Exposure to Insecticides Reduces Populations of Rhynchophorus palmarum in Oil Palm Plantations with Bud Rot Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Concentration-Mortality Bioassay
2.3. Time-Mortality Bioassay
2.4. Insecticide Effects on Reproduction
2.5. Field Assays in Palm Trees with Bud Rot Disease
2.6. Statistical Analysis
3. Results
3.1. Toxicity
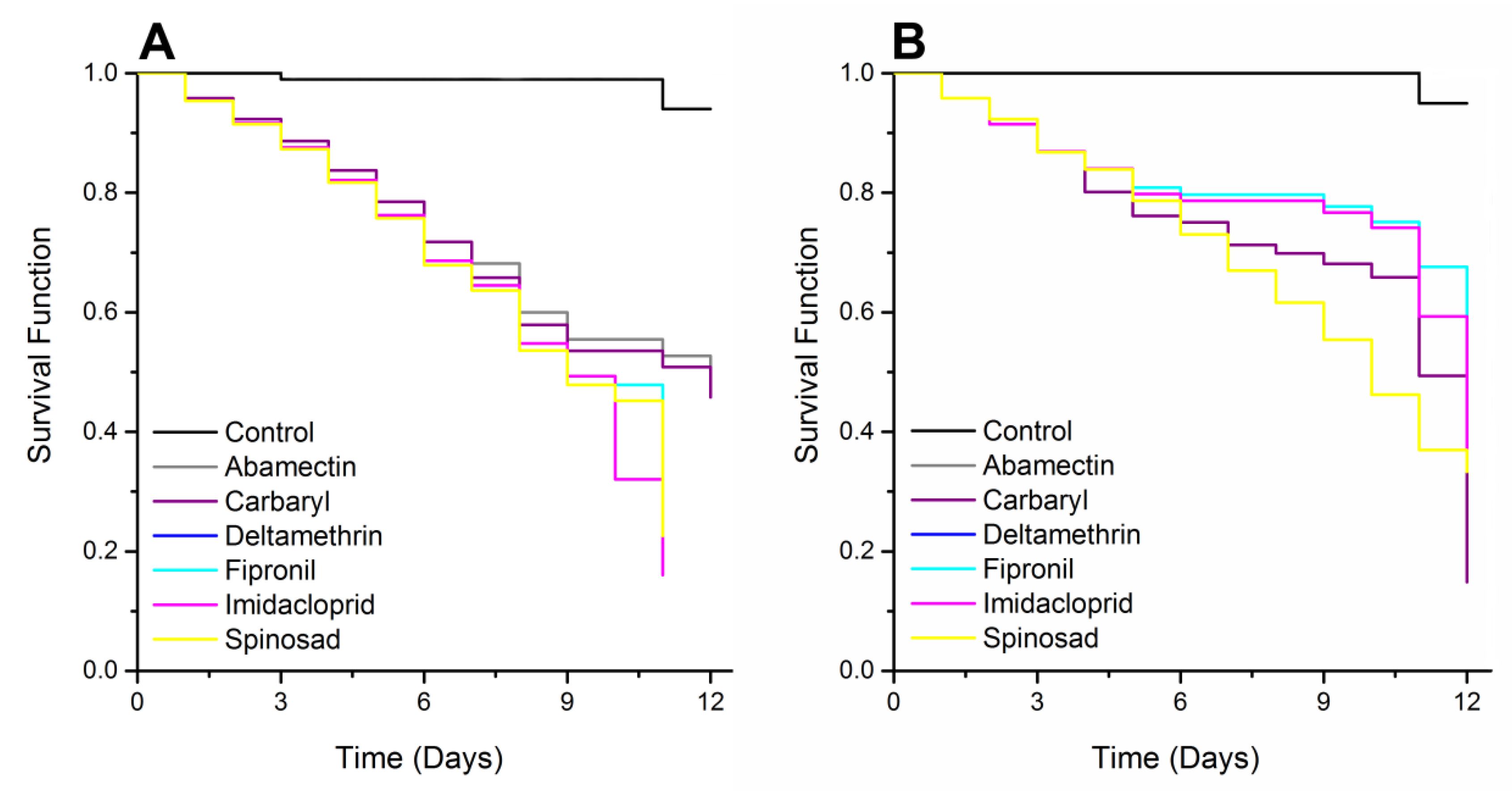
3.2. Survival Analysis
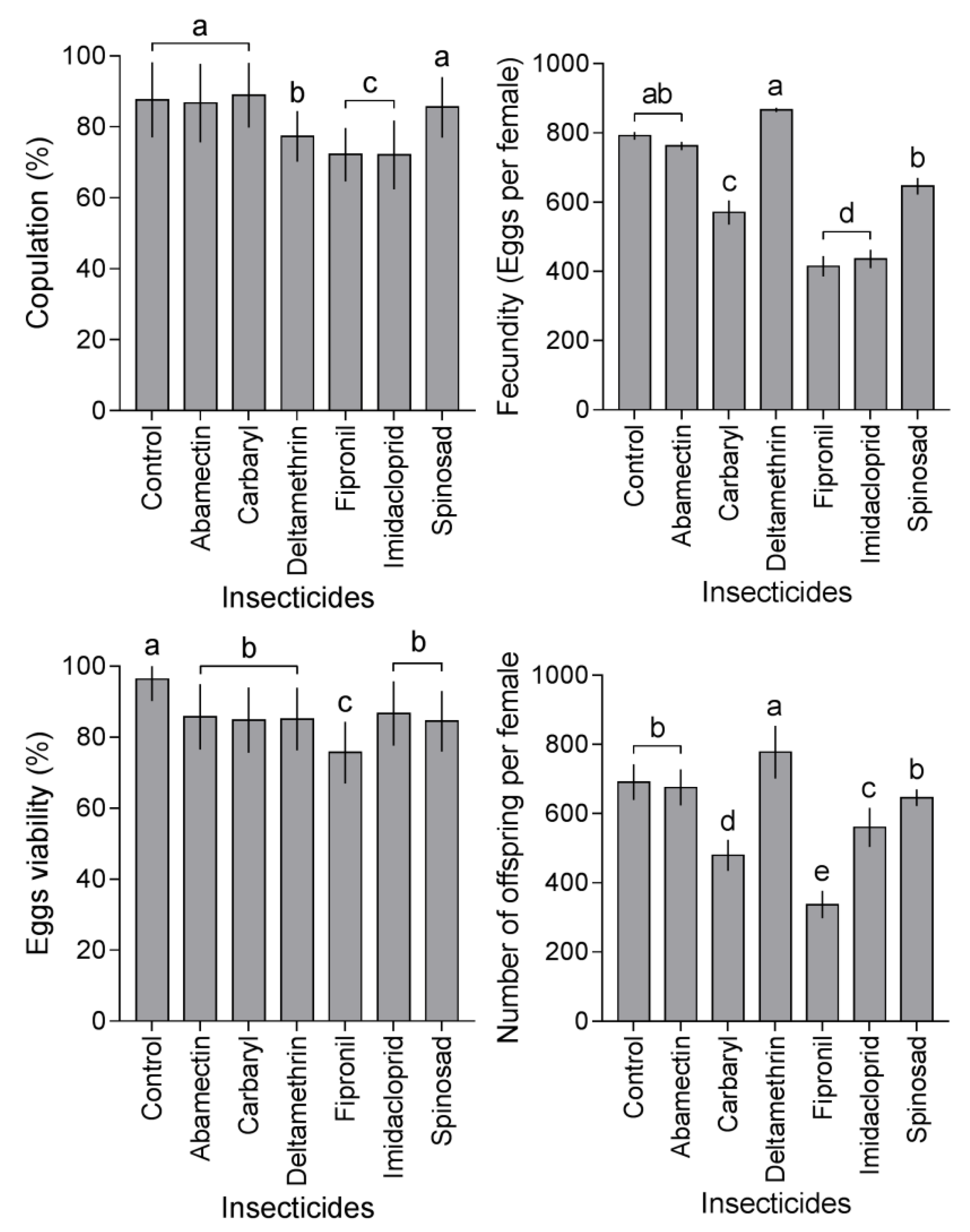
3.3. Insecticide Effects on Reproduction
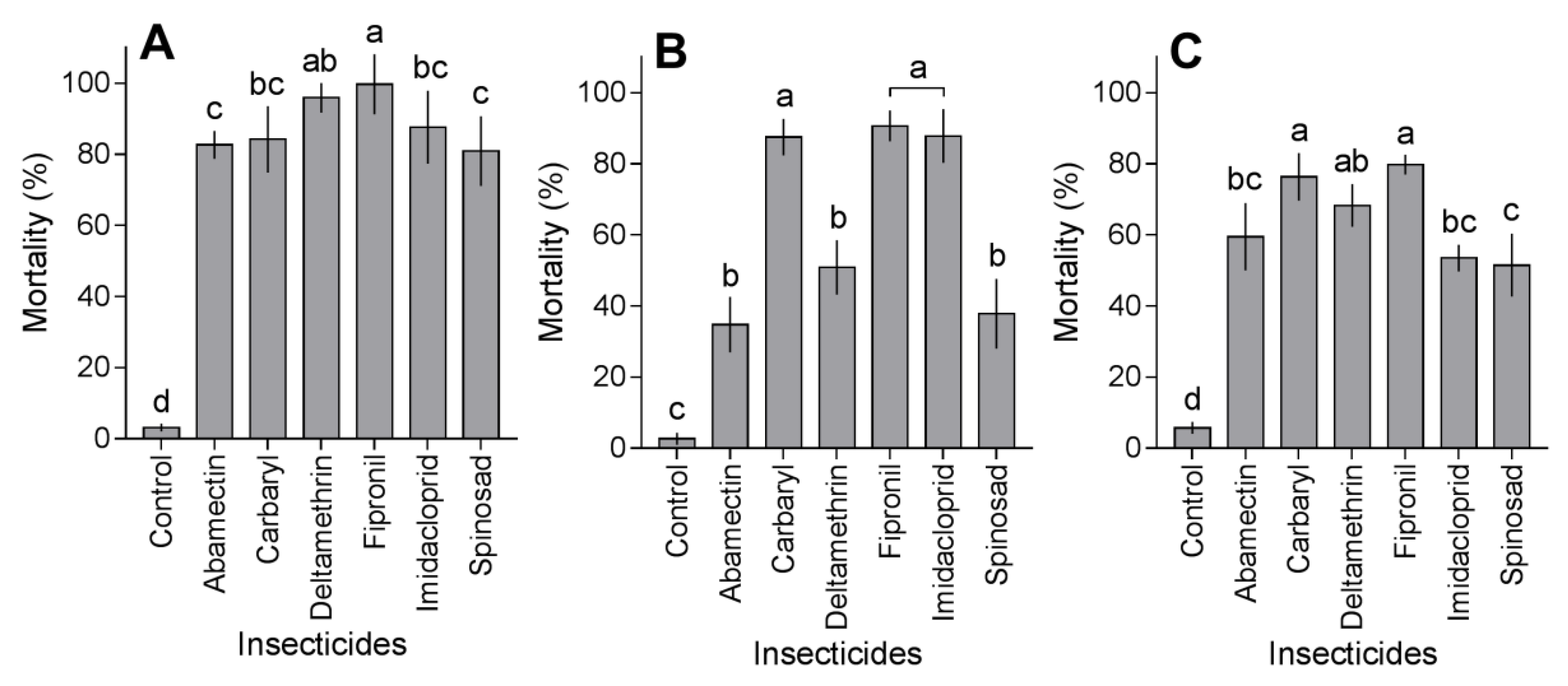
3.4. Mortality in Field Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Giblin-Davis, R.M.; Gerber, K.; Griffith, R. Laboratory rearing of Rhynchophorus cruentatus and R. palmarum (Coleoptera: Curculionidae). Fla. Entomol. 1989, 72, 480–488. [Google Scholar] [CrossRef]
- Jaffé, K.; Sánchez, P.; Cerda, H.; Hernández, J.V.; Jaffé, R.; Urdaneta, N.; Guerra, G.; Martínez, R.; Miras, B. Chemical ecology of the palm weevil Rhynchophorus palmarum (L.) (Coleoptera: Curculionidae): Attraction to host plants and to a male-produced aggregation pheromone. J. Chem. Ecol. 1993, 19, 1703–1720. [Google Scholar] [CrossRef]
- Hagley, E.A.C. The role of the palm weevil, Rhynchophorus palmarum as a vector of the red ring disease of coconuts. I. Results of preliminary investigations. J. Econ. Entomol. 1963, 56, 375–380. [Google Scholar] [CrossRef]
