Aseptic Rearing and Infection with Gut Bacteria Improve the Fitness of Transgenic Diamondback Moth, Plutella xylostella
Abstract
:1. Introduction
2. Materials and Methods
3. Results
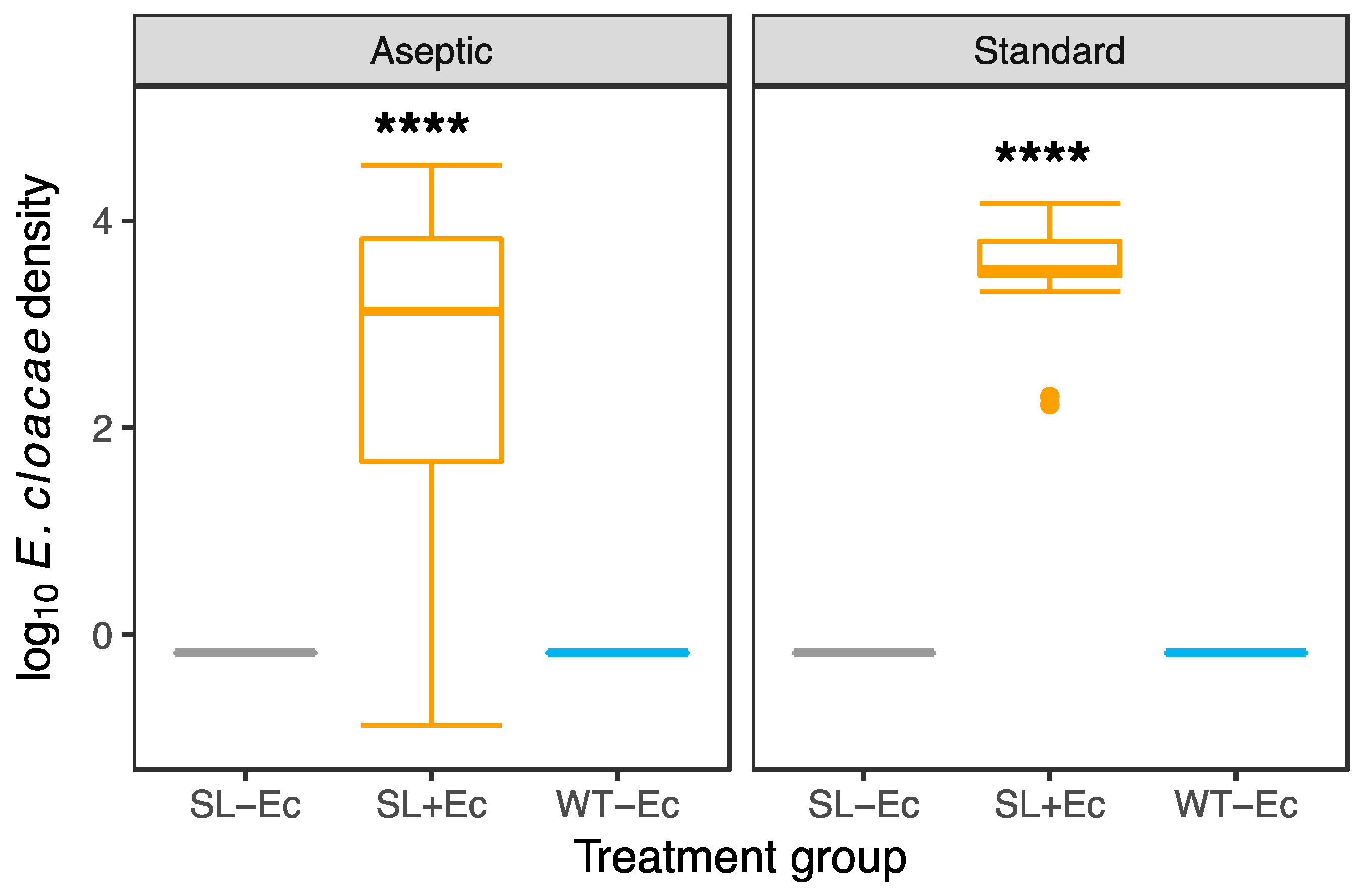
3.1. Bacteria
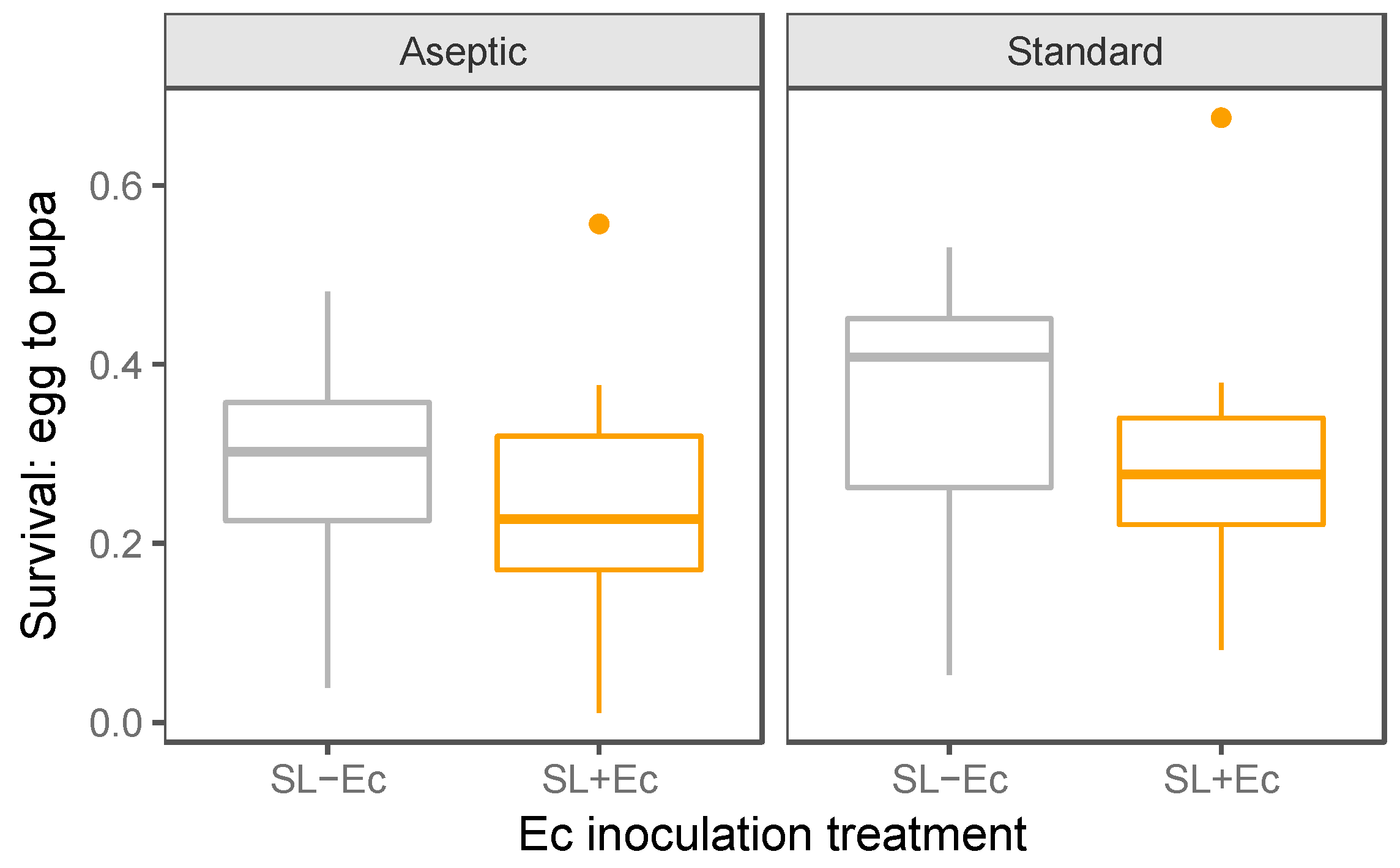
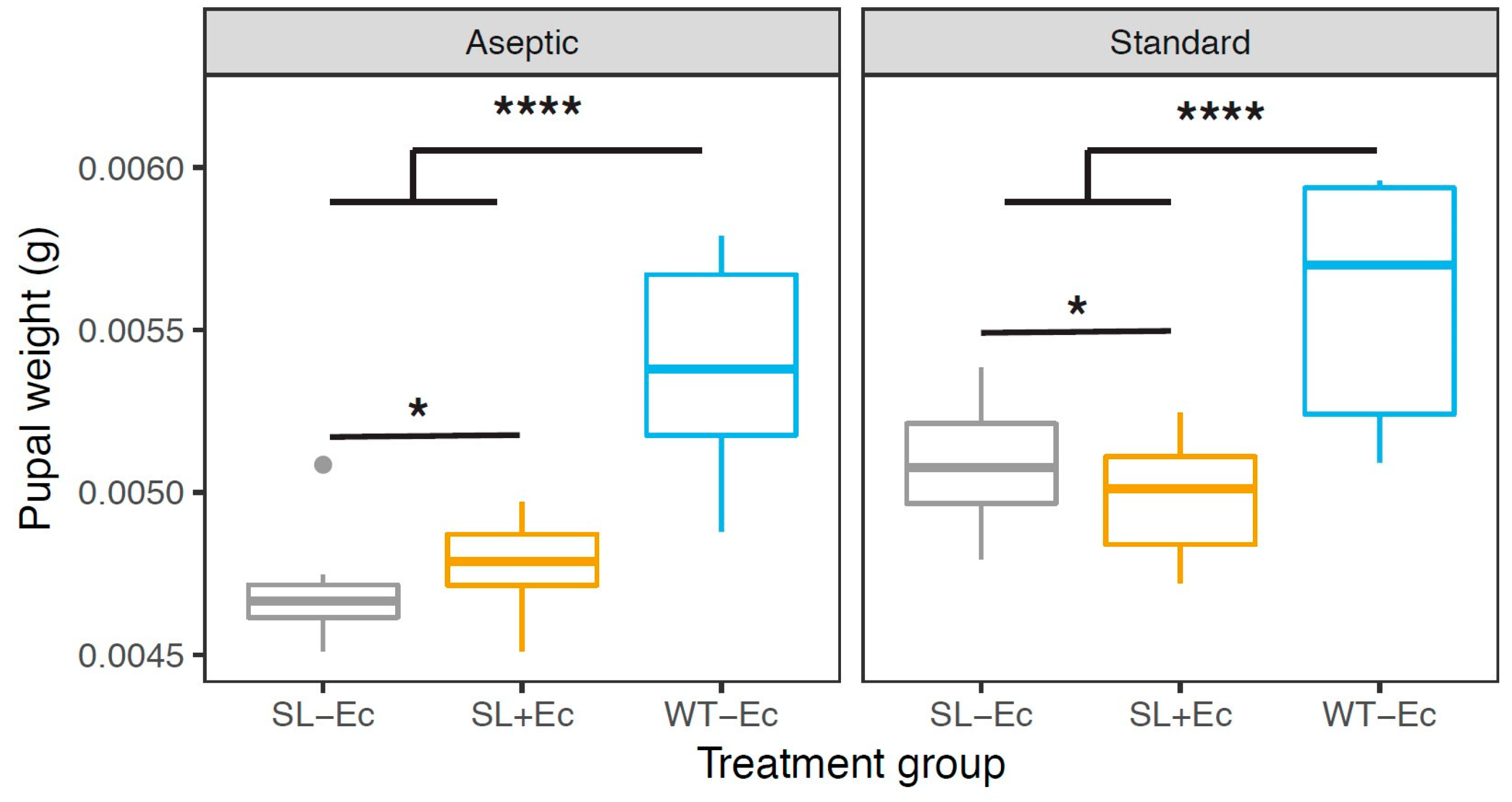
3.2. Development and Weight Gain
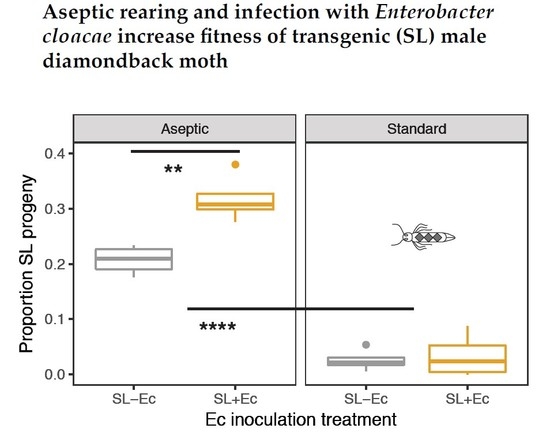
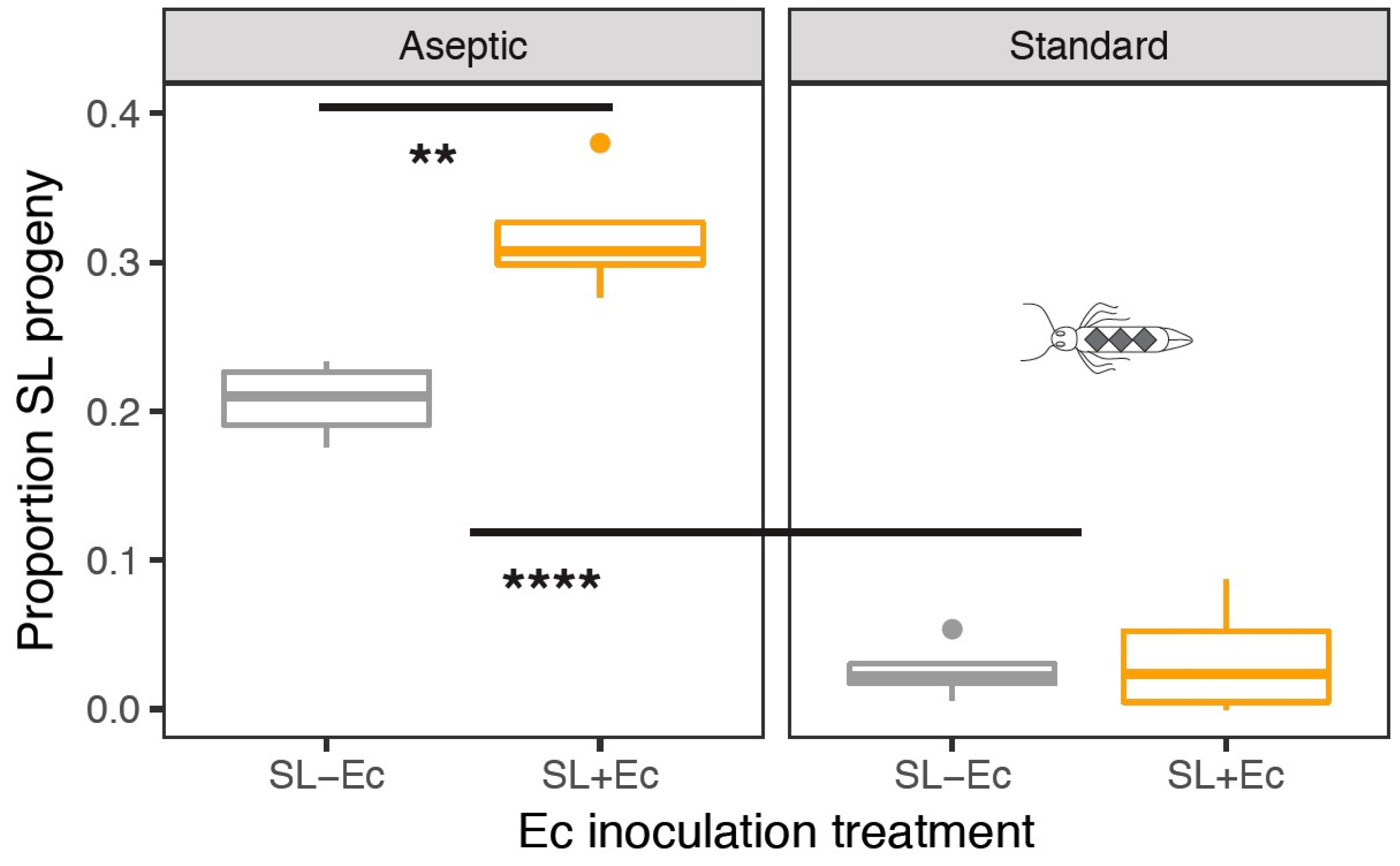
3.3. Fitness in Mate Competition Experiments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Onstad, D.W. Insect Resistance Management, 2nd ed.; Academic Press: New York, NY, USA, 2014. [Google Scholar]
- Heinrich, J.C.; Scott, M.J. A repressible female-specific lethal genetic system for making transgenic insect strains suitable for a sterile-release program. Proc. Natl. Acad. Sci. USA 2000, 97, 8229–8232. [Google Scholar] [CrossRef] [PubMed]
- Van Lenteren, J.C.; van Roermund, H.J.W.; Sutterlin, S. Biological control of greenhouse whitefly (Trialeurodes vaporariorum) with the parasitoid Encarsia formosa: How does it work? Biol. Control 1996, 6, 1–6. [Google Scholar] [CrossRef]
- Lindquist, A.W. The Use of gamma radiation for control or eradication of the screw-worm. J. Econ. Entomol. 1955, 48, 467–469. [Google Scholar] [CrossRef]
