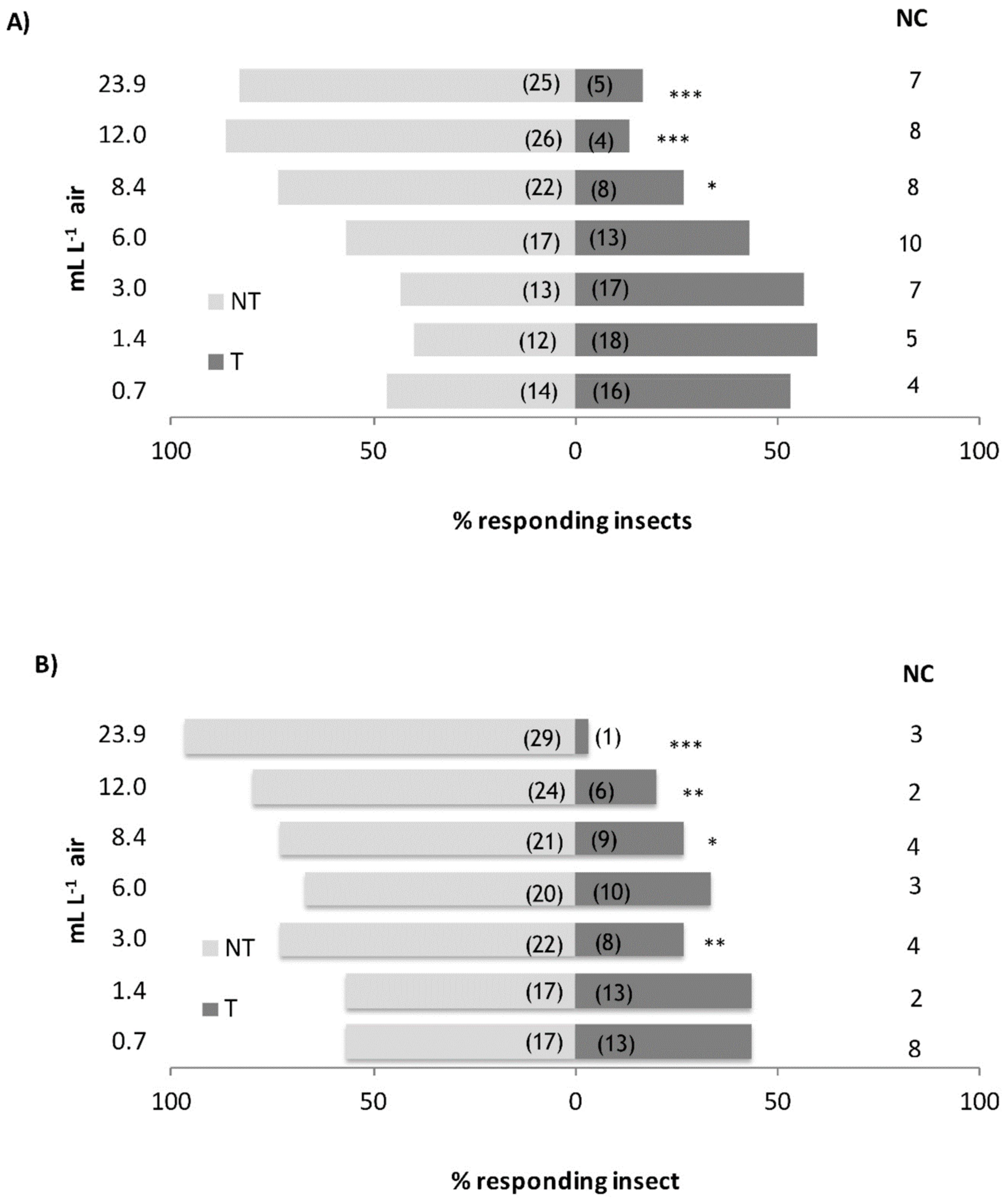
3.2. Behavioural Assays
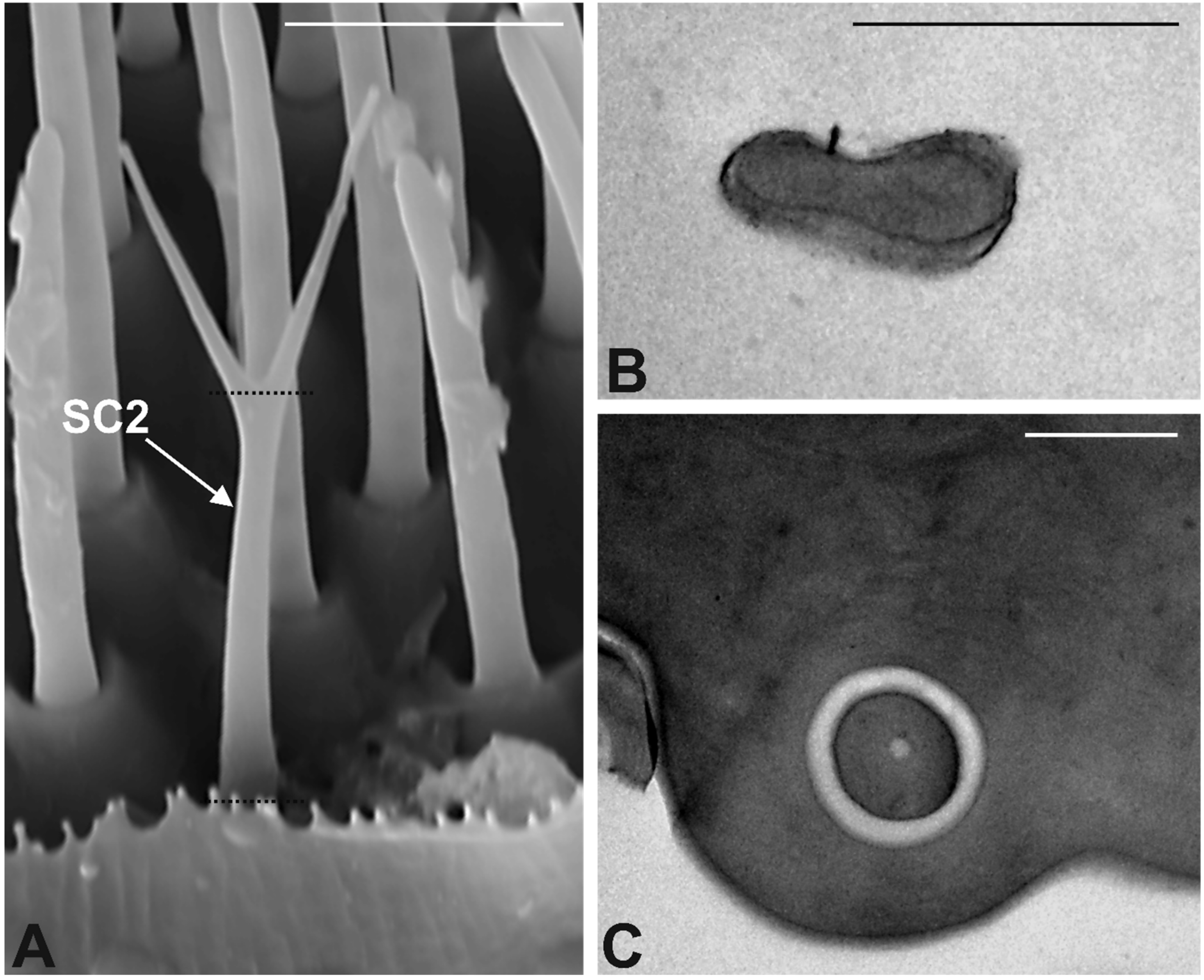
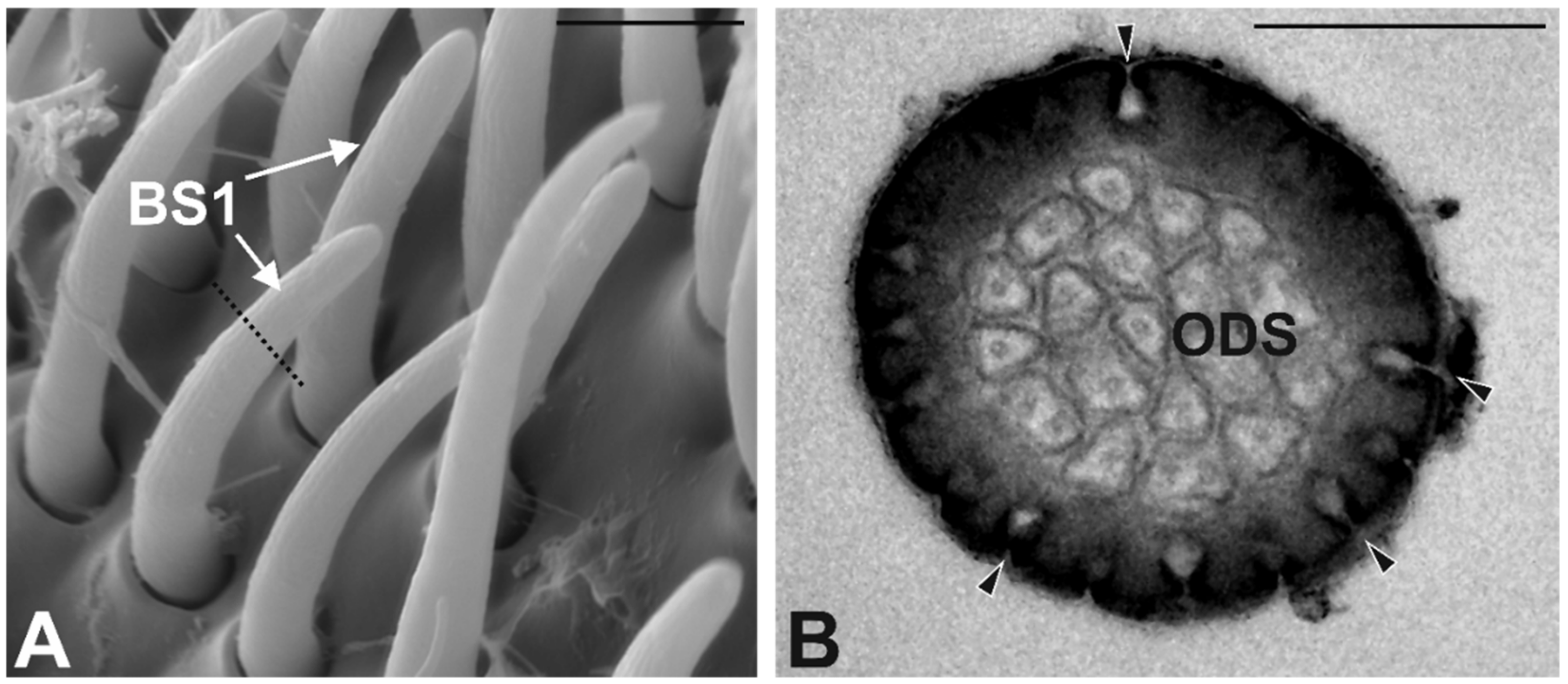
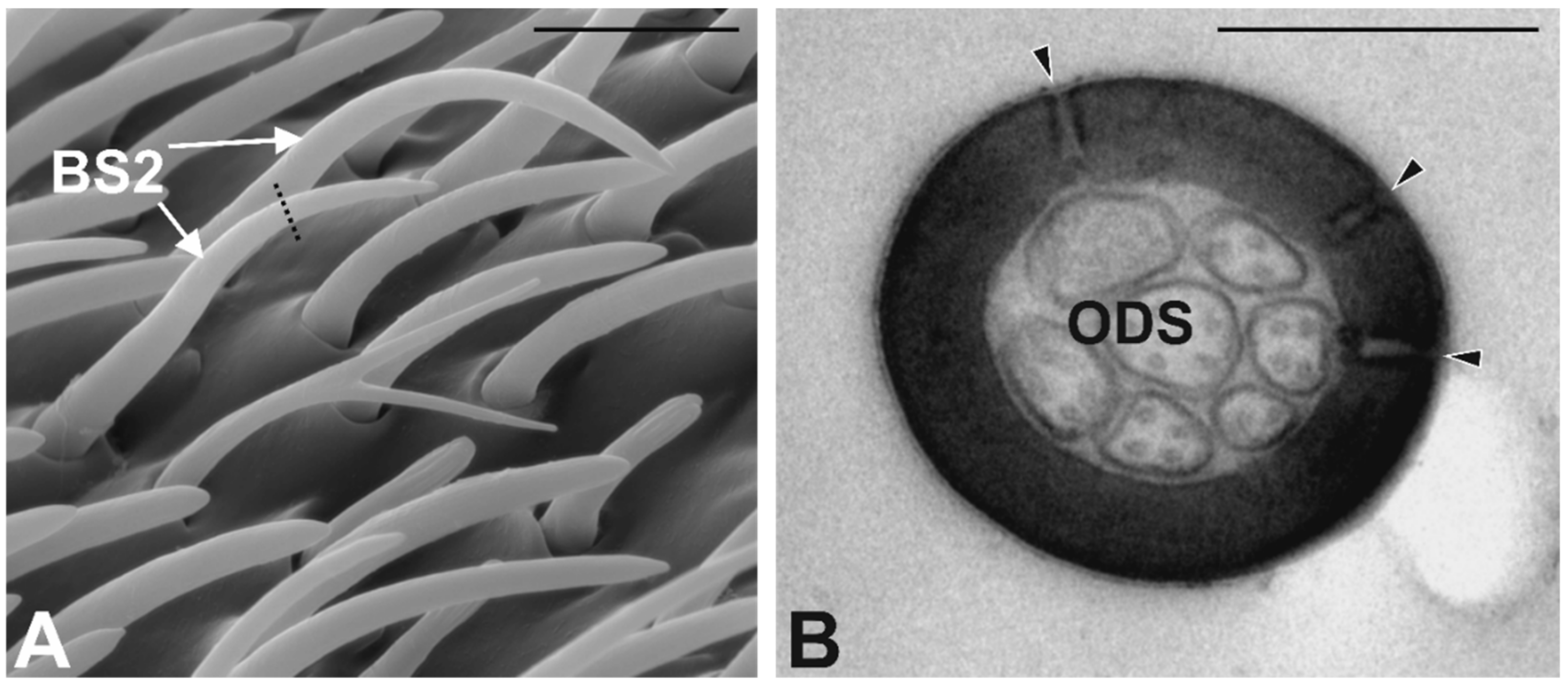
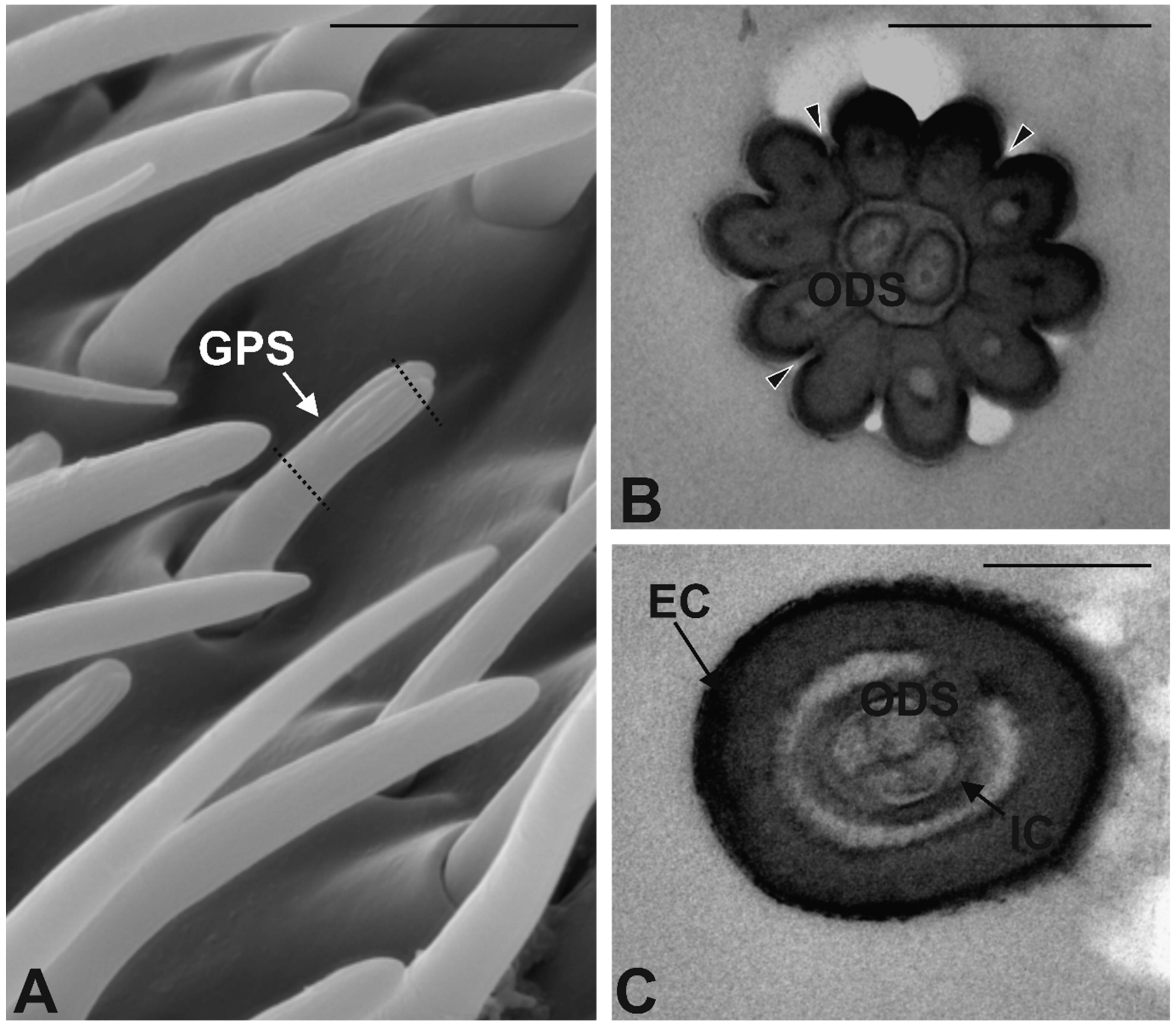
3.3. Antennal Morphology
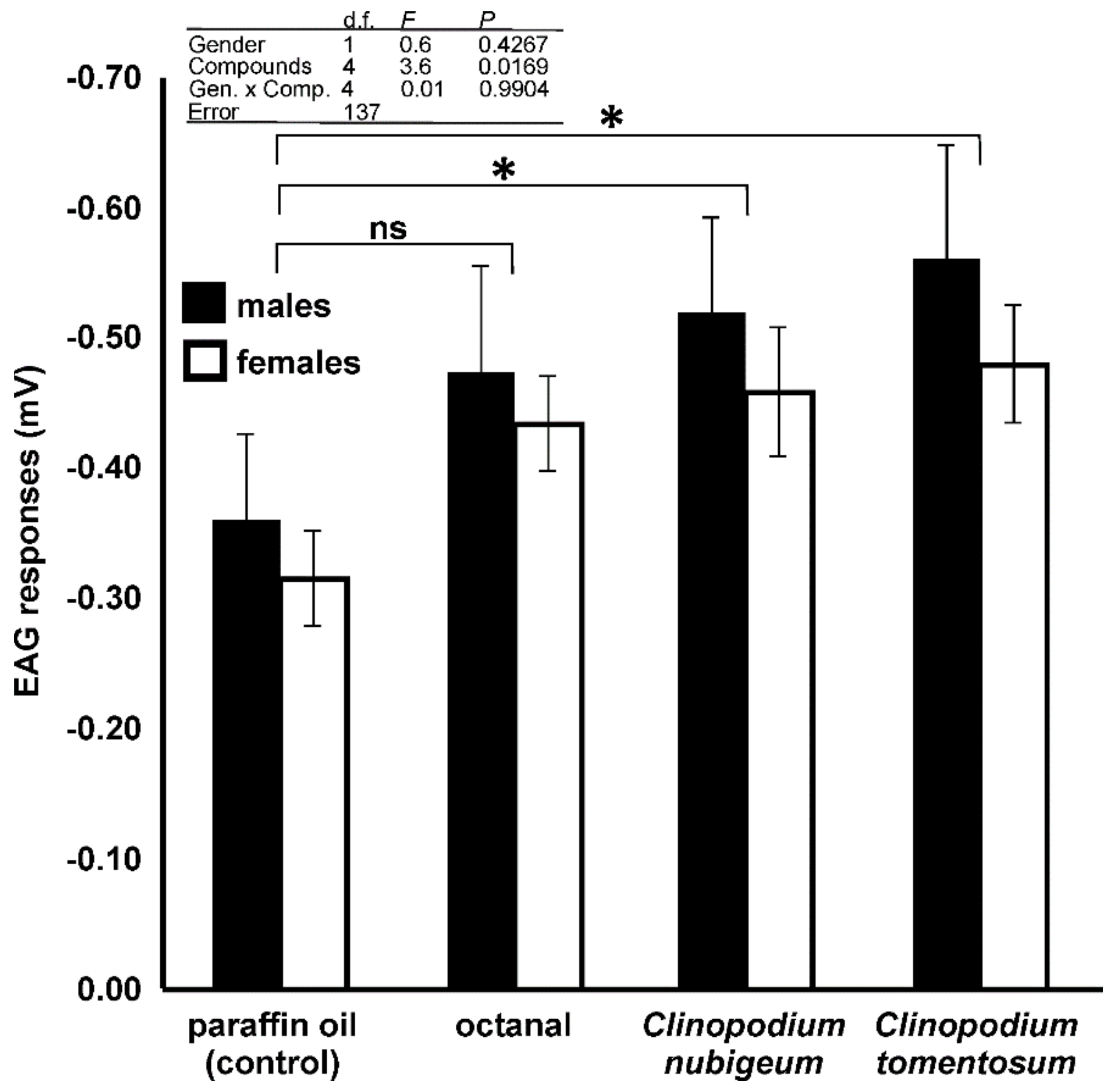
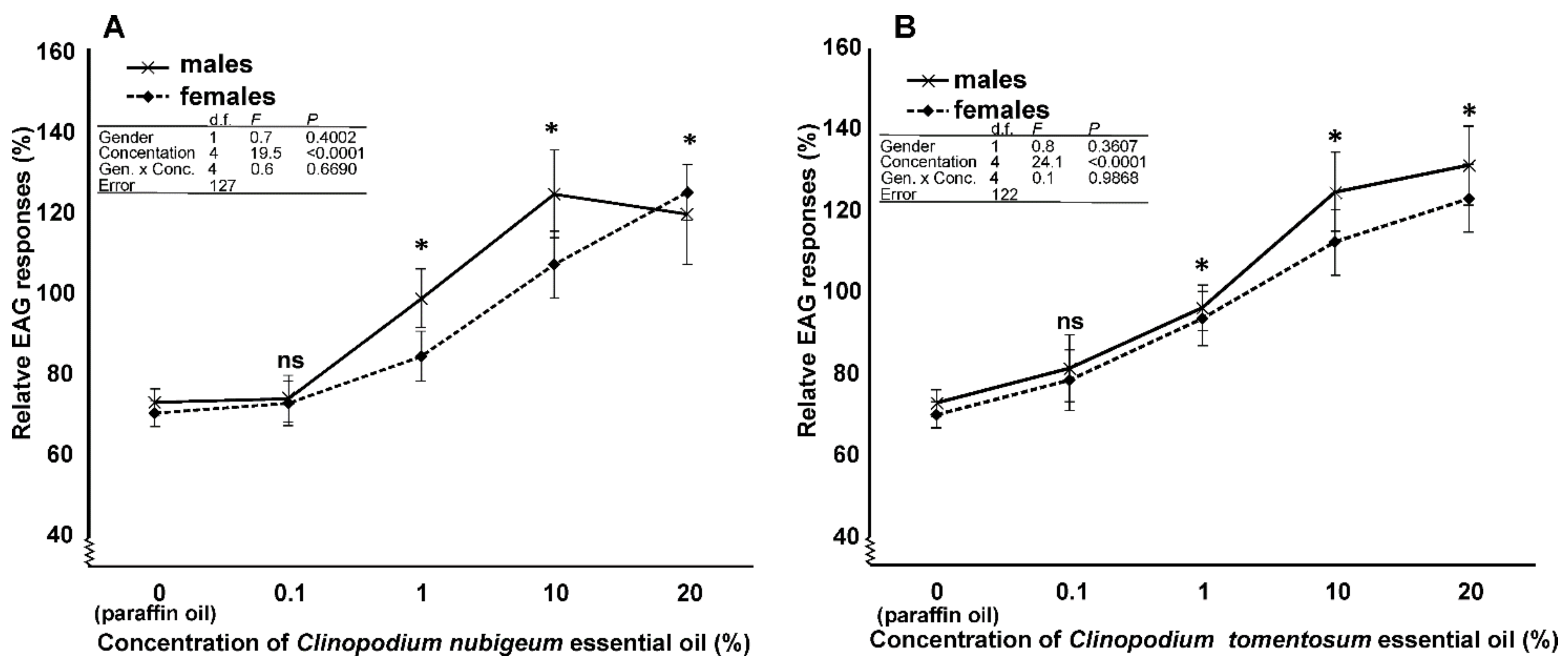
3.4. EAG Analysis
4. Discussion
4.1. Chemical Characterization of EOs
4.2. Behavioural Assays
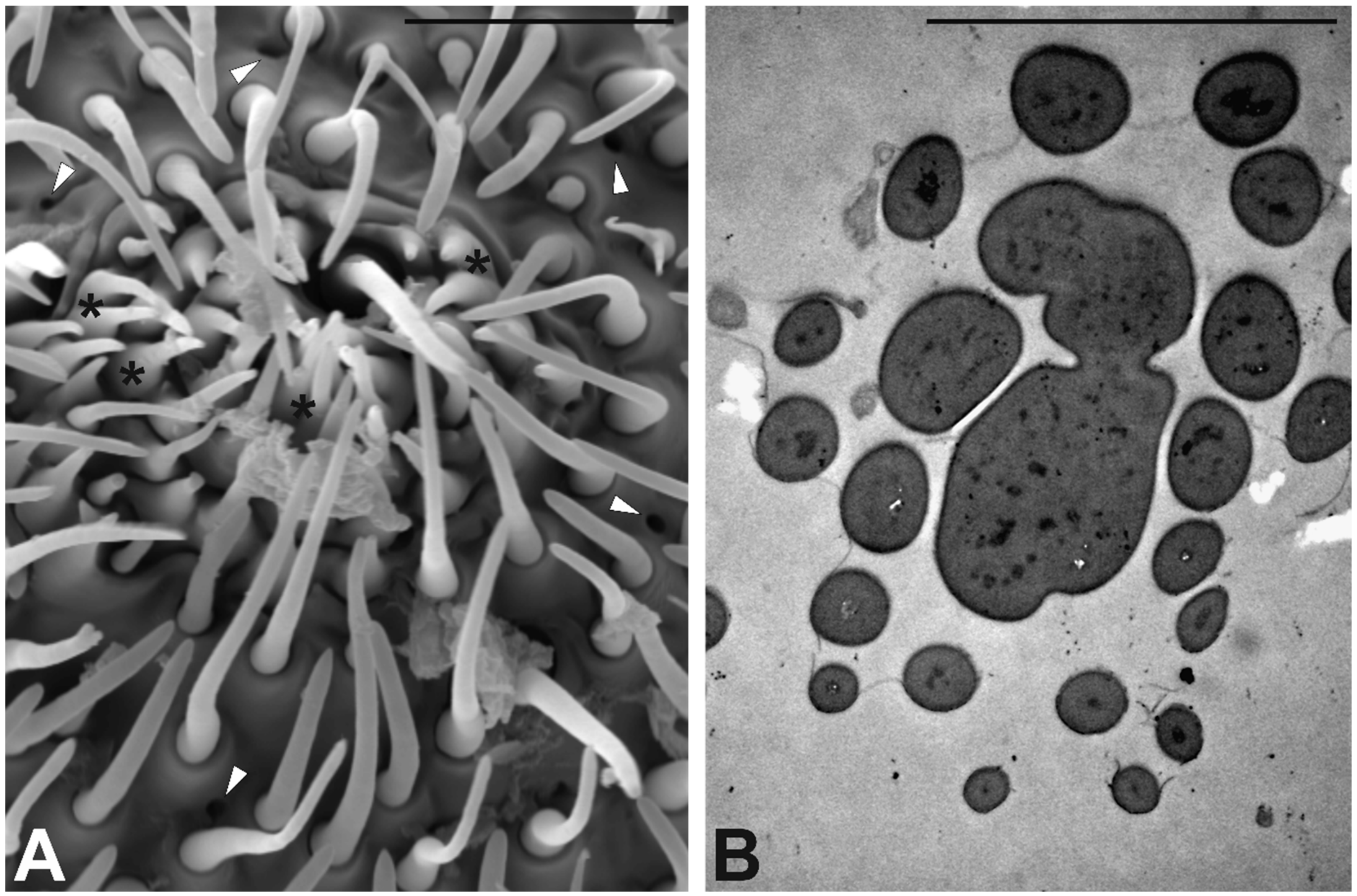
4.3. Antennal Sensilla in S. zeamais
4.4. EAG Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rees, D.P. Insects of Stored Products; CSIRO Publishing: Clayton, Australia, 2004. [Google Scholar]
- Correa, Y.D.C.G.; Faroni, L.R.A.; Haddi, K.; Oliveira, E.E.; Pereira, E.J.G. Locomotory and physiological responses induced by clove and cinnamon essential oils in the maize weevil Sitophilus zeamais. Pestic. Biochem. Physiol. 2015, 125, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Hodges, R.J.; Hall, D.R.; Mbugua, J.N.; Likhayo, P.W. The responses of Prostephanus truncatus (Coleoptera: Bostrichidae) and Sitophilus zeamais (Coleoptera: Curculionidae) to pheromone and synthetic maize volatiles as lures in crevice or flight traps. Bull. Entomol. Res. 1998, 88, 131–139. [Google Scholar] [CrossRef]
- Likhayo, P.W.; Hodges, R.J. Field monitoring Sitophilus zeamais and Sitophilus oryzae (Coleoptera: Curculionidae) using refuge and flight traps baited with synthetic pheromone and cracked wheat. J. Stored Prod. Res. 2000, 36, 341–353. [Google Scholar] [CrossRef]
- Wale, M.; Assegie, H. Efficacy of castor bean oil (Ricinus communis L.) against maize weevils (Sitophilus zeamais Mots.) in northwestern Ethiopia. J. Stored Prod. Res. 2015, 63, 38–41. [Google Scholar] [CrossRef]
- Snelson, J.T. Grain Protectants (ACIAR Monograph No. 3); Canberra Publishing and Printing Co.: Fyshwick, Australia, 1987. [Google Scholar]
- Nakakita, H.; Kawashima, K. A new method to control stored product insects using carbon dioxide with high pressure followed by sudden pressure loss. In Stored Product Protection, Proceedings of the 6th International Working Conference on Stored-Product Protection, Canberra, Australia, 17–23 April 1994; Highley, E., Wright, E.J., Banks, H.J., Champ, B.R., Eds.; Wallingford: Oxon, UK, 1994; pp. 126–129. [Google Scholar]
- Bell, C.H.; Wilson, S.M. Phosphine tolerance and resistance in Trogoderma granarium Everts (Coleoptera: Dermestidae). J. Stored Prod. Res. 1995, 31, 199–205. [Google Scholar] [CrossRef]
- Navarro, S. Modified atmospheres for the control of stored product insects and mites. In Insect Management for Food Storage and Processing, 2nd ed.; Heaps, J.W., Ed.; AACC International: St. Paul, MN, USA, 2006; pp. 105–145. [Google Scholar]
