Characterization of the Expression and Functions of Two Odorant-Binding Proteins of Sitophilus zeamais Motschulsky (Coleoptera: Curculionoidea)
Abstract
1. Introduction
2. Materials and Methods
2.1. General Odorants
2.2. Insect Rearing
2.3. RNA Extraction and cDNA Synthesis
2.4. Quantitative Real-Time PCR (qRT-PCR)
2.5. Sequence Analysis and Phylogenetic Tree Construction
2.6. Cloning of SzeaOBPs and Construction of the Expression Vector
2.7. Prokaryotic Expression and Purification of the Recombinant pET-SzeaOBP Proteins
2.8. Preparation of Multi-Clonal Antibody and Western Blot Analysis
2.9. Fluorescence Competitive Binding Assays
2.10. Behavioural Experiments Using a Y-Tube Olfactometer
2.11. RNA Interference
3. Results
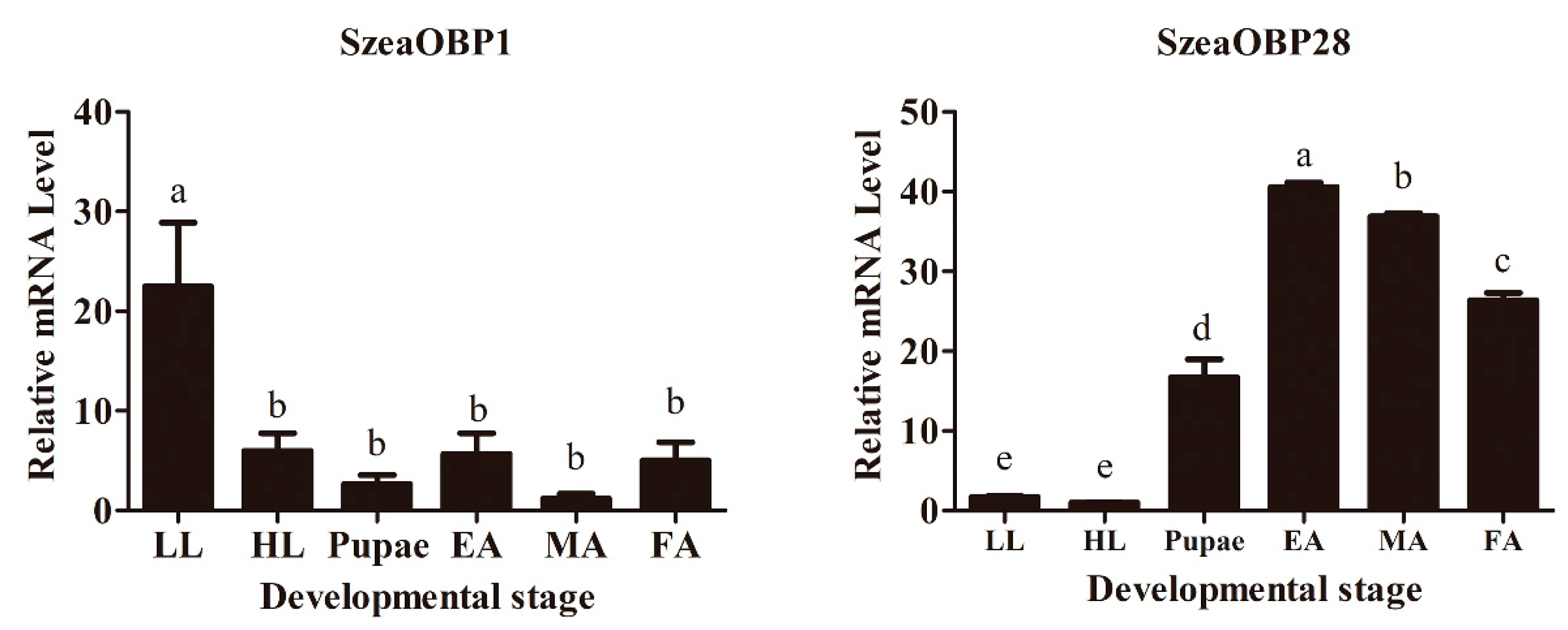
3.1. Comparison of Relative mRNA Expression
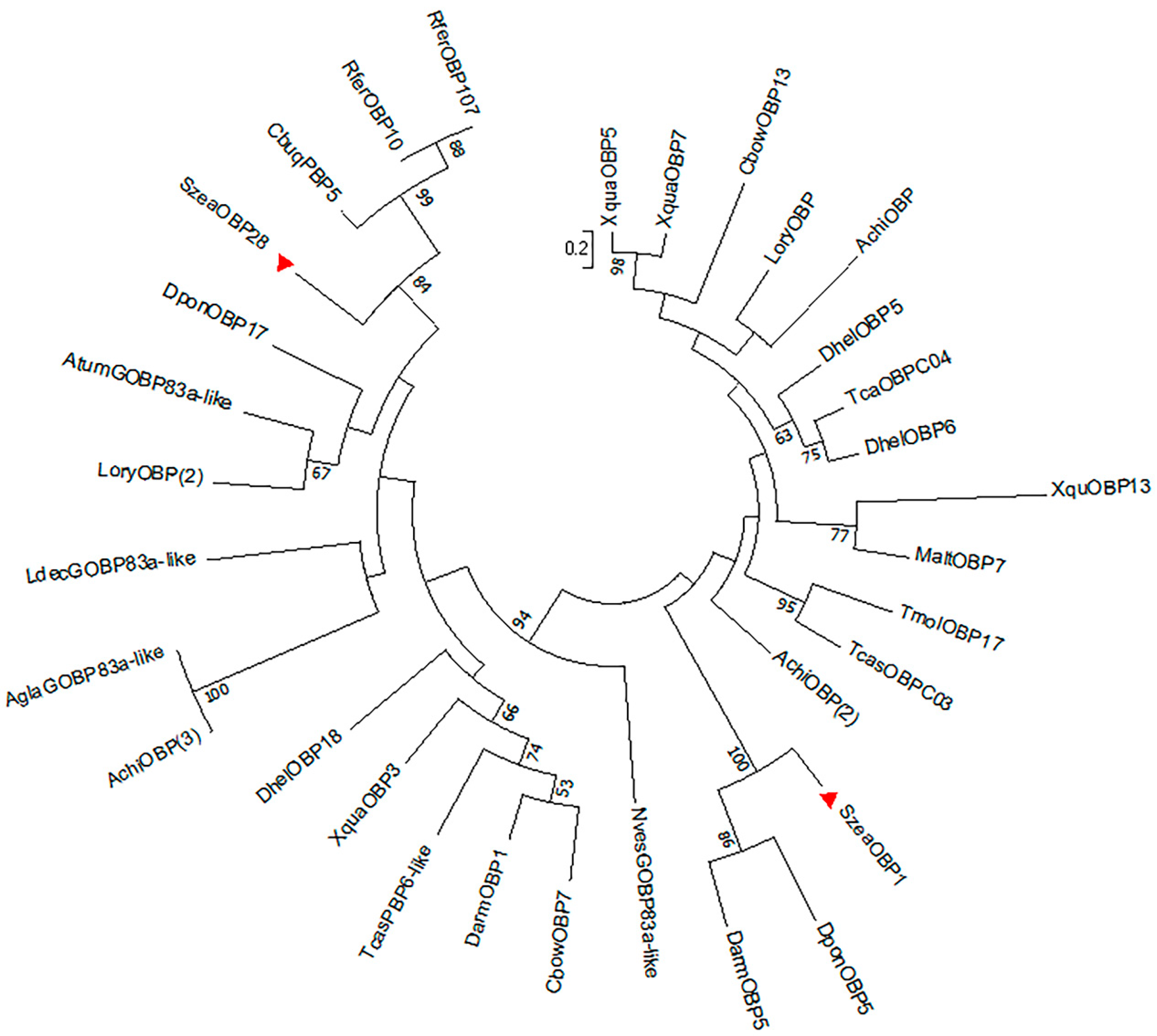
3.2. SzeaOBP Sequence Alignment and Phylogenetic Analysis
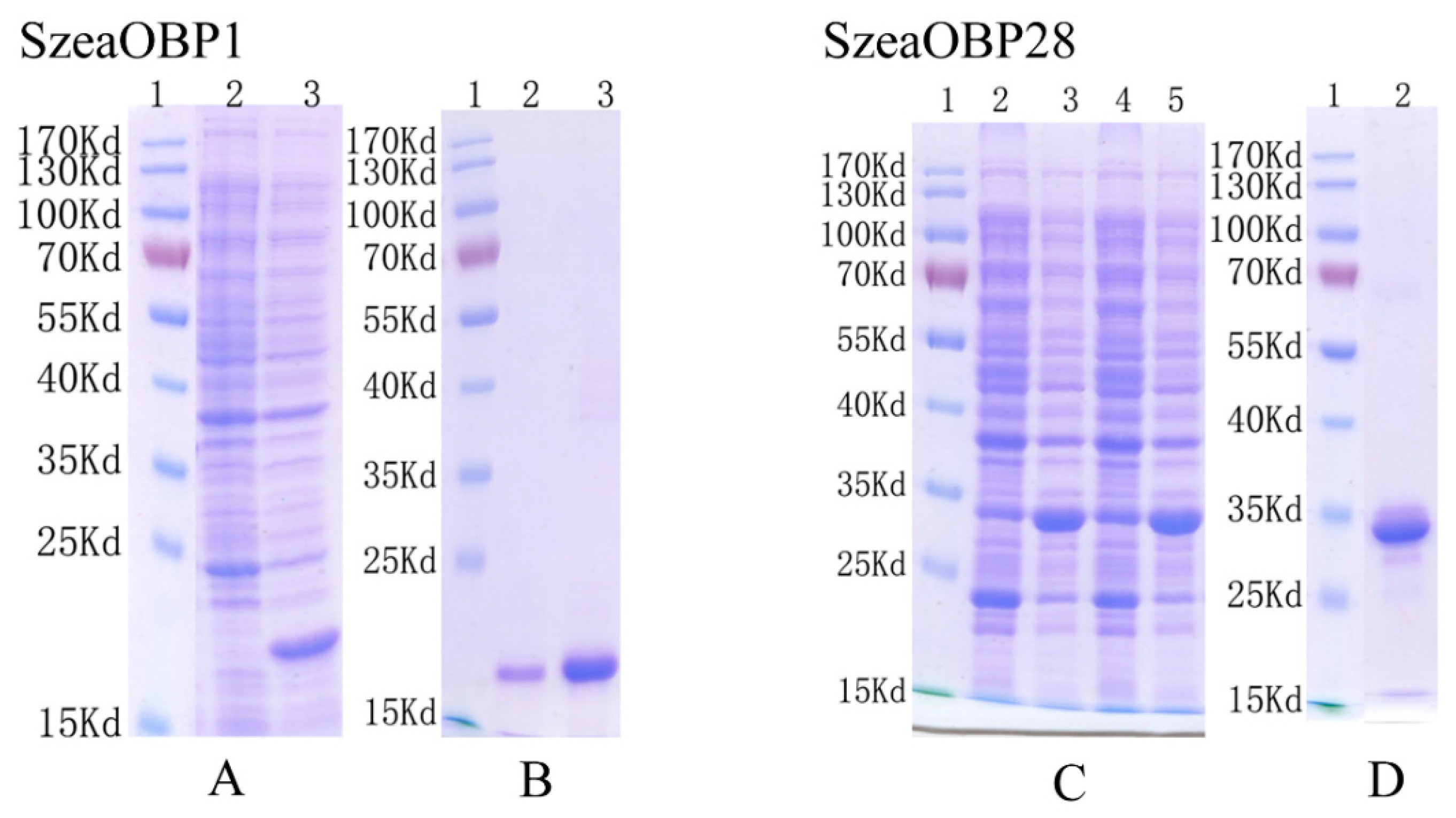
3.3. Cloning Results and Bacterial Expression
3.4. Antibody Production and Western Blot Analysis of the Two Recombinant Proteins
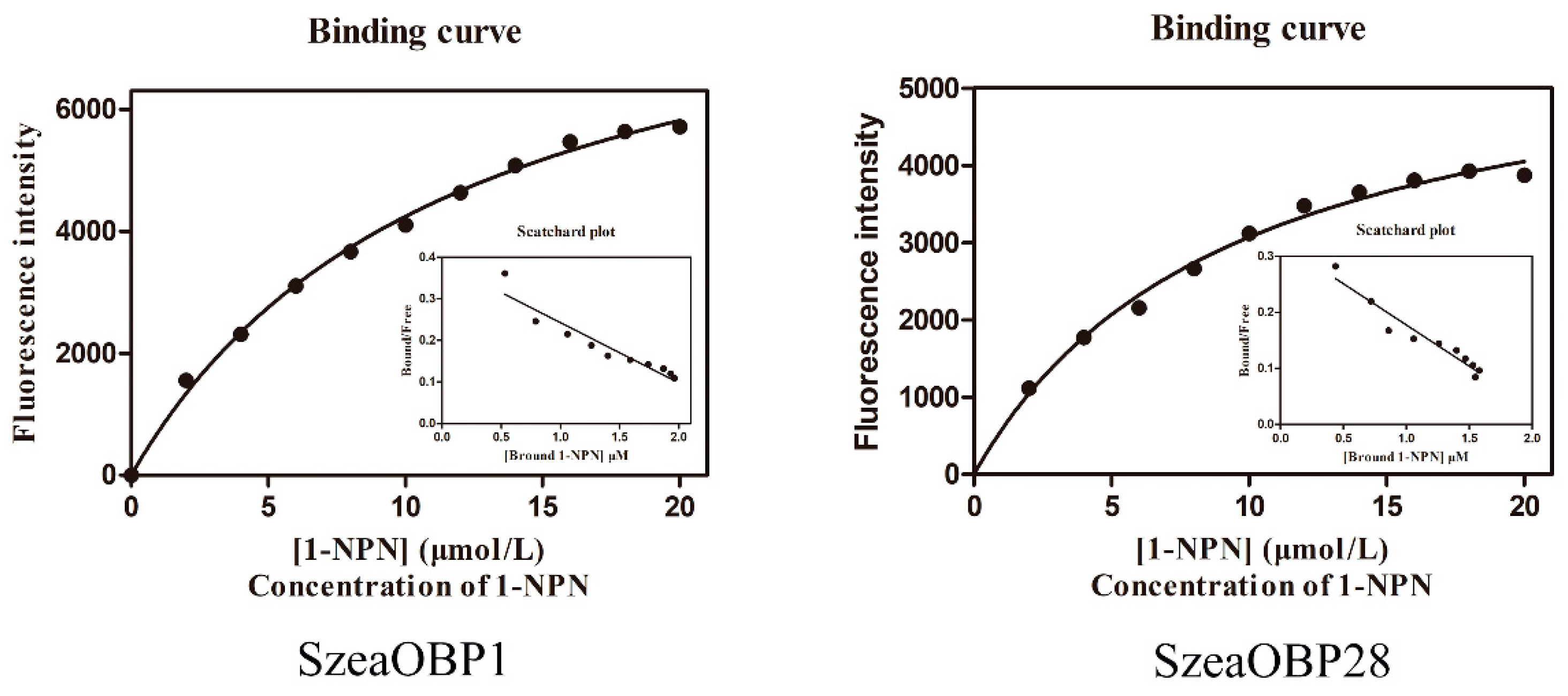
3.5. Fluorescence Competitive Binding Assays
3.6. Behavioural Response to Host Plant Volatile Components
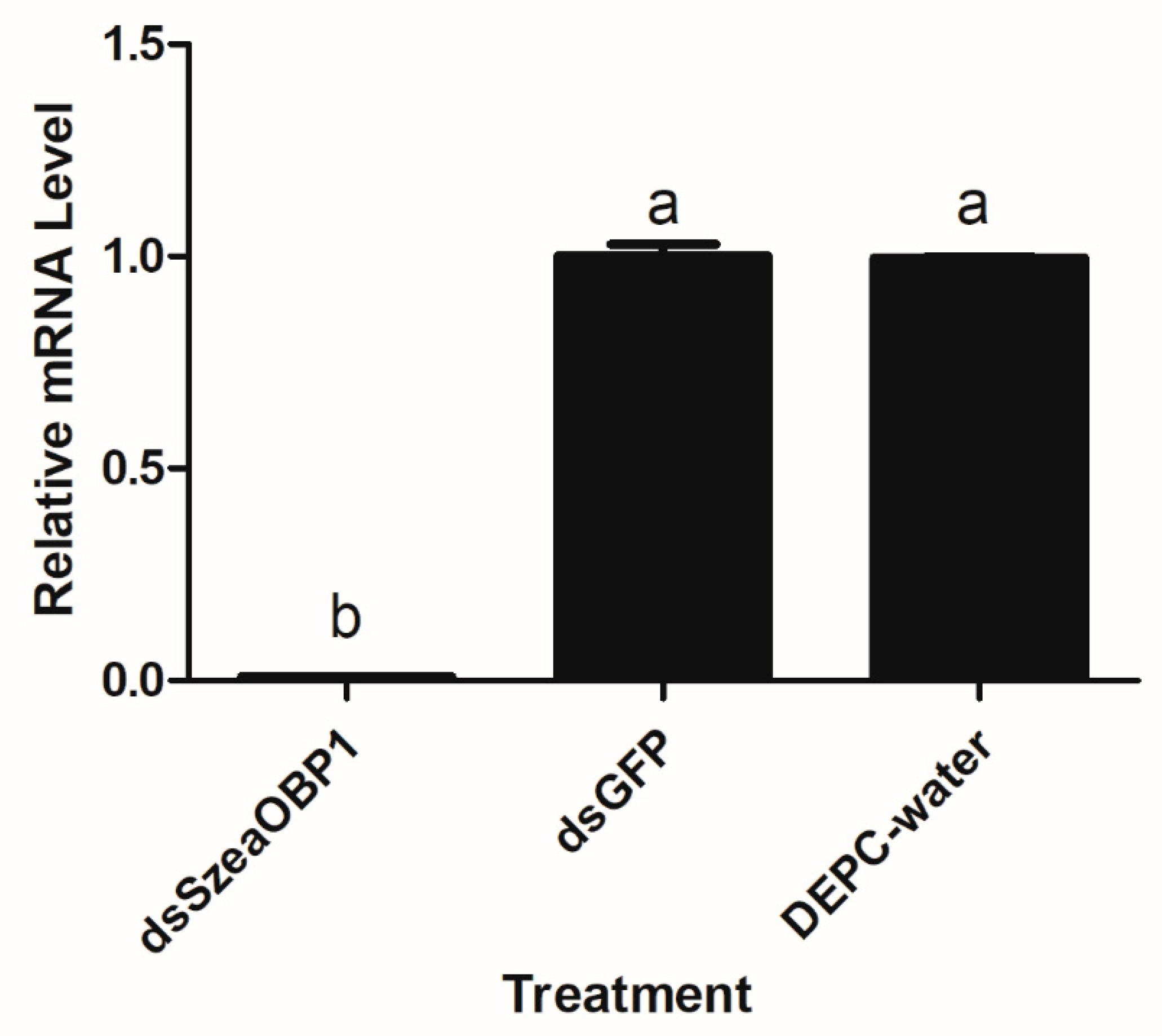
3.7. Effect of dsRNA Treatment on Sitophilus Zeamais Behaviour
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sun, L.; Li, Y.; Zhang, Z.D.; Guo, H.W.; Xiao, Q.; Wang, Q.; Zhang, Y.J. Expression patterns and ligand binding characterization of Plus-C odorant-binding protein 14 from Adelphocoris lineolatus (Goeze). Comp. Biochem. Phys. B 2019, 227, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Vogt, R.G. Odorant/pheromone metabolism in insects. Chem. Sens. 2006, 31, A7–A8. [Google Scholar]
- Vogt, R.G.; Grosse-Wilde, E.; Zhou, J.J. The Lepidoptera odorant binding protein gene family: Gene gain and loss within the GOBP/PBP complex of moths and butterflies. Insect Biochem. Mol. Biol. 2015, 62, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Vogt, R.G.; Riddiford, L.M. Pheromone binding and inactivation by moth antennae. Nature 1981, 293, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Bruce, T.J.A.; Wadhams, L.J.; Woodcock, C.M. Insect host location: A volatile situation. Trends Plant Sci. 2005, 10, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Brito, N.F.; Moreira, M.F.; Melo, A.C.A. A look inside odorant-binding proteins in insect chemoreception. J. Insect Physiol. 2016, 95, 51–65. [Google Scholar] [CrossRef]
