Abstract
Bactrocera carambolae is one of the approximately 100 sibling species of the Bactrocera dorsalis complex and considered to be very closely related to B. dorsalis. Due to their high morphological similarity and overlapping distribution, as well as to their economic impact and quarantine status, the development of reliable markers for species delimitation between the two taxa is of great importance. Here we present the complete mitochondrial genome of B. carambolae sourced from its native range in Malaysia and its invaded territory in Suriname. The mitogenome of B. carambolae presents the typical organization of an insect mitochondrion. Comparisons of the analyzed B. carambolae sequences to all available complete mitochondrial sequences of B. dorsalis revealed several species-specific polymorphic sites. Phylogenetic analysis based on Bactrocera mitogenomes supports that B. carambolae is a differentiated taxon though closely related to B. dorsalis. The present complete mitochondrial sequences of B. carambolae could be used, in the frame of Integrative Taxonomy, for species discrimination and resolution of the phylogenetic relationships within this taxonomically challenging complex, which would facilitate the application of species-specific population suppression strategies, such as the sterile insect technique.
1. Introduction
The Bactrocera dorsalis species complex consists of approximately 100 morphologically similar taxa distributed mainly in South-East Asia and Australasia [1]. Although most members within the complex present no economic interest, a small number of them are serious pests infesting many commercial fruits. Among them are the Oriental fruit fly, B. dorsalis (including the species formerly known as B. philippinensis, B. papayae and B. invadens), and the Carambola fruit fly, B. carambolae, both of which are highly destructive and invasive [2]. Hence, the clarification of their phylogenetic relationships and the development of robust species discriminating tools for the above taxa presents not only scientific, but also great economic interest as the outcome potentially affects international trade regulations and quarantine policies. In the frame of Area-Wide Integrated Pest Management (AW-IPM), availability of population-specific and species-specific markers is also critical. As examples, such markers can support the quick identification of the origin of new invasions or expansions of pests as well as the development and application of species-specific pest control methods that include mass rearing and release of laboratory insects to suppress local populations, such as the sterile insect technique (SIT), since they allow both assessing the suitability of different strains for local applications and identifying released males [3,4].
Species delimitation among the members of the B. dorsalis complex has been a long-standing issue because of their overlapping geographical distributions [1,5], overlapping host range [6], the lack of prominent discriminating morphological characteristics and the significant intraspecific morphological variations [7]. Recently, three taxa have been synonymized as one biological species: B. philippinensis (hereafter B. ‘syn. philippinensis’) was first synonymized by Drew and Romig [1] as B. papayae (hereafter B. ‘syn. papayae’) while Schutze et al. [8] subsequently synonymized both B. ‘syn. papayae’ and B. invadens (hereafter B. ‘syn. invadens’) as B. dorsalis. The latter taxonomic revision was supported by multidisciplinary evidence from morphological, morphometric, molecular/genetic, cytogenetic, behavioral/sexual compatibility and chemo-ecological studies [8,9,10,11,12,13,14,15,16,17,18,19,20,21]. However, the synonymization by Schutze et al. [8] was criticized [22] and debated [23]. The species status of B. carambolae in relation to B. dorsalis has also been explored by many researchers using multiple approaches. Data on morphology/morphometrics [1,24], certain genetic markers [12,14,21,25,26,27,28,29], mating behavior [11] and chemoecology [20,30,31,32] supported the identity of B. carambolae as a separate biological species and provided some diagnostic features for species discrimination. On the other hand, identification of morphological hybrids [33] and data from nuclear protein coding genes [14] and microsatellite analysis [34] suggest naturally occurring hybridization and gene flow between the two taxa.
Mitochondrial DNA (mtDNA) is a very popular molecular marker for evolutionary, phylogenetic and population genetic studies and is informative for analyses at several taxonomic levels [35]. Partial mitochondrial sequences have been extensively used for exploring relationships among species of the Bactrocera genus; however, they had their limitations, for instance in the discrimination among closely related members of the B. dorsalis complex [9,10,12,14,15,25,27,36,37,38,39,40,41]. On the other hand, complete mitochondrial genome sequences, which are accumulating rapidly in databases nowadays, have proven to be a valuable alternative approach for phylogeny reconstruction and molecular systematics in several insect groups [42,43,44,45,46,47,48,49,50], including Tephritidae [51,52,53,54,55,56,57,58,59,60,61,62,63,64]. Especially, when the discrimination of closely related species is attempted, the comparative analysis of complete mitogenomes can help to select the most informative mitochondrial markers/sequences for specific issues [53]. However, it is becoming more evident that factors related to mtDNA inheritance, such as bottlenecks, introgression, heteroplasmy and sweeps by reproductive symbionts, restrict the usefulness of mtDNA as a standalone marker for species delimitation [65,66,67]. Therefore, the combined use of both mitochondrial and nuclear genetic markers together with information from different disciplines is expected to provide a more accurate and indisputable species resolution under the umbrella of Integrative Taxonomy [68].
In the current study, the complete mitochondrial genome sequences of three well-characterized B. carambolae specimens originating from the native as well as from the invaded territory of the species were generated and described in detail. The new Β. carambolae mitogenomes together with the one that was already available in the databases were compared against three complete mitochondrial sequences of B. dorsalis generated in the present study and all available ones from the databases, including B. ‘syn. philippinensis’, B. ‘syn. papayae’ and B. ‘syn. invadens’, attempting to identify potential species-specific polymorphic sites throughout the mitogenome. Furthermore, a phylogenetic analysis within Bactrocera was performed focusing on the placing of the B. carambolae complete mitogenomes in comparison to B. dorsalis.
2. Materials and Methods
2.1. Insects
The B. carambolae specimens used in this study originated from Malaysia and Suriname. The Malaysian strains were collected from infested wax apples (Syzygium spp.) from the forest fringe in Raub, Pahang state, Malaysia. The emerged adults from the infested fruits were subjected to morphological identification based on three key morphological characteristics: (a) the presence of a recurved pattern on the wing costal band beyond apex R4+5, (b) the presence of a fore femoral dark spot and (c) the presence of bar-shaped bands at terga III–V [3]. Close morphological identification confirmed all flies emerged from this source was B. carambolae. Flies were laboratory reared for 2–3 generations on carambola (Averrhoa carambola) fruits (27 ± 2 °C, 85% ± 5% RH, 12 h L: 12 h D) in order to confirm the species status of the offspring and to raise a sufficient number of flies. The sample from Suriname came from a laboratory colony initiated by insects collected from carambola fruits from the districts of Paramaribo and Saramacca, Suriname, and reared on carambola fruits for 21 generations (24 °C, 85% RH, 12 h L: 12 h D) in the Carambola fruit fly unit, Department of Agricultural Research, Ministry of Agriculture, Animal Husbandry and Fisheries, Paramaribo. Pupae from the above strains were sent to the Insect Pest Control Laboratory (IPCL) of the Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture (Seibersdorf, Austria) and adults emerging from these pupae were used in the present study. In addition, B. dorsalis specimens from laboratory colonies maintained on artificial diet (25 ± 2 °C, 60% ± 5% RH, 14 h L: 10 h D) at the IPCL were used. The above colonies represented three populations originating from Saraburi (Thailand), Philippines (B. ‘syn. philippinensis’) and Kenya (B. ‘syn. invadens’). Their status has been verified by taxonomists and the insect materials have been used in several research projects [11,17,18,19,20,69].