- Rochat, D.; Malosse, C.; Lettere, M.; Ducrot, P.H.; Zagatti, P.; Renou, M.; Descoins, C. Male-produced aggregation pheromone of the American palm weevil, Rhynchophorus palmarum (L.) (Coleoptera, Curculionidae): Collection, identification, electrophysiogical activity, and laboratory bioassay. J. Chem. Ecol. 1991, 17, 2127–2141. [Google Scholar] [CrossRef]
- Plata-Rueda, A.; Martínez, L.C.; Fernandes, F.L.; Ramalho, F.S.; Zanuncio, J.C.; Serrão, J.E. Interaction between the Bud Rot disease of oil palm and Rhynchophorus palmarum (Coleoptera: Curculionidae). J. Econ. Entomol. 2016, 109, 962–965. [Google Scholar] [CrossRef] [PubMed]
- Torres, G.A.; Sarria, G.A.; Varón, F.; Coffey, M.D.; Elliott, M.L.; Martínez, G. First report of bud rot caused by Phytophthora palmivora on African oil palm in Colombia. Plant Dis. 2010, 94, 1163. [Google Scholar] [CrossRef]
- De Franqueville, H. Oil palm bud rot in Latin America. Exp. Agric. 2003, 39, 225–240. [Google Scholar] [CrossRef]
- Swinburne, T.R. Fatal yellows, bud rot and spear rot of African oil palm—A comparison of the symptoms of these diseases in Brazil, Ecuador and Colombia. Planter 1993, 69, 15–23. [Google Scholar]
- Martínez, L.C.; Plata-Rueda, A. Lepidoptera vectors of Pestalotiopsis fungal disease: First records in oil palm plantations from Colombia. Int. J. Trop. Insect Sci. 2013, 33, 239–246. [Google Scholar] [CrossRef]