- Leal, W.S. Odorant reception in insects: Roles of receptors, binding proteins, and degrading enzymes. Annu. Rev. Entomol. 2013, 58, 373–391. [Google Scholar] [CrossRef]
- Pelosi, P.; Iovinella, I.; Felicioli, A.; Dani, F.R. Soluble proteins of chemical communication: An overview across arthropods. Front. Physiol. 2014, 5, 320. [Google Scholar] [CrossRef]
- Pelosi, P.; Iovinella, I.; Zhu, J.; Wang, G.R.; Francesca, R. Beyond chemoreception: Diverse tasks of soluble olfactory proteins in insects. Biol. Rev. 2018, 93, 184–200. [Google Scholar] [CrossRef]
- Fan, J.; Francis, F.; Liu, Y.; Chen, J.L.; Cheng, D.F. An overview of odorant-binding protein functions in insect peripheral olfactory reception. Genet. Mol. Res. 2011, 10, 3056–3069. [Google Scholar] [CrossRef]
- Pimentel, D. World resources and food losses to pests. See Ref. 1991, 37, 5–11. [Google Scholar]
- Phillips, T.W.; Throne, J.E. Biorational approaches to managing stored-product insects. Annu. Rev. Entomol. 2010, 55, 375–397. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Xiao, J.J.; Zhou, L.J.; Liu, Y.; Wu, X.W.; Hua, R.M.; Wan, G.R.; Cao, H.Q. Insecticidal activity of melaleuca alternifolia essential oil and RNA-Seq analysis of Sitophilus zeamais transcriptome in response to oil fumigation. PLoS ONE 2016. [Google Scholar] [CrossRef]
- Zanan, R.; Khandagale, K.; Hinge, V.; Elangovan, M.; Henry, R.J.; Nadaf, A. Characterization of fragrance in sorghum (Sorghum bicolor (L.) Moench) grain and development of a gene-based marker for selection in breeding. Mol. Breed. 2016, 36, 146. [Google Scholar] [CrossRef]
- Muhammad, F.J.C.; Imran, P.; Faqir, M.A.; Muhammad, A.N. Characterization of sorghum and millet with special reference to fatty acid and volatile profile. Turk. J. Agric. Food Sci. Technol. 2015, 3, 515–521. [Google Scholar]
- Sansenya, S.; Hua, Y.L.; Chumanee, S. The correlation between 2-Acetyl-1-pyrroline content, biological compounds and molecular characterization to the aroma intensities of Thai local rice. J. Oleo Sci. 2018, 67, 893–904. [Google Scholar] [CrossRef]
- Yang, D.S.; Lee, K.S.; Kim, K.J.; Kays, S.J. Site of origin of volatile compounds in cooked rice. Cereal Chem. 2008, 85, 591–598. [Google Scholar] [CrossRef]
- Liyanaarachchi, G.D.; Kottearachchi, N.S.; Samarasekera, R. Volatile profiles of traditional aromatic rice varieties in Sri Lanka. J. Natl. Sci. Found. Sri Lanka 2014, 42, 87–93. [Google Scholar] [CrossRef]
- Joseph, A.M. Cereal volatiles, A Review. J. Agric. Food Chem. 1978, 26, 175–178. [Google Scholar]
