The Waggle Dance as an Intended Flight: A Cognitive Perspective
Abstract
1. Introduction
2. Results and Discussion
2.1. Functional Components and Phylogenetic Routes of the Waggle Dance
2.2. What Are Intentions and Do Honeybees Have Intentions?
- (1)
- Haldane and Spurway: “The dance is seen as a highly ritualized intention movement leading to communication which is mainly kinesthetic. The bees which are sent out by the dance fly to the appropriate goal because, by following the dancer, they have automatically carried out the intention movements for the flight. The same principle applies to other communications in which the signal is repeated, including many bird calls” ([7], p. 278).
- (2)
- C.G. Jung et al.: “This kind of message (he means: As encoded in the waggle dance) is no different in principle from the information conveyed by a human being. In the latter case we would certainly regard such behavior as a conscious and intentional act and can hardly imagine how anyone could prove in a court of law that it had taken place unconsciously. Nevertheless it would be possible to suppose that in bees the process is unconscious.” ([30], p. 94).
2.3. Where the Information Comes From: Exploring the Environment
2.4. The Memory About the Landscape as a Cognitive Map
2.5. Integration of Experienced and Communicated Locations in a Common Reference, the Cognitive Map
2.6. What Needs to Be Asked?
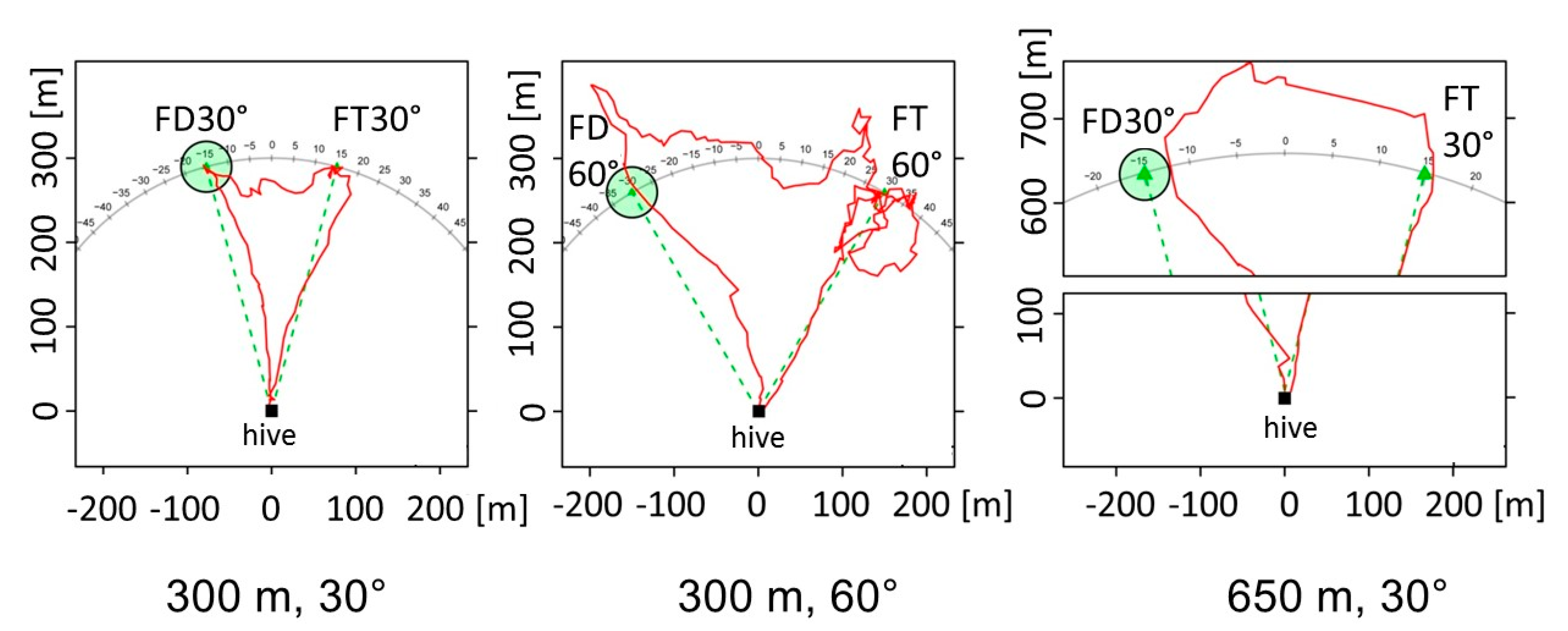
- A life history of social interactions and flight activities of individually identified bees may inform us about how dance following and, later in life, dancing depend on experience with the landscape. For example, does dance following of young bees depend on experience collected during their exploratory orientation flights in the sense that only dances are followed that indicate locations within the explored area or in the direction of the explored sector? Are dances not followed if they point into an unexplored landscape sector? In the context of these questions, new experiments should be done to solve the controversy about the famous “lake experiment” [50]. Gould and Gould [50] asked whether recruits of the waggle dance would reject heading towards an “impossible” place (a place in an unexplored area), e.g., a feeding site on a boat in a lake. They reported some evidence in this direction, but their results could not be replicated [51]. However, since the latter authors trained bees with odor at the feeding site, their controls for rejecting guidance by the odor of the feeder on the boat were not fully convincing. Tautz et al. [52] inadvertently found a similar effect as the Goulds when training across a lake in a study on the effect of different landscapes on odometry. In both “lake experiments” of [50,52], the trained bees danced less actively when the station was in the middle of the lake and thus fewer recruits arrived. When the boat reached land again, recruits reappeared but at the point on the shore nearest to the boat, not at the boat itself, indicating an expectation of where the food source could be. This would possibly indicate that the recruits did not choose an impossible location, or the dancer did not dance for an impossible location. However, again, both studies were not controlled well for this effect and no flight trajectories of the trained bees or the recruits were recorded. Tracking bees with a harmonic radar [53] will be required to solve the problem.