2.2. DNA Isolation, Amplification and Sequencing
Total genomic DNA was extracted from single flies, using either the CTAB protocol [70] or the DNeasy Blood and Tissue kit (Qiagen, Hilden, Germany) following manufacturer’s instructions for total DNA purification from animal tissues. Negative controls were included in DNA extraction. DNA quality and quantity were measured using the NanoDrop 1000 Spectrophotometer (Thermo Fischer Scientific, Waltham, MA, USA).
Each mitogenome sequence was obtained from a single specimen by standard PCR amplifications using primers that were designed based on the mitochondrial sequence of Bactrocera dorsalis (accession no NC_008748; Supplementary Table S1). Twenty-seven pairs of primers targeting overlapping fragments were designed by the Oligoexplorer and Oligoanalyzer programs (Supplementary Table S2). Approximately 30 ng of template DNA was used in each reaction of 25 μL (1× PCR buffer, 1.5 mM MgCl2, 0.2 mM of each dNTP, 0.5 μM of the appropriate primers and 1 U Taq polymerase). The BIOTAQ (BIOLINE, UK) or the One Taq (NEB, Ipswich, MA, USA) DNA polymerases were used. Amplification was performed in a SensoQuest thermocycler by the following program: initial denaturation at 94 °C for 3 min, 40 cycles of 45 section denaturation at 94 °C, 30 section primer annealing at 46–59 °C and 1 min DNA chain extension at 72 °C, and final extension at 72 for 7 min. PCR products were purified by the Nucleospin Gel and PCR Clean up kit (Macherey Nagel, Düren, Germany) or by Exonuclease I and Shrimp Alkaline Phosphatase (NEB, USA).
Sequencing reactions were performed by Macrogen Europe (Amsterdam, The Netherlands) or Eurofins Genomics (Ebersberg, Germany). Each fragment was sequenced in both directions and the sequences obtained by the forward and the reverse reactions were merged using EMBOSS Merger [71] after careful manual inspection. In cases of inconsistencies reactions were repeated. The mitogenome sequences were assembled using EMBOSS Merger [71] and submitted to GenBank (accession nos.: KT343905, MG916998, MN104217-20, Supplementary Table S1).
2.3. Sequence Analysis
Sequence annotation was manually performed by comparison to the B. dorsalis mitogenome sequence (accession no NC_008748; Supplementary Table S1). The secondary structure and the presence of specific anticodons of the 22 tRNAs were checked by tRNAscan-SE [72] (http://lowelab.ucsc.edu/tRNAscan-SE/) and MITOS [73] (http://mitos.bioinf.uni-leipzig.de/index.py). Repeats in the control region were found by the “Tandem Repeat Finder” program [74] (http://tandem.bu.edu/trf/trf.html). Multiple sequence alignments for genome annotation as well as for identification of polymorphic sites were performed by ClustalOmega (www.ebi.ac.uk) using default parameters.
2.4. Phylogenetic Analysis
Phylogenetic analysis based on concatenated COI and ND4 partial gene sequences was performed using B. dorsalis and B. carambolae sequences from different locations previously analyzed by Boykin et al. [27] (Supplementary Table S3) together with the corresponding gene fragments from the complete sequences generated in the present study (Supplementary Table S1) (dataset 1). Phylogenetic analysis based on alignments of complete mtDNA sequences was performed using all Bactrocera complete mitogenomes available (Supplementary Table S1) (dataset 2). Multiple sequence alignments were constructed by ClustalW using default parameters. Phylogenetic trees were inferred by the Maximum Likelihood (ML) method based on the Hasegawa–Kishino–Yano model (dataset 1) or the General Time Reversible (GTR) (dataset 2) model with 1000 bootstrap replicates. All the above analyses, alignments, model selection and phylogeny reconstruction, were performed in MEGA 7.0 [75,76,77]. Dataset 2 was also analyzed by maximum likelihood (ML) inference using IQ-TREE 1.4.2 [78] and, in particular, the IQ-TREE web server (http://iqtree.cibiv.univie.ac.at) [79]. The best-fit substitution model was determined by IQ-TREE (“Auto” option in the field Substitution model) including FreeRate heterogeneity in the model selection process (“Yes [+R]” option in the field FreeRate heterogeneity). To assess nodal support, 1000 ultrafast (UFBoot) [80] bootstrap replicates were performed.
3. Results and Discussion
The mitogenomes of three B. carambolae specimens, two from Malaysia (M5 and M8) and one from Suriname (S2) were analyzed. In order to further substantiate the species characterization of the specimens, we performed a phylogenetic analysis based on COI + ND4 partial mitochondrial sequences from the B. dorsalis complex previously used in several studies [10,12,27] together with the ones generated in the present study (Supplementary Tables S1 and S3). The above analysis clustered the sequences of our specimens together with the other B. carambolae and separately from all B. dorsalis sequences (Supplementary Figure S1), which, although in a context of low nodal support, supports their identification as true representatives of B. carambolae.
The mitogenomes of the M8 and S2 individuals were completely sequenced and found to be of 15,918 and 15,912 bp long, respectively. Frοm the M5mitogenome, 15,034 bp were successfully sequenced, while part from the non-coding region between the 12S rRNA and tRNAIle genes of about 900 bp in size was missing. Each mitogenome contains 13 protein-coding, two rRNA (12S and 16S rRNA) and 22 tRNA genes, and one major non-coding sequence, the control region (Figure 1; Table 1). All mitogenomes presented very high A + T content (72.64–72.75%, excluding control region) with gene arrangements identical to other Bactrocera mitogenomes [51,53,54,55,56,62,63,64,81,82,83].