- Phillips, T.W.; Jiang, X.L.; Burkholder, W.E.; Phillips, J.K.; Tran, H.Q. Behavioral responses to food volatiles by two species of stored-product coleoptera, Sitophilus oryzae (curculionidae) and Tribolium castaneum (tenebrionidae). J. Chem. Ecol. 1993, 19, 723–734. [Google Scholar] [CrossRef]
- Concepcion, J.C.T.; Ouk, S.; Riedel, A.; Calingacion, M.; Zhao, D.; Ouk, M.; Garson, M.J.; Fitzgerald, M.A. Quality evaluation, fatty acid analysis and untargeted profiling of volatiles in Cambodian rice. Food Chem. 2018, 240, 1014–1021. [Google Scholar] [CrossRef]
- Germinara, G.S.; De Cristofaro, A.; Rotundo, G. Electrophysiological and behavioral responses of Theocolax elegans (Westwood) (Hymenoptera: Pteromalidae) to cereal grain volatiles. Biomed. Res. Int. 2016. [Google Scholar] [CrossRef]
- Mugula, J.K.; Nnko, S.A.M.; Narvhus, J.A.; Sorhaug, T. Microbiological and fermentation characteristics of togwa, a Tanzanian fermented food. Int. J. Food Microbiol. 2003, 80, 187–199. [Google Scholar] [CrossRef]
- Cho, S.G.; Kays, S.J. Aroma-active compounds of wild rice (Zizania palustris L.). Food Res. 2013, 54, 1463–1470. [Google Scholar] [CrossRef]
- Toshova, T.B.; Velchev, D.I.; Subchev, M.A.; Toth, M.; Vuts, J.; Pickett, J.A.; Dewhirst, S.Y. Electrophysiological responses and field attraction of the grey corn weevil, Tanymecus (Episomecus) dilaticollis Gyllenhal (Coleoptera: Curculionidae) to synthetic plant volatiles. Chemoecology 2010, 20, 199–206. [Google Scholar] [CrossRef]
- Balakrishnan, K.; Holighaus, G.; Weissbecker, B.; Schutz, S. Electroantennographic responses of red flour beetle Tribolium castaneum Herbst (Coleoptera: Tenebrionidae) to volatile organic compounds. J. Appl. Entomol. 2017, 141, 477–486. [Google Scholar] [CrossRef]
- Wang, S.C.; Adhikari, K.; Hung, Y.C. Acceptability and preference drivers of freshly roasted peanuts. J. Food Sci. 2017, 82, 174–184. [Google Scholar] [CrossRef]
- Vidya, H.; Hemant, P.; Altafhusain, N. Comparative characterization of aroma volatiles and related gene expression analysis at vegetative and mature stages in basmati and non-basmati rice (Oryza sativa L.) cultivars. Appl. Biochem. Biotechnol. 2016, 178, 619–639. [Google Scholar]
- Da Silva, N.C.; Nascimento, C.F.; Nascimento, F.A.; de Resende, F.D.; Daniel, J.L.P.; Siqueira, G.R. Fermentation and aerobic stability of rehydrated corn grain silage treated with different doses of Lactobacillus buchneri or a combination of Lactobacillus plantarum and Pediococcus acidilactici. J. Dairy Sci. 2018, 101, 4158–4167. [Google Scholar] [CrossRef]