- Given bees’ rich navigational memory, one may ask what exactly is communicated by the waggle dance: Just the outbound vector, or the location of the goal as a spot in the environment defined by its spatial relations to landmarks? Such experiments require uncoupling the vector information from the act of heading to a goal that is not characterized by the endpoint of the vector. This will be possible by releasing the recruits not at the hive entrance, then at different places within the explored area around the hive. If recruits would fly according to the vector information from the dance in the same way as they do when released at the hive entrance, than they apply the flight instruction only. If, however, they also fly towards the real location of the dance vector endpoint, then they refer to additional information that can only derive from their map-like representation learned during exploratory orientation flight. The latter possibility would be particularly strong if one can exclude any foraging of bees in the area around the dance-indicated feeder. An additional experiment could address the question of whether recruits expect certain landscape features on their outbound flights. These expectations can either be any view-based landscape features, spatial relations of objects on the way towards the goal, or particular salient features serving bees as guides toward the goal, like ground landmarks (paths, forest edges, rivers etc.). Again, radar tracking of recruits released at other locations than the hive entrance will be necessary over distances of several hundred meters.
2.7. The Honeybee Dance—Not a Language, but a Form of Rich Symbolic Communication
3. Conclusions
Acknowledgments
Conflicts of Interest
References
- von Frisch, K. The Dance Language and Orientation of Bees; Harvard University Press: Boston, MA, USA, 1967. [Google Scholar]
- Riley, J.R.; Greggers, U.; Smith, A.D.; Reynolds, D.R.; Menzel, R. The flight paths of honeybees recruited by the waggle dance. Nature 2005, 435, 205–207. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.V. Honey bees as a model for vision, perception, and cognition. Annu. Rev. Entomol. 2010, 55, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Edrich, W. Honey bees can perform accurately directed waggle dances based solely on information from a homeward trip. J. Comp. Physiol. A 2015, 201, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Seeley, T.D.; Visscher, P.K.; Schlegel, T.; Hogan, P.M.; Franks, N.R.; Marshall, J.A. Stop signals provide cross inhibition in collective decision-making by honeybee swarms. Science 2012, 335, 108–111. [Google Scholar] [CrossRef]
- Biesmeijer, J.C.; Seeley, T.D. The use of waggle dance information by honey bees throughout their foraging careers. Behav. Ecol. Sociobiol. 2005, 59, 133–142. [Google Scholar] [CrossRef]
- Haldane, J.B.; Spurway, H. A statistical analysis of communication in “apis mellifera” and a comparison with communication in other animals. Insectes Sociaux 1954, 1, 247–283. [Google Scholar] [CrossRef]
- Dethier, V.G.; Richard, L.S.; Turner, L.H. Sensory input and central excitation and inhibition in the blowfly. J. Comp. Physiol. Psychol. 1965, 60, 303–313. [Google Scholar] [CrossRef]