- Isman, M.B. Botanical insecticides, deterrents and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Bougherra, H.H.; Bedini, S.; Flamini, G.; Cosci, F.; Belhamel, K.; Conti, B. Pistacia lentiscus essential oil has repellent effect against three major insect pests of pasta. Ind. Crops Prod. 2015, 63, 249–255. [Google Scholar] [CrossRef]
- Bedini, S.; Bougherra, H.H.; Flamini, G.; Cosci, F.; Belhamel, K.; Ascrizzi, R.; Conti, B. Repellency of anethole-and estragole-type fennel essential oils against stored grain pests: The different twins. Bull. Insectol. 2016, 69, 149–157. [Google Scholar]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Hee, S.K.; Ho, S.H. Antifeedant and growth inhibitory effects of α-pinene on the stored-product insects, Tribolium castaneum (Herbst) and Sitophilus zeamais (Motsch). Int. Pest Contr. 1998, 40, 18–20. [Google Scholar]
- Enan, E. Insecticidal activity of essential oils: Octopaminergic sites of action. Comp. Biochem. Physiol. Part C Toxicol. Pharmcol. 2001, 130, 325–337. [Google Scholar] [CrossRef]
- Regnault-Roger, C.; Vincent, C.; Thor, J. Essential oils in insect control: Low-risk products in a high-stakes world. Annu. Rev. Entomol. 2012, 57, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Bedini, S.; Flamini, G.; Ascrizzi, R.; Venturi, F.; Ferroni, G.; Bader, A.; Girardi, J.; Conti, B. Essential oils sensory quality and their bioactivity against the mosquito Aedes albopictus. Sci. Rep. 2018, 8, 17857. [Google Scholar] [CrossRef] [PubMed]
- Bedini, S.; Flamini, G.; Cosci, F.; Ascrizzi, R.; Echeverria, M.C.; Gomez, E.V.; Guidi, L.; Landi, M.; Lucchi, A.; Conti, B. Toxicity and oviposition deterrence of essential oils of Clinopodium nubigenum and Lavandula angustifolia against the myiasis-inducing blowfly Lucilia sericata. PLoS ONE 2019, 14, e0212576. [Google Scholar] [CrossRef] [PubMed]
- Conti, B.; Canale, A.; Cioni, P.L.; Flamini, G. Repellence of essential oils from tropical and Mediterranean Lamiaceae against Sitophilus zeamais. Bull. Insectol. 2010, 63, 197–202. [Google Scholar]
- Pavela, R. History, presence and perspective of using plant extracts as commercial botanical insecticides and farm products for protection against insects—A review. Plant Prot. Sci. 2016, 52, 229–241. [Google Scholar]
- Benelli, G.; Flamini, G.; Canale, A.; Molfetta, I.; Cioni, P.L.; Conti, B. Repellence of Hyptis suaveolens whole essential oil and major constituents against adults of the granary weevil Sitophilus granarius. Bull. Insectol. 2012, 65, 177–183. [Google Scholar]