- Martínez, L.C.; Plata-Rueda, A.; Serrao, J.E.; Zanuncio, J.C. Life history traits and damage potential of an invasive pest Acharia fusca (Lepidoptera: Limacodidae) on oil palm. Ann. Entomol. Soc. Am. 2014, 107, 1086–1093. [Google Scholar] [CrossRef]
- Martínez, L.C.; Plata-Rueda, A.; Zanuncio, J.C.; Ribeiro, G.T.; Serrao, J.E. Effects of temperature on the development of Stenoma impressella (Lepidoptera: Elachistidae) on oil palm in Colombia. Fla. Entomol. 2014, 97, 1805–1811. [Google Scholar] [CrossRef]
- Gitau, C.W.; Gurr, G.M.; Dewhurst, C.F.; Fletcher, M.J.; Mitchell, A. Insect pests and insect-vectored diseases of palms. Austral Entomol. 2009, 48, 328–342. [Google Scholar] [CrossRef]
- Oehlschlager, A.C.; Chinchilla, C.; Castillo, G.; Gonzalez, L. Control of red ring disease by mass trapping of Rhynchophorus palmarum (Coleoptera: Curculionidae). Fla. Entomol. 2002, 85, 507–513. [Google Scholar] [CrossRef]
- Oehlschlager, A.C. Palm weevil pheromones–discovery and use. J. Chem. Ecol. 2016, 42, 617–630. [Google Scholar] [CrossRef]
- Moura, J.I.L.; Mariau, D.; Delabie, J.H.C. Eficiência de Paratheresia menezesi Townsend (Diptera: Tachinidae) no controle biológico natural de Rhynchophorus palmarum (L.) (Coleoptera: Curculionidae). Oléagineux 1993, 48, 219–223. [Google Scholar]
