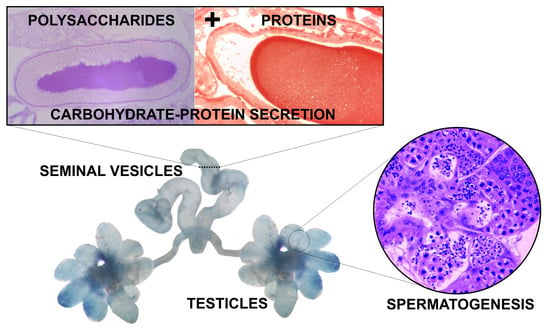
A Glycoproteinaceous Secretion in the Seminal Vesicles of the Termite Coptotermes gestroi (Isoptera: Rhinotermitidae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Morphology of the Reproductive System
2.3. Histology and Histochemistry
2.4. Morphometry of the Testes
2.5. Transmission Electron Microscopy (TEM)
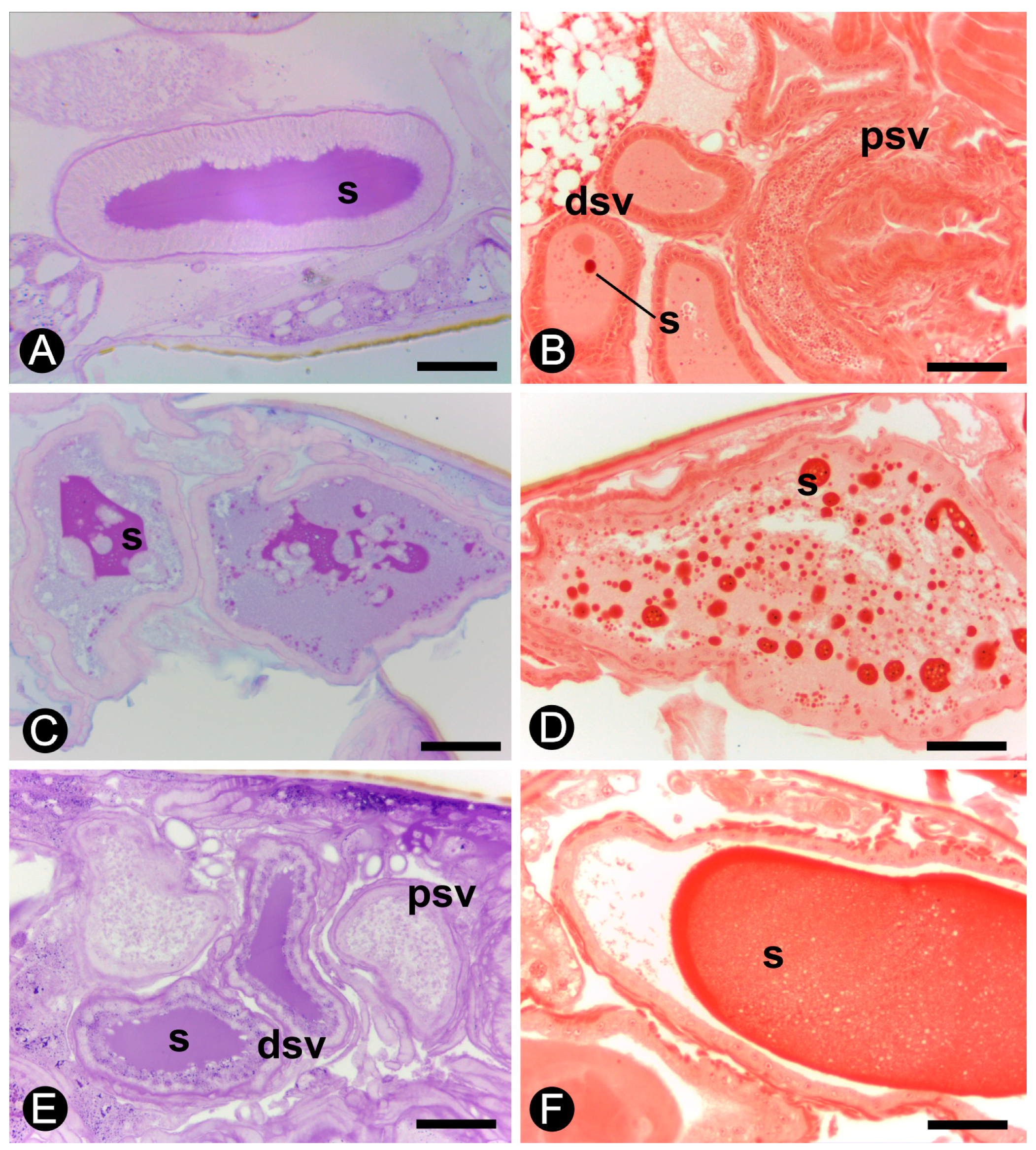
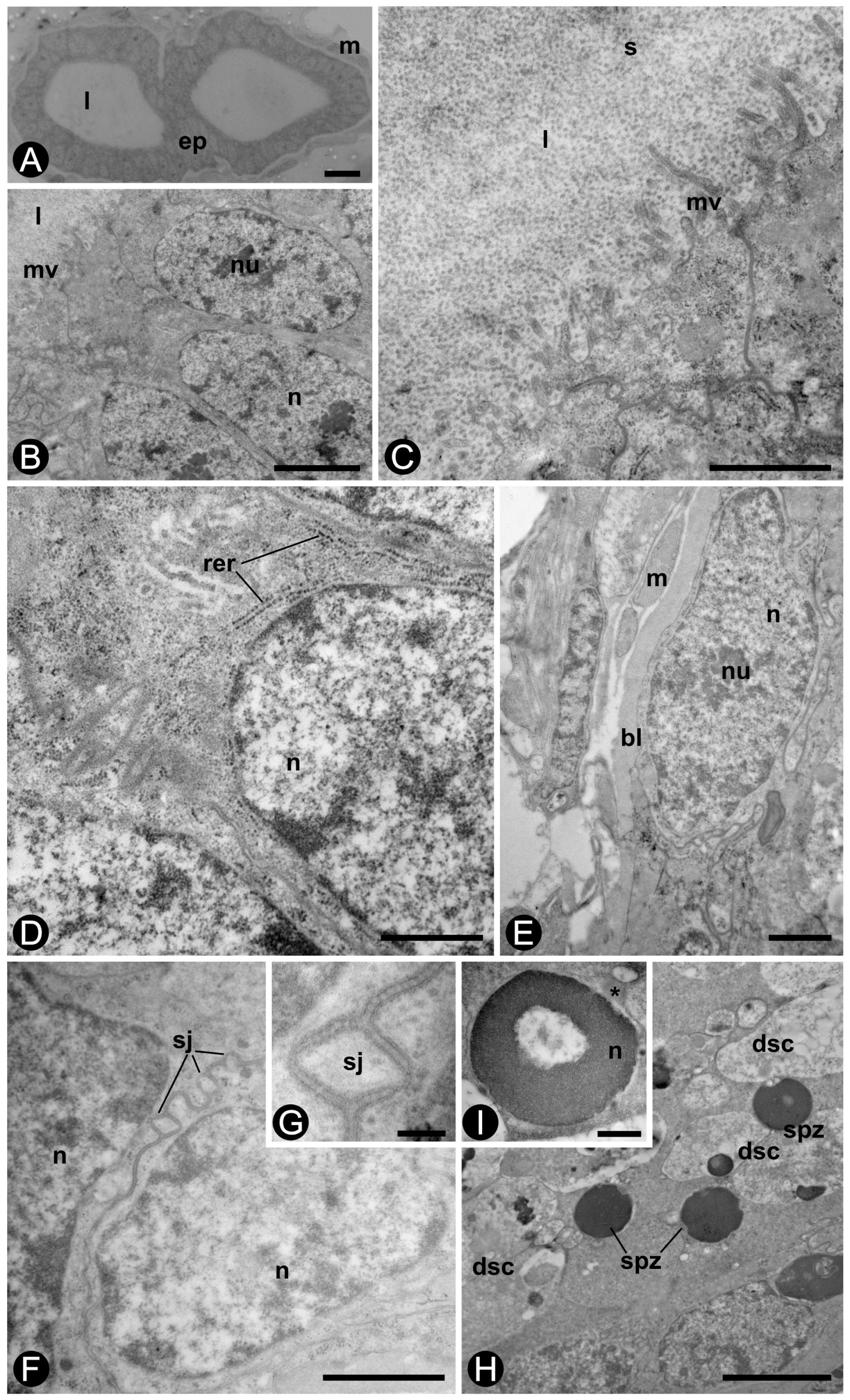
3. Results
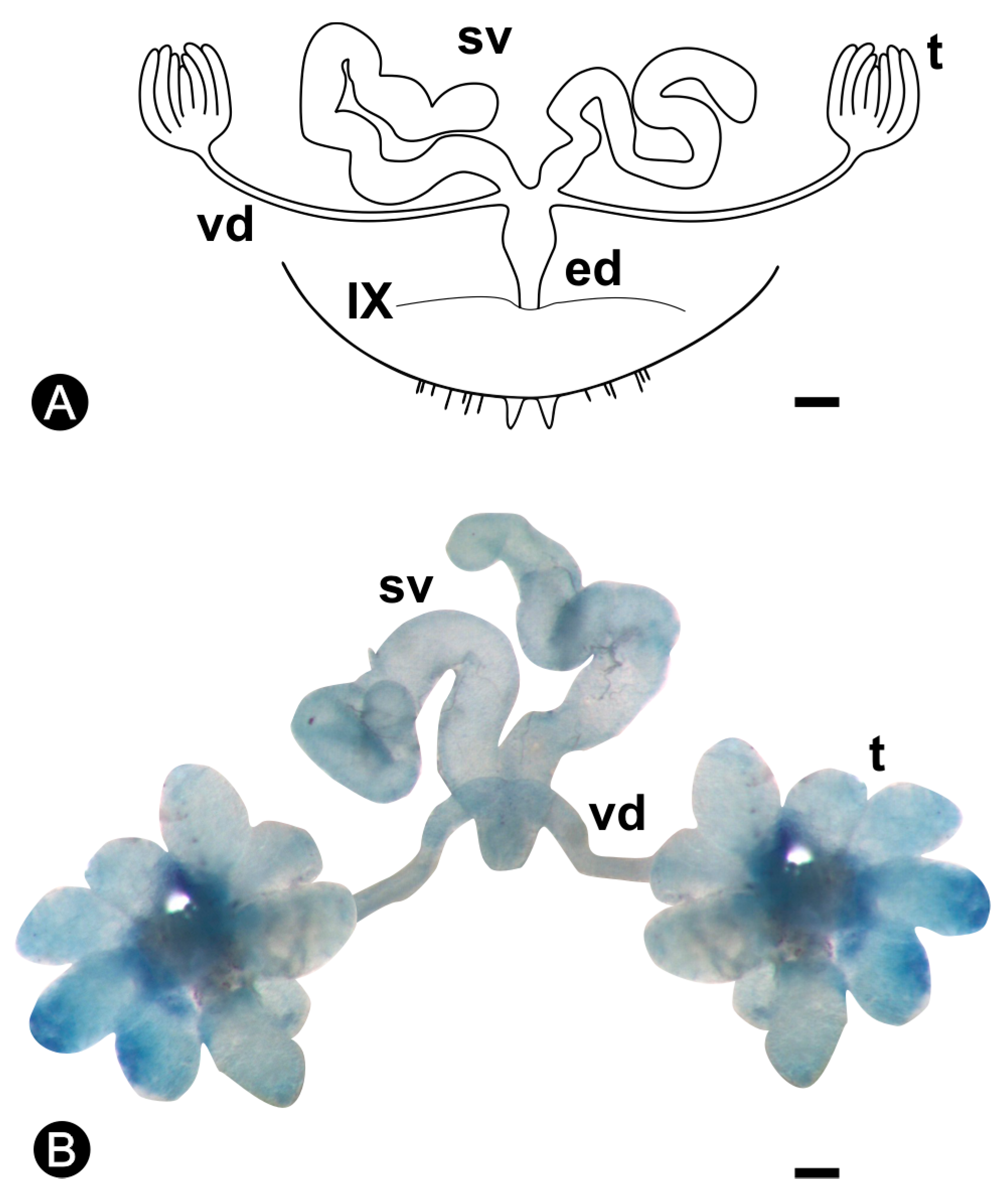
3.1. Morphology of the Reproductive System
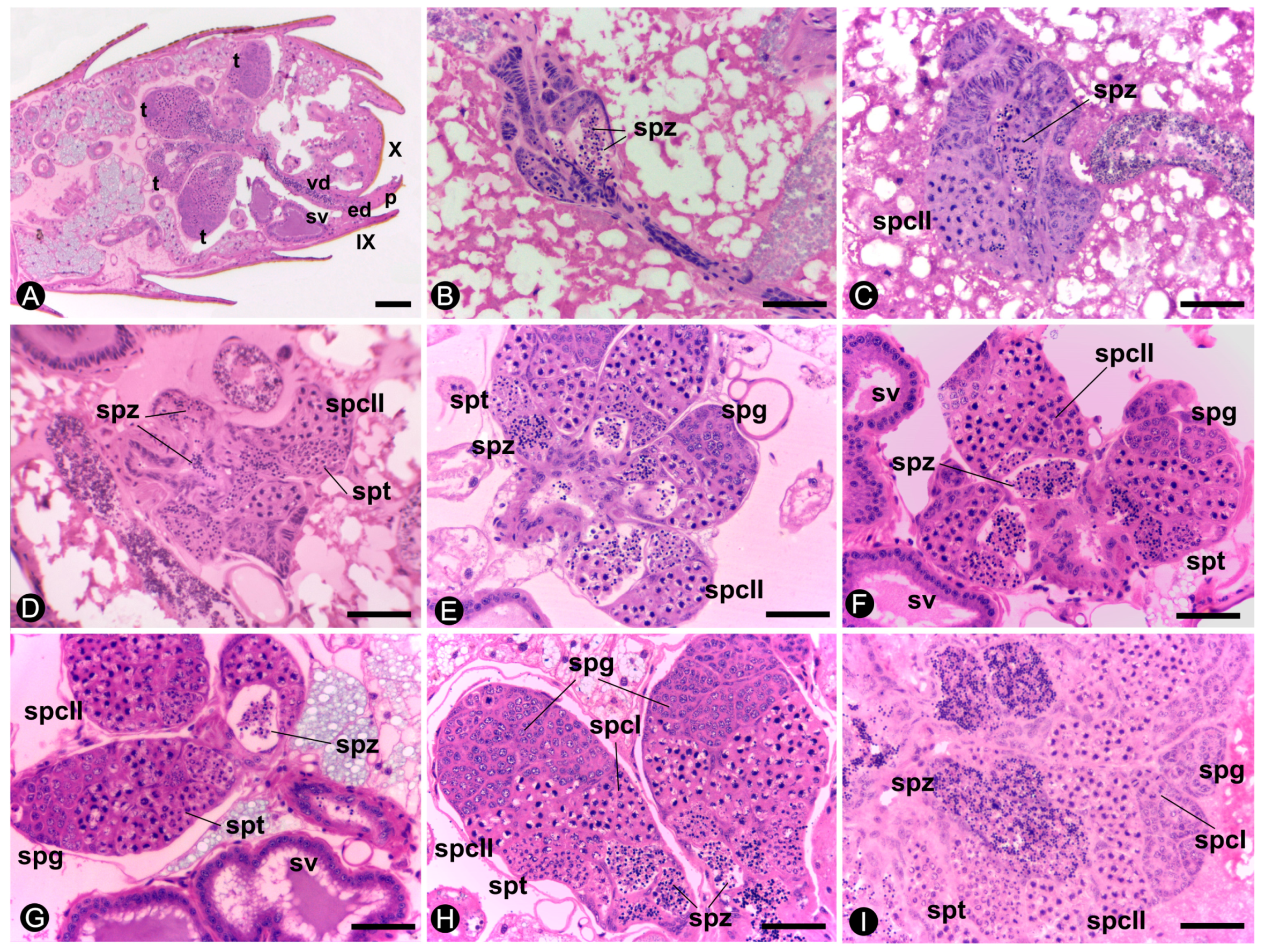
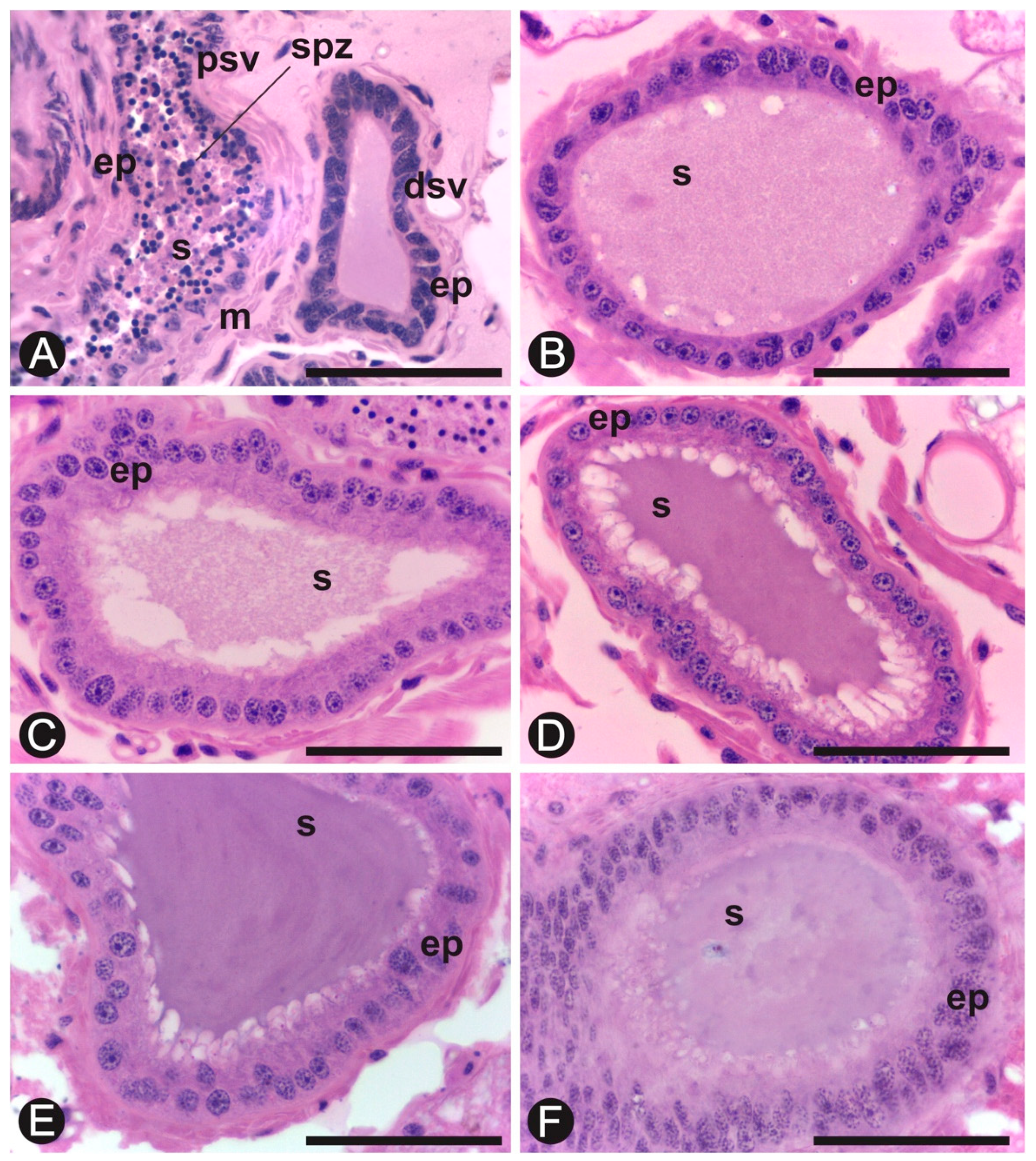
3.2. Histology and Histochemistry
3.3. Morphometry of the Testes
3.4. Transmission Electron Microscopy (TEM)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Takematsu, Y.; Yoshimura, T.; Yusuf, S.; Yanase, Y.; Kambara, K.; Tashiro, A.; Doi, S.; Takahashi, M.; Sukartana, P.; Inoue, T. Termite