- Wang, Y.L.; Chen, Q.; Zhao, H.B.; Ren, B.Z. Identification and comparison of candidate olfactory genes in the olfactory and non olfactory organs of Elm Pest Ambrostoma quadriimpressum (Coleoptera: Chrysomelidae) based on transcriptome analysis. PLoS ONE 2016, 11, e0147144. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C (T)) Method. Method Enzymol. 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Tang, Q.Y.; Zhang, C.X. Data Processing System (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect Sci. 2013, 20, 254–260. [Google Scholar] [CrossRef]
- Petersen, T.N.; Brunak, S.; Heijne, G.V.; Nielsen, H. SignalP 4.0: Discriminating signal peptides from transmembrane regions. Nature Methods 2011, 8, 785–786. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Yusuf, A.A.; Crewe, R.M.; Pirk, C.W. Olfactory detection of prey by the termite-raiding ant Pachycondyla analis. J. Insect Sci. 2014, 14, 53. [Google Scholar]
- Takabayashi, T.; Dicke, M. Response of predatory mites with different rearing histories to volatiles of uninfested plants. Entomol. Exp. Appl. 1992, 64, 187–193. [Google Scholar] [CrossRef]
- Caetano, R.L.; Carrico, C.; Freitas, D.M.D.; Pinto, Z.T. Ultrastructure of sensilla on antennae and maxillary palps in three Mesembrinellidae species. Rev. Chil. Hist Nat. 2018. [Google Scholar] [CrossRef]
- Shen, C.; Zhang, Y.; Xia, D.S.; Wang, J.; Tang, Q.F. Sensilla on the Antennal Funiculus of the Maize Weevil, Sitophilus zeamais (Motschulsky) (Coleoptera: Curculionidae): A Morphological Investigation. Coleopt. Bull. 2019, 73, 443–450. [Google Scholar] [CrossRef]
- Carey, A.F.; Wang, G.R.; Su, C.Y.; Zwiebel, L.J.; Carlson, J.R. Odorant reception in the malaria mosquito Anopheles gambiae. Nature 2010, 464, 66–71. [Google Scholar] [CrossRef]
- Wang, G.R.; Carey, A.F.; Carlson, J.R.; Zwiebel, L.J. Molecular basis of odor coding in the malaria vector mosquito Anopheles gambiae. Proc. Natl. Acad. Sci. USA 2010, 107, 4418–4423. [Google Scholar] [CrossRef]
- Morrison, W.R.; Allen, M.; Leskey, T.C. Behavioural response of the invasive Halyomorpha halys (Hemiptera: Pentatomidae) to host plant stimuli augmented with semiochemicals in the field. Agr. For. Entomol. 2018, 20, 62–72. [Google Scholar] [CrossRef]
- Weber, D.C.; Morrison, W.R.; Khrimian, A.; Rice, K.B.; Leskey, T.C.; Rodriguez-Saona, C.; Nielsen, A.L.; Blaauw, B.R. Chemical ecology of Halyomorpha halys: Discoveries and applications. J. Pest Sci. 2017, 90, 989–1008. [Google Scholar] [CrossRef]
