Evaluation of Purdue Improved Crop Storage Triple Layer Hermetic Storage Bag against Prostephanus truncatus (Horn) (Coleoptera: Bostrichidae) and Sitophilus zeamais (Motsch.) (Coleoptera: Curculionidae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Experiment Set-up
- (i)
- PICS + U: PICS bag + untreated maize, which simulated farmer practice of storing un-infested maize grain;
- (ii)
- PICS + I: PICS bag + untreated maize + Prostephanus truncatus + Sitophilus zeamais; which simulated farmer practice of storing already infested maize grain with low levels of insect infestation;
- (iii)
- PP+A: Polypropylene bag + maize treated with Actellic Super dust;
- (iv)
- PP+U: Polypropylene bag + untreated maize;
- (v)
- Jute+A: Jute bag + maize treated with Actellic Super dust;
- (vi)
- Jute+U: Jute bag + untreated maize.
2.2. Insects
2.3. Data Collection
2.4. Statistical Analysis
3. Results
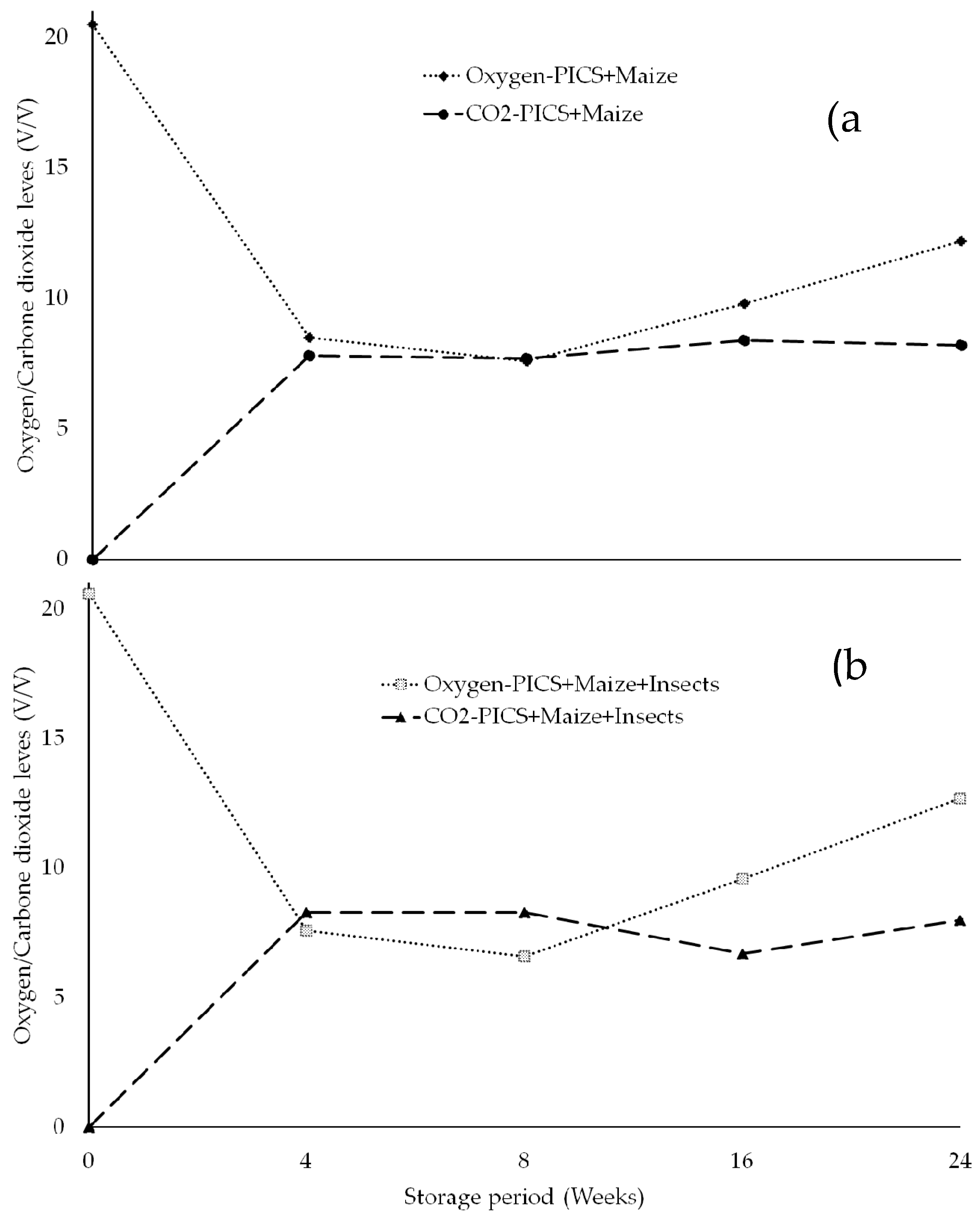
3.1. Gas Composition Levels
3.2. Effect of Treatments on Grain Moisture Content Level
3.3. Effect of Treatments on Weight of Dust
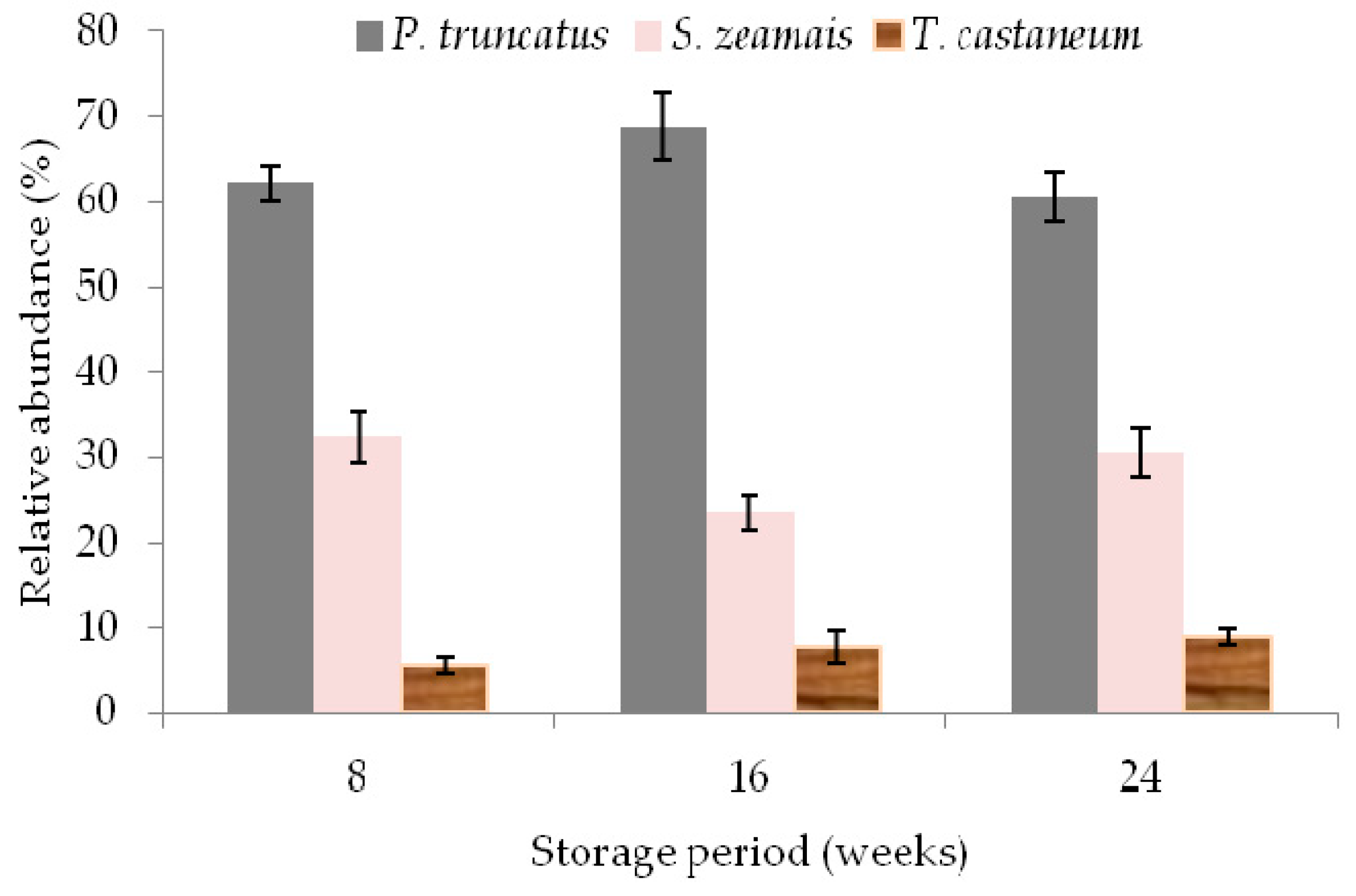
3.4. Effect of Treatments on Number of Live Insects
3.5. Effect of Treatment on Grain Damage and Weight Loss
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tefera, T.; Kanampiu, F.; DeGroote, H.; Hellin, J.; Mugo, S.; Kimenju, S.; Beyene, Y.; Boddupalli, P.M.; Shiferaw, B.; Banziger, M. The metal silo: An effective grain storage technology for reducing postharvest insect and pathogen losses in maize while improving smallholder farmers’ food security in developing countries. Crop Prot. 2011, 30, 240–245. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. Maize Production in Sub-Saharan Africa 2013/2014 Production Year. 2014. Available online: http://faostat3.fao.org/browse/Q/QC/E (accessed on 16 March 2016).
- Baoua, I.B.; Amadou, L.; Ousmane, B.; Baributsa, D.; Murdock, D.D. PICS bag for postharvest storage of maize grain in West Africa. J. Stored Prod. Res. 2014, 58, 20–28. [Google Scholar] [CrossRef]
- De Lima, C.P.E. The assessment of losses due to insects and rodents in maize stored for subsistence in Kenya. Trop. Stored Prod. Inf. 1979, 38, 21–26. [Google Scholar]
