Abstract
Conservative techniques, such as ground cover management, could help promote viticulture sustainability, which is a goal of conservation biological control, by providing shelter and food sources for predatory insects. A field experiment was conducted in a Mediterranean vineyard to evaluate ground cover management impacts on predatory insect and potential grapevine pest abundance and diversity, both on the ground and in the grapevine canopy. Three different ground cover management techniques (tillage, spontaneous cover and flower-driven cover) were tested for two years (2016 and 2017). Overall, the ground cover management significantly affected the abundance of important epigeal predators, of which carabids, forficulids and staphylinids were the most captured. The carabid abundances under both the cover crop treatments were found to be approximately three times higher compared with that under the tillage treatment. In contrast, the canopy insect abundance in the vineyard was similar among the treatments for both the predators and the potential grapevine pest species. These results indicate that cover crop vegetation can be used in vineyards to enhance predatory insect abundance and may improve agroecosystem resilience.
1. Introduction
Agriculture is an important human activity that affects ecosystem sustainability. Land use intensification impacts agroecosystem functioning by reducing biodiversity and causing shifts in functional composition [1,2,3]. Grape is a major monoculture crop worldwide with high levels of habitat disturbance due to, among other things, considerable use of agrochemicals [4]. Biological control of pests is an important ecosystem service and considered a valuable alternative to chemical control, contributing to achievement of sustainable viticulture [5,6].
Insects constitute a crucial component of agroecosystem biodiversity, and they are essential in maintenance of soil structure and fertility, organic matter decomposition, seed dispersion, crop pollination and pest control [4]. Predatory insects are a very important group of natural enemies of pests, and their community structure and composition have substantial impacts on biological control effectiveness [7,8]. Although native generalist predators play an important role as phytophagous population regulators in agroecosystems, their importance has not been recognised until relatively recently [9]. Nevertheless, their conservation is the core of conservation biological control (CBC) [10,11]. Some of these generalist predators, such as ground beetles, are also used as indicators of ecological sustainability because of their clear response to habitat changes, large numbers of species, ease of capture and wide distribution [12,13]. It is worth noting that both specialist and generalist predators live together in agroecosystems. For example, in vineyards, ground beetles (Coleoptera: Carabidae), earwigs (Dermaptera: Forficulidae), rove beetles (Coleoptera: Staphylinidae), ladybirds (Coleoptera: Coccinellidae), lacewings (Neuroptera: Chrysopidae), predatory bugs (Heteroptera) and other predators were found [14,15].
Insect presence is usually positively correlated with vegetation abundance and diversity [16,17]. Therefore, creating adequate vegetation infrastructure in or around crops is a sustainable measure to increase predator abundance and diversity [18,19]. Among such agroecological infrastructures, cover crops, composed of native or sown vegetation, are ideal candidates to enhance biodiversity and promote soil conservation [20]. Thus, cover crop use can affect sustainable viticulture, because it greatly influences ecosystem services and promotes CBC goals by providing favourable microclimates, shelter and food sources for predatory insects, which need pollen, floral and extrafloral nectars [21,22,23]. Several researchers have reported an increment of abundance and/or diversity of arthropod predators, as result of this kind of ground cover management in vineyards [24,25,26]. Nevertheless, increasing vegetation diversity is no guarantee of pest control, and pest species may even take advantage of benefits provided by cover crops [27]. Therefore, plant selectivity should be carefully considered to avoid promotion of pest species.
This study is part of a research project focused on effects of different ground cover management strategies in a Mediterranean vineyard on total and functional abundance and diversity of arthropods. This study was undertaken to evaluate ground cover management impacts on predatory insect abundance and diversity in a Mediterranean vineyard. Three different ground cover management strategies were tested: (i) tillage; (ii) native vegetation; and (iii) flower mixture seeded. The overall impact on insect abundance and diversity at an order level was recently reported [14], as was their effects on predatory mites [25]. Herein, we addressed the following questions: (i) What are the predatory and potential pest insect assemblages at both the ground and grapevine canopy levels? (ii) How do ground cover management techniques impact predator and potential grapevine pest abundance and diversity? We hypothesised that cover crop use in vineyards, both composed of native vegetation or a mixture of flowering plants sown, supports abundance and diversity of natural enemies, such as insect predators, and may help meet CBC goals. In addition, we observed that cover vegetation enhanced beneficial entomofauna without promoting grapevine pests. Thus, cover crop use in vineyards may improve agroecosystem resilience.
2. Materials and Methods
2.1. Study Site and Experimental Design
This study was performed in 2 hectares of a rain-fed vineyard in La Rioja (Northern Spain) (42°26′ N, 2°30′ W). The vineyard was planted in 1995 with the “Tempranillo” variety of Vitis vinifera, with a planting pattern of 2.9 m between rows and that of 1.15 m within rows. The soil texture was characterised by loam and sandy loam with low organic matter (<1%). Three soil management techniques (9 plots in total, 3 per treatment) were analysed using a completely randomised design for two years (2016 and 2017): (i) tillage; (ii) spontaneous cover; and (iii) flower-driven cover. Each plot comprised 360 vines and an area of 1200 m2. In the tillage treatment, the most common undervine management technique used in Spanish vineyards with bare soil (15–20 cm depth) was practised. For the spontaneous cover treatment, the ground vegetation was mowed in June once every year. The weed community was dominated by annual dicotyledonous plants with relatively short and early flowering periods and was mainly characterised by Veronica hederifolia (Scrophulariaceae), Urtica dioica (Urticaceae), Bromus tectorum (Poaceae), Stellaria media (Caryophyllaceae), Hordeum murinum (Poaceae), Capsella bursa pastoris (Brassicaceae) and Papaver rhoeas (Papaveraceae). Besides, the flower-driven cover treatment was sown every year in March with “Deco Vignes Anuelles” (Nova Flore, Champigné, France) (20 kg/ha). It was characterised by Calendula officinalis (Compositae), Centaurea cyanus (Asteraceae), Cosmos bipinnatus (Asteraceae), Dahlia sp. (Asteraceae), Eschscholzia californica (Papaveraceae), and Lepidium sp. (Brassicaceae). Flowers were selected that had a good balance of high-quality nectar and pollen, bright colours and gradually bloomed throughout the vegetative cycle of the vine. Furthermore, a detailed vegetation characterisation (relative abundance and diversity values) of the spontaneous and flower-driven cover treatments can be found in [25]. Finally, the vineyard management followed environmentally friendly strategies, which included mating disruption for Lobesia botrana Den & Schiff (Lepidoptera: Tortricidae) control and no herbicide use. Moreover, pesticides were mainly applied to control Eotetranychus carpini (Oudemans) (Acari: Tetranychidae), Erysiphe necator Schwein (“powdery mildew”) and Plasmopara viticola (Berk. & M.A. Curtis) Berl & De Toni (“downy mildew”) (Supplementary Table S1).