- Dethier, V. Communication by insects: Physiology of dancing. Science 1957, 125, 331–336. [Google Scholar] [CrossRef]
- Frantsevich, L.; Gorb, S. Courtship dances in the flies of the genus lispe (diptera: Muscidae): From the fly’s viewpoint. Arch. Insect Biochem. Physiol. 2006, 62, 26–42. [Google Scholar] [CrossRef]
- Collett, T.S.; Land, M.F. Visual control of flight behaviour in the hoverfly, syritta pipiens. J. Comp. Physiol. 1975, 99, 1–66. [Google Scholar] [CrossRef]
- Blest, A. The evolution, ontogeny and quantitative control of the settling movements of some new world saturniid moths, with some comments on distance communication by honey-bees. Behaviour 1960, 188–253. [Google Scholar] [CrossRef]
- Wagner, V.A. Psycho-Biologische Untersuchungen an Hummeln Mit Bezugnahme Auf Die Frage Der Geselligkeit Im Tierreiche; E. Schweizerbart’sche Verlagsbuchhandlung (E. Nägele): Stuttgart, Germany, 1907. [Google Scholar]
- Lindauer, M.; Kerr, W.E. Die gegenseitige verständigung bei den stachellosen bienen. J. Comp. Physiol. A Neuroethol. Sens. Neuralbehav. Physiol. 1958, 41, 405–434. [Google Scholar]
- Esch, H.; Esch, I.; Kerr, W.E. Sound: An element common to communication of stingless bees and to dances of the honey bee. Science 1965, 149, 320–321. [Google Scholar] [CrossRef] [PubMed]
- Dornhaus, A.; Chittka, L. Evolutionary origins of bee dances. Nature 1999, 401, 38. [Google Scholar] [CrossRef]
- Jacobs-Jessen, U.F. Zur orientierung der hummeln und einiger anderer hymenopteren. Zeitschrift Für Vergleichende Physiologie 1959, 41, 597–641. [Google Scholar] [CrossRef]
- Nieh, J. Recruitment communication in stingless bees (hymenoptera, apidae, meliponini). Apidologie 2004, 35, 159–182. [Google Scholar] [CrossRef]
- Sanchez, D.; Nieh, J.C.; Henaut, Y.; Cruz, L.; Vandame, R. High precision during food recruitment of experienced (reactivated) foragers in the stingless bee scaptotrigona mexicana (apidae, meliponini). Naturwissenschaften 2004, 91, 346–349. [Google Scholar] [CrossRef]
- Haas, A. Das rätsel des hummeltrompeters: Lichtalarm: 1. Bericht über verhaltensstudien an einem kleinen nest von bombus bypnorum mit arbeiter-königin. Zeitschrift Für Tierpsychologie 1961, 18, 129–138. [Google Scholar]
- Pastor, K.A.; Seeley, T.D. The brief piping signal of the honey bee: Begging call or stop signal? Ethology 2005, 111, 775–784. [Google Scholar] [CrossRef]
- Nieh, J.C. The stop signal of honey bees: Reconsidering its message. Behav. Ecol. Sociobiol. 1993, 33, 51–56. [Google Scholar] [CrossRef]
- Ruttner, F. Biogeography and Taxonomy of Honeybees; Springer Science & Business Media: Berlin, Germany, 2013. [Google Scholar]
- Koeniger, N.; Koeniger, G.; Tingek, S. Honey Bees of Borneo: Exploring the Centre of Apis Diversity; Natural History Publications Borneo: Kota Kinabalu, Malaysia, 2010. [Google Scholar]
- Lindauer, M. Über die verständigung bei indischen bienen. Z. Vergl. Physiol. 1956, 38, 521–557. [Google Scholar] [CrossRef]
- Koeniger, N.; Koeniger, G. Observations and experiments on migration and dance communication of apsis dorsata in sri lanka. J. Apic. Res. 1980, 19, 21–34. [Google Scholar] [CrossRef]