- Alonso-Amelot, M.E.; Avila-Nunez, J.I. Comparison of seven methods for stored cereal losses to insects for their application in rural conditions. J. Stored Prod. Res. 2011, 47, 82–87. [Google Scholar] [CrossRef]
- Affognon, H.; Mutungi, C.; Sanginga, P.; Borgemeister, C. Unpacking Postharvest Losses in Sub-Saharan Africa: A Meta-Analysis. World Dev. 2015, 66, 49–68. [Google Scholar] [CrossRef]
- Kumar, D.; Kalita, P. Reducing postharvest losses during storage of grain crops to strengthen food security in developing countries. Foods 2017, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Muhihu, S.K.; Kibata, G.N. Developing a control programme to combat an outbreak of Prostephanus truncatus Horn (Coleoptera: Bostrichidae) in Kenya. Trop. Sci. 1985, 25, 239–248. [Google Scholar]
- Mutambuki, K.; Ngatia, C.M.; Mbugua, J.N. Postharvest technology transfer to reduce on-farm grain losses in Kitui District, Kenya. J. Agric. Sci. Technol. 2011, B1, 392–399. [Google Scholar]
- De Groote, H.; Kimenju, C.S.; Likhayo, P.; Kanampiu, F.; Tefera, T.; Hellin, J. Effectiveness of hermetic systems in controlling maize storage pests in Kenya. J. Stored Prod. Res. 2013, 53, 27–36. [Google Scholar] [CrossRef]
- Njoroge, A.W.; Affognon, H.D.; Mutungi, C.M.; Manono, J.; Lamuka, P.O.; Murdock, L.L. Triple bag hermetic storage delivers a lethal punch to Prostephanus truncatus (Horn) (Coleoptera: Bostrichidae) in stored maize. J. Stored Prod. Res. 2014, 58, 12–19. [Google Scholar] [CrossRef]
- Ng’ang’a, J.; Mutungi, C.; Imathiu, S.M.; Affognon, H. Low permeability triple-layer plastic bags prevent losses of maize caused by insects in rural on-farm stores. Food Secur. 2016, 8, 621–633. [Google Scholar] [CrossRef]
- Likhayo, P.; Bruce, A.Y.; Mutambuki, K.; Tefera, T.; Mueke, J. On-farm evaluation of hermetic technology against maize storage pests in Kenya. J. Econ. Entomol. 2016, 109, 1943–1950. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.; Schulten, G. Losses caused by insects, mites, and microorganisms. In Postharvest Grain Loss Assessment Methods; Harris, K., Lindblad, C., Eds.; American Association of Cereal Chemists: New York, NY, USA, 1978; pp. 83–95. [Google Scholar]