2.2. Insect Sampling
Insects were collected twice a month from the beginning of May to the end of September in both 2016 and 2017 (20 sampling events across both the years). The insect fauna was studied using two different sampling techniques to provide a broad understanding of the main groups of predatory and pest insects. We used pitfall traps at a ground level and vacuum sampling in the grapevine canopy. The pitfall traps consisted of a plastic bottle with a funnel, which contained 150 mL of 25% ethylene glycol, and two per plot were located along the central row under the canopy approximately 30 m apart. These were active continuously between the samplings. Additionally, the vacuum sampling was carried out with a field aspirator, InsectZooka 2888A® (BioQuip Products, Rancho Dominguez, CA, USA), for 2 min per plot. All the samples were preserved in 70% ethanol at 3 °C until insect identification. Adult predatory insects were sorted to morphospecies and in specific ground beetles to genus. Potential grapevine pests were identified to species level. The insects were identified with help of Chinery, Triplehorn and Johnson, Jeannel, Herrera and Arriabita as well as Ortuño and Marcos [28,29,30,31,32].
2.3. Data Analyses
Relative abundance (%) (proportion of collected insects from each studied taxa of the total number) was calculated for the predatory and potential grapevine pest insects collected by the pitfall and the vacuum sampling to analyse insect community assemblages. Insect data were tested for normality (Kolmogorov–Smirnov test) and homogeneity of variances (Levene’s test), and they were log (x + 1)-transformed, when homoscedasticity was violated. To test the impact of the ground management technique, the effects of each treatment on the cumulative insect abundance and diversity were analysed by two-way ANOVA and a post-hoc Tukey’s honestly significant difference (HSD) test (α = 0.05). The dependent variables were analysed with respect to the factors: year, treatment and interaction year × treatment. The ground and canopy samples were analysed separately. A single pitfall sample was constituted by a combination of two traps per plot. All the analyses were performed in SPSS 20.0 (SPSS Statistics, SPSS Inc., Chicago, IL, USA). Biodiversity was evaluated using Hill numbers (qD), also known as “effective number of species” or “true diversity”, which allows for a more accurate interpretation of results [33,34]. The order of diversity (q) represents sensitivity to common and rare species. q = 0 indicates the species richness; q = 1 indicates the exponential form of the Shannon–Wiener index (H′); and q = 2 indicates the inverse of the Simpson index (λ). Additionally, figures were prepared using GraphPad Prism for Windows 8.00 (GraphPad Inc., La Jolla, CA, USA).
3. Results
3.1. Epigeal and Canopy Insect Assemblages
In total, 3560 predatory and potential pest insects were collected during the two years of study; 87.39% and 12.61% were captured using pitfall and vacuum sampling, respectively. The predators dominated the epigeal insect assemblages compared with the potential grapevine pests (99.52% vs. 0.48%). The ground beetles, the earwigs and the rove beetles were the most representative families captured by pitfall traps (67.66%, 19.67% and 3.60%, respectively). On the contrary, the ratio of the predators to the potential grapevine pests in the grapevine canopy was 6:4, mainly because of the abundance of Empoasca vitis (Goethe) (Hemiptera: Cicadellidae) (37.64%). Most of the predatory insect families collected by vacuum sampling belonged to Aeolothripidae (Thysanoptera), Chrysopidae, Cecidomyiidae (Diptera) and Coccinellidae (34.52%, 10.47%, 8.46% and 6.01%, respectively). Each of these natural enemy families is able to support biological control of different grapevine pests.
The richness of the predator families (n = 15), composed of Carabidae, Forficulidae, Aeolothripidae, Staphylinidae, Cecidomyiidae, Chrysopidae, Coccinellidae, Reduviidae (Heteroptera), Miridae (Heteroptera), Crabronidae (Hymenoptera), Vespidae (Hymenoptera), Sphecidae (Hymenoptera), Anthocoridae (Heteroptera), Geocoridae (Heteroptera) and Asilidae (Diptera), was considerably higher compared with that of the potential grapevine pest species (n = 4). Regarding the potential grapevine pest species, E. vitis was dominant (97.17%) with respect to Altica ampelophaga Guérin-Méneville (Coleoptera: Chrysomelidae), Xylotrechus arvicola (Olivier) (Coleoptera: Cerambycidae) and Sinoxylon sexdentatum (Olivier) (Coleoptera: Bostrichidae) (1.13%, 1.13% and 0.57%, respectively).
3.2. Impact of Different Ground Cover Management Techniques on Insect Abundance
The ground cover management techniques significantly affected the important epigeal predator abundance, which was higher under the cover crop treatment compared with under the tillage treatment (Table 1; Figure 1). In addition, these abundances were greater under the spontaneous cover treatment than under the flower-driven cover treatment. Specifically, the cover crop treatments showed approximately three times higher abundance of carabids compared with the tillage treatment (Figure 1A), although forficulids were only significantly more abundant under the spontaneous cover treatment (Figure 1B). However, no significant differences were found among the treatments for staphylinids or the potential grapevine pests (Figure 1C,D).

Table 1.
Two-way ANOVA results of the total abundance of the main predator families and the total potential grapevine pests on the ground. Significant differences are highlighted in bold.
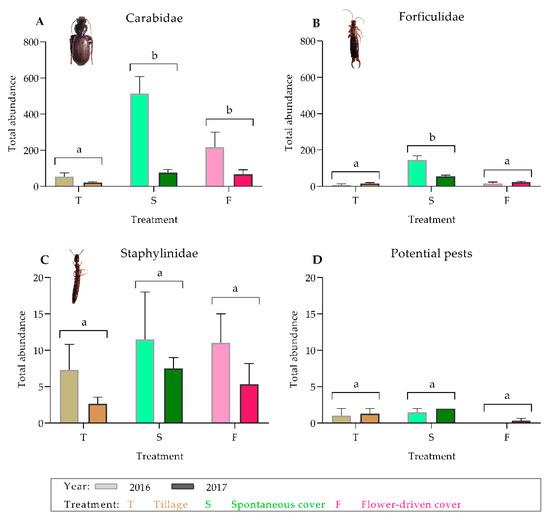
Figure 1.