assemblages in urban areas of southeast Asia-diversity and economic impacts. In Sustainable Development and Utilization of Tropical Forest Resources, 1st ed.; Imamura, Y., Umezawa, T., Hata, T., Eds.; Kyoto University: Kyoto, Japan, 2006; Volume 1, pp. 84–91. [Google Scholar]
- Milano, S.; Fontes, L.R. Cupim e Cidade: Implicações Ecológicas e Controle, 1st ed.; Milano, S., Fontes, L.R., Eds.; Conquista Artes Gráficas: São Paulo, Brazil, 2002. [Google Scholar]
- Costa-Leonardo, A.M. Cupins-Praga: Morfologia, Biologia e Controle, 1st ed.; Ana Maria Costa-Leonardo: Rio Claro, Brazil, 2002. [Google Scholar]
- Costa-Leonardo, A.M.; Arab, A. Reproductive strategy of Coptotermes gestroi (Isoptera: Rhinotermitidae). Sociobiology 2004, 44, 123–125. [Google Scholar]
- Lelis, A.T. Primeiro registro no Brasil de rainha de substituição de Coptotermes havilandi e sua implicação no controle desse cupim. Vetores e Pragas 1999, 4, 19–23. [Google Scholar]
- Noirot, C. Formation of castes in the higher termites. In Biology of Termites, 1st ed.; Krishna, K., Weesner, F.M., Eds.; Academic Press: New York, NY, USA, 1969; Volume 1, pp. 311–350. [Google Scholar]
- Noirot, C. Sexual castes and reproductive strategies in termites. In Social Insects an Evolutionary Approach to Castes and Reproduction, 1st ed.; Engels, W., Ed.; Springer: New York, NY, USA, 1990; Volume 1, pp. 5–35. [Google Scholar]
- Barsotti, R.C.; Costa-Leonardo, A.M. The caste system of Coptotermes gestroi (Isoptera, Rhinotermitidae). Sociobiology 2005, 46, 1–17. [Google Scholar]