- Tang, Q.F.; Shen, C.; Zhang, Y.; Yang, Z.P.; Han, R.R.; Wang, J. Antennal transcriptome analysis of the maize weevil Sitophilus zeamais: Identification and tissue expression profiling of candidate odorant-binding protein genes. Arch. Insect Biochem. 2019. [Google Scholar] [CrossRef]
- Tang, Q.F.; Zhang, Y.; Shen, C.; Xia, D.S. Identification and expression profiling of odorant receptor protein genes in Sitophilus zeamais (Coleoptera: Curculionoidea) using RT-qPCR. Neotrop. Entomol. 2019. [Google Scholar] [CrossRef]
- Pelletier, J.; Bozzolan, F.; Solvar, M.; François, M.C.; Jacquin-Joly, E.; Maïbèche-Coisne, M. Identification of candidate aldehyde oxidases from the silkworm Bombyx mori potentially involved in antennal pheromone degradation. Gene 2007, 404, 31–40. [Google Scholar] [CrossRef]
- Tang, B.W.; Tai, S.L.; Dai, W.; Zhang, C.N. Expression and functional analysis of two odorant binding proteins from Bradysia odoriphaga (Diptera: Sciaridae). J. Agric. Food Chem. 2019, 67, 3565–3574. [Google Scholar] [CrossRef]
- Hu, Z.D.; Feng, X.; Lin, Q.S. cDNA cloning and characterization of the carboxylesterase pxCCE016b from the diamondback moth, Plutella xylostella L. J. Intergr. Agr. 2016, 15, 1059–1068. [Google Scholar] [CrossRef]
- Jin, M.H.; Zhao, X.; Li, G.Y.; Che, X.Z.; Liu, Z.G.; Xue, C.B. Molecular characterization and bioinformatics analysis of a prophenoloxidase-1 (PPO1) in Plutella xylostella. J. Insect Sci. 2016, 8, 1–8. [Google Scholar] [CrossRef]
- Pan, Z.Z.; Xu, L.; Zhu, Y.J.; Shi, H.; Chen, Z.; Chen, M.C.; Chen, Q.X.; Liu, B. Characterization of a new CRY2Ab gene of Bacillus thuringiensis with high insecticidal activity against Plutella xylostella L. World J. Microbiol. Biotechnol. 2014, 30, 2655–2662. [Google Scholar] [CrossRef]
- Dong, X.L.; Zhai, Y.F.; Hu, M.Y.; Zhong, G.H.; Huang, W.J.; Zheng, Z.H.; Han, P.F. Proteomic and properties analysis of botanical insecticide Rhodojaponin III-Induced response of the diamondback moth, Plutella xyllostella (L.). PLoS ONE 2013, 8, e67723. [Google Scholar] [CrossRef]
- Guo, L.; Tang, B.Z.; Dong, W.; Liang, P.; Gao, X.W. Cloning, characterization and expression profiling of the cDNA encoding the ryanodine receptor in diamondback moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae). Pest Manag. Sci. 2012, 68, 1605–1614. [Google Scholar] [CrossRef]
- Xiu, C.L.; Zhang, W.; Xu, B.; Wyckhuys, K.A.G.; Cai, X.M.; Su, H.H.; Lu, Y.H. Volatiles from aphid-infested plants attract adults of the multicolored Asian lady beetle Harmonia axyridis. Biol. Control 2019, 129, 1–11. [Google Scholar] [CrossRef]