- Moura, J.I.; Toma, R.; Sgrillo, R.B.; Delabie, J.H. Natural efficiency of parasitism by Billaea rhynchophorae (Blanchard) (Diptera: Tachinidae) for the control of Rhynchophorus palmarum (L.) (Coleoptera: Curculionidae). Neotrop. Entomol. 2006, 35, 273–274. [Google Scholar] [CrossRef] [PubMed]
- Howard, F.W.; Giblin-Davis, R.; Moore, D.; Abad, R. Insects on Palms; Cabi: London, UK, 2001; 400p. [Google Scholar]
- Martínez, O.L.; Plata-Rueda, A.; Martínez, L.C. Oil palm plantations as an agroecosystem: Impact on integrated pest management and pesticide use. Outlooks Pest Manag. 2013, 24, 225–229. [Google Scholar] [CrossRef]
- Giblin-Davis, R.M.; Howard, F.W. Vulnerability of stressed palms to attack by Rhynchophorus cruentatus (Coleoptera: Curculionidae) and insecticidal control of the pest. J. Econ. Entomol. 1989, 82, 1185–1190. [Google Scholar] [CrossRef]
- Llácer, E.; Dembilio, O.; Jacas, J.A. Evaluation of the efficacy of an insecticidal paint based on chlorpyrifos and pyriproxyfen in a micro-encapsulated formulation against the red palm weevil, Rhynchophorus ferrugineus. J. Econ. Entomol. 2010, 103, 402–408. [Google Scholar] [CrossRef]
- Sparks, T.C.; Nauen, R. IRAC: Mode of action classification and insecticide resistance management. Pestic. Biochem. Physiol. 2015, 121, 122–128. [Google Scholar] [CrossRef]
- Environmental Protection Agency, EPA’s Pesticides Industry Sales and Usage, 2006 and 2007 Market Estimates. 2011. Available online: http://www.epa.gov/oppbead1/pestsales/ (accessed on 1 February 2011).