- Bedini, S.; Flamini, G.; Girardi, J.; Cosci, F.; Conti, B. Not just for beer: Evaluation of spent hops (Humulus lupulus L.) as a source of eco-friendly repellents for insect pests of stored foods. J. Pest Sci. 2015, 88, 583–592. [Google Scholar] [CrossRef]
- Bhavya, M.L.; Chandu, A.G.S.; Devi, S.S. Ocimum tenuiflorum oil, a potential insecticide against rice weevil with anti-acetylcholinesterase activity. Ind. Crops Prod. 2018, 126, 434–439. [Google Scholar] [CrossRef]
- Estrada-Reyes, R.; Martínez-Vázquez, M.; Gallegos-Solís, A.; Heinze, G.; Moreno, J. Depressant effects of Clinopodium mexicanum Benth. Govaerts (Lamiaceae) on the central nervous system. J. Ethnopharmacol. 2010, 130, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gilardoni, G.; Malagon, O.; Morocho, V.; Negri, R.; Tosi, S.; Guglielminetti, M.; Vidari, G.; Finzi, P.V. Phytochemical researches and antimicrobial activity of Clinopodium nubigenum Kunth (Kuntze) raw extracts. Braz. J. Pharmacogn. 2011, 21, 850–855. [Google Scholar] [CrossRef]
- Ali, S.A.I.; DIakite, M.M.; Ali, S.; Wang, M.Q. Morphology and ultrastructure of the antennal sensilla of Sitophilus granarius (Coleoptera: Curculionidae). Bull. Entomol. Res. 2016, 106, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Fouda, M.A.; Al-Dali, A.G.; Ghannam, I.S. Ultrastructure of Sensory Receptors on the Antennae and Mouthparts of the Adult, Sitophilus oryzae L. and Sitophilus granarius L. (Coleoptera: Curculionidae). J. Nucl. Technol. Appl. Sci. 2016, 4, 25–33. [Google Scholar]
- Abd El-Ghany, N.M.; Abd El-Aziz, S.E. External morphology of antennae and mouthpart sensillae of the granary weevil (Coleoptera: Curculionidae). J. Entomol. Sci. 2017, 52, 29–38. [Google Scholar] [CrossRef]
- Masada, Y. Analysis of Essential Oils by Gas Chromatography and Mass Spectrometry; John Wiley & Sons: New York, NY, USA, 1976. [Google Scholar]
- Swigar, A.A.; Silverstein, R.M. Monoterpenes; Aldrich Chemical Company: Milwaukee, WI, USA, 1981. [Google Scholar]
- Jennings, W.; Shibamoto, T. Qualitative Analysis of Flavor and Fragrance Volatiles by Glass Capillary Gas Chromatography; Academic Press: San Jose, CA, USA, 1982. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy; Allured Publishing Corporation: Carol Stream, IL, USA, 1995. [Google Scholar]
- Adams, R.P.; Zanoni, T.A.; Lara, A.; Barrero, A.F.; Cool, L.G. Comparisons among Cupressus arizonica Greene, C. benthamii Endl., C. lindleyi Klotz, ex Endl. and C. lusitanica Mill, using Leaf Essential Oils and DNA Fingerprinting. J. Essent. Oil Res. 1997, 9, 303–309. [Google Scholar] [CrossRef]
- Davies, N.W. Gas chromatographic retention indices of monoterpenes and sesquiterpenes on Methyl Silicon and Carbowax 20M phases. J. Chromatogr. A 1990, 503, 1–24. [Google Scholar] [CrossRef]