- Christensen, S.A.; Nemchenko, A.; Borrego, E.; Murray, I.; Sobhy, I.S.; Bosak, L.; DeBlasio, S.; Erb, M.; Robert, C.A.M.; Vaughn, K.A.; et al. The maize lipoxygenase, ZmLOX10, mediates green leaf volatile, jasmonate and herbivore-induced plant volatile production for defense against insect attack. Plant J. 2013, 74, 59–73. [Google Scholar] [CrossRef]
- Islam, J.; Dhiman, S.; Tyagi, V.; Duarah, S.; Zaman, K.; Chattopadhyay, P. Behavioural and electrophysiological responses of mosquito vectors Aedes aegypti, Anopheles stephensi and Culex quinquefasciatus to an ethyl ester: Ethyl 2-aminobenzoate. J. Insect Behav. 2017, 30, 343–358. [Google Scholar] [CrossRef]
- Morawo, T.; Fadamiro, H. Identification of key plant-associated volatiles emitted by Heliothis virescens larvae that attract the parasitoid, microplitis croceipes: Implications for parasitoid perception of odor blends. J. Chem. Ecol. 2016, 42, 1112–1121. [Google Scholar] [CrossRef]



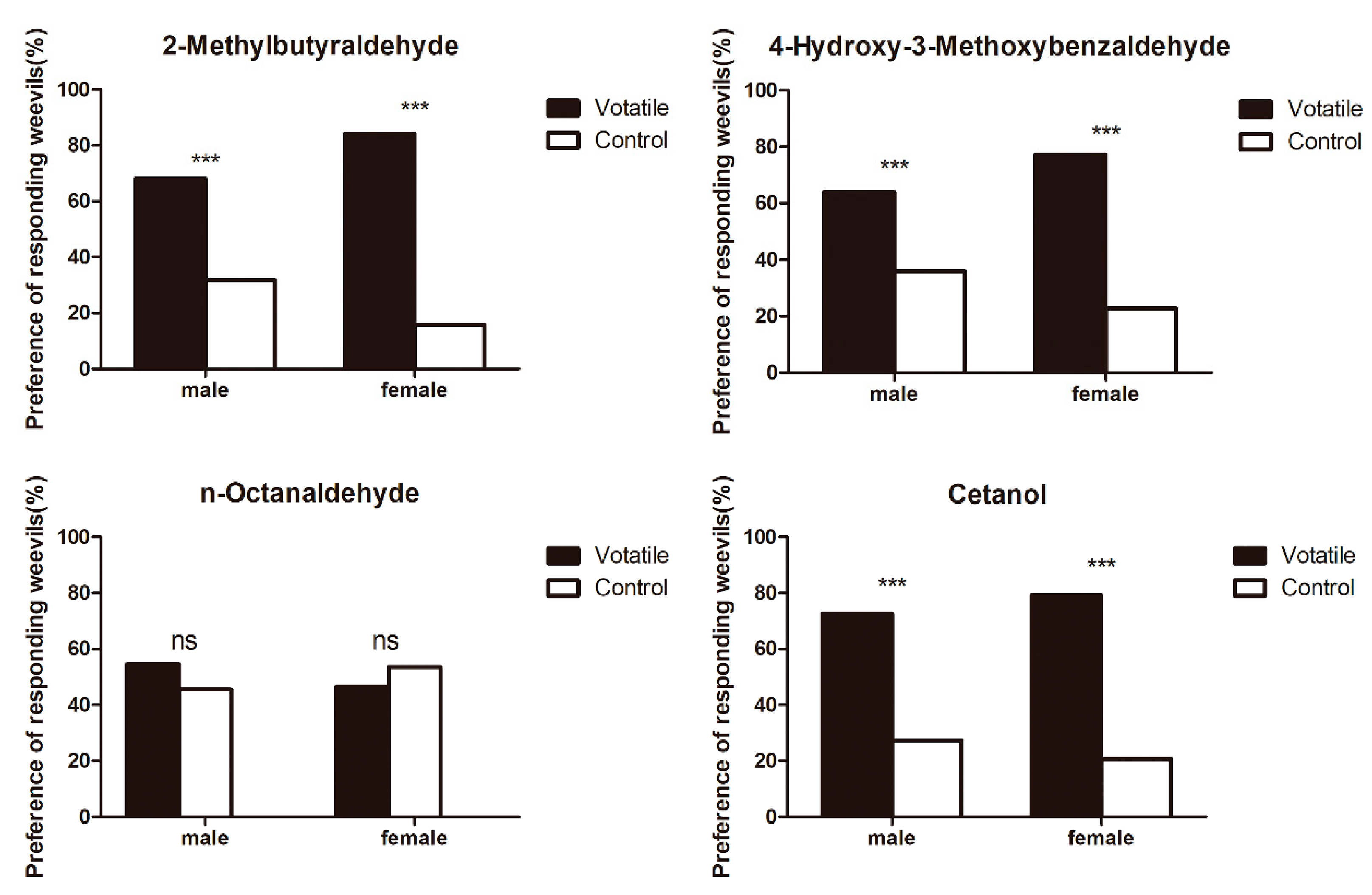

| Ligand | CAS | Purity (%) | Source | Origin | Reference | Molecular Weight | Formula |
|---|---|---|---|---|---|---|---|
| Pentadecane | 629-62-9 | 99 | Aladdin | S. bicolor | [14] | 212.41 | C15H32 |
| N-hexadecane | 544-76-3 | 98+ | Adamas-beta | S. bicolor | [15] | 226.44 | C16H34 |
| 1-Nonanol | 143-08-8 | 98 | Adamas-beta | A. hypogaea | [16,17] | 144.25 | C9H20O |
| 1-Hexanol | 111-27-3 | 98 | Adamas-beta | O. sativa, S. bicolor, S. italica | [15,16,18] | 102.17 | C6H14O |
| Capryl alcohol | 111-87-5 | 99 | Adamas-beta | S. bicolor | [15] | 130.23 | C8H18O |
| Cetanol | 36653-82-4 | 98+ | Adamas-beta | Storage grain | [19] | 242.44 | C16H34O |
| Decanal | 112-31-2 | 97 | Adamas-beta | S. bicolor | [15] | 156.27 | C10H20O |
| Valeraldehyde | 110-62-3 | 98 | Adamas-beta | S. bicolor | [14,20] | 86.13 | C5H10O |
| Heptaldehyde | 111-71-7 | 97 | Adamas-beta | O. sativa | [21] | 114.19 | C7H14O |
| 4-Hydroxy-3-methoxybenzaldehyde | 121-33-5 | 99 | Adamas-beta | T. aestivum, O. sativa, A. ativa, S. cereale, H. vulgare | [20,22] | 152.15 | C8H8O3 |
| 2-Methylbutyraldehyde | 96-17-3 | 98+ | Adamas-beta | S. bicolor, Z. mays, S. italica | [23] | 86.13 | C5H10O |
| Benzaldehyde | 100-52-7 | 99 | Adamas-beta | O. sativa | [24,25] | 106.12 | C7H6O |
| Nonanal | 124-19-6 | 95+ | Tokyo Chemical Industry | O. sativa, S. bicolor, A. hypogaea, S. italica | [26,27] | 142.24 | C9H18O |
| n-Octanaldehyde | 124-13-0 | 98 | Adamas-beta | O. sativa, A. hypogaea, | [16,26] | 128.21 | C8H16O |
