Endophytic Effects of Beauveria bassiana on Corn (Zea mays) and Its Herbivore, Rachiplusia nu (Lepidoptera: Noctuidae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment I
2.1.1. Measurement of Growth Parameters and Crop Yield
2.1.2. Vertical Transmission
2.1.3. Percentage of Seed Germination
2.2. Experiment II
Feeding Preference
2.3. Data Analysis
3. Results
3.1. Experiment I—Growth and Crop Yield in Plants Inoculated with B. bassiana
Vertical Transmission of B. bassiana and Percentage of Seed Germination
3.2. Experiment II
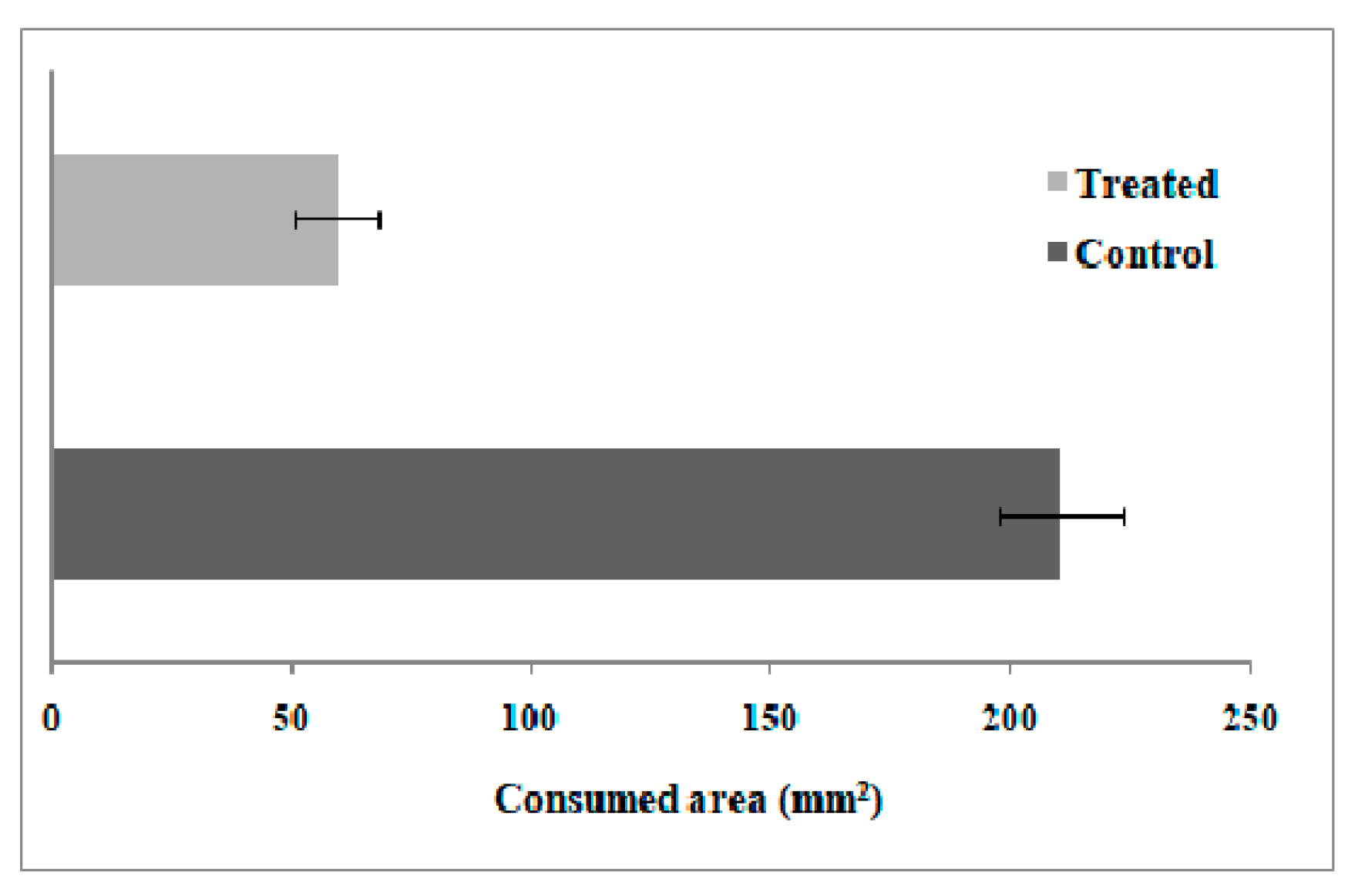
Feeding Preference
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- SAGPyA. Secretaría de Agricultura, Ganadería, Pesca y Alimentos, Agricultura, Ministerio de Agricultura, Ganadería y Pesca de Argentina. Estimaciones Agrícolas, Informes por Cultivo; 2017. Available online: https://www.sagpya.mecon.gov.ar/ (accessed on 9 September 2017).
