Molecular Cloning and Expression Analysis of Three Suppressors of Cytokine Signaling Genes (SOCS5, SOCS6, SOCS7) in the Mealworm Beetle Tenebrio molitor
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Microorganisms
2.3. Identification and In Silico Characterization of TmSOCS Genes
2.4. Cloning the TmSOCS ORF
2.5. Developmental and Tissue-Specific Expression of TmSOCS Transcripts
2.6. Immune Challenge Studies
3. Results and Discussion
3.1. Identification of TmSOCS Homologs and Molecular Characterization
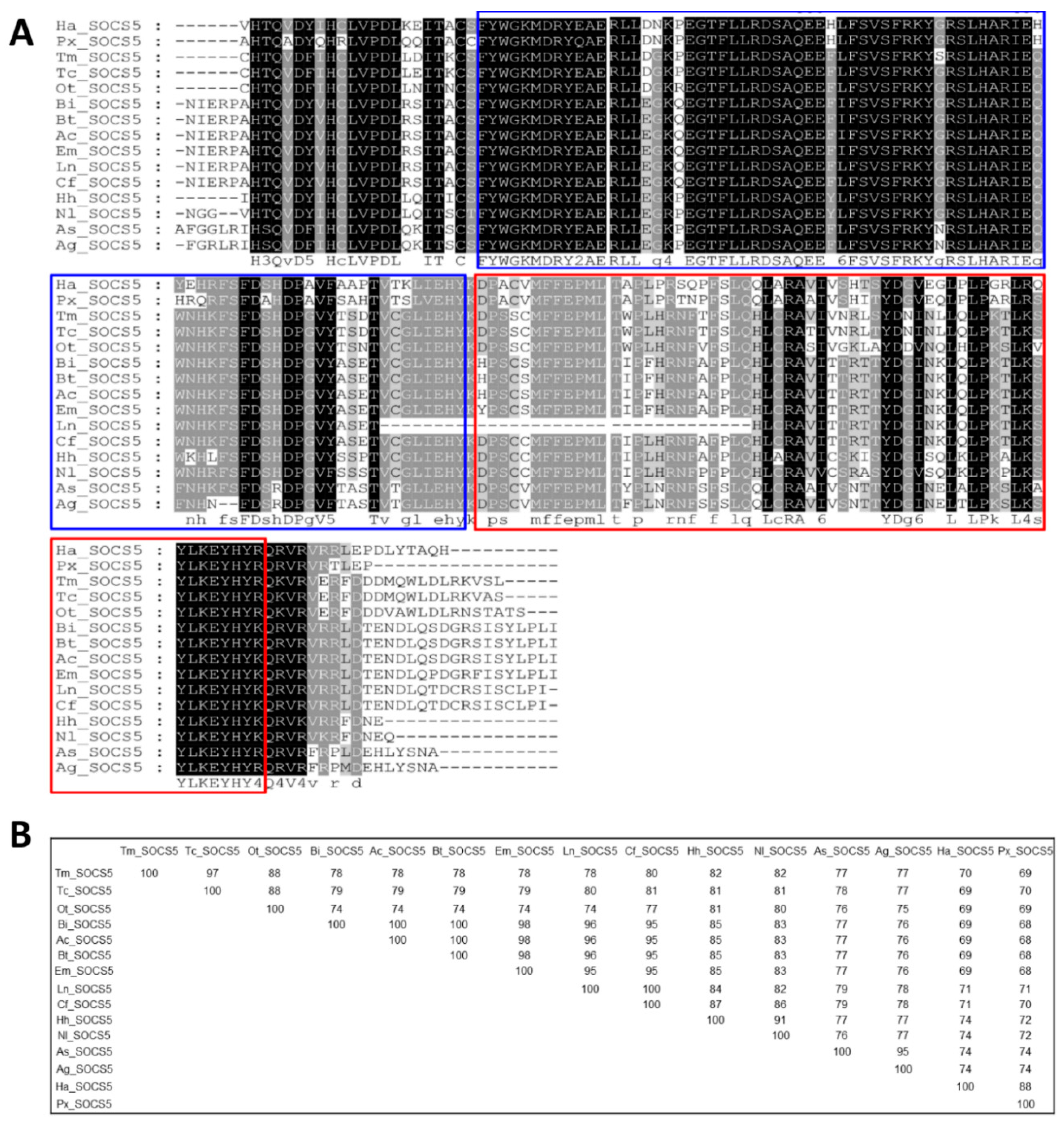
3.2. Molecular Evolutionary Relationships of the TmSOCS Proteins
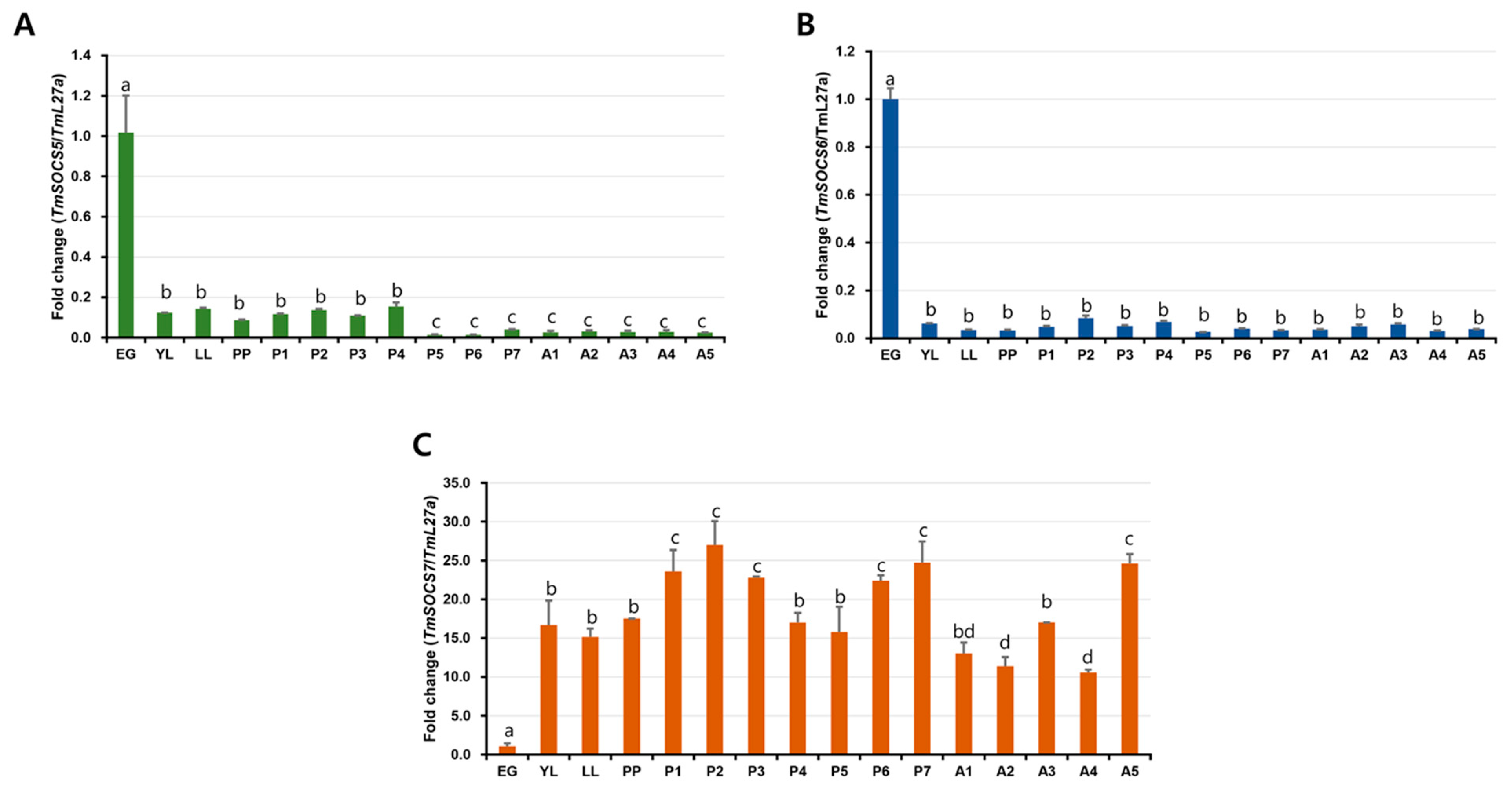
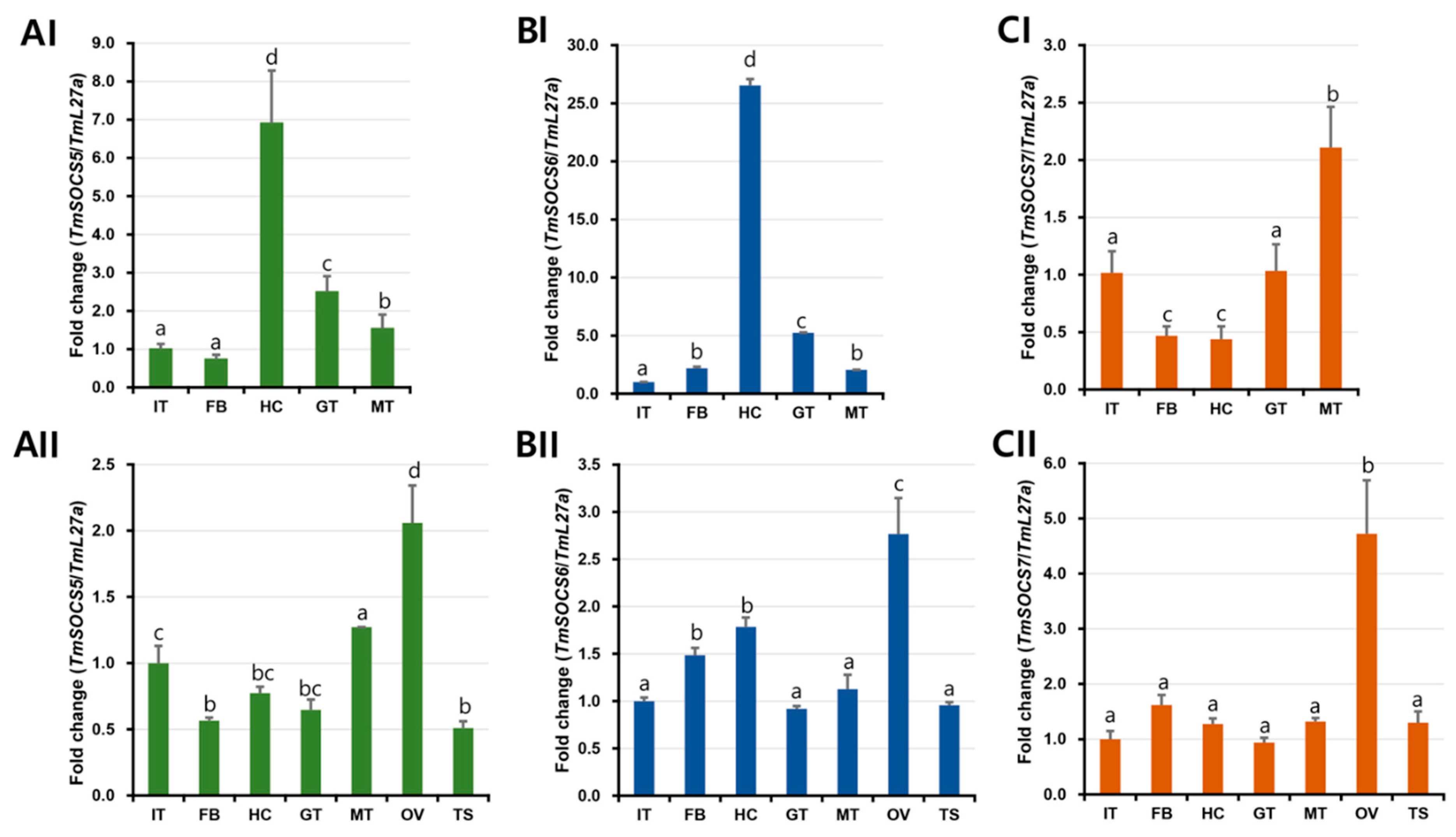
3.3. Developmental Expresion of TmSOCS Genes
3.4. Tissue-Specific Expression of T. molitor SOCS Genes
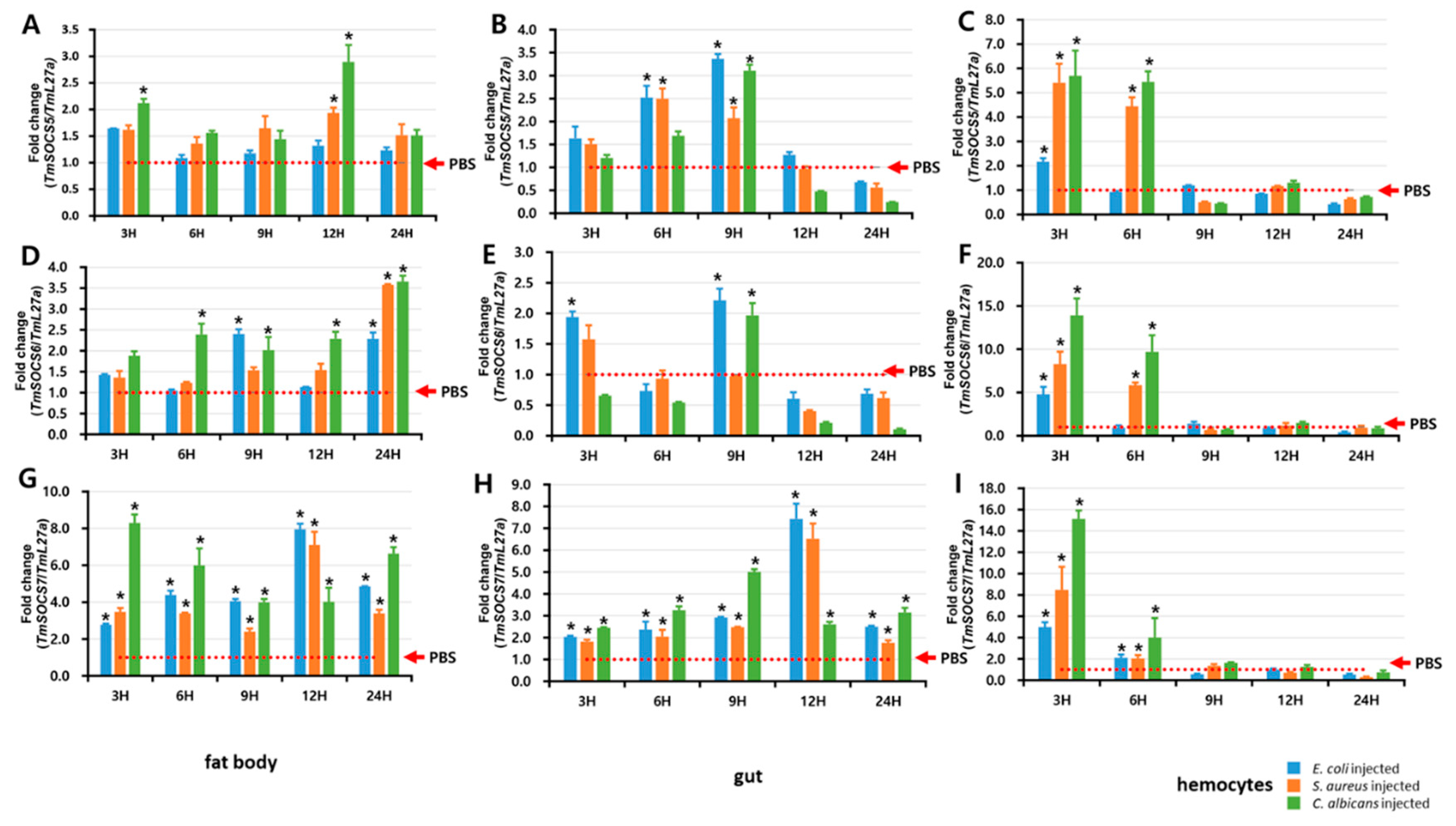
3.5. Expression of TmSOCS Genes after Immune Stimulation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ghoreschi, K.; Laurence, A.; O’Shea, J.J. Janus kinases in immune cell signaling. Immunol. Rev. 2009, 228, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.A. The Jak/STAT pathway. Cold Spring Harb. Perspect. Biol. 2012, 4, a011205. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Yu, D.H.; Feng, G.S. Shp-2 tyrosine phosphatase functions as a negative regulator of the interferon-stimulated JAK-STAT pathway. Mol. Cell. Biol. 1999, 19, 2416–2424. [Google Scholar] [CrossRef]
- Kario, E.; Marmor, M.D.; Adamsky, K.; Citri, A.; Amit, I.; Amariglio, N.; Rechavi, G.; Yarden, Y. Suppressors of cytokine signaling 4 and 5 regulate epidermal growth factor receptor signaling. J. Biol. Chem. 2005, 280, 7038–7048. [Google Scholar] [CrossRef] [PubMed]