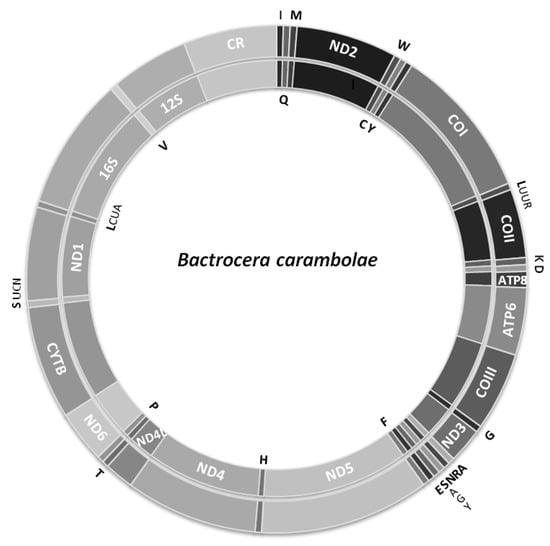
Figure 1.
The Bactrocera carambolae mitochondrial genome. Genes shown at the outer circle are encoded by the H-strand whereas those at the inner circle are encoded by the L-strand. Abbreviations as in Table 1.

Table 1.
Organization of the Bactrocera carambolae mitochondrial genome.
3.1. Protein-Coding Genes
The majority of the protein-coding genes (PCGs) are encoded by the H strand and only ND1, ND4, ND4L and ND5 are encoded by the L strand (Table 1). The initiation codons were identical to those reported for B. dorsalis PCGs [53], i.e., ATG for COII, ATP6, COIII, ND4, ND4L and CYTB; ATT for ND2, ND3, ND5 and ND6; ATA for ND1; TCG for COI and GTG for ATP8 (Table 1). The GTG initiation codon seems to be characteristic for the ATP8 gene of the species of the Bactrocera subgenus [53,54,55,56,62,63,64,83,84]. Three of the PCGs possess an incomplete termination codon (Table 1); TA for COI, which is characteristic for all Bactrocera species analyzed so far and T for ND1 and ND5 similarly to the majority of tephritids [52,53,54,55,56,57,58,59,60,61,62,63,64,81,82,83,84,85,86,87,88]. Incomplete termination codons are common in animal mitochondrial DNA and are likely to be completed by post-transcriptional polyadenylation [89].
The overlaps of seven nucleotides between ATP8 and ATP6 and ND4 and ND4L genes are the longest observed between protein-coding genes of B. carambolae. Overlaps restricted to one or two nucleotides can also be observed between ATP6 and COIII, ND3 and tRNAAla, ND6 and CYTB and CYTB and tRNASer (Table 1). Overlaps that are similar in size and position are common among tephritids [52,55,58,59,60,61,62,63,64].
3.2. RNA Genes
The 16S rRNAs of B. carambolae M5 and S2 individuals consist of 1332 nucleotides (positions: 12,776–14,107 and 12,773–14,104, respectively), while that of the M8 appears to be one nucleotide shorter (positions: 12,773–14,103) (Table 1). Similarly, the 12S rRNA genes are 790 nucleotides long for M5 and S2 and 789 for M8 (positions: 14,180–14,969, 14,177–14,966 and 14,176–14,964, respectively) (Table 1). In accordance with other insect mitogenomes, these genes are located in the L strand between the gene for tRNALeu (CUA) and the control region, and are separated by the tRNAVal gene (Table 1). The 22 tRNA genes, predicted to fold into the expected cloverleaf secondary structures, are dispersed among the protein-coding and the rRNA genes; 14 of them lie on the H and 8 on the L strand of the mtDNA (Table 1). Their positions and sizes (63–72 nucleotides) follow the typical organization for insect mtDNA.
3.3. Non-Coding Regions
Similarly, to all tephritids, the mitogenome of B. carambolae contains only one long non-coding region, i.e., the control region (D-loop), located between the 12S rRNA and the tRNAIle genes (Figure 1). Its length was found to be 949 and 948 nucleotides and its A+T content was found to be 87.14% and 87.87% for M8 and S2 individuals, respectively (Table 1).
A stretch of 22 thymidines resides at the 5′ end of the D-loop (near to the tRNAIle gene), a feature that is common among tephritid and other insect mitogenomes, and is believed to play a role in the control of transcription and/or replication [54,59,60,81,90]. The 13 nucleotide long motifs TTTAATTTTTTAA and TTAATTTTATTAA were found to be tandemly repeated four times at the same position (D-loop position 212–262) of the M8 and S2 individuals, respectively. Tandem repeats have been identified in the control regions of Bactrocera as well as in other tephritid species [54,60,81].
The longest intergenic spacer (IGS) region in the analyzed B. carambolae mitogenomes was found between the tRNAGln and tRNAMet genes with a size of 66 nucleotides for both M5 and M8 individuals and 67 nucleotides for S2 (Table 1). Although the position of the longest IGS seems to be conserved among several species of the Bactrocera subgenus [53,55,56,62,63], the sequence presents no significant similarity except within the B. dorsalis complex (the sequence identity between B. carambolae and B. dorsalis was about 97%). The second longest IGS is located between the tRNACys and tRNATyr genes and is 46 nucleotides long in all three B. carambolae specimens analyzed (Table 1). This IGS folded into secondary structures and its first 33 nucleotides could be found repeated in the D-loop region of B. carambolae, which is similar to other Bactrocera species as well as members of the B. dorsalis complex suggesting recombination events [53,81]. Yu et al. [53] reported an 11 bp insertion at the end of this spacer in a B. carambolae specimen and suggested that it could be used as a specific marker for species discrimination between B. carambolae and the other members of the complex. However, the above insertion was not observed in any of the three B. carambolae specimens analyzed. Furthermore, a short TA repeat making this IGS longer (53 compared to 46 bp) was also found in one of the B. dorsalis sequences generated in the present study (accession no KT343905). The above findings suggest that small insertions in the spacer lying between the tRNACys and tRNATyr genes are more likely to represent individual- or population- rather than species-specific polymorphisms.
3.4. Sequence Comparisons and Phylogenetic Analysis
The three B. carambolae mitogenomes analyzed here were compared to the complete mitochondrial sequences of B. carambolae (one) and B. dorsalis (six) found in GenBank and to the three additional sequences of the B. dorsalis complex generated in the present study (Supplementary Table S1). The identity scores obtained between the complete mitogenome sequences from B. carambolae and B. dorsalis ranged from 98.45% to 98.98% being imperceptibly lower than the ones observed among the B. dorsalis sequences (98.88–99.49%). The identity scores were extremely high even for the D-loop region, which is considered the most variable region of the mitogenome (95.68–98.52% between B. carambolae and B. dorsalis; 97.99–99.16% among B. dorsalis).
However, alignment of the above sequences revealed a small number (12) of positions that consistently differed between the B. carambolae and the B. dorsalis sequences (Table 2). Almost all of the above polymorphisms were found within the PCG sequences and could be potential markers for discriminating the two very closely related taxa analyzed. Nonetheless, additional data at population level is required to assess whether these polymorphisms are fixed and species-specific.