- Tamagno, S.; Greco, I.; Almeida, H.; Borras, L. Physiological differences in yield related traits between flint and dent Argentinean commercial maize genotypes. Eur. J. Agron. 2015, 68, 50–56. [Google Scholar] [CrossRef]
- Rimoldi, F.; Fogel, M.N.; Schneider, M.I.; Ronco, A.E. Lethal and sublethal effects of cypermethrin and methoxyfenozide on the larvae of Rachiplusia nu (Guenee) (Lepidoptera: Noctuidae). Invertebr. Reprod. Dev. 2012, 56, 200–208. [Google Scholar] [CrossRef]
- Sarandón, S.J. Incorporando la Agroecología en las Instituciones de Educación Agrícola: Una necesidad para la Sustentabilidad Rural. In La Agroecología en la Construcción de Alternativas Hacia la Sustentabilidad Rural; Hernández, J.M., Ed.; Ediciones Siglo, InstitutoTecnológico de Estudios Superiores de Occidente: Guadalajara, México, 2011; pp. 168–189. [Google Scholar]
- Ambethgar, V. Potential of entomopathogenic fungi in insecticide resistance management (IRM): A review. J. Biopestic. 2009, 2, 177–193. [Google Scholar]
- Kabaluk, J.T.; Ericsson, J.D. Seed treatment increases yield of field corn when applied for wireworm control. Agron. J. 2007, 99, 1377–1381. [Google Scholar] [CrossRef]
- Ownley, B.H.; Griffin, M.R.; Klingeman, W.E.; Gwinn, K.D.; Moulton, J.K.; Pereira, R.M. Beauveria bassiana: Endophytic colonization and plant disease control. J. Invertebr. Pathol. 2008, 98, 267–270. [Google Scholar] [CrossRef]
- Vega, F.E. Insect pathology and fungal endophytes. J. Invertebr. Pathol. 2008, 98, 277–279. [Google Scholar] [CrossRef]
- Vega, F.E.; Goettel, M.S.; Blackwell, M.; Chandler, D.; Jackson, M.A.; Keller, S.; Koike, M.; Maniania, N.K.; Monzón, A.; Ownley, B.H.; et al. Fungal entomopathogens: New insights on their ecology. Fungal Ecol. 2009, 2, 149–159. [Google Scholar] [CrossRef]
- Castillo Lopez, D.; Sword, G.A. The endophytic fungal entomopathogens Beauveria bassiana and Purpureocillium lilacinum enhance the growth of cultivated cotton (Gossypium hirsutum) and negatively affect survival of the cotton bollworm (Helicoverpa zea). Biol. Control 2015, 89, 53–60. [Google Scholar] [CrossRef]
- Sánchez Rodríguez, A.R.; Campillo, M.C.; Quesada-Moraga, E. Beauveria bassiana: An entomopathogenic fungus alleviates Fe chlorosis symptoms in plants grown on calcareous substrates. SciHort 2015, 197, 193–202. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, A.R.; Raya-Díaz, S.; Zamarreño, Á.M.; García-Mina, J.M.; del Campillo, M.C.; Quesada-Moraga, E. An endophytic Beauveria bassiana strain increases spike production in bread and durum wheat plants and effectively controls cotton leafworm (Spodoptera littoralis) larvae. Biol. Control 2018, 116, 90–102. [Google Scholar] [CrossRef]
- Jaber, L.R.; Enkerli, J. Effect of seed treatment duration on growth and colonization of Vicia faba by endophytic Beauveria bassiana and Metarhizium brunneum. Biol. Control 2016, 103, 187–195. [Google Scholar] [CrossRef]
- Jaber, L.R.; Enkerli, J. Fungal entomopathogens as endophytes: Can they promote plant growth? Biocontrol. Sci. Technol. 2017, 27, 28–41. [Google Scholar] [CrossRef]
- Dash, C.K.; Bamisile, B.S.; Keppanan, R.; Qasim, M.; Lin, Y.; Islam, S.U.; Wang, L. Endophytic entomopathogenic fungi enhance the growth of Phaseolus vulgaris L. (Fabaceae) and negatively affect the development and reproduction of Tetranychus urticae Koch (Acari: Tetranychidae). Microb. Pathog. 2018, 125, 385–392. [Google Scholar] [CrossRef]