- Markham, R.H.; Bosque-Perez, N.; Borgemeister, C.; Meikle, W.D. Developing pest management strategies for the maize weevil, Sitophilus zeamais, and the larger grain borer, Prostephanus truncatus, in the humid and sub-humid tropics. FAO Plant Prot. Bull. 1994, 42, 97–116. [Google Scholar]
- Rees, D.P. Insects of Stored Products; CSIRO Publishing: Collingwood, Australia, 2004. [Google Scholar]
- Hagstrum, D.W.; Phillips, T.W.; Cuperus, G. Stored Product Protection; Kansas State University: Manhattan, AR, USA, 2012; ISBN 978-0-9855003-0-6. [Google Scholar]
- Phiri, N.A.; Otieno, G. Managing Pests of Stored Maize in Kenya, Malawi and Tanzania; Survey Report; The MDG Centre East & Southern Africa: Nairobi, Kenya, 2008; 82p. [Google Scholar]
- Hertlein, M.B.; Thompson, G.D.; Subramanyam, B.; Athanassiou, C.G. Spinosad: A new natural product for stored grain protection. J. Stored Prod. Res. 2011, 47, 131–146. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. The State of the Food Insecurity in the World; Food and Agriculture Organization: Rome, Italy, 2004; Available online: www.fao.org/3/a-y5650e.pdf (accessed on 10 March 2019).
- Mboya, R.M. An investigation of the extent of infestation of stored maize by insect pests in Rungwe district, Tanzania. Food Secur. 2013, 5, 525–531. [Google Scholar] [CrossRef]
- Quezada, M.Y.; Moreno, J.; Varqueze, M.E.; Mondoza, M.; Mendeze-Albores, A.; Moreno-Martineze, E. Hermetic storage system preventing the proliferation of Prostephanus truncatus Horn and storage fungi in maize with different moisture contents. Postharvest. Biol. Technol. 2006, 39, 321–326. [Google Scholar] [CrossRef]
- Villers, P.; Navarro, S.; De Bruin, T.D. Development of hermetic storage technology in sealed flexible storage structures. In Proceedings of the 8th International Conference on Controlled Atmosphere and Fumigation in Stored Products, Chengdu, China, 21–26 September 2008. [Google Scholar]
- Jones, M.; Alexander, C.; Lowenberg-DeBoer, J. Profitability of Hermetic Purdue Improved Cowpea Storage (PICS) Bags for African Common Bean Producers Working Paper No. 11–13; Dept. of Agricultural Economics, Purdue University West Lafayette: West Lafayette, IN, USA, 2011. [Google Scholar]