Table 2.
The interspecies nucleotide polymorphisms observed in the complete mitochondrial sequences of B. dorsalis and B. carambolae used in the present study. Gene abbreviation as in Table 1 Position refers to nucleotide position within respective gene.
The ML phylogenetic analysis with the complete Bactrocera mitochondrial sequences (six generated in the present study and 20 from GenBank) (Supplementary Table S1) conducted with MEGA software (Figure 2) resulted in almost identical tree topology to the one inferred by the IQ-Tree ML algorithm (data not shown). Topologies were very similar to other recent analyses also using data of complete mitogenomes to explore phylogenetic relationships within the Bactrocera genus [54,58,63,82,83,91,92] and confirmed the very close relationship of the B. dorsalis complex members. Within the complex (Figure 2B), the B. dorsalis sequences formed a highly supported clade, while all B. carambolae sequences, though not forming a single clade, were placed outside the B. dorsalis clade. The above results suggest the differentiation of the B. carambolae mitosequence and could provide some support to the retention of B. carambolae as a separate taxon from B. dorsalis [8]. However, additional data and analyses would be required to clarify the issue of species limits between the above two taxa.
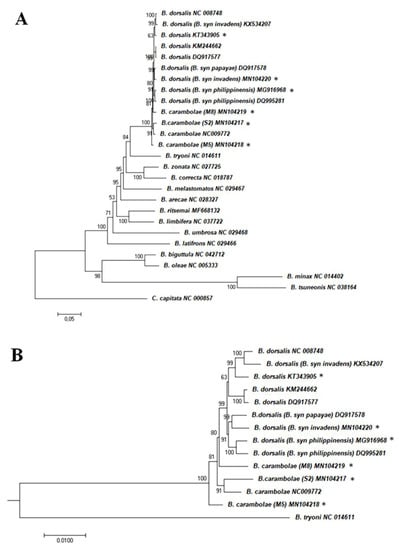
Figure 2.
Molecular phylogenetic analysis by Maximum Likelihood method. (A) Tree based on 26 Bactrocera complete mitochondrial genome sequences; (B) part of the tree depicted in (A) presenting only the clade of the B. dorsalis complex sequences. Ceratitis capitata was used as outgroup to root the tree. The evolutionary history was inferred by using the Maximum Likelihood method based on the General Time Reversible model. The percentage of trees in which the associated taxa clustered together is shown next to the branches; only the ones higher than 50 are presented. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site (scale bar = 0.05 (A) or 0.01 (B) substitutions per site). Asterisks indicate the sequences analyzed in the present study. Sequences’ accession numbers in Supplementary Table S1.
In summary, the complete mitochondrial sequence of three B. carambolae specimens is presented. These are the first published B. carambolae mitogenomes described in detail, though not the first appearing in databases. The structure and the organization of the B. carambolae mitogenomes analyzed follow the typical pattern of an insect mitochondrion. The availability of several complete B. carambolae mitogenomes allowed, through sequence alignments against all available B. dorsalis mitogenomes, the identification of potentially species-specific nucleotide polymorphisms. Phylogenetic analyses within the Bactrocera genus supported the differentiation of B. carambolae in comparison to B. dorsalis. The future disposal of additional complete mitosequences from other members of the B. dorsalis complex could enable more extensive comparative analyses, to aim for a better resolution of their evolutionary relationships and for identification of the most informative polymorphic mitochondrial regions. Nevertheless, multidisciplinary approaches, combining mitochondrial and nuclear genetic information together with data on different aspects of species biology in the frame of Integrative Taxonomy, are considered necessary for reliable identification of species boundaries within this speciose complex of destructive pests.
Supplementary Materials
The following are available online at https://www.mdpi.com/2075-4450/10/12/429/s1, Figure S1. Molecular phylogenetic analysis by Maximum Likelihood method based on concatenated partial sequences of the COI and ND4 genes of 38 B. carambolae and B. dorsalis specimens, Table S1. List of the mitogenome sequences used in the present study, Table S2. List of the primers used for the amplification of the mitogenomes of the Bactrocera carambolae and Bactrocera dorsalis specimens, Table S3. List of the GenBank accession numbers of the COI and ND4 partial sequences from B. dorsalis and B. carambolae used in the present study.
Author Contributions
Conceptualization E.D., A.A.A., A.Z. and K.B.; funding acquisition A.Z.; specimen collection and identification A.v.S.-M., S.-L.W.; Methodology and Experiments E.D., A.S., P.G., G.-A.Z., T.K., D.P., G.S.; Data analysis E.D., A.S., P.G., G.-A.Z., T.K., D.P., G.S., A.A.A.; writing—original draft preparation E.D., A.A.A., K.B.; writing—review and editing, E.D., A.v.S.-M., S.-L.W., A.A.A., K.B., A.Z.; all authors have read and approved the final article for submission.
Funding
The present study has been funded by the Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture through Coordinated Research Projects (CRP) and Special Service Agreements (SSA).