- Horowitz, A.R.; Mendelson, Z.; Ishaaya, I. Effect of abamectin mixed with mineral oil on the sweetpotato whitefly (Homoptera: Aleyrodidae). J. Econ. Entomol. 1997, 90, 349–353. [Google Scholar] [CrossRef]
- Huang, J.F.; Tian, M.; Lv, C.J.; Li, H.Y.; Zhong, G.H. Preliminary studies on induction of apoptosis by abamectin in Spodoptera frugiperda (Sf9) cell line. Pestic. Biochem. Physiol. 2011, 100, 256–263. [Google Scholar] [CrossRef]
- Martínez, L.C.; Plata-Rueda, A.; Zanuncio, J.C.; Serrão, J.E. Comparative toxicity of six insecticides on the rhinoceros beetle (Coleoptera: Scarabaeidae). Fla. Entomol. 2014, 97, 1056–1062. [Google Scholar] [CrossRef]
- Zinkl, J.G.; Shea, P.J.; Nakamoto, R.J.; Callman, J. Brain cholinesterase activity of rainbow trout poisoned by carbaryl. Bull. Environ. Contam. Toxicol. 1987, 38, 29–35. [Google Scholar] [CrossRef]
- He, F. Neurotoxic effects of insecticides-current and future research: A review. Neurotoxicology 2000, 21, 829–835. [Google Scholar]
- Pope, C.; Karanth, S.; Liu, J. Pharmacology and toxicology of cholinesterase inhibitors: Uses and misuses of a common mechanism of action. Environ. Toxicol. Pharmacol. 2005, 19, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Bloomquist, J.R.; Miller, T.A. Sodium channel neurotoxins as probes of the knockdown resistance mechanism. Neurotoxicology 1986, 7, 217–224. [Google Scholar]
- Soderlund, D.M.; Bloomquist, J.R. Neurotoxic actions of pyrethroid insecticides. Annu. Rev. Entomol. 1989, 34, 77–96. [Google Scholar] [CrossRef]
- Athanassiou, G.G.; Papagregoriou, A.S.; Buchelos, C.T. Insecticidal and residual effect of three pyrethroids against Sitophilus oryzae (L.) (Coleoptera: Curculionidae) on stored wheat. J. Stored Prod. Res. 2004, 40, 289–297. [Google Scholar] [CrossRef]
- Cole, L.M.; Nicholson, R.A.; Casida, J.E. Action of phenylpyrazole insecticides at the GABAgated chloride channel. Pestic. Biochem. Physiol. 1993, 46, 47–54. [Google Scholar] [CrossRef]
- Hainzl, D.; Casida, J.E. Fipronil insecticide: Novel photochemical desulfinylation with retention of neurotoxicity (insecticide action and environmental persistence). Proc. Natl. Acad. Sci. USA 1996, 93, 12764–12767. [Google Scholar] [CrossRef] [PubMed]
- Durham, E.W.; Siegfried, B.D.; Scharf, M.E. In vivo and in vitro metabolism of fipronil by larvae of the European corn borer Ostrinia nubilalis. Pest Manag. Sci. 2002, 58, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Buckingham, S.D.; Kleier, D.; Rauh, J.J.; Grauso, M.; Sattelle, D.B. Neonicotinoids: Insecticides acting on insect nicotinic acetylcholine receptors. Trends Pharmacol. Sci. 2001, 22, 573–580. [Google Scholar] [CrossRef]
- Llácer, E.; Negre, M.; Jacas, J.A. Evaluation of an oil dispersion formulation of imidacloprid as a drench against Rhynchophorus ferrugineus (Coleoptera, Curculionidae) in young palm trees. Pest Manag. Sci. 2012, 68, 878–882. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.C.; Plata-Rueda, A.; Gonçalves, W.G.; Freire, A.F.P.A.; Zanuncio, J.C.; Bozdoğan, H.; Serrão, J.E. Toxicity and cytotoxicity of the insecticide imidacloprid in the midgut of the predatory bug, Podisus nigrispinus. Ecotoxicol. Environ. Saf. 2019, 167, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Salgado, V.L. Studies on the mode of action of spinosad: Insect symptoms and physiological correlates. Pestic. Biochem. Physiol. 1998, 60, 91–102. [Google Scholar] [CrossRef]