- Dyer, F.C.; Seeley, T.D. Dance dialects and foraging range in three asian honey bee species. Behav. Ecol. Sociobiol. 1991, 28, 227–233. [Google Scholar] [CrossRef]
- Birukov, G. Photomenotaktische transpositionen bei geotrupes silvaticus. Rev. Swiss Zool. 1953, 60, 535–554. [Google Scholar]
- Jander, R. Insect orientation. Annu. Rev. Entomol. 1963, 8, 95–114. [Google Scholar] [CrossRef]
- Jung, C.G.; Jung, C.; Von Franz, M.-L.; Henderson, J.L.; Jacobi, J.; Jaffe, A. Man and His Symbols; Dell: Round Rock, TX, USA, 1964. [Google Scholar]
- von Frisch, K.; Lindauer, M. Himmel und erde in konkurrenz bei der orientierung der bienen. Naturwiss 1954, 41, 245–253. [Google Scholar] [CrossRef]
- Menzel, R. Navigation and communication in insects. In Learning and Memory: A Comprehensive Reference; Byrne, J.H., Ed.; Academic Press: Amsterdam, The Netherlands, 2017; Volume 1, pp. 389–410. [Google Scholar]
- Capaldi, E.A.; Smith, A.D.; Osborne, J.L.; Fahrbach, S.E.; Farris, S.M.; Reynolds, D.R.; Edwards, A.S.; Martin, A.; Robinson, G.E.; Poppy, G.M.; et al. Ontogeny of orientation flight in the honeybee revealed by harmonic radar. Nature 2000, 403, 537–540. [Google Scholar] [CrossRef]
- Degen, J.; Kirbach, A.; Reiter, L.; Lehmann, K.; Norton, P.; Storms, M.; Koblofsky, M.; Winter, S.; Georgieva, P.B.; Nguyen, H.; et al. Exploratory behaviour of honeybees during orientation flights. Anim. Behav. 2015, 102, 45–57. [Google Scholar] [CrossRef]
- Degen, J.; Kirbach, A.; Reiter, L.; Lehmann, K.; Norton, P.; Storms, M.; Koblofsky, M.; Winter, S.; Georgieva, P.B.; Nguyen, H.; et al. Honeybees learn landscape features during exploratory orientation flights. Curr. Biol. 2016, 26, 2800–2804. [Google Scholar] [CrossRef]
- Degen, J.; Hovestadt, T.; Storms, M.; Menzel, R. Exploratory behavior of re-orienting foragers differs from other flight patterns of honeybees. PLoS ONE 2018, 13, e0202171. [Google Scholar] [CrossRef]
- Menzel, R.; Brandt, R.; Gumbert, A.; Komischke, B.; Kunze, J. Two spatial memories for honeybee navigation. Proc. R. Soc. Lond. B Biol. Sci. 2000, 267, 961–968. [Google Scholar] [CrossRef]
- Menzel, R.; De Marco, R.J.; Greggers, U. Spatial memory, navigation and dance behaviour in apis mellifera. J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 2006, 192, 889–903. [Google Scholar] [CrossRef]
- Menzel, R.; Greggers, U.; Smith, A.; Berger, S.; Brandt, R.; Brunke, S.; Bundrock, G.; Hulse, S.; Plumpe, T.; Schaupp, F.; et al. Honey bees navigate according to a map-like spatial memory. Proc. Natl. Acad. Sci. USA 2005, 102, 3040–3045. [Google Scholar] [CrossRef] [PubMed]
- Cruse, H.; Wehner, R. No need for a cognitive map: Decentralized memory for insect navigation. PLoS Comput. Biol. 2011, 7, e1002009. [Google Scholar] [CrossRef]
- Cheeseman, J.F.; Winnebeck, E.C.; Millar, C.D.; Kirkland, L.S.; Sleigh, J.; Goodwin, M.; Pawley, M.D.; Bloch, G.; Lehmann, K.; Menzel, R.; et al. General anesthesia alters time perception by phase shifting the circadian clock. Proc. Natl. Acad. Sci. USA 2012, 109, 7061–7066. [Google Scholar] [CrossRef]
- Cheeseman, J.F.; Millar, C.D.; Greggers, U.; Lehmann, K.; Pawley, M.D.; Gallistel, C.R.; Warman, G.R.; Menzel, R. Way-finding in displaced clock-shifted bees proves bees use a cognitive map. Proc. Natl. Acad. Sci. USA 2014, 111, 8949–8954. [Google Scholar] [CrossRef]