- Seif, F.; Khoshmirsafa, M.; Aazami, H.; Mohsenzadegan, M.; Sedighi, G.; Bahar, M. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun. Signal. 2017, 15, 23. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, S. Ubiquitin-mediated regulation of JAK-STAT signaling in embryonic stem cells. Jakstat 2012, 1, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Starr, R.; Willson, T.A.; Viney, E.M.; Murray, L.J.; Rayner, J.R.; Jenkins, B.J.; Gonda, T.J.; Alexander, W.S.; Metcalf, D.; Nicola, N.A.; et al. A family of cytokine-inducible inhibitors of signaling. Nature 1997, 387, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Hilton, D.J.; Richardson, R.T.; Alexander, W.S.; Viney, E.M.; Willson, T.A.; Sprigg, N.S.; Starr, R.; Nicholson, S.E.; Metcalf, D.; Nicola, N.A. Twenty proteins containing a C-terminal SOCS box form five structural classes. Proc. Natl. Acad. Sci. USA 1998, 95, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Liongue, C.; Sertori, R.; Ward, A.C. Evolution of cytokine receptor signaling. J. Immunol. 2016, 197, 11–18. [Google Scholar] [CrossRef]
- Li, L.; Gronning, L.M.; Anderson, P.O.; Li, S.; Edvardsen, K.; Johnston, J.; Kioussis, D.; Shepherd, P.R.; Wang, P. Insulin induces SOCS-6 expression and its binding to the p85 monomer of phosphoinositide 3-kinase, resulting in improvement in glucose metabolism. J. Biol. Chem. 2004, 279, 34107–34114. [Google Scholar] [CrossRef] [PubMed]