- Alphey, L.; Benedict, M.; Bellini, R.; Clark, G.G.; Dame, D.A.; Service, M.W.; Dobson, S.L. Sterile-insect methods for control of mosquito-borne diseases: An analysis. Vector-Borne Zoon. Dis. 2010, 10, 295–311. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, M. In vivo mass production of insect viruses for use as pesticides. In Microbial and Viral Pesticides; Kurstak, E., Ed.; Marcel Dekker: New York, NY, USA, 1982; pp. 463–492. [Google Scholar]
- Rumpold, B.A.; Schluter, O.K. Potential and challenges of insects as an innovative source for food and feed production. Innov. Food Sci. Emerg. Technol. 2013, 17, 1–11. [Google Scholar] [CrossRef]
- Dyck, V.A.; Hendrichs, J.; Robinson, A.S. Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management; Springer: Dordrecht, The Netherlands, 2005. [Google Scholar]
- Morlan, H.B.; McCray, E.M., Jr.; Kilpatrick, J.W. Field tests with sexually sterile males for control of Aedes aegypti. Mosquito News 1962, 22, 295–300. [Google Scholar]
- Gossen, M.; Bujard, H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. USA 1992, 89, 5547–5551. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.D.; Donnelly, C.A.; Wood, R.J.; Alphey, L.S. Insect population control using a dominant, repressible, lethal genetic system. Science 2000, 287, 2474–2476. [Google Scholar] [CrossRef]
- Fu, G.; Condon, K.C.; Epton, M.J.; Gong, P.; Jin, L.; Condon, G.C.; Morrison, N.I.; Dafa’alla, T.H.; Alphey, L. Female-specific insect lethality engineered using alternative splicing. Nat. Biotechnol. 2007, 25, 353–357. [Google Scholar] [CrossRef]