Acknowledgments
We would like to thank Carlos Caceres and the Plant Pests group of the Insect Pest Control Laboratory of the Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture for providing biological material. We would also like to thank Ilias Kappas for his valuable help with the phylogenetic analyses.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Drew, R.A.I.; Romig, M.C. Tropical Fruit Flies of South-East Asia (Tephritidae: Dacinae); CABI: Wallingford, UK, 2013. [Google Scholar]
- Clarke, A.R.; Armstrong, K.F.; Carmichael, A.E.; Milne, J.R.; Roderick, G.K.; Yeates, D.K. Invasive phytophagous pests arising through a recent tropical evolutionary radiation: The Bactrocera dorsalis complex of fruit flies. Ann. Rev. Entomol. 2005, 50, 293–319. [Google Scholar] [CrossRef]
- Krafsur, E.S. Role of Population Genetics in the Sterile Insect Technique. In Sterile Insect Technique; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 389–406. [Google Scholar]
- Hendrichs, J.; Teresa Vera, M.; De Meyer, M.; Clarke, A.R. Resolving cryptic species complexes of major tephritid pests. Zookeys 2015, 540, 5–39. [Google Scholar] [CrossRef]
- Drew, R.A.I.; Hancock, D.L. The Bactrocera dorsalis complex of fruit flies (Diptera: Tephritidae: Dacinae) in Asia. Bull. Entomol. Res. Suppl. Ser. 1994, 2, 1–68. [Google Scholar] [CrossRef]
- Allwood, A.J.; Chinajariyawong, A.; Drew, R.A.I.; Hameck, E.L.; Hancock, D.L.; Hengsawad, J.C.; Jipanin, M.; Kon Krong, C.; Kritsaneepaiboon, S.; Leong, C.T.S.; et al. Host plant records for fruit flies (Diptera: Tephritidae) in South East Asia. Raffles Bull. Zool. 1999, 47 (Suppl. 7), 1–92. [Google Scholar]
- Leblanc, L.; San Jose, M.; Barr, N.; Rubinoff, D. A phylogenetic assessment of the polyphyletic nature and intraspecific color polymorphism in the Bactrocera dorsalis complex (Diptera, Tephritidae). Zookeys 2015, 540, 339–367. [Google Scholar] [CrossRef]
- Schutze, M.K.; Aketarawong, N.; Amornsak, W.; Armstrong, K.F.; Augustinos, A.A.; Barr, N.; Bo, W.; Bourtzis, K.; Boykin, L.M.; Caceres, C.; et al. Synonymization of key pest species within the Bactrocera dorsalis species complex (Diptera: Tephritidae): Taxonomic changes based on a review of 20 years of integrative morphological, molecular, cytogenetic, behavioural and chemoecological data. Syst. Entomol. 2015, 40, 456–471. [Google Scholar] [CrossRef]
- Khamis, F.M.; Masiga, D.K.; Mohamed, S.A.; Salifu, D.; de Meyer, M.; Ekesi, S. Taxonomic identity of the invasive fruit fly pest, Bactrocera invadens: Concordance in morphometry and DNA barcoding. PLoS ONE 2012, 7, e44862. [Google Scholar] [CrossRef]
- Schutze, M.K.; Krosch, M.N.; Armstrong, K.F.; Chapman, T.A.; Englezou, A.; Chomic, A.; Cameron, S.L.; Hailstones, D.; Clarke, A.R. Population structure of Bactrocera dorsalis s.s., B. papayae and B. philippinensis (Diptera: Tephritidae) in southeast Asia: Evidence for a single species hypothesis using mitochondrial DNA and wing-shape data. BMC Evol. Biol. 2012, 12, 130. [Google Scholar] [CrossRef]
- Schutze, M.K.; Jessup, A.; Ul-Haq, I.; Vreysen, M.J.; Wornoayporn, V.; Vera, M.T.; Clarke, A.R. Mating compatibility among four pest members of the Bactrocera dorsalis fruit fly species complex (Diptera: Tephritidae). J. Econ. Entomol. 2013, 106, 695–707. [Google Scholar] [CrossRef]
- Schutze, M.K.; Mahmood, K.; Pavasovic, A.; Bo, W.; Newman, J.; Clarke, A.R.; Krosch, M.N.; Cameron, S.L. One and the same: Integrative taxonomic evidence that Bactrocera invadens (Diptera: Tephritidae) is the same species as the Oriental fruit fly Bactrocera dorsalis. Syst. Entomol. 2015, 40, 472–486. [Google Scholar] [CrossRef]
- Krosch, M.N.; Schutze, M.K.; Armstrong, K.F.; Boontop, Y.; Boykin, L.M.; Chapman, T.A.; Englezou, A.; Cameron, S.L.; Clarke, A.R. Piecing together an integrative taxonomic puzzle: Microsatellite, wing shape and aedeagus length analyses of Bactrocera dorsalis s.l. (Diptera: Tephritidae) find no evidence of multiple lineages in a proposed contact zone along the Thai/Malay Peninsul. Syst. Entomol. 2013, 38, 2–13. [Google Scholar] [CrossRef]
- San Jose, M.; Leblanc, L.; Geib, S.M.; Rubinoff, D. An Evaluation of the Species Status of Bactrocera invadens and the Systematics of the Bactrocera dorsalis (Diptera: Tephritidae) Complex. Ann. Entomol. Soc. Am. 2013, 106, 1–11. [Google Scholar] [CrossRef]
- Frey, J.E.; Guillén, L.; Frey, B.; Samietz, J.; Rull, J.; Aluja, M. Developing diagnostic SNP panels for the identification of true fruit flies (Diptera: Tephritidae) within the limits of COI-based species delimitation. BMC Evol. Biol. 2013, 13, 106. [Google Scholar] [CrossRef]
- Aketarawong, N.; Isasawin, S.; Thanaphum, S. Evidence of weak genetic structure and recent gene flow between Bactrocera dorsalis s.s. and B. papayae, across Southern Thailand and West Malaysia, supporting a single target pest for SIT applications. BMC Genet. 2014, 15, 70. [Google Scholar] [CrossRef]
- Augustinos, A.A.; Drosopoulou, E.; Gariou-Papalexiou, A.; Bourtzis, K.; Mavragani-Tsipidou, P.; Zacharopoulou, A. The Bactrocera dorsalis species complex: Comparative cytogenetic analysis in support of Sterile Insect Technique applications. BMC Genet. 2014, 15 (Suppl. 2), S16. [Google Scholar] [CrossRef]
- Augustinos, A.A.; Drosopoulou, E.; Gariou-Papalexiou, A.; Asimakis, E.D.; Cáceres, C.; Tsiamis, G.; Bourtzis, K.; Mavragani-Tsipidou, P.; Zacharopoulou, A. Cytogenetic and symbiont analysis of five members of the B. dorsalis complex (Diptera, tephritidae): No evidence of chromosomal or symbiont-based speciation events. Zookeys 2015, 540, 273–298. [Google Scholar]