- Beadle, G.W.; Ephrussi, B. Differentiation of eye pigments in Drosophila as studied by transplantation. Genetics 1936, 21, 225–247. [Google Scholar]
- Statsoft Inc. Statistica (Data Analysis Software System), Version 6; StatSoft, Italia S.r.l: Vigonza (PD), Italy, 2001. [Google Scholar]
- Sokal, R.; Rohlf, F.J. Biometry; Freeman W.H.: New York, NY, USA, 1998. [Google Scholar]
- Noriega, P.F.; De los Ángeles Mosquera, T.; Osorio, E.A.; Guerra, P.; Fonseca, A. Clinopodium nubigenum (Kunth) Kuntze essential oil: Chemical composition, antioxidant activity, and antimicrobial test against respiratory pathogens. J. Pharmacogn. Phyther. 2018, 10, 149–157. [Google Scholar]
- Ruiz, S.; Malagón, O.; Zaragoza, T.; Valarezo, E. Composition of the Essential Oils of Artemisia sodiroi Hieron., Siparuna eggersii Hieron., Tagetes filifolia Lag. and Clinopodium nubigenum (Kunth) Kuntze from Loja Ecuador. J. Essent. Oil Bear. Plants 2010, 13, 676–691. [Google Scholar] [CrossRef]
- Benzo, M.; Gilardoni, G.; Gandini, C.; Caccialanza, G.; Finzi, P.V.; Vidari, G.; Abdo, S.; Layedra, P. Determination of the threshold odor concentration of main odorants in essential oils using gas chromatography–olfactometry incremental dilution technique. J. Chromatogr. A 2007, 1150, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, R.F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Saïd, I.; Tauban, D.; Renou, M.; Mori, K.; Rochat, D. Structure and function of the antennal sensilla of the palm weevil Rhynchophorus palmarum (Coleoptera, Curculionidae). J. Insect Physiol. 2003, 49, 857–872. [Google Scholar] [CrossRef]
- Romani, R.; Rosi, M.C.; Isidoro, N.; Bin, F. The role of the antennae during courtship behaviour in the parasitic wasp Trichopria drosophilae. J. Exp. Biol. 2008, 211, 2486–2491. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Nakagawa, T.; Mitsuno, H.; Mori, H.; Endo, Y.; Tanoue, S.; Yasukochi, Y.; Touhara, K.; Nishioka, T. Identification and functional characterization of a sex pheromone receptor in the silkmoth Bombyx mori. Proc. Natl. Acad. Sci. USA 2004, 101, 16653–16658. [Google Scholar] [CrossRef]
- Brézot, P.; Tauban, D.; Renou, M. Sense organs on the antennal flagellum of the green stink bug, Nezara viridula (L.) (Heteroptera: Pentatomidae): Sensillum types and numerical growth during the post-embryonic development. Int. J. Insect Morphol. Embryol. 1996, 25, 427–441. [Google Scholar] [CrossRef]
- Chinta, S.; Dickens, J.C.; Baker, G.T. Morphology and distribution of antennal sensilla of the tarnished plant bug, Lygus lineolaris (Palisot de beauvois) (Hemiptera: Miridae). Int. J. Insect Morphol. Embryol. 1997, 26, 21–26. [Google Scholar] [CrossRef]