Effects of soil management on the total abundance of the predator families on the ground: (A) Carabidae; (B) Forficulidae; (C) Staphylinidae; and (D) potential grapevine pests on the ground. Values are mean (± standard error). The left bar of each couplet represents data in 2016, and the right bar represents data in 2017. Different letters indicate significant differences between the treatments, by two-way ANOVA and a Tukey’s HSD test (α = 0.05).
For the ground beetles, 20 morphospecies that belonged to nine genera on the ground were identified (Table 2; Supplementary Table S2; Figure 2). The most common carabid morphospecies was Nebria sp1. (15.39%), which was present along with Steropus sp1. (15.06%), Brachinus sp1. (14.68%) and Amara sp1. (10.55%). The other carabid morphospecies had relative abundances of less than 10%. In addition, Harpalus and Ophonus were the genera with the most morphospecies identified (n = 5). Moreover, Nebria and Harpalus were significantly more abundant under the spontaneous cover treatment compared with those under the other treatments. Only the Amara abundance was significantly greater under both the cover crop treatments. Finally, no significant differences were found among the treatments for Steropus, Brachinus and Ophonus.

Table 2.
Two-way ANOVA results of the total abundance of the Carabidae genera captured on the ground. Significant differences are highlighted in bold.
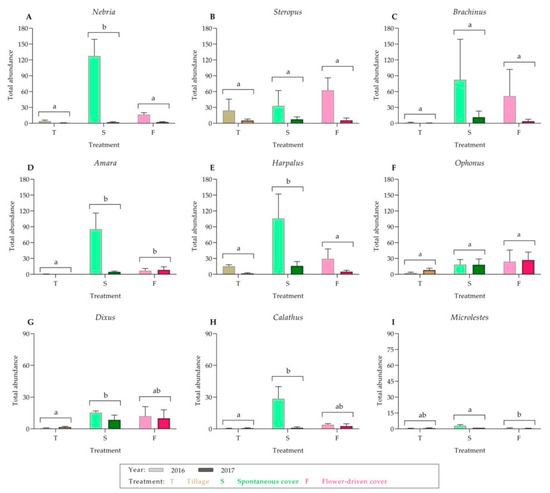
Figure 2.
Carabidae genera captured on the ground: (A) Nebria; (B) Steropus; (C) Brachinus; (D) Amara; (E) Harpalus; (F) Ophonus; (G) Dixus; (H) Calathus; and (I) Microlestes. Values are mean (± standard error). The left bar of each couplet represents data in 2016, and the right bar represents data in 2017. Different letters indicate significant differences between the treatments, by two-way ANOVA and a Tukey’s HSD test (α = 0.05).
In contrast to the epigeal fauna, the canopy insect abundance in the vineyard was similar among the treatments for both the predators and the potential grapevine pest species (Table 3; Figure 3). For the predatory insects, higher abundances of Chrysopidae, Cecidomyiidae and Coccinellidae were observed under both the cover crop treatments compared with those under the tillage treatment, although these differences were not statistically significant.

Table 3.
Two-way ANOVA results of the total abundance of the main predator families and the total potential grapevine pests in the canopy. Significant differences are highlighted in bold.
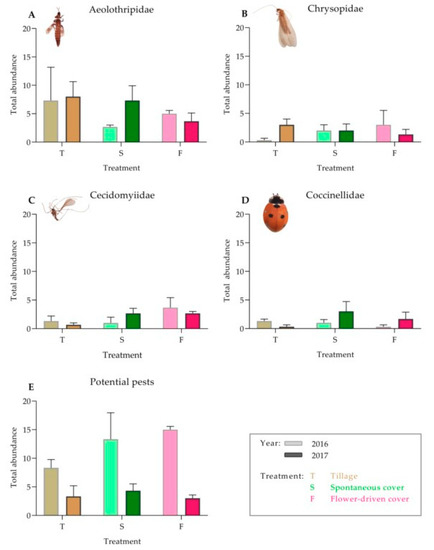
Figure 3.
Effect of soil management on the total abundance of the predator families in the canopy: (A) Aeolothripidae; (B) Chrysopidae; (C) Cecidomyiidae; (D) Coccinellidae; and (E) potential grapevine pests in the grapevine canopy. Values are mean (± standard error). The left bar of each couplet represents data in 2016, and the right bar represents data in 2017.
We observed similar trends in the population dynamics of the predators and the potential grapevine pests between the years both on the ground and in the grapevine canopy, with a higher abundance at the beginning of the grapevine vegetative cycle (Figure 4 and Figure 5; Supplementary Table S3). The greatest epigeal predator abundance was observed at the beginning of June, which coincides with the grape phenological stage 17-H (separate floral buttons). The abundance of the epigeal predators collected in 2016 at the same phenological stage was nearly two times higher than that in 2017. In fact, half of the sampling dates significantly differed among years (Supplementary Table S3). For almost all the samplings, the spontaneous cover treatment showed the highest epigeal predator abundance values, which were statistically significant except in August (Figure 4; Supplementary Table S3). In contrast, the grapevine canopy predator and potential grapevine pest abundances were quite similar between the years, and no significant differences were recorded among the treatments, except for one sampling date (31 May 2017), in the case of the potential grapevine pests (Figure 4 and Figure 5; Supplementary Table S3). The potential grapevine pests and the predaceous insects showed overlap in habitat use during the fruit-growing season.
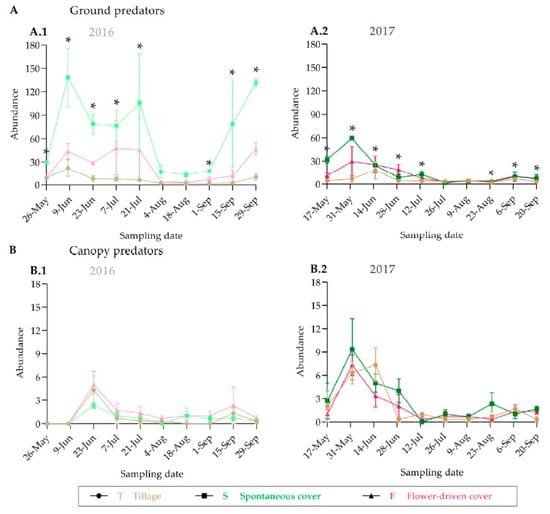
Figure 4.
Population dynamics of predators on the ground (A) and in the grapevine canopy (B). An asterisk indicates significant differences between treatments, by ANOVA and Tukey-HSD test (α = 0.05).