- Hee, A.; Ooi, Y.-S.; Wee, S.-L.; Tan, K.-H. Comparative sensitivity to methyl eugenol of four putative Bactrocera dorsalis complex sibling species: Further evidence that they belong to one and the same species B. dorsalis. ZooKeys 2015, 540, 313–321. [Google Scholar] [CrossRef]
- Vaničkova, Â.L.; Nagy, R.; Pompeiano, A.; Kalinova, Â.B. Epicuticular chemistry reinforces the new taxonomic classification of the Bactrocera dorsalis species complex (Diptera: Tephritidae,Dacinae). PLoS ONE 2017, 12, e0184102. [Google Scholar] [CrossRef]
- Dupuis, J.R.; Bremer, F.T.; Kauwe, A.; San Jose, M.; Leblanc, L.; Rubinoff, D.; Geib, S.M. HiMAP: Robust phylogenomics from highly multiplexed amplicon sequencing. Mol. Ecol. Resour. 2018, 18, 1000–1019. [Google Scholar] [CrossRef]
- Drew, R.A.I.; Romig, M.C. Keys to the Tropical Fruit Flies of South-East Asia; CABI: Wallingford, UK, 2016. [Google Scholar]
- Schutze, M.K.; Bourtzis, K.; Cameron, S.; Clarke, A.R.; de Meyer, M.; Hee, A.K.W.; Hendrichs, J.; Krosch, M.; Mwatawala, M. Integrative taxonomy versus taxonomic authority without peer review: The case of the Oriental fruit fly, Bactrocera dorsalis (Tephritidae). Syst. Entomol. 2017, 42, 609–620. [Google Scholar] [CrossRef]
- Iwahashi, O. Distinguishing between the two sympatric species Bactrocera carambolae and B. papayae (Diptera: Tephritidae) based on aedeagal length. Ann. Entomol. Soc. Am. 1999, 92, 639–643. [Google Scholar] [CrossRef]
- Armstrong, K.F.; Ball, S.L. DNA barcodes for biosecurity: Invasive species identification. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 1813–1823. [Google Scholar] [CrossRef]
- Armstrong, K.; Cameron, C. Species identification of tephritids across a broad taxonomic range using ribosomal DNA. In Area-Wide Control of Fruit Flies and Other Insect Pests; Tan, K.H., Ed.; Penerbit Universiti Sains Malaysia: Pulau Pinang, Malaysia, 2000; pp. 703–710. [Google Scholar]
- Boykin, L.M.; Schutze, M.K.; Krosch, M.N.; Chomič, A.; Chapman, T.A.; Englezou, A.; Armstrong, K.F.; Clarke, A.R.; Hailstones, D.; Cameronet, S.L. Multi-gene phylogenetic analysis of south-east Asian pest members of the Bactrocera dorsalis species complex (Diptera: Tephritidae) does not support current taxonomy. J. Appl. Entomol. 2014, 138, 235–253. [Google Scholar] [CrossRef]
- San Jose, M.; Doorenweerda, C.; Leblanc, L.; Barr, N.; Geib, S.; Rubinoff, D. Incongruence between molecules and morphology: A seven-gene phylogeny of Dacini fruit flies paves the way for reclassification (Diptera: Tephritidae). Mol. Phylogen. Evol. 2018, 121, 139–149. [Google Scholar] [CrossRef]
- Krosch, M.N.; Schutze, M.K.; Strutt, F.; Clarke, A.R.; Cameron, S.L. A transcriptome-based analytical workflow for identifying loci for species diagnosis: A case study with Bactrocera fruit flies (Diptera: Tephritidae). Austral Entomol. 2019, 58, 395–408. [Google Scholar] [CrossRef]
- Fletcher, M.T.; Kitching, W. Chemistry of fruit flies. Chem. Rev. 1995, 95, 789–828. [Google Scholar] [CrossRef]
- Wee, S.L.; Tan, K.H. Temporal accumulation of phenylpropanoids in male fruit flies, Bactrocera dorsalis and B. carambolae (Diptera: Tephritidae) following methyl eugenol consumption. Chemoecology 2007, 17, 81–85. [Google Scholar] [CrossRef]
- Wee, S.L.; Hee, A.K.W.; Tan, K.H. Comparative sensitivity to and consumption of methyl eugenol in three Bactrocera dorsalis (Diptera: Tephritidae) complex sibling species. Chemoecology 2002, 12, 193–197. [Google Scholar] [CrossRef]
- Wee, S.L.; Tan, K.H. Evidence of natural hybridization between two sympatric sibling species of Bactrocera dorsalis complex based on pheromone analysis. J. Chem. Ecol. 2005, 31, 845–858. [Google Scholar] [CrossRef]
- Aketarawong, N.; Isasawin, S.; Sojikul, P.; Thanaphum, S. Gene flow and genetic structure of Bactrocera carambolae (Diptera, Tephritidae) among geographical differences and sister species, B. dorsalis, inferred from microsatellite DNA data. Zookeys 2015, 540, 239–272. [Google Scholar] [CrossRef]
- Avise, J.C. Phylogeography: The History and Formation of Species; Harvard University Press: Cambridge, UK, 2000. [Google Scholar]
- Muraji, M.; Nakahara, S. Phylogenetic relationships among fruit flies, Bactrocera (Diptera, Tephritidae), based on the mitochondrial rDNA sequences. Insect Mol. Biol. 2001, 10, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.T.; Kambhampati, S.; Armstrong, K.A. Phylogenetic relationships among Bactrocera species (Diptera: Tephritidae) inferred from mitochondrial DNA sequences. Mol. Phylogenet. Evol. 2003, 26, 8–17. [Google Scholar] [CrossRef]
- Smith, P.T.; Kambhampati, S.; Armstrong, K.A. Phylogenetic relationships and character evolution among selected species of Bactrocera (Diptera: Tephritidae) based on multiple mitochondrial genes. Insect Syst. Evol. 2005, 36, 343–359. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, Y.H.; Wu, W.X.; Le Wang, Z. Molecular phylogeny of Bactrocera species (Diptera: Tephritidae: Dacini) inferred from mitochondrial sequences of 16S rDNA and COI sequences. Fla. Entomol. 2010, 93, 369–377. [Google Scholar]
- San Jose, M.; Doorenweerd, C.; Leblanc, L.; Barr, N.; Geib, S.; Rubinoff, D. Tracking the Origins of Fly Invasions; Using Mitochondrial Haplotype Diversity to Identify Potential Source Populations in Two Genetically Intertwined Fruit Fly Species (Bactrocera carambolae and Bactrocera dorsalis [Diptera: Tephritidae]). J. Econ. Entomol. 2018, 111, 2914–2926. [Google Scholar] [CrossRef]
- Kunprom, C.; Pramual, P. DNA barcode variability and host plant usage of fruit flies (Diptera: Tephritidae) in Thailand. Genome 2016, 59, 792–804. [Google Scholar] [CrossRef]