- Albino, E.; Costa-Leonardo, A.M. Nymphs in foraging populations of Coptotermes gestroi (Isoptera: Rhinotermitidae). Anim. Biol. 2011, 61, 427–439. [Google Scholar] [CrossRef]
- Hartke, T.R.; Baer, B. The mating biology of termites: A comparative review. Anim. Behav. 2011, 82, 927–936. [Google Scholar] [CrossRef]
- Grassé, P.P. Termitologia; Masson: Paris, France, 1982. [Google Scholar]
- Baccetti, B.; Dallai, R.; Rosati, F.; Giusti, F.; Bernini, F.; Selmi, G. The spermatozoon of Arthropoda. XXVI. The spermatozoon of Isoptera, Embioptera and Dermaptera. J. Microsc. 1974, 21, 159–172. [Google Scholar]
- Dallai, R.; Gottardo, M.; Beutel, R.G. Structure and evolution of insect sperm: New interpretations in the age of plylogenomics. Annu. Rev. Entomol. 2016, 61, 1–23. [Google Scholar] [CrossRef]
- Roonwal, M.L. External genitalia of termites (Isoptera). J. Zool. Soc. India 1955, 7, 107–114. [Google Scholar]
- Weesner, F.M. The reproductive system. In Biology of Termites, 1st ed.; Krishna, K., Weesner, F.M., Eds.; Academic Press: New York, NY, USA, 1969; Volume 3, pp. 125–160. [Google Scholar]
- Klass, K.D. The male abdomen of the relic termite Mastotermes darwiniensis Insecta: Isoptera: Mastotermitidae. Zool. Anz. 2000, 239, 231–262. [Google Scholar]
- Klass, K.D.; Thorne, B.L.; Lenz, M. The male postabdomen of Stolotermes inopinus: A termite with unusually well-developed external genitalia (Dictyoptera: Isoptera: Stolotermitinae). Acta Zool. 2000, 81, 121–130. [Google Scholar] [CrossRef]
- Baccetti, B.; Dallai, R. The spermatozoon of Arthropoda. XXX. The multiflagellate spermatozoon in the termite Mastotermes darwiniensis. J. Cell Biol. 1978, 76, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Baccetti, B.; Dallai, R.; Callaini, G. The spermatozoon of Arthropoda: Zootermopsis nevadensis and isopteran sperm phylogeny. Int. J. Inver. Rep. 1981, 3, 87–89. [Google Scholar] [CrossRef]
- Costa-Leonardo, A.M.; Barsotti, R.C. Swarming and incipient colonies of Coptotermes havilandi (Isoptera, Rhinotermitidae). Sociobiology 1998, 31, 131–142. [Google Scholar]
- Junqueira, L.C.U.; Junqueira, L.M.M.S. Técnicas Básicas de Citologia e Histologia, 1st ed.; Editora Santos: São Paulo, Brazil, 1983. [Google Scholar]
- Vidal, B.C.; Mello, M.L.S. Biologia Celular, 1st ed.; Atheneu: São Paulo, Brazil, 1986. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2019. Available online: http://www.R-project.org/ (accessed on 26 April 2019).