- De Bruin, T.; Villers, P.; Wagh, A.; Navarro, S. Worldwide use of hermetic storage for the preservation of agricultural products. In Proceedings of the 9th International Controlled Atmosphere and Fumigation Conference, Antalya, Turkey, 15–19 October 2012. [Google Scholar]
- Fintrac. Smallholder grain storage in sub-Saharan Africa: A case study on hermetic storage technology commercialization in Kenya. Fintrac Top. Pap. Vol. 2016, 3, 1–8. [Google Scholar]
- Murdock, L.I.; Margam, V.; Baoua, I.; Balfe, S.; Shade, R.E. Death by desiccation: Effects of hermetic storage on cowpea bruchids. J. Stored Prod. Res. 2012, 49, 166–170. [Google Scholar] [CrossRef]
- Baoua, I.B.; Amadou, L.; Margam, V.; Murdock, L.I. Comparative evaluation of six storage methods for postharvest preservation of cowpea grain. J. Stored Prod. Res. 2012, 49, 171–175. [Google Scholar] [CrossRef]
- Kharel, K.; Mason, L.J.; Williams, S.B.; Murdock, L.L. A time-saving method for sealing Purdue Improved Crop Storage (PICS) bags. J. Stored Prod. Res. 2018, 77, 106–111. [Google Scholar] [CrossRef]
- Baoua, I.B.; Margam, V.; Amadou, L.; Murdock, L.I. Performance of triple bagging hermetic technology for postharvest storage of cowpeas in Niger. J. Stored Prod. Res. 2012, 51, 81–85. [Google Scholar] [CrossRef]
- Hell, K.; Ognakossan, E.; Lamboni, Y. PICS hermetic storage bags ineffective in controlling infestations of Prostephanus truncatus and Dinoderus spp. in traditional cassava chips. J. Stored Prod. Res. 2014, 58, 53–58. [Google Scholar] [CrossRef]
- Ognakossan, K.E.; Tounou, A.K.; Lamboni, Y.; Hell, K. Postharvest insect infestation in maize grain stored in woven polypropylene and in hermetic bags. Int. J. Trop. Insect Sci. 2013, 33, 71–81. [Google Scholar] [CrossRef]
- Chigoverah, A.A.; Mvumi, B.M. Efficacy of metal silos and hermetic bags against stored-maize insect pests under simulated smallholder farmer conditions. J. Stored Prod. Res. 2016, 69, 179–189. [Google Scholar] [CrossRef]
- Denloye, A.A.; Tesilim, K.O.; Negbenebor, H.; Makanjuola, W.A. Assessment of the efficacy of Actellic and Sumithion in protecting grains from insect infestation during storage. J. Entomol. 2008, 5, 24–30. [Google Scholar]