- Crouse, G.D.; Sparks, T.C.; Schoonover, J.; Gifford, J.; Dripps, J.; Bruce, T.; Larson, L.L.; Garlich, J.; Hatton, C.; Hill, R.L.; et al. Recent advances in the chemistry of spinosyns. Pest Manag. Sci. 2001, 57, 177–185. [Google Scholar] [CrossRef]
- Orr, N.; Shaffner, A.J.; Richey, K.; Crouse, G.D. Novel mode of action of spinosad: Receptor binding studies demonstrating lack of interaction with known insecticidal target sites. Pestic. Biochem. Physiol. 2009, 95, 1–5. [Google Scholar] [CrossRef]
- Martínez, G.; Arias, N.A.; Sarria, G.A.; Torres, G.A.; Varón, F.; Noreña, C.; Salcedo, S.; Aya, H.; Ariza, J.G.; Aldana, R.; et al. Manejo Integrado de la pudrición del cogollo (PC) de la palma de aceite (No. D-1768); Centro de Investigación en Palma de Aceite, Cenipalma [Colombia] Federación Nacional de Cultivadores de Palma de Aceite, Fedepalma [Colombia] Servicio Nacional de Aprendizaje, SENA Sociedad de Agricultores de Colombia, SAC: Bogotá, Colombia, 2009; pp. 1–24. [Google Scholar]
- Finney, D.J. Probit Analysis; Cambridge University Press: Cambridge, UK, 1964; 333p. [Google Scholar]
- Originlab Corporation. OriginPro V. 9.1.0 SR2 B87; Originlab Corporation: Northampton, MA, USA, 2013; 210p. [Google Scholar]
- SAS Institute. The SAS System for Windows, Release 9.0; SAS Institute: Cary, NC, USA, 2002; 222p. [Google Scholar]
- Plata-Rueda, A.; Martínez, L.C.; Dos Santos, M.H.; Fernandes, F.L.; Wilcken, C.F.; Soares, M.A.; Serrão, J.E.; Zanuncio, J.C. Insecticidal activity of garlic essential oil and their constituents against the mealworm beetle, Tenebrio molitor Linnaeus (Coleoptera: Tenebrionidae). Sci. Rep. 2017, 7, 46406. [Google Scholar] [CrossRef] [PubMed]
- Amaral, K.D.; Martínez, L.C.; Lima, M.A.P.; Serrão, J.E.; Della Lucia, T.M.C. Azadirachtin impairs egg production in Atta sexdens leaf-cutting ant queens. Environ. Pollut. 2018, 243, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.C.; Plata-Rueda, A.; Zanuncio, J.C.; Serrão, J.E. Bioactivity of six plant extracts on adults of Demotispa neivai (Coleoptera: Chrysomelidae). J. Insect Sci. 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.C.; Plata-Rueda, A.; Agudelo, O. Efficacy of insecticides on Leptopharsa gibbicarina Froeschner (Hemiptera: Tingidae) applied by root Absorption technique in oil palm. Persian Gulf Crop Prot. 2013, 2, 10–17. [Google Scholar]
- Clark, D.C.; Haynes, K.F. Sublethal effects of cypermethrin on chemical communication, courtship, and oviposition in the cabbage looper (Lepidoptera: Noctuidae). J. Econ. Entomol. 1992, 85, 1771–1778. [Google Scholar] [CrossRef]
- Knight, A.L.; Flexner, L. Disruption of mating in codling moth (Lepidoptera: Tortricidae) by chlorantranilipole, an anthranilic diamide insecticide. Pest Manag. Sci. 2007, 63, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Liu, S.; Gu, J.; Wang, X.; Liang, X.; Liu, Z. Sublethal effects of four insecticides on the reproduction and wing formation of brown planthopper, Nilaparvata lugens. Pest Manag. Sci. 2009, 65, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Alford, R.A.; Holmes, J.A. Sublethal effects of carbaryl, aminocarb, fenitrothion, and Bacillus thuringiensis on the development and fecundity of the spruce budworm (Lepidoptera: Tortricidae). J. Econ. Entomol. 1986, 79, 31–34. [Google Scholar] [CrossRef]