- Ballard, J.W.O. Comparative genomics of mitochondrial DNA in members of the Drosophila melanogaster subgroup. J. Mol. Evol. 2000, 51, 48–63. [Google Scholar] [CrossRef]
- Ballard, J.W.O. Comparative genomics of mitochondrial DNA in Drosophila simulans. J. Mol. Evol. 2000, 51, 64–75. [Google Scholar] [CrossRef]
- Yukuhiro, K.; Sezutsu, H.; Itoh, M.; Shimizu, K.; Banno, Y. Significant levels of sequence divergence and gene rearrangements have occurred between the mitochondrial genomes of the wild mulberry silkmoth, Bombyx mandarina, and its close relative, the domesticated silkmoth, Bombyx mori. Mol. Biol. Evol. 2002, 19, 1385–1389. [Google Scholar] [CrossRef]
- Friedrich, M.; Muqim, N. Sequence and phylogenetic analysis of the complete mitochondrial genome of the flour beetle Tribolium castanaeum. Mol. Phylogenet. Evol. 2003, 26, 502–512. [Google Scholar] [CrossRef]
- Cameron, S.L.; Lambkin, C.L.; Barker, S.C.; Whiting, M.F. A mitochondrial genome phylogeny of Diptera: Whole genome sequence data accurately resolve relationships over broad timescales with high precision. Syst. Entomol. 2007, 32, 40–59. [Google Scholar] [CrossRef]
- Wan, X.; Kim, M.I.; Kim, M.J.; Kim, I. Complete mitochondrial genome of the free-living earwig, Challia fletcheri (Dermaptera: Pygidicranidae) and phylogeny of Polyneoptera. PLoS ONE 2012, 7, e42056. [Google Scholar] [CrossRef]
- Nelson, L.A.; Lambkin, C.L.; Batterham, P.; Wallman, J.F.; Dowton, M.; Whiting, M.F.; Yeates, D.K.; Cameron, S.L. Beyond barcoding: A mitochondrial genomics approach to molecular phylogenetics and diagnostics of blowflies (Diptera: Calliphoridae). Gene 2012, 511, 131–142. [Google Scholar] [CrossRef]
- Cameron, S.L. Insect mitochondrial genomics: Implications for evolution and phylogeny. Annu. Rev. Entomol. 2014, 59, 95–117. [Google Scholar] [CrossRef]
- Chen, M.M.; Li, Y.; Chen, M.; Wang, H.; Li, Q.; Xia, R.X.; Zeng, C.Y.; Li, Y.P.; Liu, Y.Q.; Qin, L. Complete mitochondrial genome of the atlas moth, Attacus atlas (Lepidoptera: Saturniidae) and the phylogenetic relationship of Saturniidae species. Gene 2014, 545, 95–101. [Google Scholar] [CrossRef]
- Nardi, F.; Carapelli, A.; Dallai, R.; Frati, F. The mitochondrial genome of the olive fly Bactrocera oleae: Two haplotypes from distant geographical locations. Insect Mol. Biol. 2003, 12, 605–611. [Google Scholar] [CrossRef]
- Nardi, F.; Carapelli, A.; Boore, J.L.; Roderick, G.K.; Dallai, R.; Frati, F. Domestication of olive fly through a multi-regional host shift to cultivated olives: Comparative dating using complete mitochondrial genomes. Mol. Phylogenet. Evol. 2010, 57, 678–686. [Google Scholar] [CrossRef]
- Yu, D.J.; Xu, L.; Nardi, F.; Li, J.G.; Zhang, R.J. The complete nucleotide sequence of the mitochondrial genome of the oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae). Gene 2007, 396, 66–74. [Google Scholar] [CrossRef]
- Choudhary, J.S.; Naaz, N.; Prabhakar, C.S.; Rao, M.S.; Das, B. The mitochondrial genome of the peach fruit fly, Bactrocera zonata (Saunders) (Diptera: Tephritidae): Complete DNA sequence, genome organization, and phylogenetic analysis with other tephritids using next generation DNA sequencing. Gene 2015, 569, 191–202. [Google Scholar] [CrossRef]
- Yong, H.S.; Song, S.L.; Lim, P.E.; Chan, K.G.; Chow, W.L.; Eamsobhana, P. Complete mitochondrial genome of Bactrocera arecae (Insecta: Tephritidae) by next-generation sequencing and molecular phylogeny of Dacini tribe. Sci. Rep. 2015, 5, 15155. [Google Scholar] [CrossRef]
- Yong, H.S.; Song, S.L.; Lim, P.E.; Eamsobhana, P.; Suana, I.W. Complete mitochondrial genome of three Bactrocera fruit flies of subgenus Bactrocera (Diptera: Tephritidae) and their phylogenetic implications. PLoS ONE 2016, 11, 1–13. [Google Scholar] [CrossRef]
- Yong, H.S.; Song, S.L.; Lim, P.E.; Eamsobhana, P.; Suana, I.W. Differentiating sibling speciesof Zeugodacus caudatus (Insecta: Tephritidae) by complete mitochondrial genome. Genetica 2016, 144, 513–521. [Google Scholar] [CrossRef]
- Yong, H.S.; Song, S.L.; Lim, P.E.; Eamsobhana, P. Complete mitochondrial genome of Zeugodacus tau (Insecta: Tephritidae) and differentiation of Z. tau species complex by mitochondrial cytochrome c oxidase subunit I gene. PLoS ONE 2017, 12, e0189325. [Google Scholar] [CrossRef]
- Jiang, F.; Pan, X.; Li, X.; Yu, Y.; Zhang, J.; Jiang, H.; Dou, L.; Zhu, S. The first complete mitochondrial genome of Dacus longicornis (Diptera: Tephritidae) using next-generation sequencing and mitochondrial genome phylogeny of Dacini tribe. Sci. Rep. 2016, 6, 36426. [Google Scholar] [CrossRef]
- Drosopoulou, Ε.; Pantelidou, C.; Gariou-Papalexiou, A.; Augustinos, A.A.; Chartomatsidou, T.; Kyritsis, G.A.; Bourtzis, K.; Mavragani-Tsipidou, P.; Zacharopoulou, Α. The chromosomes and the mitogenome of Ceratitis fasciventris (Diptera: Tephritidae): Two genetic approaches towards the Ceratitis FAR species complex resolution. Sci. Rep. 2017, 7, 4877. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, Y.; Feng, S.; He, J.; Zhao, Z.; Bai, Z.; Liu, L.; Zhang, R.; Li, Z. The mitochondrial genome of the wolfberry fruit fly, Neoceratitis asiatica (Becker) (Diptera: Tephritidae) and the phylogeny of Neoceratitis Hendel genus. Sci. Rep. 2017, 7, 16612. [Google Scholar] [CrossRef]
- Song, S.L.; Yong, H.S.; Suana, I.W.; Lima, P.E. Complete mitochondrial genome of Bactrocera ritsemai (Insecta: Tephritidae) and phylogenetic relationship with its congeners and related tephritid taxa. J. Asia Pac. Entomol. 2018, 212, 52–257. [Google Scholar] [CrossRef]
- Suana, W.; Song, S.L.; Yong, H.S.; Lim, P.E. Complete mitochondrial genome of Bactrocera limbifera (Insecta:Tephritidae) and phylogenetic relationship with its congeners. J. Trop. Entomol. 2018, 7, 1–10. [Google Scholar]