- Romani, R.; Rossi Stacconi, M.V. Mapping and ultrastructure of antennal chemosensilla of the wheat bug Eurygaster maura. Insect Sci. 2009, 16, 193–203. [Google Scholar] [CrossRef]
- Adams, J.R.; Holpert, P.E.; Forgash, J. Electron microscopy of the contact chemoreceptors of the stable fly, Stomoxys calcitrans (Diptera: Muscidae). Ann. Entomol. Soc. Am. 1965, 58, 909–917. [Google Scholar] [CrossRef]
- Felt, B.T.; Vande Berg, J.S. Ultrastructure of the blowfly chemoreceptor sensillum (Phormia regina). J. Morphol. 1976, 150, 763–778. [Google Scholar] [CrossRef] [PubMed]
- McIver, S.; Siemicki, R. Fine structure of tarsal sensilla of Aedes aegypti (L.) (Diptera: Culicidae). J. Morphol. 1978, 155, 137–156. [Google Scholar] [CrossRef] [PubMed]
- Myers, J. The structure of the antennae of the Florida queen butterfly, Danaus gilippus berenice (Cramer). J. Morphol. 1968, 125, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.C.; Schoonhoven, L.M. Tarsal contact chemosensory hairs of the large white butterfly Pieris brassicae and their possible role in oviposition behavior. Entomol. Exp. Appl. 1973, 16, 343–357. [Google Scholar]
- Graffal, K.P. An ultrastructural study of the tips of four classical bimodal sensilla with one mechanosensitive and several chemosensitive receptor cells. Zoomorphology 1979, 92, 273–291. [Google Scholar] [CrossRef]
- Bartlet, E.; Isidoro, N.; Williams, H. Antennal glands in Psylliodes chrysocephala and their possible role in reproductive behaviour. Physiol. Entomol. 1994, 19, 241–250. [Google Scholar] [CrossRef]
- Merivee, E.; Ploomi, A.; Luik, A.; Rahi, M.; Sammelselg, V. Antennal sensilla of the ground beetle Platynus dorsalis (Pontoppidan 1763) (Coleoptera, Carabidae). Microsc. Res. Tech. 2001, 55, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Merivee, E.; Ploomi, A.; Rahi, M.; Bresciani, J.; Ravn, H.P.; Luik, A.; Sammelselg, V. Antennal sensilla of groud beetle Bembidion properans Steph. (Coleoptera Carabidae). Micron 2002, 33, 429–440. [Google Scholar] [CrossRef]
- Di Giulio, A.; Maurizi, E.; Stacconi, M.V.R.; Romani, R. Functional structure of antennal sensilla in the myrmecophilous beetle Paussus favieri (Coleoptera, Carabidae, Paussini). Micron 2012, 43, 705–719. [Google Scholar] [CrossRef]
- Merivee, E.; Renou, M.; Mand, M.; Luik, A.; Heidemaa, M.; Ploomi, A. Electrophysiological responses to salts from antennal chaetoid taste sensilla of the ground beetle Pterostichus aethiops. J. Insect Physiol. 2004, 50, 1001–1013. [Google Scholar] [CrossRef]