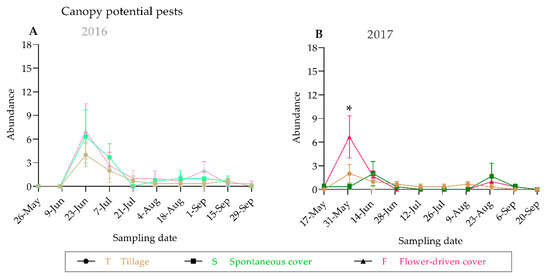
Figure 5.
Population dynamics of potential grapevine pests in the grapevine canopy in 2016 (A) and 2017 (B). An asterisk indicates significant differences between treatments, by ANOVA and Tukey-HSD test (α = 0.05).
3.3. Effects of Different Ground Cover Management Techniques on Insect Diversity
The observed predatory insect diversity was higher on the ground than in the grapevine canopy; however, the opposite was true for the potential pests (Table 4; Supplementary Table S4). Of the families studied, Carabidae showed the highest diversity values, both on the ground and in the grapevine canopy. The highest carabid richness was recorded under the spontaneous cover treatment. The effective number of the ground beetles calculated for three diversity levels (0D, 1D and 2D) increased as the order of diversity (q) decreased, which denoted a high degree of dominance in the community; decreases, as q increased, were stronger under the cover crop treatments, where the ratio between 2D and 0D was around 3. However, this trend was not observed for the grapevine canopy predators, where the number of the common species (2D) was quite similar to the richness (0D). Additionally, the flower-driven cover treatment showed greater, although not significantly different, diversity of cecidomyiids than the other treatments. Moreover, there was higher diversity of potential pests in the grapevine canopy, although we only found differences at the ground level.

Table 4.
Hill numbers of the predatory and the potential pest insects, both on the ground and at the canopy level. Data are shown as mean (± standard error).
4. Discussion
The grapevines occur at the centre of complex communities, with a wide range of insects at both the ground and canopy levels. We expected that the ground cover management influences the insect predatory population both on the ground and in the grapevine canopy, but we only observed a significant effect on some epigeal predator taxa. Nevertheless, we cannot compare results between the ground and canopy levels because of using two different techniques for sampling.
4.1. Epigeal Predators
The vineyard, where this study was conducted, supported a diverse predatory insect assemblage. The ground beetles were the most abundant and diverse insects captured on the ground, which is consistent with the findings reported by Kromp et al. [12,35] in other crops. Earwigs were the second most abundant but only represented by one species, Forficula auricularia Linnaeus, 1758 (Dermaptera: Forficulidae), which is also known as the European earwig and is an important omnivorous predator in vineyards. The spontaneous cover vegetation had a significant impact on the abundance of both the families compared with the tillage treatment, which is consistent with the findings of Danne et al., Irvin et al. and Sharley et al. [36,37,38]; this could be explained by tilling effects, such as habitat disturbance, litter layer removal, microclimate condition alterations as well as shelter and food availability reduction [39], which have strong impacts on insects that live on the soil surface. However, no differences among the treatments were observed for the rove beetles, one of the most ecologically important predaceous insects in agroecosystems; this is consistent with the observations of Bohac [40], who reported that agricultural measures, such as tillage, have a lower influence on staphylinids compared with others factors (e.g., landscape factors).
Carabids are considered an ecologically important family of natural enemies of pests [12] and key contributors to biocontrol in agroecosystems [41] because of their broad diet, which allows them to persist and prevent pest outbreaks despite seasonal disturbance [42]. Most carabids are polyphagous predators, and both larval and adult forms are able to feed on pests such as lepidopteran larvae, aphids and slugs [43]. In addition, some species can also feed on leaves, seeds, fruits and fungi [43].
The ground beetle communities in the studied vineyard were dominated (>85%) by six genera: Harpalus, Nebria, Steropus, Brachinus, Ophonus and Amara. Three genera (Harpalus, Ophonus and Amara) belong to the tribe Harpalini and are well known as true granivores [44]. Several species of Harpalus are known to be involved in seed regulation in vineyards without any seed preferences [45]. However, specific affinities have been reported for Ophonus and Amara, such as Apiaceae and Poaeceae, respectively [46,47]. Both the plant families were recorded in the spontaneous cover treatment (0.10% and 14.80%, respectively) [14]. This higher relative abundance of Poaceae might be positively correlated with a higher abundance of Amara under the spontaneous cover treatment. In addition, most of the Carabidae genera were more abundant under both the cover crop treatments than under the tillage treatment, even if differences were only significant in the case of Amara. Amara may have been more abundant, because it is a spermophagous genus [48], and seeds retained on the surface of the cover crop treatments may provide an important source of food. However, several carabid genera were only significantly more abundant under the spontaneous cover treatment, such as Nebria, Harpalus, Dixus and Calathus; this finding is consistent with those of other studies [49,50], which reported that ground beetles do not directly feed on floral resources and that native vegetation may increase food availability for them.
There are various factors, such as ground beetle body size, mobility and trophic levels, which are often considered to be potentially essential in carabid responses to habitat quality [51,52]. In the studied vineyard, the most abundant genera were Harpalus and Nebria, and both were significantly more abundant under the spontaneous cover treatment. Harpalus may be abundant, because weeds and variety of seeds of grasses provide a great amount of their food sources. Alternatively, Nebria may be abundant as a result of native cover vegetation effects on microclimatic conditions (temperature and humidity) and shelter, because they have hygrophilic and photophobic tendencies [32]. Alternatively, some authors suggested that larger carabids (size ≥ 15 mm) are negatively associated with disturbed habitats [52,53,54,55]. However, no differences were found among the treatments relative to the abundance of Steropus (large carabids) in the studied vineyard, potentially because this genus is able to tolerate a wide range of environments [56]. Conversely, these differences were observed in the abundance of Microlestes (small carabids), which were more abundant under the treatment with less disturbance. Nevertheless, Microlestes abundance did not differ under the tillage treatment compared with under the cover crop treatments, potentially because they are able to tolerate sunlight and sudden changes in humidity and temperature [32].