- Russo, M.L.; Pelizza, S.A.; Vianna, M.F.; Allegrucci, N.; Cabello, M.N.; Toledo, A.V.; Mourelos, C.; Scorsetti, A.C. Effect of endophytic entomopathogenic fungi on soybean Glycine max (L.) Merr. growth and yield. J. King Saud Univ. Sci. 2018. [Google Scholar] [CrossRef]
- Vega, F.E. The use of fungal entomopathogens as endophytes in biological control: A review. Mycologia 2018, 110, 4–30. [Google Scholar]
- Cherry, A.J.; Banito, A.; Djegui, D.; Lomer, C. Suppression of the stem-borer Sesamia calamistis (Lepidoptera: Noctuidae) in maize following seed dressing, topical application and stem injection with African isolates of Beauveria bassiana. Int. J. Pest. Manag. 2004, 50, 67–73. [Google Scholar] [CrossRef]
- Bing, L.; Lewis, L.C. Suppression of Ostrinia nubilalis (Hübner) (Lepidoptera: Pyralidae) by endophytic Beauveria bassiana (Balsamo) Vuillemin. Environ. Entomol. 1991, 20, 1207–1211. [Google Scholar] [CrossRef]
- Ramírez-Rodríguez, D.; Sanchez-Peña, S.R. Recovery of endophytic Beauveria bassiana on a culture medium based on cetyltrimethylammonium bromide. Biocontrol. Sci. Technol. 2016, 26, 570–575. [Google Scholar] [CrossRef]
- Russo, M.L.; Pelizza, S.A.; Cabello, M.N.; Stenglein, S.A.; Scorsetti, A.C. Endophytic colonisation of tobacco, corn, wheat and soybeans by the fungal entomopathogen Beauveria bassiana (Ascomycota, Hypocreales). Biocontrol Sci. Technol. 2015, 25, 475–480. [Google Scholar] [CrossRef]
- Renuka, S.; Ramanujam, B.; Poornesha, B. Endophytic ability of different isolates of entomopathogenic fungi Beauveria bassiana (Balsamo) Vuillemin in stem and leaf tissues of maize (Zea mays L.). Indian J. Microbiol. 2016, 56, 126–133. [Google Scholar] [CrossRef]
- Pelizza, S.A.; Eliades, L.A.; Scorsetti, A.C.; Cabello, M.N.; Lange, C.E. Entomopathogenic fungi from Argentina for the control of Schistocerca cancellata (Orthoptera: Acrididae) nymphs: Fungal pathogenicity and enzyme activity. Biocontrol. Sci. Technol. 2012, 22, 1119–1129. [Google Scholar] [CrossRef]
- Russo, M.L.; Scorsetti, A.C.; Vianna, M.F.; Allegrucci, N.; Ferreri, N.A.; Cabello, M.N.; Pelizza, S.A. Effects of endophytic Beauveria bassiana (Ascomycota: Hypocreales) on biological, reproductive parameters and food preference of the soybean pest Helicoverpa gelotopoeon. J. King Saud Univ. Sci. 2018. [Google Scholar] [CrossRef]
- Ayala-Zermeño, M.A.; Gallou, A.; Berlanga-Padilla, A.M.; Serna-Domínguez, M.G.; Arredondo-Bernal, H.C.; Montesinos-Matías, R. Characterisation of entomopathogenic fungi used in the biological control programme of Diaphorinacitri in Mexico. Biocontrol. Sci. Technol. 2015, 25, 1192–1207. [Google Scholar] [CrossRef]
- Greene, G.L.; Leppla, N.C.; Dickerson, W.A. Velvetbean caterpillar: A rearing procedure and artificial medium. J. Econ. Entomol. 1976, 69, 487–488. [Google Scholar] [CrossRef]
- Klein. Available online: http://trigoklein.com.ar/estacion-meteorologica/ (accessed on 10 July 2017).
- Distéfano, S.G.; Lenzi, L.; Gadbán, L.C.; Fuentes, F. Intensidad y pérdida de rendimiento en cultivares de soja por “mancha ojo de rana”. INTA Informe de ActualizaciónTécnica 2010, 17, 53–64. [Google Scholar]
- Lauer, J. Methods for Calculating Corn Yield. 2002. 33. Available online: http://corn.agronomy.wisc.edu/AA/pdf (accessed on 20 February 2019).