- Martens, N.; Uzan, G.; Wery, M.; Hooghe, R.; Hooghe-Peters, E.L.; Gertler, A. Suppressor of cytokine signaling 7 inhibits prolactin, growth hormone, and leptin signaling by interacting with STAT5 or STAT3 and attenuating their nuclear translocation. J. Biol. Chem. 2005, 280, 13817–13823. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wangkahart, E.; Secombes, C.J.; Wang, T. Insights into the evolution of the suppressors of cytokine signaling (SOCS) gene family in vertebrates. Mol. Biol. Evol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Karsten, P.; Hader, S.; Zeidler, M.P. Cloning and expression of Drosophila SOCS36E and its potential regulation by the JAK/STAT pathway. Mech. Dev. 2002, 117, 343–346. [Google Scholar] [CrossRef]
- Rawlings, J.S.; Rennebeck, G.; Harrison, S.M.W.; Xi, R.; Harrison, D.A. Two Drosophila suppressors of cytokine signaling (SOCS) differentially regulate JAK and EGFR pathway activities. BMC Cell Biol. 2004, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Choi, J.-Y.; Oh, C.; Kang, D.-H.; Choi, S.-Y.; Heo, G.-J.; Lee, J.; De Zoysa, M. Molecular cloning and characterization of SOCS-2 from Manila clam Ruditapes philippinarum. Fish Shellfish Immunol. 2014, 36, 453–458. [Google Scholar] [CrossRef]
- De Zoysa, M.; Lee, J. Suppressor of cytokine signaling 2 (SOCS-2) homologue in disk abalone: Cloning, sequence characterization and expression analysis. Fish Shellfish Immunol. 2009, 26, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, J.; Zhang, H.; Gai, Y.; Wang, L.; Li, F.; Yang, J.; Qiu, L.; Song, L. The involvement of suppressors of cytokine signaling 2 (SOCS2) in immune defense responses of Chinese mitten crab Eriocheir sinensis. Dev. Comp. Immunol. 