- Chapman, R.F. The Insects: Structure and Function, 4th ed.; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Springhetti, A.D.; Oddone, P. Sugli organi genitali maschili delle Rhinotermitidæ (Isoptera). Insect. Soc. 1963, 10, 143–152. [Google Scholar] [CrossRef]
- Ye, Y.; Jones, S.C.; Ammar, E. Reproductive characteristics of imagos of Reticulitermes flavipes (Isoptera: Rhinotermitidae). Ann. Entomol. Soc. Am. 2009, 102, 889–894. [Google Scholar] [CrossRef]
- Baccetti, B. The evolution of the acrosomal complex. In The Spermatozoon, 1st ed.; Fawcett, D.W., Bedford, J.M., Eds.; Urban and Schwarzenberg: Munich, Germany, 1979; Volume 1, pp. 305–329. [Google Scholar]
- Jamieson, B.G.M. The Ultrastructure and Phylogeny of Insect Spermatozoa; Cambridge University Press: Cambridge, UK, 1987. [Google Scholar]
- Oguchi, K.; Shimoji, H.; Hayashi, Y.; Miura, T. Reproductive organ development along the caste differentiation pathways in the dampwood termite Hodotermopsis sjostedti. Insect. Soc. 2016, 63, 519–529. [Google Scholar] [CrossRef]
- Wu, J.; Su, X.; Kong, X.; Liu, M.; Xing, L. Multiple male and female reproductive strategies and the presence of a polyandric mating system in the termite Reticulitermes labralis (Isoptera: Rhinotermitidae). Sociobiology 2013, 60, 459–465. [Google Scholar] [CrossRef][Green Version]
- Barbosa, J.R.C.; Constantino, R. Polymorphism in the neotropical termite Serritermes serrifer. Entomol. Exp. Appl. 2017, 163, 43–50. [Google Scholar] [CrossRef]
- Lenz, M.; Barrett, R.A. Neotenic formation in field colonies of Coptotermes lacteus (Froggatt) in Australia, with comments on the roles of neotenics in the genus Coptotermes (Isoptera: Rhinotermitidae). Sociobiology 1982, 7, 47–59. [Google Scholar]
- Laranjo, L.T.; Haifig, I.; Costa-Leonardo, A.M. Morphology of the male reproductive system during post-embryonic development of the termite Silvestritermes euamignathus (Isoptera: Termitidae). Zool. Anz. 2018, 272, 20–28. [Google Scholar] [CrossRef]
- Haifig, I.; Costa-Leonardo, A.M. Caste differentiation pathways in the Neotropical termite Silvestritermes euamignathus (Isoptera: Termitidae). Entomol. Sci. 2016, 19, 174–179. [Google Scholar] [CrossRef]
- Laranjo, L.T.; Costa-Leonardo, A.M. First record of intersex in neotenic reproductives of the termite Cryptotermes brevis (Isoptera: Kalotermitidae). Entomol. Sci. 2016, 20, 142–149. [Google Scholar] [CrossRef]
- Laranjo, L.T.; Silva, I.B.; Costa-Leonardo, A.M. Development and comparative morphology of the reproductive system in different aged males of the drywood termite Cryptotermes brevis (Blattaria, Isoptera, Kalotermitidae). Protoplasma 2019. [Google Scholar] [CrossRef] [PubMed]
- Weesner, F.M. The reproductive systems of young primary reproductives of Tenuirostritermes tenuirostris (Desneux). Insect. Soc. 1955, 2, 323–345. [Google Scholar] [CrossRef]