- Jin, L.; Walker, A.S.; Fu, G.; Harvey-Samuel, T.; Dafa’alla, T.; Miles, A.; Marubbi, T.; Granville, D.; Humprey-Jones, N.; O’Connell, S.; et al. Engineered female- specific lethality for control of pest Lepidoptera. ACS Synth. Biol. 2013, 2, 160–166. [Google Scholar] [CrossRef]
- Horn, C.; Schmid, B.G.; Pogoda, F.S.; Wimmer, E.A. Fluorescent transformation markers for insect transgenesis. Insect Biochem. Mol. Boil. 2002, 32, 1221–1235. [Google Scholar] [CrossRef]
- Carvalho, D.O.; McKemey, A.R.; Garziera, L.; Lacroix, R.; Donnelly, C.A.; Alphey, L.; Malavasi, A.; Capurro, M.L. Suppression of a field population of Aedes aegypti in Brazil by sustained release of transgenic male mosquitoes. PLoS Negl. Trop. Dis. 2015, 9, e0003864. [Google Scholar] [CrossRef]
- Harris, A.F.; McKemey, A.R.; Nimmo, D.; Curtis, Z.; Black, I.; Morgan, S.A.; Neira Oviedo, M.; Lacroix, R.; Naish, N.; Morrison, N.; et al. Successful suppression of a field mosquito population by release of engineered male mosquitoes. Nat. Biotechnol. 2012, 30, 828–830. [Google Scholar] [CrossRef]
- Gorman, K.; Young, J.; Pineda, L.; Márquez, R.; Sosa, N.; Bernal, D.; Torres, R.; Soto, Y.; Lacroix, R.; Naish, N.; et al. Short-term suppression of Aedes aegypti using genetic control does not facilitate Aedes albopictus. Pest Manag. Sci. 2016, 72, 618–628. [Google Scholar] [CrossRef]
- Phuc, K.H.; Andreasen, M.H.; Burton, R.S.; Vass, C.; Epton, M.J.; Pape, G.; Guoliang, F.; Condon, K.C.; Scaife, S.; Donnelly, C.A.; et al. Late-acting dominant lethal genetic systems and mosquito control. BMC Biol. 2007, 5, 11. [Google Scholar] [CrossRef]