- Merivee, E.; Märtmann, H.; Must, A.; Milius, M.; Williams, I.; Mänd, M. Electrophysiological responses from neurons of antennal taste sensilla in the polyphagous predatory ground beetle Pterostichus oblongopunctatus (Fabricius 1787) to plant sugars and amino acids. J. Insect Physiol. 2008, 54, 1213–1219. [Google Scholar] [CrossRef]
- Zacharuk, R.Y. Antennae and sensilla. In Comprehensive Insect Physiology, Biochemistry and Pharmacology; Kerkut, G.A., Gilbert, L.I., Eds.; Pergamon Press: Oxford, UK, 1985; Volume 6, pp. 1–69. [Google Scholar]
- Palma, R.; Mutis, A.; Isaacs, R.; Quiroz, A. Type and Distribution of Sensilla in the Antennae of the Red Clover Root Borer, Hylastinus obscurus. J. Insect Sci. 2013, 13, 1–10. [Google Scholar] [CrossRef]
- Yan, S.C.; Meng, Z.J.; Peng, L.; Liu, D. Antennal sensilla of the pine weevil Pissodes nitidus Roel. (Coleoptera: Curculionidae). Microsc. Res. Tech. 2011, 74, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Pophof, B. Olfactory responses recorded from sensilla coeloconica of the silkmoth Bombyx mori. Physiol. Entomol. 1997, 22, 239–248. [Google Scholar] [CrossRef]
- Diehl, P.A.; Vlimant, M.; Guerenstein, P.; Guerin, P.M. Ultrastructure and receptor cell responses of the antennal grooved peg sensilla of Triatoma infestans (Hemiptera: Reduviidae). Arthropod. Struct. Dev. 2003, 31, 271–285. [Google Scholar] [CrossRef]
- Pophof, B.; Stange, G.; Abrell, L. Volatile organic compounds as signals in a plant-herbivore system: Electrophysiological responses in olfactory sensilla of the moth Cactoblastis cactorum. Chem. Senses 2005, 30, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Altner, H.; Prillinger, L. Ultrastructure of invertebrate chemo-, thermo-, and hygroreceptors and its functional significance. Int. Rev. Cytol. 1980, 67, 69–139. [Google Scholar]
- Quennedey, A. Insect Epidermal Gland Cells: Ultrastructure and Morphogenesis. In Microscopic Anatomy of Invertebrates; Harrison, F.W., Locke, M., Eds.; Wiley-Liss: New York, NY, USA, 1998; pp. 177–207. [Google Scholar]
- Romani, R.; Isidoro, N.; Riolo, P.; Bin, F. Antennal glands in male bees: Structures for sexual communication by pheromones? Apidolologie 2003, 34, 603–610. [Google Scholar] [CrossRef]
- Di Giulio, A.; Rossi Stacconi, M.V.; Romani, R. Fine structure of the antennal glands of the ant nest beetle Paussus favieri (Coleoptera, Carabidae Paussini). Arthropod. Struct. Dev. 2009, 38, 293–302. [Google Scholar] [CrossRef]
- Germinara, G.S.; Rotundo, G.; De Cristofaro, A. Repellence and fumigant toxicity of propionic acid against adults of Sitophilus granarius (L.) and S. oryzae (L.). J. Stored Prod. Res. 2007, 43, 229–233. [Google Scholar] [CrossRef]



| Constituents | l.r.i. a | Relative Abundance (%) | |
|---|---|---|---|