4.2. Grapevine Canopy Predators
The studied cover crop treatments did not significantly affect the grapevine canopy predaceous insect abundance. Cover vegetation in vineyards can provide shelter, nectar, alternative prey and pollen, which support insect populations [57]. Several authors have reported that floral nectar and pollen also are highly attractive to lacewings and coccinellids [58], but differences among the treatments were not found in this study. Chrysoperla carnea (Stephens, 1836) (Neuroptera: Chrysopidae) was the main lacewing captured; it is a polyphagous predator in its larval form but only feeds on sugary substances and pollen in its adult form. Similarly, the larvae of several cecidomyiid species are predators, especially of aphids, and can also attack mealybugs, mites and other small arthropods, whereas adults feed on floral sources. The Cecidomyiid abundances were around two and three times higher under the spontaneous cover and flower-driven cover treatments, respectively, compared with that under the tillage treatment, although not significantly. Among Coleoptera, the most abundant predatory insect family was Coccinellidae, which was mostly represented by Coccinella (Coccinella) septempunctata Linnaeus, 1758, Scymnus (Scymnus) interruptus (Goeze, 1777), Adonia variegata (Goeze, 1777), Coccidula rufa (Herbst, 1783) and Propylea quatuordecimpunctata (Linnaeus, 1758). Most of these insects attack aphids, although they can also feed on the eggs of lepidopterans, such as L. botrana. Ladybirds may be more abundant under both the spontaneous cover and flower-driven cover treatments compare with that under the tillage treatment, because C. septempunctata lives in the herbaceous layer, which is less than half a meter in length, and some authors [59] have reported that Centaurea cyanus Linnaeus, 1753 (Asteraceae) is positively correlated with their presence. The Coccinellid abundance was nearly two times higher under the spontaneous cover treatment than under the tillage treatment, but not significantly. Besides, we did not observe differences between the spontaneous cover and flower-driven cover treatments in relation with the ladybirds abundance. These results are in line with the published paper by Burgio et al. [24] in vineyard but in contrast with other authors [58,60] that reported a positive effect of flowering plants on Coccinellidae.
4.3. Pest Assemblages
The presence of the potential grapevine pests was negligible at the ground level, but they did occur in the grapevine canopy. The main pest in Mediterranean and European vineyards, L. botrana, was not captured in this study. This indicates that mating disruption, in addition to being an environmentally friendly technique, is efficient to control this pest. However, we recorded the dominance of E. vitis, which is a polyphagous cicadellid. It is considered a secondary pest, which can be found on both grapevines and weeds [61]. Species of coccinellids, neuropterans (e.g., C. carnea) and heteropterans (e.g., Orius spp. (Hemiptera: Anthocoridae)) have been cited as predators of E. vitis. Otherwise, only three coleopterans occasionally were captured in vineyards and are considered secondary pests (A. ampelophaga, X. arvicola, and S. sexdentatum). No significant differences among the treatments were found relative to the potential pest abundance. Therefore, although diverse cover vegetation can support many phytophagous insects, according to the data reported by Sáenz-Romo et al. and Siemann et al. [14,62], it does not seem to enhance potential grapevine pest species.
4.4. Insect Population Dynamics
With respect to the population dynamics, the epigeal predator abundance showed strong annual variability, possibly due to abiotic factors (mainly temperature and relative humidity), which were harsher in 2017 than in 2016 [25]. Nevertheless, almost no differences were found at the grapevine canopy level; this may be explained by microclimatic conditions, which are more favourable because of the grapevine leaves effect.
Alternatively, although the total epigeal predator abundance was significantly higher under the spontaneous cover treatment on almost all the sampling dates, it was observed that the grass mowing, carried out in the beginning of June, caused the population decline. This finding is consistent with those of Rouabah et al., Thorbek and Bilde as well as Woodcock et al. [63,64,65], who reported that reduction in vegetation height has a clear impact on abundance of carabid and staphylinid beetles. Furthermore, reduction of epigeal predators in mid-summer may be caused by temporarily depressed ground beetle activity densities due to high nightly temperatures [43]. Moreover, according to Sáenz-Romo et al. and Rebek et al. [14,66], spontaneous vegetation biomass can attract predaceous insects and alternative prey in vineyards, even when flowers are not in bloom. Thus, spontaneous vegetation cover in vineyards might be associated with providing benefits to predaceous insects throughout the growing period, which is consistent with the findings reported by Thomson and Hoffmann [67].
4.5. Diversity Values
Most agroecosystem biodiversity resides in the soil [68], and this is particularly true for insects. Even though intensification of agricultural practices such as tilling has been reported to be important drivers of biodiversity loss in agroecosystems [69,70], no significant differences were found among the treatments in most of the predatory families studied. Nevertheless, it was observed that the spontaneous cover treatment increased the carabid richness (0D). This result confirms the possibility that carabid morphospecies richness is positively correlated with higher vegetation diversity, which was also reported by other researchers [45,52,71,72]. Thereby, a cover crop canopy seems to be a key factor that influences both abundance and diversity of epigeal predators such as ground beetles. According to Melnychuck et al. [73], epigeal predator diversity tends to be higher under an herbaceous cover of grasses, because spring growth provides early coverage, as was observed in the studied vineyard.
5. Conclusions
Overall, the insect communities were influenced by the ground cover management techniques in the studied vineyard. It impacted the insect predators on the ground but not in the grapevine canopy. The cover crop vegetation enhanced beneficial entomofauna, especially carabids and forficulids, without promoting potential grapevine pest species. In particular, the spontaneous cover vegetation increased both the abundance and the diversity of ground beetles. More specifically, it significantly impacted the abundance of the carnivorous genus Nebria in comparison with the tillage and flower-driven treatments. Thus, in fact, establishment of long-term vegetation cover could improve agroecosystem resilience, and management of spontaneous cover vegetation seems to be the most interesting strategy for implementing CBC in vineyards.
Supplementary Materials
The following are available online at https://www.mdpi.com/2075-4450/10/12/421/s1, Table S1: Pesticide treatments applied to the pest and disease control, Table S2: Size, relative abundance and statistical results (two-way ANOVA) of Carabidae morphospecies abundance. Significant differences are highlighted in bold, Table S3: Two-way ANOVA results of the population dynamics of the predators both on the ground and in the canopy and the potential pests in the grapevine canopy. Significant differences are highlighted in bold, Table S4: Two-way ANOVA results of predator biodiversity values. Significant differences are highlighted in bold.
Author Contributions
Conceptualization, M.G.S.-R., V.S.M.-M. and I.P.-M.; methodology, V.S.M.-M. and I.P.-M.; formal analysis, M.G.S.-R., V.S.M.-M. and I.P.-M.; investigation, M.G.S.-R. and A.V.-B.; writing of the original draft preparation, M.G.S.-R.; writing of review and editing, M.G.S.-R., H.M.-G., R.C.-H., V.S.M.-M. and I.P.-M.; visualization, M.G.S.-R.; supervision, V.S.M.-M. and I.P.-M.; project administration, S.I.-P., E.M.-V., V.S.M.-M. and I.P.-M.; funding acquisition, M.G.S.-R., A.V.-B., S.I.-P., E.M.-V., V.S.M.-M. and I.P.-M.