- Likhayo, P.; Tefera, T.; Mugo, S.; Mueke, J. Combined effect of hermetic bag and insect resistant variety for the control of larger grain borer and maize weevil in stored maize. Int. J. Sci. Res. 2015, 4, 2305–2313. [Google Scholar]
- Meikle, W.G.; Markham, R.H.; Nansen, C.; Holst, N.; Degbey, P.; Azoma, K.; Korie, S. Pest management in traditional maize stores in West Africa: A farmer’s perspective. J. Econ. Entomol. 2002, 95, 1079–1088. [Google Scholar] [CrossRef]
- Compton, J.A.F.; Floyd, S.; Magrath, P.A.; Addo, S.; Gbedevi, S.R.; Agbo, B.; Bokor, G.; Amekupe, S.; Motey, Z.; Penni, H.; et al. Involving grain traders in determining the effect of postharvest insect damage on the price of maize in African markets. Crop Prot. 1998, 17, 483–489. [Google Scholar] [CrossRef]
- Kadjo, D.; Ricker-Gilbert, J.; Alexander, C. Estimating price discounts for low-quality maize in Sub-Saharan Africa: Evidence from Benin. World Dev. 2016, 77, 115–128. [Google Scholar] [CrossRef]
- Boxall, R. Damage and loss caused by the larger grain borer Prostephanus truncatus. Integr. Pest Manag. Rev. 2002, 7, 105–121. [Google Scholar] [CrossRef]
- Abdullahi, C.; Muhamad, R.; Dzolkhifli, O.; Sinniah, U.R. Damage potential of Tribolium casteneum (Herbst) (Coleoptera: Tenebrionidae) on cocoa beans: Effect of initial adult population density and post infestation storage time. J. Stored Prod. Res. 2018, 75, 1–9. [Google Scholar] [CrossRef]

| Treatments | Storage Period (Weeks) | |||
|---|---|---|---|---|
| 0 | 8 | 16 | 24 | |
| PICS + U | 12.5 ± 0.3a | 12.7 ± 0.6a | 12.6 ± 0.2bc | 12.8 ± 0.5ab |
| PICS + I | 12.9 ± 0.3a | 13.0 ± 0.2a | 12.9 ± 0.1c | 13.3 ± 0.2b |
| PP + A | 12.7 ± 0.2a | 12.3 ± 0.1a | 12.1 ± 0.2ab | 12.1 ± 0.3ab |
| PP + U | 12.5 ± 0.1a | 12.6 ± 0.0a | 12.6 ± 0.1bc | 12.0 ± 0.2ab |
| Jute + A | 13.0 ± 0.3a | 12.8 ± 0.1a | 12.2 ± 0.2ab | 11.8 ± 0.1a |
| Jute + U | 12.4 ± 0.4a | 12.4 ±0.0a | 11.7 ± 0.2a | 12.6 ± 0.3ab |
| Mean | 12.6 | 12.6 | 12.3 | 12.4 |
| F5, 15-value | 0.88 | 1.15 | 10.59 | 3.47 |
| P-value | 0.518 | 0.376 | <0.001 | <0.001 |
| Treatments | Storage Period (Weeks) | |||