2010, 34, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Dai, L.; Yu, Y.; Wang, D.; Peng, T.; Liu, C. A role of suppressor of cytokine signaling 2 in the regulation of ecdysteroid signaling pathway in Procambarus clarkii. J. Exp. Zool. 2016, 325A, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Song, X.; Zhang, Z.; Li, H.; Lu, K.; Yin, B.; He, J.; Li, C. Shrimp with knockdown of LvSOCS2, a negative feedback loop regulator of JAK/STAT pathway in Litopenaeus vannamei, exhibit enhanced resistance against WSSV. Dev. Comp. Immunol. 2016, 65, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Xu, Q.; Lu, M.; Wang, F.; Liu, Z.; Liu, D.; Yang, W.; Yi, Q.; Wang, L.; Song, L. The involvement of suppressor of cytokine signaling 6 (SOCS6) in immune response of Chinese mitten crab Eriocheir sinensis. Fish Shellfish Immunol. 2018, 72, 502–509. [Google Scholar] [CrossRef]
- Abbas, M.N.; Zhu, B.-J.; Kausar, S.; Dai, L.-S.; Sun, Y.-X.; Tian, J.W.; Liu, C.-L. Suppressor of cytokine signaling 2-12 regulates antimicrobial peptides and ecdysteroid signaling pathways in Bombyx mori (Dazao). J. Insect Physiol. 2017, 103, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.N.; Kausar, S.; Sun, Y.-X.; Tian, J.W.; Zhu, B.-J.; Liu, C.-L. Suppressor of cytokine signaling 6 can enhance epidermal growth factor receptor signaling pathway in Bombyx mori (Dazao). Dev. Comp. Immunol. 2018, 81, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Yaffe, H.; Buxdorf, K.; Shapira, I.; Ein-Gedi, S.; Zvi, M.M.; Fridman, E.; Moshelion, M.; Levy, M. LogSpin: A simple, economical and fast method for RNA isolation from infected or healthy plants and other eukaryotic tissues. BMC Res. Notes 2012, 5, 45. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.H.; Kim, Y.J.; Park, K.B.; Seong, J.H.; Kim, S.G.; Park, S.; Noh, M.Y.; Lee, Y.S.; Han, Y.S. TmCactin plays an important role in Gram-negative and -positive bacterial infection by regulating expression of 7AMP genes in Tenebrio molitor. Sci. Rep. 2017, 7, 46459. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Zhang, Y.; Liu, Y.; Xiang, Z.; Qu, F.; Lu, Z. Cloning and characterization of three suppressors of cytokine signaling (SOCS) genes from the Pacific oyster, Crassostrea gigas. Fish Shellfish Immunol. 2015, 44, 525–532. [Google Scholar] [CrossRef]
- Croker, B.A.; Kiu, H.; Nicholson, S.E. SOCS regulation of the JAK/STAT signaling pathway. Semin. Cell Dev. Biol. 2008, 19, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.A.; Baganizi, D.R.; Sahu, R.; Singh, S.R.; Dennis, V.A. SOCS proteins as regulators of inflammatory responses induced by bacterial infections: A review. Front. Microbiol. 2017, 8, 2431. [Google Scholar] [CrossRef]
- Alexander, W.S.; Hilton, D.J. The role of suppressors of cytokine signaling (SOCS) proteins in regulation of the immune response. Ann. Rev. Immunol. 2004, 22, 503–529. [Google Scholar] [CrossRef] [PubMed]
- Bullock, A.N.; Rodriguez, M.C.; Debreczeni, J.E.; Songyang, Z.; Knapp, S. Structure of the SOCS4-Elongin B/C complex reveals a distinct SOCS box interface and the molecular basis for SOCS-dependent EGFR degradation. Structure 2007, 15, 1493–1504. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.-P.; Chandrasekharan, I.R.; Low, A.; Speed, T.A.; Nicholson, S.E.; Norton, R.S. The N-terminal domains of SOCS proteins: A conserved region in the disordered N-termini of SOCS4 and 5. Proteins 2011, 80, 946–957. [Google Scholar] [CrossRef]