- Irvin, N.; Hoddle, M.S.; O’Brochta, D.A.; Carey, B.; Atkinson, P.W. Assessing fitness costs for transgenic Aedes aegypti expressing the GFP marker and transposase genes. Proc. Natl. Acad. Sci. USA 2004, 101, 891–896. [Google Scholar] [CrossRef]
- Hoffman, A.A.; Ross, P.A. Rates and patterns of laboratory adaptation in (mostly) insects. J. Econ. Entomol. 2018, 111, 501–509. [Google Scholar] [CrossRef]
- Zhou, L.; Alphey, N.; Walker, A.S.; Travers, L.M.; Hasan, F.; Morrison, N.; Bonsall, M.B.; Raymond, B.R. Combining the high- dose/refuge strategy and self- limiting transgenic insects in resistance management—a test in experimental mesocosms. Evol. Appl. 2018, 11, 727–738. [Google Scholar] [CrossRef]
- Raymond, B.; Johnston, P.R.; Wright, D.J.; Ellis, R.J.; Crickmore, N.; Bonsall, M.B. A mid-gut microbiota is not required for the pathogenicity of Bacillus thuringiensis to diamondback moth larvae. Environ. Microbiol. 2009, 11, 2556–2563. [Google Scholar] [CrossRef]
- Leftwich, P.T.; Bolton, M.; Chapman, T. Evolutionary biology and genetic techniques for insect control. Evol. Appl. 2015, 9, 212–230. [Google Scholar] [CrossRef]
- Behar, A.; Jurkevitch, E.; Yuval, B. Bringing back the fruit into fruit fly–bacteria interactions. Mol. Ecol. 2008, 17, 1375–1386. [Google Scholar] [CrossRef] [PubMed]
- Ben Ami, E.; Yuval, B.; Jurkevitch, E. Manipulation of the microbiota of mass-reared Mediterranean fruit flies Ceratitis capitata (Diptera: Tephritidae) improves sterile male sexual performance. ISME J. 2010, 4, 8–37. [Google Scholar] [CrossRef]