Funding
This research was funded by the Ministry of Economy and Competitiveness (AGL2014-53336R). M.G.S.-R. and A.V.-B. were supported by fellowships from the University of La Rioja (Spain) (FPI-UR 2015 and 2018, respectively). R.C.-H. is currently supported by a Ramón y Cajal award (RYC-2016-19939).
Acknowledgments
We thank Vicente Sáenz Romo for editing the artwork for the graphical abstract and the figures. We thank Mallory Eckstut from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript and three anonymous referees for their valuable comments.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Allan, E.; Bossdorf, O.; Dormann, C.F.; Prati, D.; Gossner, M.M.; Tscharntke, T.; Blüthgen, N.; Bellach, M.; Birkhofer, K.; Boch, S.; et al. Interannual variation in land-use intensity enhances grassland multidiversity. Proc. Natl. Acad. Sci. USA 2014, 111, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, B.J.; Duffy, J.E.; Gonzalez, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Narwani, A.; Mace, G.M.; Tilman, D.; Wardle, D.A.; et al. Biodiversity loss and its impact on humanity. Nature 2012, 486, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Tscharntke, T.; Klein, A.M.; Kruess, A.; Steffan-Dewenter, I.; Thies, C. Landscape perspectives on agricultural intensification and biodiversity—Ecosystem service management. Ecol. Lett. 2005, 8, 857–874. [Google Scholar] [CrossRef]
- Provost, C.; Pedneault, K. The organic vineyard as a balanced ecosystem: Improved organic grape management and impacts on wine quality. Sci. Hortic. 2016, 208, 43–56. [Google Scholar] [CrossRef]
- Samways, M.J. Insect Diversity Conservation; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Altieri, M. The ecological role of biodiversity in agroecosystems. Agric. Ecosyst. Environ. 1999, 74, 19–31. [Google Scholar] [CrossRef]
- Griffin, J.N.; Byrnes, J.E.K.; Cardinale, B.J. Effects of predator richness on prey suppression: A meta-analysis. Ecology 2013, 94, 2180–2187. [Google Scholar] [CrossRef]
- Rusch, A.; Birkhofer, K.; Bommarco, R.; Smith, H.G.; Ekbom, B. Predator body sizes and habitat preferences predict predation rates in an agroecosystem. Basic Appl. Ecol. 2015, 16, 250–259. [Google Scholar] [CrossRef]
- Jacas, J.A.; Urbaneja, A. Control. Biológico de Plagas Agrícolas; Phytoma: Valencia, Spain, 2008. [Google Scholar]
- Symondson, W.O.C.; Glen, D.M.; Ives, A.R.; Langdon, C.J.; Wiltshire, C.W. Dynamics of the relationship between a generalist predator and slugs over five years. Ecology 2002, 83, 137–147. [Google Scholar] [CrossRef]
- Thies, C.; Haenke, S.; Scherber, C.; Bengtsson, J.; Bommarco, R.; Clement, L.W.; Ceryngier, P.; Dennis, C.; Emmerson, M.; Gagic, V.; et al. The relationship between agricultural intensification and biological control: Experimental tests across Europe. Ecol. Appl. 2011, 21, 2187–2196. [Google Scholar] [CrossRef]
- Kromp, B. Carabid beetles in sustainable agriculture: A review on pest control efficacy, cultivation impacts and enhancement. Agric. Ecosyst. Environ. 1999, 74, 187–228. [Google Scholar] [CrossRef]
- Pearce, J.L.; Venier, L.A. The use of ground beetles (Coleoptera: Carabidae) and spiders (Araneae) as bioindicators of sustainable forest management: A review. Ecol. Indic. 2006, 6, 780–793. [Google Scholar] [CrossRef]
- Sáenz-Romo, M.G.; Veas-Bernal, A.; Martínez-García, H.; Campos-Herrera, R.; Ibáñez-Pascual, S.; Martínez-Villar, E.; Pérez-Moreno, I.; Marco-Mancebón, V.S. Ground cover management in a Mediterranean vineyard: Impact on insect abundance and diversity. Agric. Ecosyst. Environ. 2019, 283, 106571. [Google Scholar] [CrossRef]
- Thomson, L.J.; Hoffmann, A.A. Vegetation increases the abundance of natural enemies in vineyards. Biol. Control 2009, 49, 259–269. [Google Scholar] [CrossRef]
- Letourneau, D.K.; Armbrecht, I.; Salguero Rivera, B.; Montoya Lerma, J.; Jimenez Carmona, E.; Constanza Daza, M. Does plant diversity benefit agroecosystems? A synthetic review. Ecol. Appl. 2011, 21, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Soliveres, S.; Van Der Plas, F.; Manning, P.; Prati, D.; Gossner, M.M.; Renner, S.C.; Alt, F.; Arndt, H.; Baumgartner, V.; Binkenstein, J.; et al. Biodiversity at multiple trophic levels is needed for ecosystem multifunctionality. Nature 2016, 536, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Hatt, S.; Lopes, T.; Boeraeve, F.; Chen, J.; Francis, F. Pest regulation and support of natural enemies in agriculture: Experimental evidence of within field wildflower strips. Ecol. Eng. 2017, 98, 240–245. [Google Scholar] [CrossRef]
- Tschumi, M.; Albrecht, M.; Collatz, J.; Dubsky, V.; Entling, M.H.; Najar-Rodriguez, A.J.; Jacot, K. Tailored flower strips promote natural enemy biodiversity and pest control in potato crops. J. Appl. Ecol. 2011, 53, 1169–1176. [Google Scholar] [CrossRef]
- Garcia, L.; Celette, F.; Gary, C.; Ripoche, A.; Valdés-Gómez, H.; Metay, A. Management of service crops for the provision of ecosystem services in vineyards: A review. Agric. Ecosyst. Environ. 2018, 251, 158–170. [Google Scholar] [CrossRef]
- Altieri, M.A.; Nicholls, C.I.; Wilson, H.; Miles, A. Habitat Management in Vineyards. A Growers Manual for Enhancing Natural Enemies; Laboratory of Agroecology, College of Natural Resources University of California, Berkeley: Berkeley, CA, USA, 2010. [Google Scholar]