| 3-Methylbutanal | 590-86-3 | 98 | Adamas-beta | O. sativa, S. bicolor, Z. mays, S. italica | [23] | 86.13 | C5H10O |
| Isobutyraldehyde | 78-84-2 | 98+ | Tokyo Chemical Industry | O. sativa, S. bicolor, Z. mays, S. italica | [23] | 72.11 | C4H8O |
| 1-Pentanecarbaldehyde | 66-25-1 | 98 | Adamas-beta | S. bicolor, O. sativa, A. hypogaea, S. italica | [14,16,27] | 100.16 | C6H12O |
| 6-Methyl-5-hepten-2-one | 110-93-0 | 98 | Adamas-beta | O. sativa | [28] | 126.2 | C8H14O |
| Acetone | 67-64-1 | 98 | Adamas-beta | S. bicolor | [14] | 58.08 | C3H6O |
| 2-Pentanone | 107-87-9 | 99 | Adamas-beta | O. sativa | [21] | 86.13 | C5H10O |
| Palmitic acid | 57-10-3 | 98+ | Adamas-beta | S. bicolor, S. italica | [15] | 256.42 | C16H32O2 |
| Linoleic acid | 60-33-3 | 95+ | Aladdin | O. sativa, S. bicolor, S. italica | [15,21] | 280.45 | C18H32O2 |
| Acetic acid (glacial) | 64-19-7 | 99 | Adamas-beta | Z. mays | [29] | 60.05 | C2H4O2 |
| Maltol (3-hydroxy-2-methyl-4-pyrone) | 118-71-8 | 99 | Adamas-beta | T. aestivum, O. sativa, A. sativa, S. cereale, H. vulgare | [20] | 126.11 | C6H6O3 |
| Myrcene (stabilized with BHT) | 123-35-3 | 75+ | Adamas-beta | Storage grain | [19] | 136.23 | C10H16 |
| Bis(2-methoxyethyl) phthalate | 117-82-8 | 95 | Adamas-beta | Storage grain | [19] | 282.29 | C14H18O6 |
| Methanol | 67-56-1 | HPLC | Adamas-beta | - | - | 32.04 | CH4O |
| N-Phenyl-1-naphthylamine | 90-30-2 | 99 | Adamas-beta | - | - | 219.28 | C16H13N |
| Primer Name | Forward (5’–3’) | Reverse (5’–3’) | Product Length (bp) |
|---|---|---|---|
| For prokaryotic expression | |||
| SzeaOBP1 | CGGGATCCGGAACAAACGACCCCCAA (BamH1) | CGCTCGAGAAGATTGTGGTCCTCTTTAA (Xhol) | 345 |
| SzeaOBP28 | CGGGATCCATGTTTGACGAATCCACATT (BamH1) | CGCTCGAGAACCATCATATAGGCTTCTG (Xhol) | 372 |
| For qRT-PCR | |||
| SzeaOBP1 | CGACTCCTTCTACCACCAC | AACACTGAACCGCATCTC | 206 |
| SzeaOBP28 | AATCAAAGTGGAGCAA | TCAAAGCGTCAGGAAT | 175 |
| β-actin | GGGGCGAATACTGTGAGAAA | AGCAGGTTCAAAAGGCTCAA | 192 |
| Ligand | SzeaOBP1 | SzeaOP28 | ||
|---|---|---|---|---|
| IC50 (μM) | Ki (μM) | IC50 (μM) | Ki (μM) | |
| Alkanes | ||||
| Pentadecane | - | - | - | - |
| N-Hexadecane | - | - | - | - |
| Alcohols | ||||
| 1-Nonanol | 8.131 ± 0.274 | 6.944 ± 0.234 | - | - |
| 1-Hexanol | 5.470 ± 0.186 | 4.671 ± 0.159 | - | - |
| Capryl alcohol | 7.814 ± 0.233 | 6.673 ± 0.199 | - | - |
| Cetanol | 3.218 ± 0.162 | 2.748 ± 0.139 | - | - |
| Aldehyde | ||||
| Decanal | - | - | - | - |
| Valeraldehyde | 5.715 ± 0.121 | 4.880 ± 0.103 | - | - |