- Vojoudi, S.; Saber, M.; Hejazi, M.J.; Talaei-Hassanloui, R. Toxicity of chlorpyrifos, spinosad and abamectin on cotton bollworm, Helicoverpa armigera and their sublethal effects on fecundity and longevity. Bull. Insectol. 2011, 64, 189–193. [Google Scholar]
- Widiarta, I.N.; Matsumura, M.; Suzuki, Y.; Nakasuji, F. Effects of sublethal doses of imidacloprid on the fecundity of green leafhoppers, Nephotettix spp. (Hemiptera: Cicadellidae) and their natural enemies. Appl. Entomol. Zool. 2001, 36, 501–507. [Google Scholar] [CrossRef][Green Version]
- Hui, W.; Juan, W.; Li, H.S.; Dai, H.G.; Gu, X.J. Sub-lethal effects of fenvalerate on the development, fecundity, and juvenile hormone esterase activity of diamondback moth, Plutella xylostella (L.). Agric. Sci. China 2010, 9, 1612–1622. [Google Scholar]
- Charmillot, P.J.; Pasquier, D.; Salamin, C.; Ter-Hovannesyan, A. Ovicidal and larvicidal effectiveness of insecticides applied by dipping apples on the small fruit tortrix Grapholita lobarzewskii. Pest Manag. Sci. 2007, 63, 677–681. [Google Scholar] [CrossRef]
- Hoffmann, E.J.; Middleton, S.M.; Wise, J.C. Ovicidal activity of organophosphate, oxadiazine, neonicotinoid and insect growth regulator chemistries on the northern strain plum curculio, Conotrachelus nenuphar. J. Insect Sci. 2008, 8. [Google Scholar] [CrossRef]
- Van Eerd, L.L.; Hoagland, R.E.; Zablotowicz, R.M.; Hall, J.C. Pesticide metabolism in plants and microorganisms. Weed Sci. 2003, 51, 472–495. [Google Scholar] [CrossRef]
- Buchholz, A.; Nauen, R. Translocation and translaminar bioavailability of two neonicotinoid insecticides after foliar application to cabbage and cotton. Pest Manag. Sci. 2002, 58, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Aljabr, A.M.; Rizwan-ul-Haq, M.; Hussain, A.; Al-Mubarak, A.I.; AL-Ayied, H.Y. Establishing midgut cell culture from Rhynchophorus ferrugineus (Olivier) and toxicity assessment against ten different insecticides. In Vitro Cell. Dev. Biol.-Anim. 2014, 50, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Fettig, C.J.; Allen, K.K.; Borys, R.R.; Christopherson, J.; Dabney, C.P.; Eager, T.J.; Gibson, K.E.; Hebertson, E.G.; Long, D.F.; Munson, A.S.; et al. Effectiveness of bifenthrin (Onyx) and carbaryl (Sevin SL) for protecting individual, high-value conifers from bark beetle attack (Coleoptera: Curculionidae: Scolytinae) in the western United States. J. Econ. Entomol. 2006, 99, 1691–1698. [Google Scholar] [CrossRef] [PubMed]
- Verghese, A.; Nagaraju, D.K.; Vasudev, V.; Jayanthi, P.K.; Madhura, H.S.; Stonehouse, J.M. Effectiveness of insecticides of synthetic, plant and animal origin against the mango stone weevil, Sternochetus mangiferae (Fabricius) (Coleoptera: Curculionidae). Crop Prot. 2005, 24, 633–636. [Google Scholar] [CrossRef]
- Fettig, C.J.; Grosman, D.M.; Munson, A.S. Efficacy of abamectin and tebuconazole injections to protect lodgepole pine from mortality attributed to mountain pine beetle (Coleoptera: Curculionidae) attack and progression of blue stain fungi. J. Entomol. Sci. 2013, 48, 270–278. [Google Scholar] [CrossRef]