- Chandrasekharan, I.R.; Mohanty, B.; Linossi, E.M.; Dagley, L.F.; Leung, E.W.; Murphy, J.M.; Babon, J.J.; Nicholson, S.E.; Norton, R.S. Structure and Functional characterization of the conserved JAK interaction region in the intrinsically disordered N-terminus of SOCS5. Biochemistry 2015, 54, 4672–4682. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.N.; Min, C.H.; Kim, H.S.; Lee, H.; Yoon, K.A.; Park, S.Y.; Lee, E.S.; Yoon, S. The nuclear localization of SOCS6 requires the N-terminal region and negatively regulates Stat3 protein levels. Biochem. Biophys. Res. Commun. 2007, 360, 333–338. [Google Scholar] [CrossRef]
- Chen, M.-S.; Liu, S.; Wang, H.; Cheng, X.; El Bouhssini, M.; Whitworth, R.J. Massive shift in gene expression during transitions between developmental stages of the Gall Midge, Mayetiola destructor. PLoS ONE 2016, 11, e0155616. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Zeng, F. Identification of development-related genes in the ovaries of adult Harmonia axyridis (Pallas) lady beetles using a time-series analysis by RNA-seq. Sci. Rep. 2016, 6, 39109. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, R.; Gupta, K.; Kajla, M.; Kumar, S.; Gakhar, S.K.; Kakani, P.; Choudhury, T.P.; Gupta, L. Molecular characterization of SOCS gene and its expression analysis on Plasmodium berghei infection in Anopheles culicifacies. Acta Trop. 2015, 152, 170–175. [Google Scholar] [CrossRef]
- Monahan, A.J.; Starz-Gaiano, M. Socs36E limits STAT signaling via Cullin2 and a SOCS-box independent mechanism in the Drosophila egg chamber. Mech. Dev. 2015, 138, 313–327. [Google Scholar] [CrossRef]