| Clinopodium nubigenum | Clinopodium tomentosum | ||
| α-thujene | 931 | 0.9 | - b |
| α-pinene | 941 | 0.5 | 0.2 |
| camphene | 954 | tr c | - |
| sabinene | 976 | 0.4 | 0.2 |
| 1-octen-3-ol | 980 | tr | - |
| β-pinene | 982 | 0.5 | 0.4 |
| myrcene | 993 | 0.3 | 0.3 |
| 3-octanol | 993 | 0.8 | - |
| α-phellandrene | 1005 | 0.2 | - |
| δ-3-carene | 1011 | tr | - |
| α-terpinene | 1018 | 1.0 | - |
| p-cymene | 1027 | 9.1 | - |
| limonene | 1032 | 1.5 | 0.9 |
| 1,8-cineole | 1034 | tr | 1.1 |
| (E)-β-ocimene | 1052 | tr | - |
| γ-terpinene | 1062 | 5.3 | - |
| cis-sabinene hydrate | 1070 | 0.6 | - |
| terpinolene | 1088 | tr | - |
| trans-sabinene hydrate | 1095 | 0.1 | - |
| linalool | 1101 | 0.1 | 0.4 |
| nonanal | 1102 | 0.1 | - |
| 1-octen-3-yl acetate | 1111 | 0.9 | 0.2 |
| β-thujone | 1118 | tr | - |
| 3-octanol acetate | 1124 | 1.0 | - |
| cis-verbenol | 1142 | - | 0.1 |
| isopulegol | 1146 | tr | - |
| menthone | 1154 | - | 1.4 |
| citronellal | 1155 | 1.8 | - |
| isomenthone | 1164 | 6.4 | 48.4 |
| isopulegone | 1177 | 3.5 | 0.8 |
| 4-terpineol | 1178 | tr | - |
| α-terpineol | 1189 | 0.2 | 0.2 |
| myrtenal | 1194 | - | 0.2 |
| decanal | 1204 | tr | - |
| citronellol | 1230 | 1.3 | - |
| pulegone | 1237 | 25.4 | 34.3 |
| neral | 1240 | - | 0.1 |
| piperitone | 1252 | 0.9 | 4.0 |
| methyl citronellate | 1261 | - | 0.8 |
| geranial | 1271 | tr | 1.1 |
| isobornyl acetate | 1285 | tr | 0.1 |
| trans-sabinyl acetate | 1291 | - | 0.1 |
| carvacrol | 1298 | 32.9 | - |
| citronellyl acetate | 1350 | 0.6 | - |
| piperitenone | 1351 | - | 2.1 |
| eugenol | 1358 | 0.8 | - |
| piperitone oxide | 1363 | 0.1 | - |
| piperitenone oxide | 1363 | - | 1.1 |
| α-copaene | 1376 | 0.3 | - |
| β-cubebene | 1390 | tr | - |
| β-caryophyllene | 1420 | - | 0.5 |
| γ-muurolene | 1477 | - | 0.5 |
| germacrene D | 1478 | 0.2 | - |
| bicyclogermacrene | 1495 | 0.9 | - |
| (E,E)-α-farnesene | 1507 | tr | - |
| δ-cadinene | 1524 | 0.5 | - |
| spathulenol | 1576 | 0.5 | - |
| manoyl oxide | 1988 | - | 0.8 |
| Monoterpene hydrocarbons | 19.7 | 1.9 | |
| Oxygenated monoterpenes | 74.0 | 96.2 | |
| Sesquiterpene hydrocarbons | 1.8 | 1.0 | |
| Oxygenated sesquiterpenes | 0.5 | - | |
| Oxygenated diterpenes | - | 0.8 | |
| Phenylpropanoids | 0.8 | - | |
| Other non-terpene derivatives | 2.8 | 0.2 | |
| Total identified (%) | 99.6 | 100.0 | |
| EO | RC50 a | Slope | Intercept | χ2 (df) | p |
|---|---|---|---|---|---|
| C. nubigenum | 11.216 (8.762−15.168) b | 3.109 ± 0.643 | −3264 ± 0.658 | 4.51 (5) | 0.478 |
| C. tomentosum | 6.579 (4.349−10.833) | 1.497 ± 0.306 | −1.225 ± 0.259 | 3.64 (5) | 0.603 |
| Concentration a | Latency Time | Permanence Time | ||
|---|---|---|---|---|