- Gurr, G.M.; Wratten, S.D.; Altieri, M.A. Ecological Engineering for Pest Management: Advances in Habitat Manipulation for Arthropods; CABI Publishing: Wallingford, UK, 2004. [Google Scholar]
- Landis, D.A.; Wratten, S.D.; Gurr, G.M. Habitat management to conserve natural enemies of arthropod pests in agriculture. Annu. Rev. Entomol. 2000, 45, 175–201. [Google Scholar] [CrossRef]
- Burgio, G.; Marchesini, E.; Reggiani, N.; Montepaone, G.; Schiatti, P.; Sommaggio, D. Habitat management of organic vineyard in Northern Italy: The role of cover plants management on arthropod functional biodiversity. Bull. Entomol. Res. 2016, 106, 759–768. [Google Scholar] [CrossRef]
- Sáenz-Romo, M.G.; Martínez-García, H.; Veas-Bernal, A.; Carvajal-Montoya, L.D.; Martínez-Villar, E.; Ibáñez-Pascual, S.; Marco-Mancebón, V.S.; Pérez-Moreno, I. Effect of ground-cover management on predatory mites (Acari: Phytoseiidae) in a Mediterranean vineyard. Vitis 2019, in press. [Google Scholar]
- Sommaggio, D.; Peretti, E.; Burgio, G. The effect of cover plants management on soil invertebrate fauna in vineyard in Northern Italy. BioControl 2018, 63, 795–806. [Google Scholar] [CrossRef]
- Karp, D.S.; Chaplin-Kramer, R.; Meehan, T.D.; Martin, E.A.; DeClerck, F.; Grab, H.; Gratton, C.; Hunt, L.; Larsen, A.E.; Martínez-Salinasc, A.; et al. Crop pests and predators exhibit inconsistent responses to surrounding landscape composition. Proc. Natl. Acad. Sci. USA 2018, 115, E7863–E7870. [Google Scholar] [CrossRef] [PubMed]
- Chinery, M. Guía de Campo de los Insectos de España y de Europa; Omega: Biel, Switzerland, 2010. [Google Scholar]
- Triplehorn, C.A.; Johnson, N.F. Borror and Delong’s Introduction to the Study of Insects, 7th ed.; Brooks, Cole: Belmont, CA, USA, 2005. [Google Scholar]
- Jeannel, R. Coleopteres Carabiques. Faune de France. Vol. 39+40; Lechevalier: Paris, France, 1941. [Google Scholar]
- Herrera, J.; Arricibita, F.J. Los carábidos de Navarra España (Coleoptera: Carabidae). Entomonograph 1990, 12, 241. [Google Scholar]
- Ortuño, V.M.; Marcos, J.M. Los Caraboidea (Insecta: Coleoptera) de la Comunidad Autónoma del País Vasco (Tomo 1); Dpto. de Medio Ambiente y Ordenación del Territorio, Servicio Central de Publicaciones del Gobierno Vasco: Vitoria-Gasteiz, Spain, 2003. [Google Scholar]
- Hill, M.O. Diversity and evenness: A unifying notation and its consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef]
- Jost, L. Entropy and diversity. Oikos 2006, 113, 363–375. [Google Scholar] [CrossRef]
- Holland, J.M.; Reynolds, C.J.M. The impact of soil cultivation on arthropod (Coleoptera and Araneae) emergence on arable land. Pedobiologia 2003, 47, 181–191. [Google Scholar] [CrossRef]
- Danne, A.; Thomson, L.J.; Sharley, D.J.; Penfold, C.M.; Hoffmann, A.A. Effects of native grass cover crops on beneficial and pest invertebrates in australian vineyards. Environ. Entomol. 2010, 39, 970–978. [Google Scholar] [CrossRef]
- Irvin, N.A.; Bistline-East, A.; Hoddle, M.S. The effect of an irrigated buckwheat cover crop on grapevine productivity, and beneficial insect and grape pest abundance in southern California. Biol. Control 2016, 93, 72–83. [Google Scholar] [CrossRef]
- Sharley, D.J.; Hoffmann, A.A.; Thomson, L.J. The effects of soil tillage on beneficial invertebrates within the vineyard. Agric. For. Entomol. 2008, 10, 233–243. [Google Scholar] [CrossRef]
- Stinner, B.R.; House, G.J. Arthropods and other invertebrates in conservation-tillage agriculture. Annu. Rev. Entomol. 1990, 35, 229–318. [Google Scholar] [CrossRef]
- Bohac, J. Staphylinid beetles as bioindicators. Agric. Ecosyst. Environ. 1999, 74, 357–372. [Google Scholar] [CrossRef]
- Ågren, G.I.; Stenberg, J.A.; Björkman, C. Omnivores as plant bodyguards: A model of the importance of plant quality. Basic Appl. Ecol. 2012, 13, 441–448. [Google Scholar] [CrossRef]
- Eubanks, M.D. Predaceous herbivores and herbivorous predators: The biology of omnivores and the ecology of omnivore-prey interactions. In The Ecology of Predator-Prey Interactions; Barbosa, P., Castellanos, I., Eds.; Oxford University Press: New York, NY, USA, 2005; pp. 3–16. [Google Scholar]
- Lövei, G.L.; Sunderland, K.D. Ecology and behavior of ground beetles (Coleoptera: Carabidae). Annu. Rev. Entomol. 1996, 41, 231–256. [Google Scholar] [CrossRef] [PubMed]
- Kotze, D.J.; Brandmayr, P.; Casale, A.; Dauffy-Richard, E.; Dekoninck, W.; Koivula, M.J.; Lövei, G.L.; Mossakowski, D.; Noordijk, J.; Paarmann, W.; et al. Forty years of carabid beetle research in Europe–from taxonomy, biology, ecology and population studies to bioindication, habitat assessment and conservation. Zookeys 2011, 100, 55–148. [Google Scholar] [CrossRef]
- Rusch, A.; Binet, D.; Delbac, L.; Thiéry, D. Local and landscape effects of agricultural intensification on Carabid community structure and weed seed predation in a perennial cropping system. Landsc. Ecol. 2016, 31, 2163–2174. [Google Scholar] [CrossRef]
- Hurka, K. Carabidae of the Czech. and Slovak Republics—Illustrated Key; Kabourek: Zlín, Czech Republic, 1996; pp. 1–565. [Google Scholar]
- Zetto Brandmayr, T. Spermophagous (seed-eating) ground beetles: First comparison of the diet and ecology of the Harpaline genera Harpalus and Ophonus (Coleoptera: Carabidae). In The Role of Ground Beetles in Ecological and Environmental Studies; Stork, N., Ed.; Intercept: Andover, MA, USA, 1990; pp. 307–316. [Google Scholar]