| Heptaldehyde | 5.707 ± 0.130 | 4.874 ± 0.111 | - | - |
| 4-Hydroxy-3-methoxybenzaldehyde | 4.368 ± 0.203 | 3.730 ± 0.173 | 24.680 ± 2.340 | 20.23 ± 1.918 |
| 2-Methylbutyraldehyde | 4.916 ± 0.102 | 4.198 ± 0.087 | - | - |
| Benzaldehyde | 5.198 ± 0.092 | 4.439 ± 0.079 | - | - |
| Nonanal | 5.179 ± 0.111 | 4.423 ± 0.095 | 58.855 ± 1.342 | 48.242 ± 1.100 |
| n-Octanaldehyde | 3.769 ± 0.120 | 3.219 ± 0.103 | - | - |
| 3-Methylbutanal | 7.617 ± 0.191 | 6.505 ± 0.163 | - | - |
| Isobutyraldehyde | 4.987 ± 0.162 | 4.259 ± 0.138 | - | - |
| 1-Pentanecarbaldehyde | 6.067 ± 0.154 | 5.181 ± 0.132 | 59.42 ± 1.214 | 48.70 ± 0.852 |
| Ketones | ||||
| 6-Methyl-5-hepten-2-one | 6.501 ± 0.150 | 5.552 ± 0.128 | 45.845 ± 1.232 | 37.578 ± 1.010 |
| Acetone | 5.320 ± 0.201 | 4.543 ± 0.172 | 48.555 ± 0.861 | 39.824 ± 0.731 |
| 2-Pentanone | 11.62 ± 0.312 | 9.923 ± 0.267 | - | - |
| Acids | ||||
| Palmitic Acid | 28.28 ± 2.082 | 24.150 ± 1.778 | 49.746 ± 1.724 | 40.776 ± 1.414 |
| Linoleic acid | - | - | - | - |
| Acetic acid (glacial) | 18.43 ± 0.840 | 15.739 ± 0.717 | - | - |
| Others | ||||
| Maltol (3-hydroxy-2-methyl-4-pyrone) | 6.179 ± 0.390 | 5.277 ± 0.333 | - | - |
| Myrcene (stabilized with BHT) | 35.45 ± 0.931 | 30.27 ± 0.798 | - | - |
| Bis(methylglycol) phthalate | 4.762 ± 0.147 | 4.067 ± 0.125 | 38.865 ± 0.200 | 31.857 ± 0.164 |
| (+)-a-Pinene | - | - | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Shen, C.; Xia, D.; Wang, J.; Tang, Q. Characterization of the Expression and Functions of Two Odorant-Binding Proteins of Sitophilus zeamais Motschulsky (Coleoptera: Curculionoidea). Insects 2019, 10, 409. https://doi.org/10.3390/insects10110409
Zhang Y, Shen C, Xia D, Wang J, Tang Q. Characterization of the Expression and Functions of Two Odorant-Binding Proteins of Sitophilus zeamais Motschulsky (Coleoptera: Curculionoidea). Insects. 2019; 10(11):409. https://doi.org/10.3390/insects10110409
Chicago/Turabian StyleZhang, Ying, Chen Shen, Daosong Xia, Jian Wang, and Qingfeng Tang. 2019. "Characterization of the Expression and Functions of Two Odorant-Binding Proteins of Sitophilus zeamais Motschulsky (Coleoptera: Curculionoidea)" Insects 10, no. 11: 409. https://doi.org/10.3390/insects10110409
APA StyleZhang, Y., Shen, C., Xia, D., Wang, J., & Tang, Q. (2019). Characterization of the Expression and Functions of Two Odorant-Binding Proteins of Sitophilus zeamais Motschulsky (Coleoptera: Curculionoidea). Insects, 10(11), 409. https://doi.org/10.3390/insects10110409