- Magnusson, K.; Mendes, A.M.; Windbichler, N.; Papathanos, P.A.; Nolan, T.; Dottorini, T.; Rizzi, E.; Christophides, G.K.; Crisanti, A. Transcription regulation of sex-biased genes during ontogeny in the malaria vector Anopheles gambiae. PLoS ONE 2011, 6, e21572. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xiao, Z.Z.; Sun, L. Suppressor of cytokine signaling 3 inhibits head kidney macrophage activation and cytokine expression in Scophthalmus maximus. Dev. Comp. Immunol. 2011, 35, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Silver, D.L.; Geisbrecht, E.R.; Montell, D.J. Requirement for JAK/STAT signaling throughout border cell migration in Drosophila. Development 2005, 132, 3483–3492. [Google Scholar] [CrossRef] [PubMed]
- Maehr, T.; Vecino, J.L.G.; Wadsworth, S.; Wang, T.H.; Secombes, C.J. Four CISH paralogues are present in rainbow trout Oncorhynchus mykiss: Differential expression and modulation during immune responses and development. Mol. Immunol. 2014, 62, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, S.; Yamada, N.; Kumazaki, M.; Yasui, Y.; Iwasaki, J.; Naito, S.; Akao, Y. Socs7, a target gene of microRNA-145, regulates interferon-β induction through STAT3 nuclear translocation in bladder cancer cells. Cell Death Dis. 2013, 4, e482. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.X.; Sun, L. Comparative analysis of the expression patterns of eight suppressors of cytokine signaling in tongue sole, Cynoglossus semilaevis. Fish Shellfish Immunol. 2016, 55, 595–601. [Google Scholar] [CrossRef]
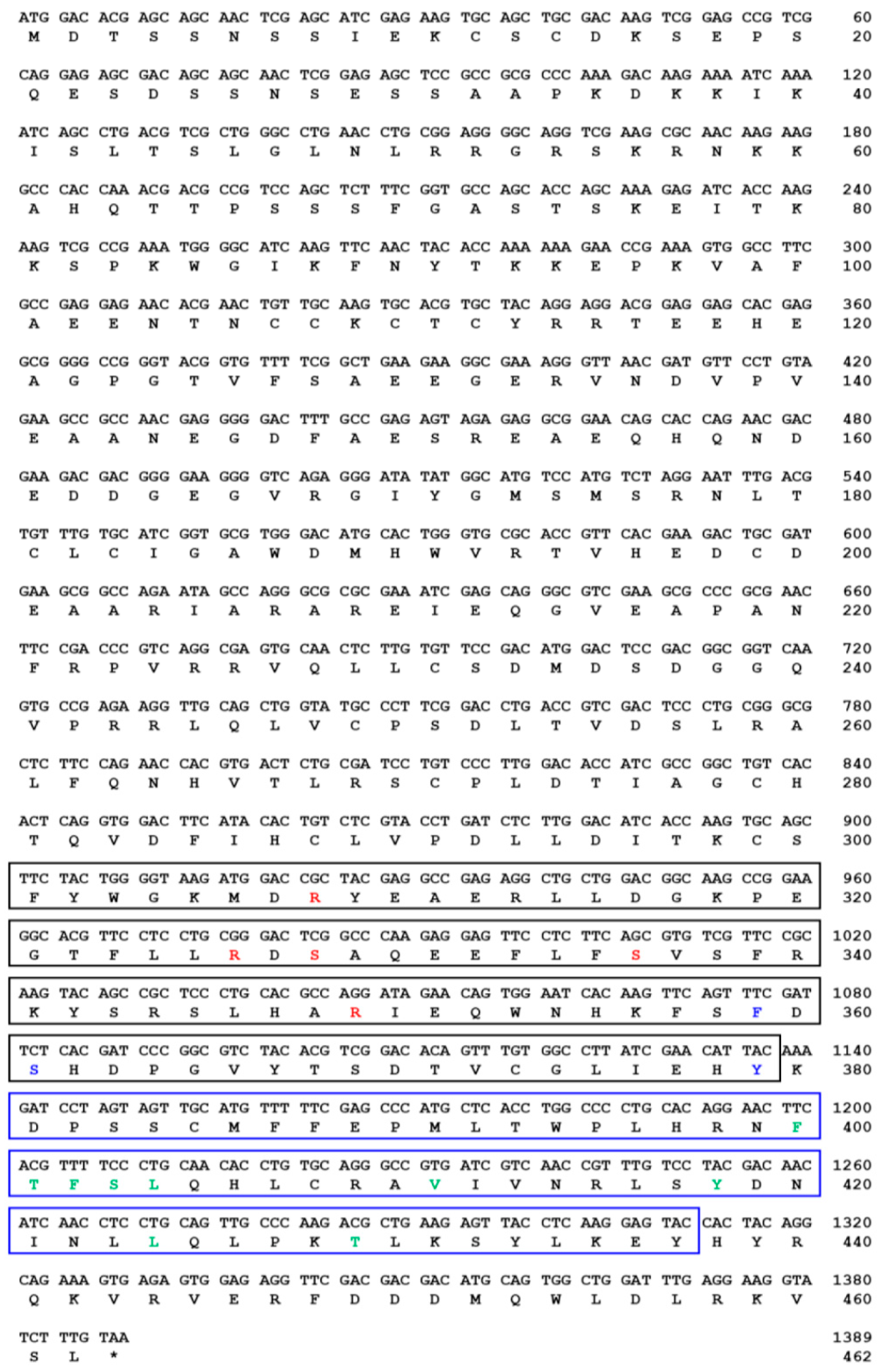
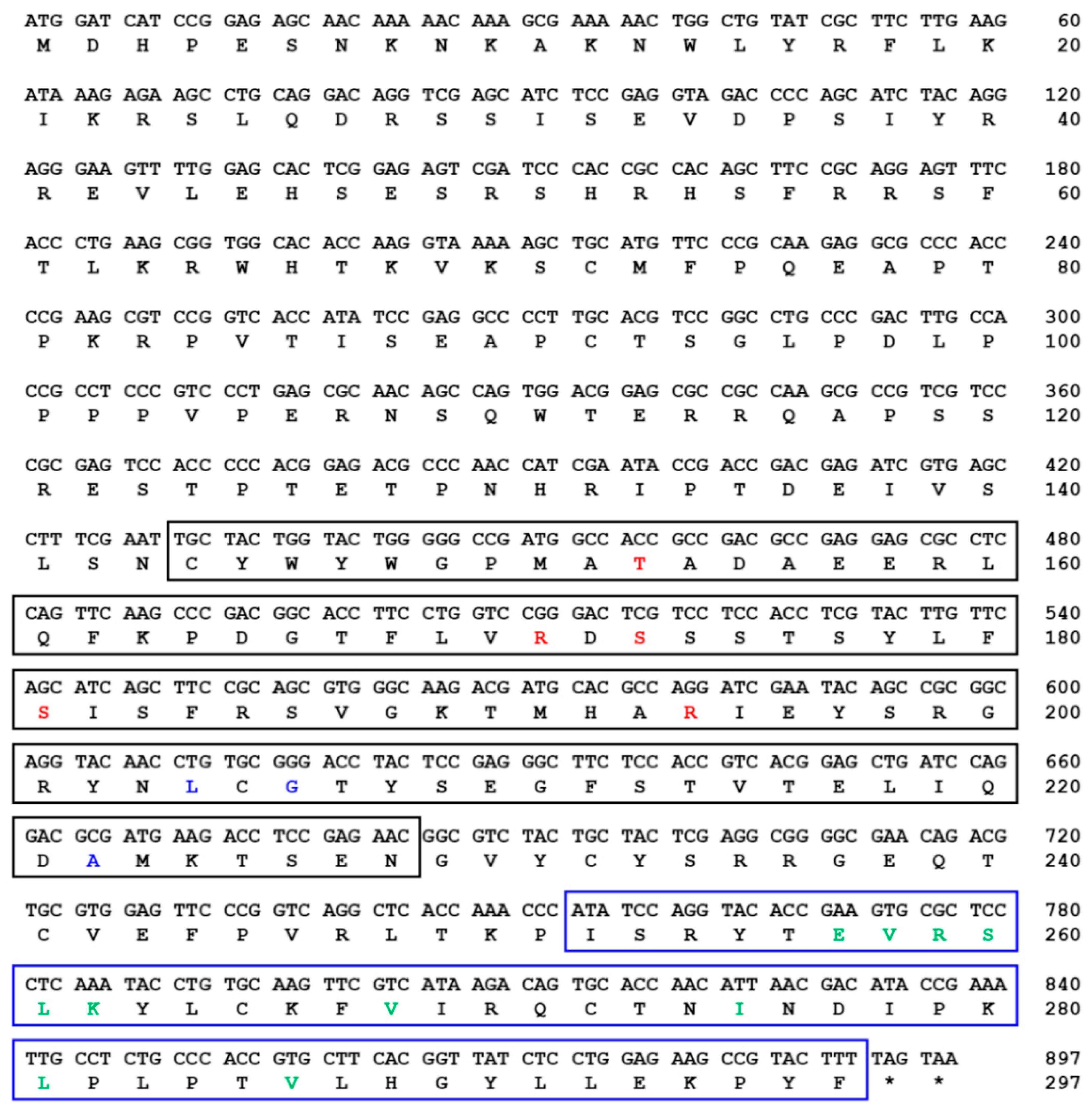
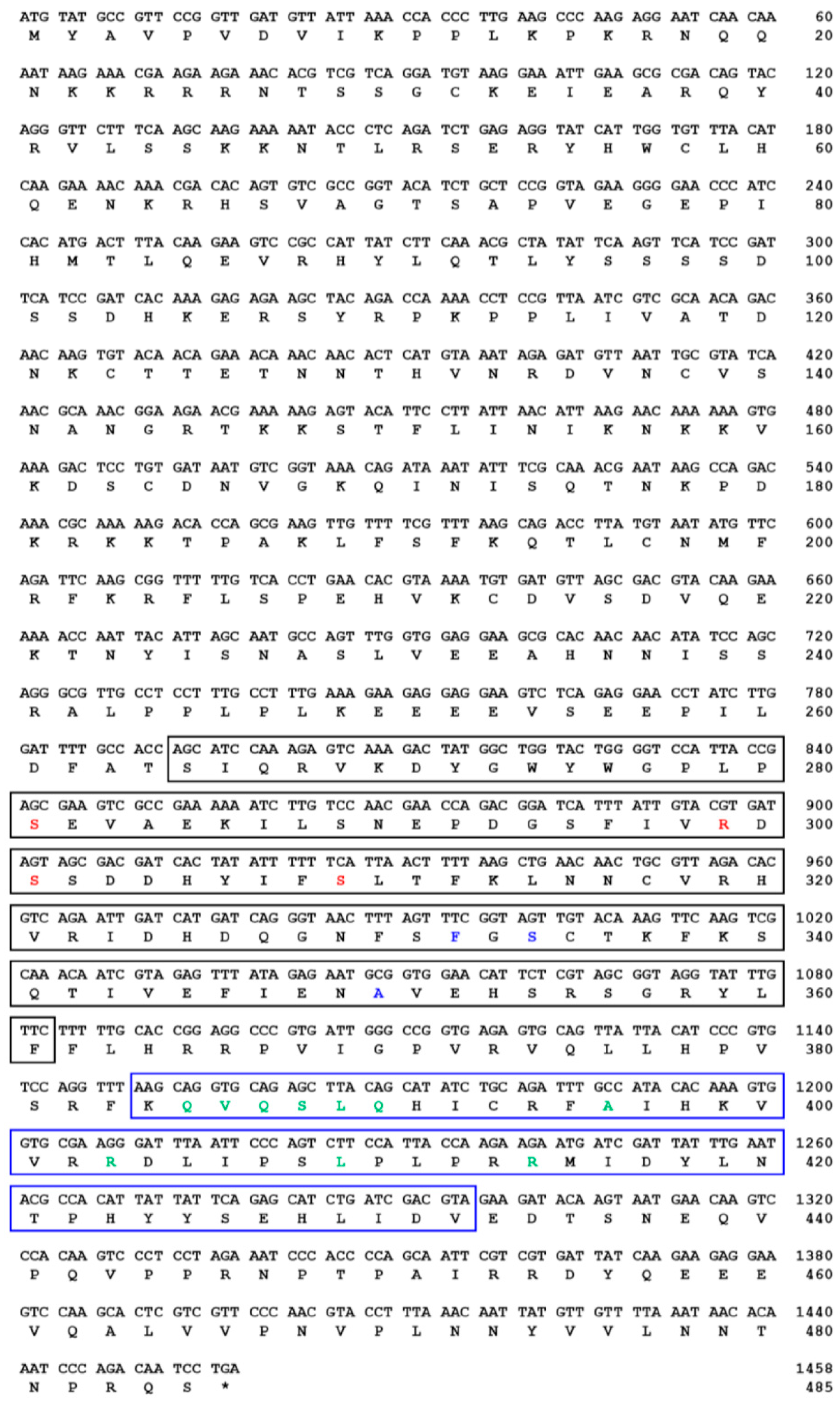
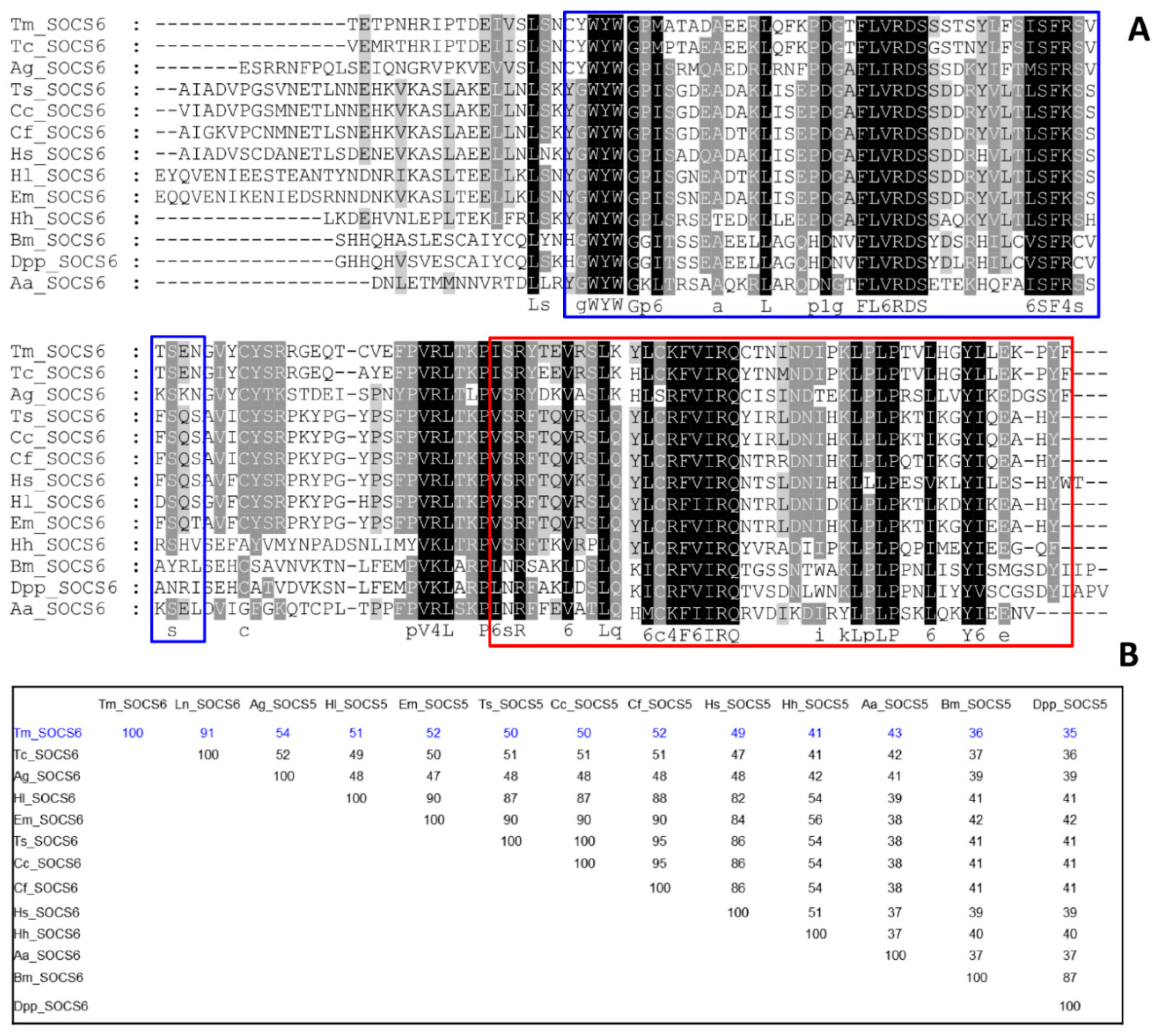
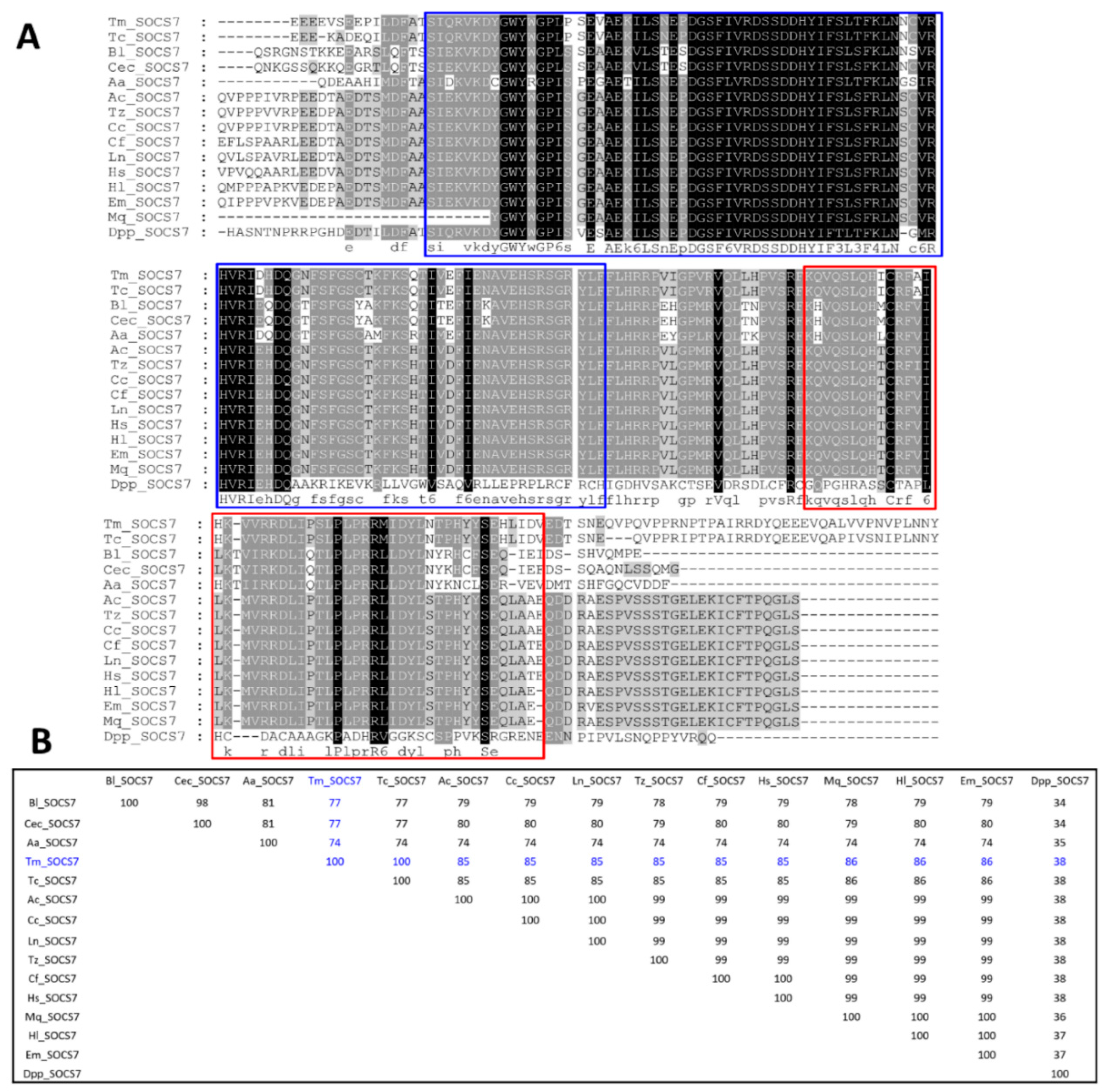
 indicates T. molitor SOCS5, SOCS6, and SOCS7 proteins.
indicates T. molitor SOCS5, SOCS6, and SOCS7 proteins.
 indicates T. molitor SOCS5, SOCS6, and SOCS7 proteins.