- Getchell, A.I.; Subramanyam, B. Immediate and delayed mortality of Rhyzopertha dominica (Coleoptera: Bostrichidae) and Sitophilus oryzae (Coleoptera: Curculionidae) adults exposed to spinosad-treated commodities. J. Econ. Entomol. 2008, 101, 1022–1027. [Google Scholar] [CrossRef]
- Grosman, D.M.; Upton, W.W. Efficacy of systemic insecticides for protection of loblolly pine against southern pine engraver beetles (Coleoptera: Curculionidae: Scolytinae) and wood borers (Coleoptera: Cerambycidae). J. Econ. Entomol. 2006, 99, 94–101. [Google Scholar] [CrossRef] [PubMed]
| Insecticides | Lethal Concentration | Estimated Value (mg mL−1) | Confidence Interval (mg mL−1) | Slope (± SE) | χ2 |
|---|---|---|---|---|---|
| Abamectin | 25 | 0.22 | 0.06–0.39 | 1.21 (±0.11) | 8.731 |
| 50 | 0.33 | 0.26–0.51 | |||
| 75 | 0.43 | 0.19–1.57 | |||
| 90 | 0.52 | 0.35–2.96 | |||
| Carbaryl | 25 | 0.17 | 0.06–0.19 | 3.15 (±2.23) | 46.71 |
| 50 | 0.24 | 0.08–0.33 | |||
| 75 | 0.41 | 0.31–0.52 | |||
| 90 | 0.56 | 0.46–0.74 | |||
| Deltamethrin | 25 | 0.06 | 0.02–0.18 | 3.11 (±1.95) | 21.28 |
| 50 | 0.17 | 0.03–0.29 | |||
| 75 | 0.28 | 0.14–0.46 | |||
| 90 | 0.38 | 0.25–0.65 | |||
| Fipronil | 25 | 0.35 | 0.32–0.37 | 3.94 (±1.49) | 64.71 |
| 50 | 0.42 | 0.40–0.44 | |||
| 75 | 0.50 | 0.48–0.52 | |||
| 90 | 0.57 | 0.55–0.60 | |||
| Imidacloprid | 25 | 0.39 | 0.20–0.49 | 2.94 (±1.92) | 34.61 |
| 50 | 0.66 | 0.56–0.84 | |||
| 75 | 0.94 | 0.38–1.92 | |||
| 90 | 1.19 | 0.96–1.78 | |||
| Spinosad | 25 | 0.26 | 0.15–0.37 | 1.37 (±0.19) | 10.22 |
| 50 | 0.54 | 0.19–0.62 | |||
| 75 | 0.91 | 0.78–1.04 | |||
| 90 | 1.35 | 1.21–1.53 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez, L.C.; Plata-Rueda, A.; Rodríguez-Dimaté, F.A.; Campos, J.M.; Santos Júnior, V.C.d.; Rolim, G.D.S.; Fernandes, F.L.; Silva, W.M.; Wilcken, C.F.; Zanuncio, J.C.; et al. Exposure to Insecticides Reduces Populations of Rhynchophorus palmarum in Oil Palm Plantations with Bud Rot Disease. Insects 2019, 10, 111. https://doi.org/10.3390/insects10040111
Martínez LC, Plata-Rueda A, Rodríguez-Dimaté FA, Campos JM, Santos Júnior VCd, Rolim GDS, Fernandes FL, Silva WM, Wilcken CF, Zanuncio JC, et al. Exposure to Insecticides Reduces Populations of Rhynchophorus palmarum in Oil Palm Plantations with Bud Rot Disease. Insects. 2019; 10(4):111. https://doi.org/10.3390/insects10040111
Chicago/Turabian StyleMartínez, Luis Carlos, Angelica Plata-Rueda, Francisco Andrés Rodríguez-Dimaté, Juliana Mendonça Campos, Valdeir Celestino dos Santos Júnior, Gabriela Da Silva Rolim, Flavio Lemes Fernandes, Wiane Meloni Silva, Carlos Frederico Wilcken, José Cola Zanuncio, and et al. 2019. "Exposure to Insecticides Reduces Populations of Rhynchophorus palmarum in Oil Palm Plantations with Bud Rot Disease" Insects 10, no. 4: 111. https://doi.org/10.3390/insects10040111
APA StyleMartínez, L. C., Plata-Rueda, A., Rodríguez-Dimaté, F. A., Campos, J. M., Santos Júnior, V. C. d., Rolim, G. D. S., Fernandes, F. L., Silva, W. M., Wilcken, C. F., Zanuncio, J. C., & Serrão, J. E. (2019). Exposure to Insecticides Reduces Populations of Rhynchophorus palmarum in Oil Palm Plantations with Bud Rot Disease. Insects, 10(4), 111. https://doi.org/10.3390/insects10040111