|---|---|---|---|---|
| 0 | 8 | 16 | 24 | |
| PICS + U | 2.6 ± 0.1a | 2.7 ± 0.1a | 2.9 ± 0.1a | 2.9 ± 0.1a |
| PICS + I | 2.5 ± 0.6a | 3.0 ± 0.7a | 3.1 ± 0.6a | 3.4 ± 0.7a |
| PP + A | 3.3 ± 0.1a | 3.2 ± 0.5a | 7.0 ± 1.6a | 18.1 ± 1.4a |
| PP + U | 3.3 ± 0.2a | 3.5 ± 0.6a | 9.5 ± 4.3a | 100.0 ± 13.8b |
| Jute + A | 2.3 ± 0.1a | 2.5 ± 0.2a | 6.0 ± 0.9a | 33.9 ± 1.8a |
| Jute + U | 2.4 ± 0.2a | 3.7 ± 0.5a | 57.2 ± 17.0b | 116.9 ± 9.6b |
| Mean | 2.7 | 3.1 | 14.3 | 45.9 |
| F5,15-value | 2.14 | 1.23 | 8.49 | 51.00 |
| P-value | 0.116 | 0.341 | <0.001 | <0.001 |
| Treatments | Storage Period (Weeks) | |||
|---|---|---|---|---|
| 0 | 8 | 16 | 24 | |
| PICS + U | 1 ± 0a | 0 ± 0a | 2 ± 1a | 15 ± 3a |
| PICS + I | 0 ± 0a | 1 ± 1ab | 1 ± 1a | 17 ± 5a |
| PP + A | 0 ± 0a | 9 ± 4c | 22 ± 2b | 171 ± 1b |
| PP + U | 0 ± 0a | 3 ± 1abc | 50 ± 1c | 545 ± 11d |
| Jute + A | 0 ± 0a | 7 ± 1c | 43 ± 6b | 136 ± 19b |
| Jute + U | 0 ± 0a | 5 ± 1bc | 431 ± 3d | 327 ± 2c |
| Mean | 0 | 4 | 91 | 202 |
| F5,15-value | 2.25 | 8.93 | 30.00 | 42.88 |
| P-value | 0.103 | <0.001 | <0.01 | <0.001 |
| Treatments | Storage Period (Weeks) | ||||
|---|---|---|---|---|---|
| 0 | 8 | 16 | 24 | ||
| PICS + U | 4.8 ± 0.2a | 6.0 ± 0.5a | 4.3 ± 0.7a | 8.8 ± 0.9a | |
| PICS + I | 6.0 ± 0.1a | 7.7 ± 0.3ab | 8.4 ± 0.4b | 11.6 ± 1.3a | |
| PP + A | 5.4 ± 0.4a | 8.9 ± 0.4b | 4.9 ± 0.9c | 93.8 ± 2.7b | |
| PP + U | 4.9 ± 0.4a | 8.5 ± 0.1ab | 40.7 ± 1.2d | 95.9 ± 1.0b | |
| Jute + A | 5.8 ± 0.5a | 9.2 ± 1.1b | 38.0 ± 1.8d | 92.6 ± 3.2b | |
| Jute + U | 6.8 ± 1.0a | 7.9 ± 0.6ab | 73.8 ± 1.8e | 97.8 ± 0.4b | |
| Mean | 5.6 | 8.0 | 31.7 | 66.7 | |
| F5,15-value | 1.73 | 3.58 | 390.62 | 629.76 | |
| P-value | 0.187 | 0.025 | <0.001 | <0.001 | |
| Treatments | Storage Period (Weeks) | |||
|---|---|---|---|---|
| 0 | 8 | 16 | 24 | |
| PICS + U | 0.5 ± 0.2a | 0.5 ± 0.0ab | 0.6 ± 0.2a | 2.4 ± 1.2a |
| PICS + I | 0.3 ± 0.0a | 0.4 ± 0.0a | 1.1 ± 0.2a | 2.9 ± 1.0a |
| PP + A | 0.4 ± 0.1a | 0.8 ± 0.1abc | 11.4 ± 0.6b | 39.7 ± 1.2b |
| PP + U | 0.6 ± 0.2a | 1.1 ± 0.2bc | 16.6 ± 1.1c | 40.6 ± 0.5b |
| Jute + A | 0.5 ± 0.1a | 1.6 ± 0.2c | 15.4 ± 1.1bc | 39.2 ± 1.4b |
| Jute + U | 0.5 ± 0.1a | 1.1 ± 0.3abc | 29.5 ± 1.7d | 41.5 ± 0.2b |