- Jorgensen, H.B.; Toft, S. Role of granivory and insectivory in the life cycle of the carabid beetle Amara similata. Ecol. Entomol. 1997, 22, 7–15. [Google Scholar] [CrossRef]
- Norris, R.F.; Kogan, M. Interactions between weeds, arthropod pests, and natural enemies in managed ecosystems. Weed Sci. 2000, 48, 94–158. [Google Scholar] [CrossRef]
- Zangger, A.; Lys, J.A.; Nentwig, W. Increasing the availability of food and the reproduction of Poecilus cupreus in a cereal field by strip-management. Entomol. Exp. Appl. 1994, 71, 11–120. [Google Scholar] [CrossRef]
- Eyre, M.D.; Luff, M.L.; Leifert, C. Crop, field boundary, productivity and disturbance influences on ground beetles (Coleoptera: Carabidae) in the agroecosystem. Agric. Ecosyst. Environ. 2013, 165, 60–67. [Google Scholar] [CrossRef]
- Ribera, I.; DoléDec, S.; Downie, I.S.; Foster, G.N. Effect of land disturbance and stress on species traits of ground beetle assemblages. Ecology 2001, 82, 1112–1129. [Google Scholar] [CrossRef]
- Cole, L.J.; McCracken, D.I.; Dennis, P.; Downie, I.S.; Griffin, A.L.; Foster, G.N.; Murphy, K.J.; Waterhouse, T. Relationships between agricultural management and ecological groups of ground beetles (Coleoptera: Carabidae) on Scottish farmland. Agric. Ecosyst. Environ. 2002, 93, 323–336. [Google Scholar] [CrossRef]
- Lövei, G.L.; Magura, T. Body size changes in ground beetle assemblages—a reanalysis of Braun et al. (2004)’s data. Ecol. Entomol. 2006, 31, 411–414. [Google Scholar] [CrossRef]
- Purtauf, T.; Dauber, J.; Wolters, V. The response of carabids to landscape simplification differs between trophic groups. Oecologia 2005, 142, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Lindroth, C.H.; Bangsholt, F. The Carabidae (Coleoptera) of Fennoscandia and Denmark; Brill Archive: Leiden, The Netherlands, 1985. [Google Scholar]
- Gurr, G.M.; Wratten, S.D.; Landis, D.A.; You, M. Habitat management to suppress pest populations: Progress and prospects. Annu. Rev. Entomol. 2017, 62, 91–109. [Google Scholar] [CrossRef] [PubMed]
- Freeman Long, R.; Corbett, A.; Lamb, C.; Reberg-Horton, C.; Chandler, J.; Stimmann, M. Beneficial insects move from flowering plants to nearby crops. Calif. Agric. 1998, 52, 23–26. [Google Scholar] [CrossRef]
- Kopta, T.; Pokluda, R.; Psota, V. Attractiveness of flowering plants for natural enemies. Hortic. Sci. 2012, 39, 89–96. [Google Scholar]
- Pemberton, R.W.; Vandenberg, N.J. Extrafloral nectar feeding by ladybird beetles (Coleoptera: Coccinellidae). Proc. Entomol. Soc. Wash. 1993, 95, 139–151. [Google Scholar]
- Pérez Marín, J.L. Plagas y Enfermedades del Viñedo en La Rioja; Gobierno de La Rioja: Logroño, Spain, 2013. [Google Scholar]
- Siemann, E.; Tilman, D.; Haarstad, J.; Ritchie, M. Experimental tests of the dependence of arthropod diversity on plant diversity. Am. Nat. 1998, 152, 738–750. [Google Scholar] [CrossRef]
- Rouabah, A.; Villerd, J.; Amiaud, B.; Plantureux, S.; Lasserre-Joulin, F. Response of carabid beetles diversity and size distribution to the vegetation structure within differently managed field margins. Agric. Ecosyst. Environ. 2015, 200, 21–32. [Google Scholar] [CrossRef]
- Thorbek, P.; Bilde, T. Reduced numbers of generalist arthropod predators after crop management. J. Appl. Ecol. 2004, 41, 526–538. [Google Scholar] [CrossRef]
- Woodcock, B.A.; Potts, S.G.; Tscheulin, T.; Pilgrim, E.; Ramsey, A.J.; Harrison-Cripps, J.; Brown, V.K.; Tallowin, J.R. Responses of invertebrate trophic level, feeding guild and body size to the management of improved grassland field margins. J. Appl. Ecol. 2009, 46, 920–929. [Google Scholar] [CrossRef]
- Rebek, E.J.; Sadof, C.S.; Hanks, L.M. Manipulating the abundance of natural enemies in ornamental landscapes with floral resource plants. Biol. Control 2005, 33, 203–216. [Google Scholar] [CrossRef]
- Thomson, L.J.; Hoffmann, A.A. Spatial scale of benefits from adjacent woody vegetation on natural enemies within vineyards. Biol. Control 2013, 64, 57–65. [Google Scholar] [CrossRef]
- Young, I.M.; Crawford, J.W. Interaction and self-organization on the soil–microbe complex. Science 2004, 304, 1634–1637. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.A.; Sutherland, W.J. Post-war changes in arable farming and biodiversity in Great Britain. J. Appl. Ecol. 2002, 39, 157–176. [Google Scholar] [CrossRef]
- Schmidt, M.H.; Roschewitz, I.; Thies, C.; Tscharntke, T. Differential effects of landscape and management on diversity and density of ground-dwelling farmland spiders. J. Appl. Ecol. 2005, 42, 281–287. [Google Scholar] [CrossRef]
- Franin, K.; Barić, B.; Kuštera, G. The role of ecological infrastructure on beneficial arthropods in vineyards. Span. J. Agric. Res. 2016, 14. [Google Scholar] [CrossRef]
- Gaigher, R.; Samways, M.J. Surface active arthropods in organic vineyards, integrated vineyards and natural habitat in the Cape Floristic Region. J. Insect Conserv. 2010, 14, 595–605. [Google Scholar] [CrossRef]
- Melnychuk, N.A.; Olfert, O.; Youngs, B.; Gillott, C. Abundance and diversity of Carabidae (Coleoptera) in different farming systems. Agric. Ecosyst. Environ. 2003, 95, 69–72. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).