- Noirot, C.; Quennedey, A. Fine structure of insect epidermal glands. Annu. Rev. Entomol. 1974, 19, 61–80. [Google Scholar] [CrossRef]
- Noirot, C.; Quennedey, A. Glands, gland cells, glandular units: Some comments on terminology and classification. Ann. Soc. Entomol. Fr. 1991, 27, 123–128. [Google Scholar]
- Geyer, J.W.C. The reproductive organs of certain termites, with notes on the hermaphrodites of Neotermes. Entomol. Mem. Dept. Agr. Union S. Afr. 1951, 2, 231–325. [Google Scholar]
- Spiegel, C.N.; Bretas, J.A.C.; Peixoto, A.A.; Vigoder, F.M.; Bruno, R.V.; Sores, M.J. Fine structure of the male reproductive system and reproductive behavior of Lutzomyia longipalpis sandflies (Diptera: Psychodidae: Phlebotominae). PLoS ONE 2013, 8, e74989. [Google Scholar] [CrossRef]
- Avila, F.W.; Sirot, L.K.; LaFlamme, B.B.; Rubinstein, C.D.; Wolfner, M.F. Insect seminal fluid proteins: Identification and function. Ann. Rev. Entomol. 2011, 56, 21–40. [Google Scholar] [CrossRef]
- King, M.; Eubel, H.; Millar, A.H.; Baer, B. Proteins within the seminal fluid are crucial to keep sperm viable in the honeybee Apis mellifera. J. Insect Physiol. 2011, 57, 409–414. [Google Scholar] [CrossRef]
- Wolfner, M.F. Tokens of love: Functions and regulation of Drosophila male accessory gland products. Insect Biochem. Molec. 1997, 27, 179–192. [Google Scholar] [CrossRef]
- Gillott, C. Male acessory gland secretions: Modulators of female reproductive physiology and behavior. Ann. Rev. Entomol. 2003, 48, 163–184. [Google Scholar] [CrossRef]
| Stage | Number of Individuals (n) | Testicular Area (μm2) |
|---|---|---|
| Nymph 3 | 3 | 9618.00 ± 129.61 a |
| Nymph 4 | 3 | 10204.30 ± 217.90 a |
| Nymph 5 | 3 | 20893.45 ± 155.91 b |
| Alate | 6 | 28478.40 ± 446.12 c |
| Neotenic | 3 | 34415.66 ± 121.50 d |
| 6-month king | 3 | 38402.90 ± 743.36 e |
| 1-year king | 6 | 40024.95 ± 171.60 e |
| 4-year king | 3 | 76808.26 ± 421.89 f |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laranjo, L.T.; Haifig, I.; Costa-Leonardo, A.M. A Glycoproteinaceous Secretion in the Seminal Vesicles of the Termite Coptotermes gestroi (Isoptera: Rhinotermitidae). Insects 2019, 10, 428. https://doi.org/10.3390/insects10120428
Laranjo LT, Haifig I, Costa-Leonardo AM. A Glycoproteinaceous Secretion in the Seminal Vesicles of the Termite Coptotermes gestroi (Isoptera: Rhinotermitidae). Insects. 2019; 10(12):428. https://doi.org/10.3390/insects10120428
Chicago/Turabian StyleLaranjo, Lara T., Ives Haifig, and Ana Maria Costa-Leonardo. 2019. "A Glycoproteinaceous Secretion in the Seminal Vesicles of the Termite Coptotermes gestroi (Isoptera: Rhinotermitidae)" Insects 10, no. 12: 428. https://doi.org/10.3390/insects10120428
APA StyleLaranjo, L. T., Haifig, I., & Costa-Leonardo, A. M. (2019). A Glycoproteinaceous Secretion in the Seminal Vesicles of the Termite Coptotermes gestroi (Isoptera: Rhinotermitidae). Insects, 10(12), 428. https://doi.org/10.3390/insects10120428