- Chandler, J.A.; Lang, J.M.; Bhatnagar, S.; Eisen, J.A.; Kopp, A. Bacterial communities of diverse Drosophila species: Ecological context of a host-microbe model system. PLoS Genet. 2011, 7, e1002272. [Google Scholar] [CrossRef]
- Dillon, R.J.; Dillon, V.M. The gut bacteria of insects: Nonpathogenic interactions. Annu. Rev. Entomol. 2004, 49, 71–92. [Google Scholar] [CrossRef]
- Engel, P.; Moran, N.A. The gut microbiota of insects: Diversity in structure and function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar] [CrossRef]
- Cruden, D.L.; Markovertz, A.J. Microbial ecology of the cockroach gut. Ann. Rev. Microbiol. 1987, 41, 617–643. [Google Scholar] [CrossRef]
- Xia, X.; Gurr, G.M.; Vasseur, L.; Zheng, D.; Zhong, H.; Qin, B.; Lin, J.; Wang, Y.; Song, F.Q.; Li, Y.; et al. Metagenomic Sequencing of Diamondback Moth Gut Microbiome Unveils Key Holobiont Adaptations for Herbivory. Front. Microbiol. 2017, 8, 663. [Google Scholar] [CrossRef]
- Dillon, R.J.; Vennard, C.T.; Charnley, A.K. Pheromones: Exploitation of gut bacteria in the locust. Nature 2000, 403, 851. [Google Scholar] [CrossRef]
- Ridley, E.V.; Wong, A.C.-N.; Westmiller, S.; Douglas, A.E. Impact of the resident microbiota on the nutritional phenotype of Drosophila melanogaster. PLoS ONE 2012, 7, e36765. [Google Scholar] [CrossRef] [PubMed]