indicates T. molitor SOCS5, SOCS6, and SOCS7 proteins.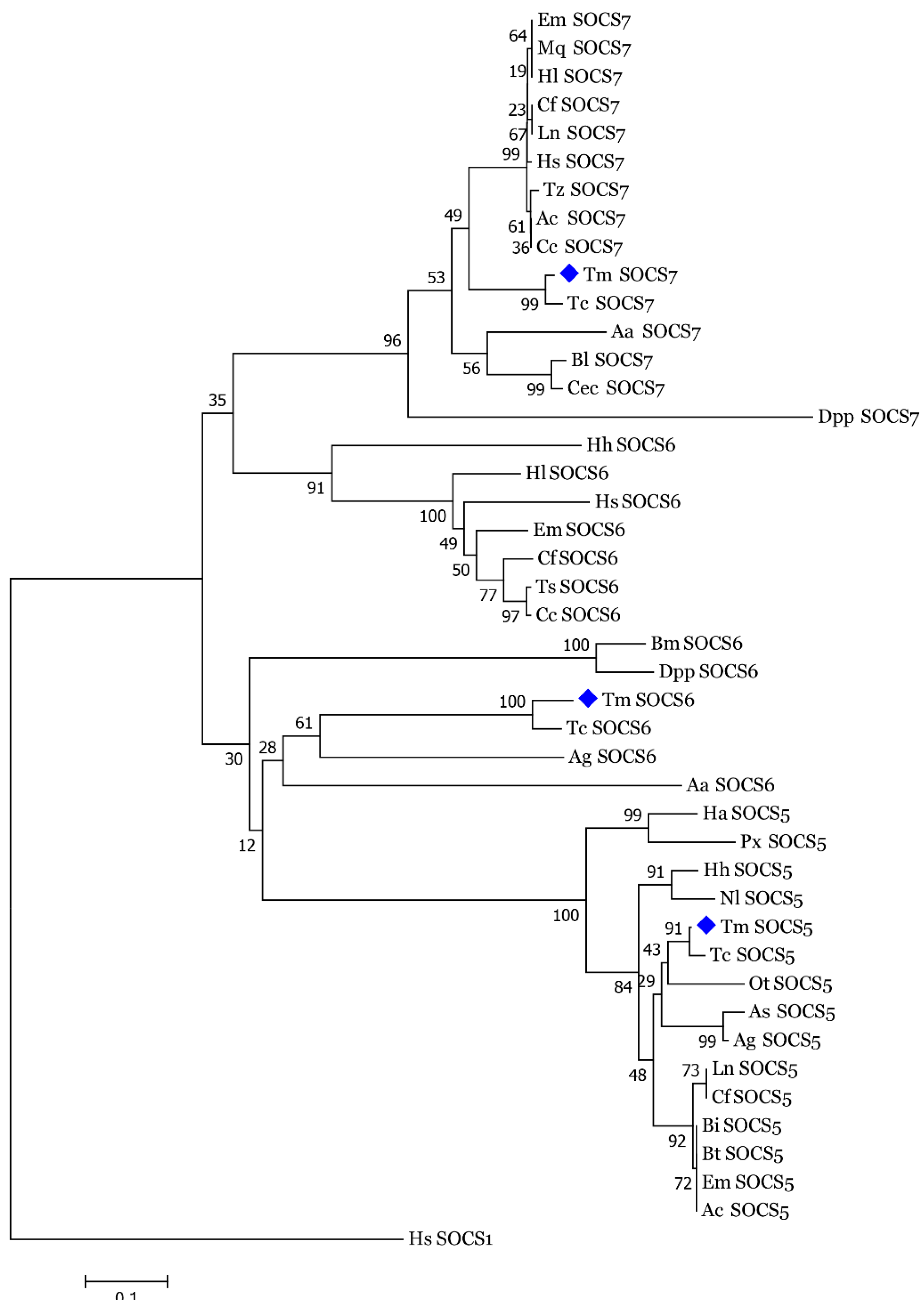
| Primer Name | Sequences (5′→3′) |
|---|---|
| TmSOCS5-cloning_Fw TmSOCS5-cloning_Rv | CCCCCTGAGATGTGATTTCC AACACCGCACATGAAAACAA |
| TmSOCS5-qPCR_Fw TmSOCS5-qPCR_Rv | CGCGCCCAAAGACAAGAAAATC TTTGGTGGGCCTTCTTGTTG |
| TmSOCS5-qPCR_Fw2 TmSOCS5-qPCR_Rv2 | TCACGTTTTCCCTGCAACAC TGCAGGAGGTTGATGTTGTC |
| TmSOCS6-cloning_Fw TmSOCS6-cloning_Rv | AGTGTCGGTTGTGCGTGGT GCGCGATTACTAAAAGTACGG |
| TmSOCS6-qPCR_Fw TmSOCS6-qPCR_Rv | TAAAGAGAAGCCTGCAGGACAG TCCGAGTGCTCCAAAACTTC |
| TmSOCS7-cloning_Fw TmSOCS7-cloning_Rv | CAGTGTCTCACGATACGCTTTC AGTCTCAGGATTGTCTGGGATT |
| TmSOCS7-qPCR_Fw TmSOCS7-qPCR_Rv | ATTGAAGCGCGACAGTACAG AAGTCATGTGGATGGGTTCCC |
| TmL27a_qPCR_Fw TmL27a_qPCR_Rev | TCATCCTGAAGGCAAAGCTCCAGT AGGTTGGTTAGGCAGGCACCTTTA |
| M13_Fwd M13_Rev | GTAAAACGACGGCCAGT CAGGAAACAGCTATGAC |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patnaik, B.B.; Kim, B.B.; Jo, Y.H.; Bang, I.S. Molecular Cloning and Expression Analysis of Three Suppressors of Cytokine Signaling Genes (SOCS5, SOCS6, SOCS7) in the Mealworm Beetle Tenebrio molitor. Insects 2019, 10, 76. https://doi.org/10.3390/insects10030076
Patnaik BB, Kim BB, Jo YH, Bang IS. Molecular Cloning and Expression Analysis of Three Suppressors of Cytokine Signaling Genes (SOCS5, SOCS6, SOCS7) in the Mealworm Beetle Tenebrio molitor. Insects. 2019; 10(3):76. https://doi.org/10.3390/insects10030076
Chicago/Turabian StylePatnaik, Bharat Bhusan, Bo Bae Kim, Yong Hun Jo, and In Seok Bang. 2019. "Molecular Cloning and Expression Analysis of Three Suppressors of Cytokine Signaling Genes (SOCS5, SOCS6, SOCS7) in the Mealworm Beetle Tenebrio molitor" Insects 10, no. 3: 76. https://doi.org/10.3390/insects10030076
APA StylePatnaik, B. B., Kim, B. B., Jo, Y. H., & Bang, I. S. (2019). Molecular Cloning and Expression Analysis of Three Suppressors of Cytokine Signaling Genes (SOCS5, SOCS6, SOCS7) in the Mealworm Beetle Tenebrio molitor. Insects, 10(3), 76. https://doi.org/10.3390/insects10030076