| Mean | 0.5 | 0.9 | 12.4 | 27.7 |
| F5,15 -value | 1.64 | 42.13 | 76.20 | 11.27 |
| P-value | 0.209 | <0.001 | <0.001 | <0.001 |
| Number of Live Insects and Weight of Dust | Number of Live Insects and Grain Damage | Number of Live Insects and Weight Loss | ||||
|---|---|---|---|---|---|---|
| Treatment | Correlation | P-Value | Correlation | P-Value | Correlation | P-Value |
| PICS + U | 0.133 | 0.623 | 0.682 | 0.004 | 0.834 | <0.001 |
| PICS + I | 0.317 | 0.231 | 0.700 | 0.002 | 0.854 | <0.001 |
| PP + A | 0.855 | <0.001 | 0.920 | <0.001 | 0.922 | <0.001 |
| PP + U | 0.861 | <0.001 | 0.972 | <0.001 | 0.961 | <0.001 |
| Jute + A | 0.841 | <0.001 | 0.917 | <0.001 | 0.935 | <0.001 |
| Jute + U | 0.971 | <0.001 | 0.954 | <0.001 | 0.963 | <0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mutambuki, K.; Affognon, H.; Likhayo, P.; Baributsa, D. Evaluation of Purdue Improved Crop Storage Triple Layer Hermetic Storage Bag against Prostephanus truncatus (Horn) (Coleoptera: Bostrichidae) and Sitophilus zeamais (Motsch.) (Coleoptera: Curculionidae). Insects 2019, 10, 204. https://doi.org/10.3390/insects10070204
Mutambuki K, Affognon H, Likhayo P, Baributsa D. Evaluation of Purdue Improved Crop Storage Triple Layer Hermetic Storage Bag against Prostephanus truncatus (Horn) (Coleoptera: Bostrichidae) and Sitophilus zeamais (Motsch.) (Coleoptera: Curculionidae). Insects. 2019; 10(7):204. https://doi.org/10.3390/insects10070204
Chicago/Turabian StyleMutambuki, Kimondo, Hippolyte Affognon, Paddy Likhayo, and Dieudonne Baributsa. 2019. "Evaluation of Purdue Improved Crop Storage Triple Layer Hermetic Storage Bag against Prostephanus truncatus (Horn) (Coleoptera: Bostrichidae) and Sitophilus zeamais (Motsch.) (Coleoptera: Curculionidae)" Insects 10, no. 7: 204. https://doi.org/10.3390/insects10070204
APA StyleMutambuki, K., Affognon, H., Likhayo, P., & Baributsa, D. (2019). Evaluation of Purdue Improved Crop Storage Triple Layer Hermetic Storage Bag against Prostephanus truncatus (Horn) (Coleoptera: Bostrichidae) and Sitophilus zeamais (Motsch.) (Coleoptera: Curculionidae). Insects, 10(7), 204. https://doi.org/10.3390/insects10070204