- Akello, J.; Sikora, R. Systemic acropedal influence of endophyte seed treatment on Acyrthosiphon pisum and Aphis fabae offspring development and reproductive fitness. Biol. Control 2012, 61, 215–221. [Google Scholar] [CrossRef]
- Posadas, J.B.; Comerio, R.M.; Mini, J.; Nussenbaum, A.L.; Lecuona, R.E. A novel dodine-free selective medium based on the use of cetyltrimethyl ammonium bromide (CTAB) to isolate Beauveria bassiana, Metarhizium anisopliae sensulato and Paecilomyces lilacinus from soil. Mycologia 2012, 104, 974–980. [Google Scholar] [CrossRef]
- Allegrucci, N.; Velazquez, M.S.; Russo, M.L.; Pérez, M.E.; Scorsetti, A.C. Endophytic colonisation of tomato by the entomopathogenic fungus Beauveria bassiana: The use of different inoculation techniques and their effects on the tomato leafminer Tuta absoluta (Lepidoptera: Gelechiidae). J. Plant Prot. Res. 2017, 54, 205–211. [Google Scholar] [CrossRef]
- Luna, M.J.; Iannone, N. Efecto de la chinche de los cuernos “Dichelops furcatus” (F.) sobre la calidad de la semilla de soja. Rev. Facul. Agron. La Plata 2013, 112, 141–145. [Google Scholar]
- ISTA International Seed Testing Association. Rules for Seed Testing, Zürich. 2007. Available online: https://www.seedtest.org/upload/cms/user/ISTAMethodValidationforSeedTestinV1.01.pdf (accessed on 17 April 2017).
- Crawford, K.M.; Land, J.M.; Rudgers, J.A. Fungal endophytes of native grasses decrease insect herbivore preference and performance. Oecologia 2010, 164, 431–444. [Google Scholar] [CrossRef]
- Bailer, W. Writing ImageJ Plugins. A Tutorial. Version 1.71. 2006. Available online: https://media.ijm.fr/fileadmin/www.ijm.fr/MEDIA/imagerie/fichiers/tut_pluginwb.pdf (accessed on 10 October 2017).
- InfoStat. InfoStat Versión 1.1; Grupo InfoStat, FCA, Universidad Nacional de Córdoba: Córdoba, Argentina, 2009. [Google Scholar]
- Akello, J.; Dubois, T.; Coyne, D.; Kyamanywa, S. The effects of Beauveria bassiana dose and exposure duration on colonization and growth of tissue cultured banana (Musa sp.) plants. Biol. Control 2009, 49, 6–10. [Google Scholar] [CrossRef]
- Qayyum, M.A.; Wakil, W.; Arif, M.J.; Sahi, S.T.; Dunlap, C.A. Infection of Helicoverpa armigera by endophytic Beauveria bassiana colonizing tomato plants. Biol. Control 2015, 90, 200–207. [Google Scholar] [CrossRef]
- Akutse, K.S.; Maniania, N.K.; Fiaboe, K.K.M.; Van Den Berg, J.; Ekesi, S. Endophytic colonization of Vicia faba and Phaseolus vulgaris (Fabaceae) by fungal pathogens and their effects on the life-history parameters of Liriomyza huidobrensis(Diptera: Agromyzidae). Fungal. Ecol. 2013, 6, 293–301. [Google Scholar] [CrossRef]
- Gurulingappa, P.; Sword, G.A.; Murdoch, G.; McGee, P.A. Colonization of crop plants by fungal entomopathogens and their effects on two insect pests when in planta. Biol. Control 2010, 55, 34–41. [Google Scholar] [CrossRef]
- Liao, X.; Lovett, B.; Fang, W.; St Leger, R.J. Metarhizium robertsii produces indole-3-acetic acid, which promotes root growth in Arabidopsis and enhances virulence to insects. Microbiology 2017, 163, 980–991. [Google Scholar] [CrossRef]
- Joseph, E.; Cario, S.; Simon, A.; Worle, M.; Mazzeo, R.; Junier, P.; Job, D. Protection of metal artifacts with the formation of metal-oxalates complexes by Beauveria bassiana. Front. Microbiol. 2012, 2, 270. [Google Scholar] [CrossRef]