- Zhang, Y.; Feng, S.; Zeng, Y.; Ning, H.; Liu, L.; Zhao, Z.; Jiang, F.; Li, Z. The first complete mitochondrial genome of Bactrocera tsuneonis (Miyake) (Diptera: Tephritidae) by next-generation sequencing and its phylogenetic implications. Int. J. Biol. Macromol. 2018, 118, 1229–1237. [Google Scholar] [CrossRef]
- Rubinoff, D.; Cameron, S.; Will, K. A genomic perspective on the shortcomings of mitochondrial DNA for “Barcoding” Identification. J. Hered. 2006, 97, 581–594. [Google Scholar] [CrossRef]
- Meier, R.; Shiyang, K.; Vaidya, G.; Ng, P.K.L. DNA barcoding and taxonomy in diptera: A tale of high intraspecific variability and low identification success. Syst. Biol. 2006, 55, 715–728. [Google Scholar] [CrossRef]
- Kodandaramaiah, U.; Simonsen, T.J.; Bromilow, S.; Wahlberg, N.; Sperling, F. Deceptive single-locus taxonomy and phylogeography: Wolbachia -associated divergence in mitochondrial DNA is not reflected in morphology and nuclear markers in a butterfly species. Ecol. Evol. 2013, 3, 5167–5176. [Google Scholar] [CrossRef]
- Schlick-Steiner, B.C.; Steiner, F.M.; Seifert, B.; Stauffer, C.; Crozier, R.H. Integrative taxonomy: A multisource approach to exploring biodiversity. Annu. Rev. Entomol. 2010, 55, 421–438. [Google Scholar] [CrossRef]
- Schutze, M.K.; Dammalage, T.; Jessup, A.; Vreysen, M.J.B.; Wornoayporn, V.; Clarke, A.R. Effects of laboratory colonization on Bactrocera dorsalis (Diptera, Tephritidae) mating behaviour: ‘what a difference a year makes’. Zookeys 2015, 540, 369–383. [Google Scholar] [CrossRef]
- Doyle, J.J. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Rice, P.; Longden, I.; Bleasby, A. EMBOSS: The European molecular biology open software suite. Trends Genet. 2000, 16, 276–277. [Google Scholar] [CrossRef]
- Lowe, T.M.; Eddy, S.R. tRNAscan-SE: A program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997, 25, 955–964. [Google Scholar] [CrossRef]
- Bernt, M.; Donath, A.; Jühling, F.; Externbrink, F.; Florentz, C.; Fritzsch, G.; Pütz, J.; Middendorf, M.; Stadler, P.F. MITOS: Improved de novo metazoan mitochondrial genome annotation. Mol. Phylogenet. Evol. 2013, 69, 313–319. [Google Scholar] [CrossRef]
- Benson, G. Tandem repeats finder: A program to analyze DNA sequences. Nucleic Acids Res. 1999, 27, 573–580. [Google Scholar] [CrossRef]
- Hasegawa, M.; Kishino, H.; Yano, T. Dating the human-ape split by a molecular clock of mitochondrial DNA. J. Mol. Evol. 1985, 22, 160–174. [Google Scholar] [CrossRef]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Trifinopoulos, J.; Nguyen, L.T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef]
- Minh, B.Q.; Nguyen, M.A.T.; von Haeseler, A. Ultrafast approximation for phylogenetic bootstrap. Mol. Biol. Evol. 2013, 30, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Nardi, F.; Hull-Sanders, H.; Wan, X.; Liu, Y. The complete nucleotide sequence of the mitochondrial genome of Bactrocera minax (Diptera: Tephritidae). PLoS ONE 2014, 9, e100558. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.J.; Jiang, L.H.; Wei, C.Y.; Liu, R.S.; Liu, X.L.; Li, J.G.; Xue, H.J. The status of Bactrocera invadens Drew, Tsuruta & White (Diptera: Tephritidae) inferred from complete mitochondrial genome analysis. Mitochondrial DNA B 2016, 1, 680–681. [Google Scholar]
- Liu, J.H.; Xu, J.; Li, Y.H.; Dan, W.; Pan, Y. Complete mitochondrial genome of the guava fruit fly, Bactrocera correcta (Diptera: Tephritidae). Mitochondrial DNA 2016, 27, 4553–4554. [Google Scholar] [CrossRef]
- Zhang, K.J.; Liu, L.; Rong, X.; Zhang, G.H.; Liu, H.; Liu, Y.H. The Complete Mitochondrial Genome of Bactrocera diaphora (Diptera: Tephritidae). Mitochondrial DNA B 2016, 27, 4314–4315. [Google Scholar] [CrossRef]
- Liu, J.H.; Liu, P.F.; Liu, L.L.; Wang, Q.M.; Zhang, Y.X.; Ruan, S.Q. Complete mitochondrial genome of stripped fruit fly, Bactrocera (Zeugodacus) scutellata (Diptera: Tephritidae) from Anshun, Southwest China. Mitochondrial DNA B 2017, 2, 387–388. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Kim, M.J.; Kim, J.S.; Kim, I. Complete mitochondrial genome of the pumpkin fruit fly, Bactrocera depressa (Diptera: Tephritidae). Mitochondrial DNA B 2017, 2, 85–87. [Google Scholar] [CrossRef]
- Isaza, J.P.; Alzate, J.F.; Canal, N.A. Complete mitochondrial genome of the Andean morphotype of Anastrepha fraterculus (Wiedemann) (Diptera: Tephritidae). Mitochondrial DNA B 2017, 2, 210–211. [Google Scholar] [CrossRef]
- Wu, P.F.; Roques, A.; Xiong, Z.P.; Xu, L.; Huang, Y.Y.; Pan, Y.Z. The complete mitochondrial genome of the melon fly Bactrocera cucurbitae (Diptera: Tephritidae). Mitochondrial DNA 2013, 24, 6–7. [Google Scholar] [CrossRef]
- Ojala, D.; Montoya, J.; Attardi, G. tRNA punctuation model of RNA processing in human mitochondria. Nature 1981, 290, 470–474. [Google Scholar] [CrossRef]
- Zhang, D.X.; Hewitt, G.M. Insect mitochondrial control region: A review of its structure, evolution and usefulness in evolutionary studies. Biochem. Syst. Ecol. 1997, 25, 99–120. [Google Scholar] [CrossRef]
- Tan, M.; Zhang, R.; Xiang, C.; Zhou, X. The complete mitochondrial genome of the pumpkin fruit fly, Bactrocera tau (Diptera: Tephritidae). Mitochondrial DNA B 2016, 27, 2502–2503. [Google Scholar] [CrossRef]
- Da Costa, L.T.; Powell, C.; van Noort, S.; Costa, C.; Sinno, M.; Caleca, V.; Rhode, C.; Kennedy, R.J.; van Staden, M.; van Asch, B. The complete mitochondrial genome of Bactrocera biguttula (Bezzi) (Diptera: Tephritidae) and phylogenetic relationships with other Dacini. Int. J. Biol. Macromol. 2018, 126, 130–140. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).