- Leftwich, P.T.; Clarke, N.V.E.; Hutchings, M.I.; Chapman, T. Gut microbiomes and reproductive isolation in Drosophila. Proc. Natl. Acad. Sci. USA 2012, 114, 12767–12772. [Google Scholar] [CrossRef]
- Watanabe, K.; Abe, K.; Sato, M. Biological control of an insect pest by gut-colonizing Enterobacter cloacae transformed with ice nucleation gene. J. Appl. Microbiol. 2000, 88, 90–97. [Google Scholar] [CrossRef]
- Lauzon, C.R.; Sjogren, R.E.; Wright, S.E.; Prokopy, R.J. Attraction of Rhagoletis pomonella (Diptera: Tephritidae) flies to odor of bacteria: Apparent confinement to specialized members of Enterobacteriaceae. Environ. Entomol. 1998, 27, 853–857. [Google Scholar] [CrossRef]
- Martins, S.; Naish, N.; Walker, A.S.; Morrison, N.I.; Scaife, S.; Dafa’alla, T.; Alphey, L. Germline transformation of the diamondback moth, Plutella xylostella L.; using the piggyBac transposable element. Insect Mol. Biol. 2012, 21, 414–421. [Google Scholar] [CrossRef]
- Augustinos, A.A.; Kyritsis, G.A.; Papadopoulos, N.T.; Abd-Alla, A.M.; Cáceres, C.; Bourtzis, K. Exploitation of the medfly gut microbiota for the enhancement of sterile insect technique: Use of Enterobacter sp. in larval diet-based probiotic applications. PLoS ONE 2015, 10, e0136459. [Google Scholar] [CrossRef]
- Hammer, T.J.; Janzen, D.H.; Hallwachs, W.; Jaffe, S.P.; Fierer, N. Caterpillars lack a resident gut microbiome. Proc. Natl. Acad. Sci. USA 2017, 114, 9641–9646. [Google Scholar] [CrossRef]