- Jirakkakul, J.; Cheevadhanarak, S.; Punya, J.; Chutrakul, C.; Senachak, J.; Buajarern, T.; Amnuaykanjanasin, A. Tenellin acts as an iron chelator to prevent iron-generated reactive oxygen species toxicity in the entomopathogenic fungus Beauveria bassiana. FEMS Microbiol. Lett. 2015, 362, 1–8. [Google Scholar] [CrossRef]
- Martinuz, A.; Schouten, A.; Menjivar, R.D.; Sikora, R.A. Effectiveness of systemic resistance toward Aphis gossypii (Hom., Aphididae) as induced by combined applications of the endophytes Fusarium oxysporum Fo162 and Rhizobium etli G12. Biol. Control 2012, 62, 206–212. [Google Scholar] [CrossRef]
- Powell, W.A.; Klingeman, W.E.; Ownley, B.H.; Gwinn, K.D.; Dee, M.; Flanagan, P.C. Endophytic Beauveria bassiana in tomatoes yields mycosis in tomato fruitworm larvae. Hort Sci. 2007, 42, 933. [Google Scholar]
- Vianna, F.; Pelizza, S.; Russo, L.; Allegrucci, N.; Scorsetti, A. Endophytic Beauveria bassiana (Ascomycota: Hypocreales) alters Helicoverpa gelotopoeon (D.) (Lepidoptera: Noctuidae) life cycle and reproductive parameters. J. Plant Prot. Res. 2018, 58, 321–327. [Google Scholar]
- Shrivastava, G.; Ownley, B.H.; Augé, R.M.; Toler, H.; Dee, M.; Vu, A.; Chen, F. Colonization by arbuscular mycorrhizal and endophytic fungi enhanced terpene production in tomato plants and their defense against a herbivorous insect. Symbiosis 2015, 65, 65–74. [Google Scholar] [CrossRef]
| Parameter | Treated | Control |
|---|---|---|
| Height (m) | 2.16 ± 0.17 a | 1.90 ± 0.18 b |
| Number of leaves | 22.13 ± 0.34 a | 19.06 ± 0.34 b |
| First cob appearance (cm) | 72.06 ± 1.01 a | 66.5 ± 1.66 b |
| Number of cobs/plant | 1.76 ± 0.11 a | 1.2 ± 0.40 b |
| Number of nodes where the first cob appears | 5.16 ± 0.13 a | 4.36 ± 0.15 b |
| Number of grain rows/cob | 14.3 ± 0.25 a | 11.8 ± 0.28 b |
| Number of grains/row | 34.5 ± 0.78 a | 26.1 ± 1.2 b |
| Seed weight/plant (gr) | 205.16 ± 3.2 a | 98.8 ± 3.8 b |
| Yield (Kg/ha) | 15,357.19 ± 35.8 a | 7222.08 ± 38.7 b |
| Seed germination (%) | 89 ± 2 a | 77 ± 5.5 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, M.L.; Scorsetti, A.C.; Vianna, M.F.; Cabello, M.; Ferreri, N.; Pelizza, S. Endophytic Effects of Beauveria bassiana on Corn (Zea mays) and Its Herbivore, Rachiplusia nu (Lepidoptera: Noctuidae). Insects 2019, 10, 110. https://doi.org/10.3390/insects10040110
Russo ML, Scorsetti AC, Vianna MF, Cabello M, Ferreri N, Pelizza S. Endophytic Effects of Beauveria bassiana on Corn (Zea mays) and Its Herbivore, Rachiplusia nu (Lepidoptera: Noctuidae). Insects. 2019; 10(4):110. https://doi.org/10.3390/insects10040110
Chicago/Turabian StyleRusso, María Leticia, Ana Clara Scorsetti, María Florencia Vianna, Marta Cabello, Natalia Ferreri, and Sebastian Pelizza. 2019. "Endophytic Effects of Beauveria bassiana on Corn (Zea mays) and Its Herbivore, Rachiplusia nu (Lepidoptera: Noctuidae)" Insects 10, no. 4: 110. https://doi.org/10.3390/insects10040110
APA StyleRusso, M. L., Scorsetti, A. C., Vianna, M. F., Cabello, M., Ferreri, N., & Pelizza, S. (2019). Endophytic Effects of Beauveria bassiana on Corn (Zea mays) and Its Herbivore, Rachiplusia nu (Lepidoptera: Noctuidae). Insects, 10(4), 110. https://doi.org/10.3390/insects10040110