- Menzel, R.; Kirbach, A.; Haass, W.-D.; Fischer, B.; Fuchs, J.; Koblofsky, M.; Lehmann, K.; Reiter, L.; Meyer, H.; Nguyen, H.; et al. A common frame of reference for learned and communicated vectors in honeybee navigation. Curr. Biol. 2011, 21, 645–650. [Google Scholar] [CrossRef]
- Beutler, R. Zeit und raum im leben der bienen. Naturwissenschaften 1950, 37, 102–105. [Google Scholar] [CrossRef]
- Lau, C.W.; Nieh, J.C. Honey bee stop-signal production: Temporal distribution and effect of feeder crowding. Apidologie 2010, 41, 87–95. [Google Scholar] [CrossRef]
- Nieh, J.C. A negative feedback signal that is triggered by peril curbs honey bee recruitment. Curr. Biol. 2010, 20, 310–315. [Google Scholar] [CrossRef]
- Johnson, B.R.; Nieh, J.C. Modeling the adaptive role of negative signaling in honey bee intraspecific competition. J. Insect Behav. 2010, 23, 459–471. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kietzman, P.M.; Visscher, P.K. The anti-waggle dance: Use of the stop signal as negative feedback. Front. Ecol. Evol. 2015, 3, 14. [Google Scholar] [CrossRef]
- Jack-McCollough, R.T.; Nieh, J.C. Honeybees tune excitatory and inhibitory recruitment signalling to resource value and predation risk. Anim. Behav. 2015, 110, 9–17. [Google Scholar] [CrossRef]
- Gould, J.L.; Gould, C.G. The insect mind: Physics or metaphysics. In Animal Mind-Human Mind; Griffin, D.R., Ed.; Springer: New York, NY, USA, 1982; pp. 269–298. [Google Scholar]
- Wray, M.K.; Klein, B.A.; Mattila, H.R.; Seeley, T.D. Honeybees do not reject dances for “implausible” locations: Reconsidering the evidence for cognitive maps in insects. Anim. Behav. 2008, 76, 261–279. [Google Scholar] [CrossRef]
- Tautz, J.; Zhang, S.W.; Spaethe, J.; Brockmann, A.; Si, A.; Srinivasan, M. Honeybee odometry: Performance in varying natural terrain. PLoS Biol. 2004, 2, E211. [Google Scholar] [CrossRef]
- Riley, J.R.; Smith, A.D.; Reynolds, D.R.; Edwards, A.S.; Osborne, J.L.; Williams, I.H.; Carreck, N.L.; Poppy, G.M. Tracking bees with harmonic radar. Nature 1996, 379, 29–30. [Google Scholar] [CrossRef]
- Campbell, R.; MacSweeney, M.; Waters, D. Sign language and the brain: A review. J. Deaf Stud. Deaf Educ. 2008, 13, 3–20. [Google Scholar] [CrossRef]
- Lindauer, M. Kommunikation im bienenstaat–arbeitsteilung, nahrungssuche, wohnungssuche. Nova Acta Leopold. Leipz. 1981, 54, 414–430. [Google Scholar]
- Hockett, C.F. Logical Considerations in the Study of Animal Communication; American Institute of Biological Sciences: Washington, DC, USA, 1960. [Google Scholar]
- Sebeok, T.A. How Animals Communicate; Indiana University Press: Indiana Bloomington, IN, USA, 1977. [Google Scholar]
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menzel, R. The Waggle Dance as an Intended Flight: A Cognitive Perspective. Insects 2019, 10, 424. https://doi.org/10.3390/insects10120424
Menzel R. The Waggle Dance as an Intended Flight: A Cognitive Perspective. Insects. 2019; 10(12):424. https://doi.org/10.3390/insects10120424
Chicago/Turabian StyleMenzel, Randolf. 2019. "The Waggle Dance as an Intended Flight: A Cognitive Perspective" Insects 10, no. 12: 424. https://doi.org/10.3390/insects10120424
APA StyleMenzel, R. (2019). The Waggle Dance as an Intended Flight: A Cognitive Perspective. Insects, 10(12), 424. https://doi.org/10.3390/insects10120424