- Matthews, A.; Mikonranta, L.; Raymond, B. Shifts along the parasite-mutualism continuum are opposed by fundamental trade-offs. Proc. R. Soc. B 2019, (in press). [Google Scholar] [CrossRef]
- Hochberg, M.E.; Gomulkiewicz, R.; Holt, R.D.; Thompson, J.N. Weak sinks could cradle mutualistic symbiosis: Strong sources should harbour parasitic symbiosis. J. Evol. Biol. 2000, 13, 213–222. [Google Scholar] [CrossRef]
- De Vries, E.J.; Jacobs, G.; Sabelis, M.W.; Meneken, S.B.J.; Breeuwer, J.A.J. Diet-dependant effects of gut bacteria on their insect host: The symbiosis of Erwinia sp. and western follower thrips. Proc. R. Soc. Lond. B 2004, 271, 2171–2178. [Google Scholar] [CrossRef]
- Schmeisser, C.; Steele, H.; Streit, W.R. Metagenomics, biotechnology with non-culturable microbes. Appl. Microbiol. Biotechnol. 2007, 75, 955–962. [Google Scholar] [CrossRef]
- Boman, H.G.; Hultmark, D. Cell-free immunity in insects. Annu. Rev. Microbiol. 1987, 41, 103–126. [Google Scholar] [CrossRef]
- Behar, A.; Yuval, B.; Jurkevitch, E. Enterobacteria-mediated nitrogen fixation in natural populations of the fruit fly Ceratitis capitata. Mol. Ecol. 2005, 14, 2637–2643. [Google Scholar] [CrossRef]
- Hageman, R.V.; Burris, R.H. Nitrogenase and nitrogenase reductase associate and dissociate with each catalytic cycle. Proc. Natl. Acad. Sci. USA 1978, 75, 2699–2702. [Google Scholar] [CrossRef]
- Dillon, R.; Charnley, K. Mutualism between the desert locust Schistocerca gregaria and its gut microbiota. Res. Microbiol. 2002, 153, 503–509. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef]