| C. nubigenum | C. tomentosum | C. nubigenum | C. tomentosum | |
| 0.7 | 120.00 ± 13.36 b | 107.23 ± 16.08 | 131.10 ± 11.29 | 107.23 ± 14.77 |
| 1.4 | 118.17 ± 14.61 | 100.23 ± 15.17 | 116.50 ± 11.42 | 136.70 ± 18.84 |
| 3.0 | 125.87 ± 13.44 | 100.27 ± 11.2 | 123.07 ± 9.25 | 116.33 ± 14.37 |
| 6.0 | 111.80 ± 13.39 | 120.70 ± 11.72 | 123.07 ± 14.34 | 121.30 ± 15.45 |
| 8.4 | 111.80 ± 13.39 | 132.70 ± 14.70 | 123.63 ± 14.34 | 148.57 ± 14.45 |
| 12.0 | 138.80 ± 15.81 | 110.80 ± 15.71 | 143.80 ± 14.66 | 130.83 ± 15.52 |
| 23.9 | 95.47 ± 12.33 | 126.57 ± 15.06 | 154.93 ± 14.45 | 150.43 ± 19.24 |
| Sensilla Type | N. (mean ± SE) | Lenght (μm) (mean ± SE) | Diameter (μm) (mean ± SE) | Pores | Wall | Neurons | Tip |
|---|---|---|---|---|---|---|---|
| CS1 | 17 ± 1 | 27 ± 1.8 | 1.2 ± 0.04 | Uniporous | Single Thick | 6 | Round |
| CS2 | 40 ± 3 | 12 ± 0.5 | 0.86 ± 0.04 | Aporous | Single Thick | 1 | Sharp branched |
| BS1 | 208 ± 13 | 12 ± 0.4 | 1 ± 0.03 | Multiporous | Single Thin | 2–3 | Round |
| BS2 | 102 ± 8 | 22 ± 1.4 | 0.9 ± 0.02 | Multiporous | Single Thick | 2–3 | Round |
| GPS | 40 ± 7 | 6.8 ± 0.2 | 0.83 ± 0.01 | Multiporous | Double walled | 3–4 | Finger-like |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romani, R.; Bedini, S.; Salerno, G.; Ascrizzi, R.; Flamini, G.; Echeverria, M.C.; Farina, P.; Conti, B. Andean Flora as a Source of New Repellents against Insect Pests: Behavioral, Morphological and Electrophysiological Studies on Sitophilus zeamais (Coleoptera: Curculionidae). Insects 2019, 10, 171. https://doi.org/10.3390/insects10060171
Romani R, Bedini S, Salerno G, Ascrizzi R, Flamini G, Echeverria MC, Farina P, Conti B. Andean Flora as a Source of New Repellents against Insect Pests: Behavioral, Morphological and Electrophysiological Studies on Sitophilus zeamais (Coleoptera: Curculionidae). Insects. 2019; 10(6):171. https://doi.org/10.3390/insects10060171
Chicago/Turabian StyleRomani, Roberto, Stefano Bedini, Gianandrea Salerno, Roberta Ascrizzi, Guido Flamini, Maria Cristina Echeverria, Priscilla Farina, and Barbara Conti. 2019. "Andean Flora as a Source of New Repellents against Insect Pests: Behavioral, Morphological and Electrophysiological Studies on Sitophilus zeamais (Coleoptera: Curculionidae)" Insects 10, no. 6: 171. https://doi.org/10.3390/insects10060171
APA StyleRomani, R., Bedini, S., Salerno, G., Ascrizzi, R., Flamini, G., Echeverria, M. C., Farina, P., & Conti, B. (2019). Andean Flora as a Source of New Repellents against Insect Pests: Behavioral, Morphological and Electrophysiological Studies on Sitophilus zeamais (Coleoptera: Curculionidae). Insects, 10(6), 171. https://doi.org/10.3390/insects10060171