- Davie, L.C.; Jones, T.M.; Elgar, M.A. The role of chemical communication in sexual selection: Hair-pencil displays in the diamondback moth, Plutella xylostella. Anim. Behav. 2010, 79, 391–399. [Google Scholar] [CrossRef]
- Harvey-Samuel, T.; Ant, T.; Gong, H.; Morrison, N.I.; Alphey, L. Population-level effects of fitness costs associated with repressible female-lethal transgene insertions in two pest insects. Evol. Appl. 2014, 7, 597–606. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Somerville, J.; Zhou, L.; Raymond, B. Aseptic Rearing and Infection with Gut Bacteria Improve the Fitness of Transgenic Diamondback Moth, Plutella xylostella. Insects 2019, 10, 89. https://doi.org/10.3390/insects10040089
Somerville J, Zhou L, Raymond B. Aseptic Rearing and Infection with Gut Bacteria Improve the Fitness of Transgenic Diamondback Moth, Plutella xylostella. Insects. 2019; 10(4):89. https://doi.org/10.3390/insects10040089
Chicago/Turabian StyleSomerville, Jasmine, Liqin Zhou, and Ben Raymond. 2019. "Aseptic Rearing and Infection with Gut Bacteria Improve the Fitness of Transgenic Diamondback Moth, Plutella xylostella" Insects 10, no. 4: 89. https://doi.org/10.3390/insects10040089
APA StyleSomerville, J., Zhou, L., & Raymond, B. (2019). Aseptic Rearing and Infection with Gut Bacteria Improve the Fitness of Transgenic Diamondback Moth, Plutella xylostella. Insects, 10(4), 89. https://doi.org/10.3390/insects10040089