Neuroethology of the Waggle Dance: How Followers Interact with the Waggle Dancer and Detect Spatial Information
Abstract
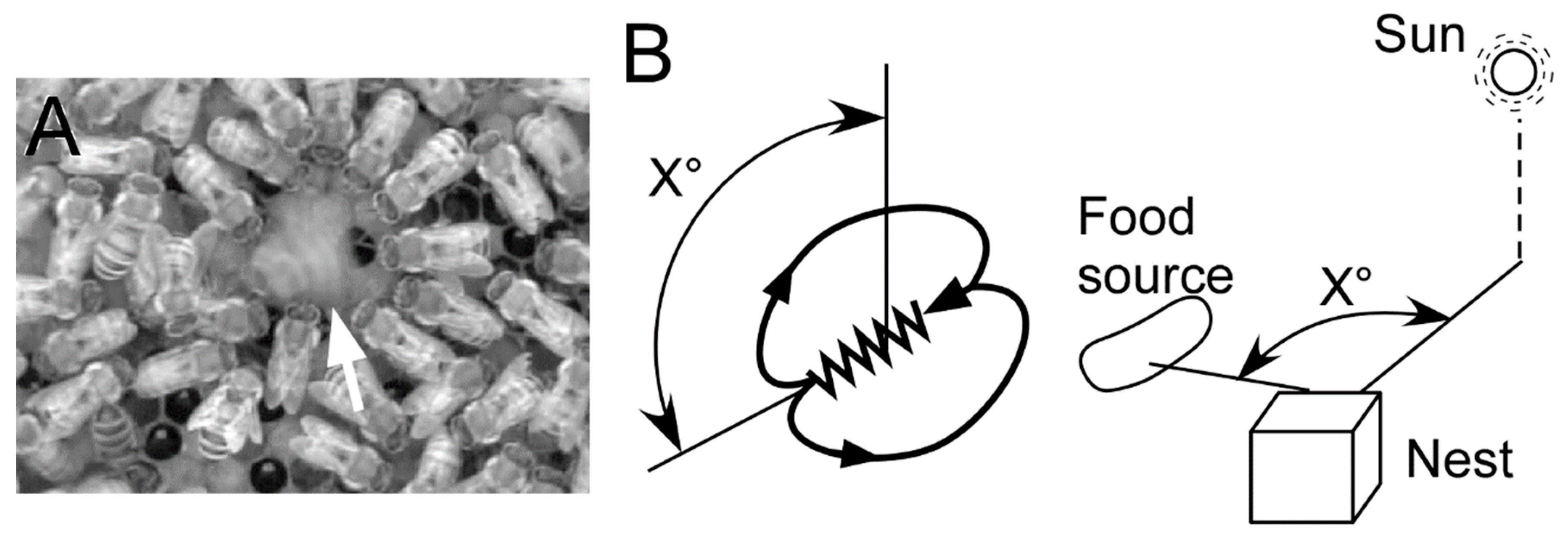
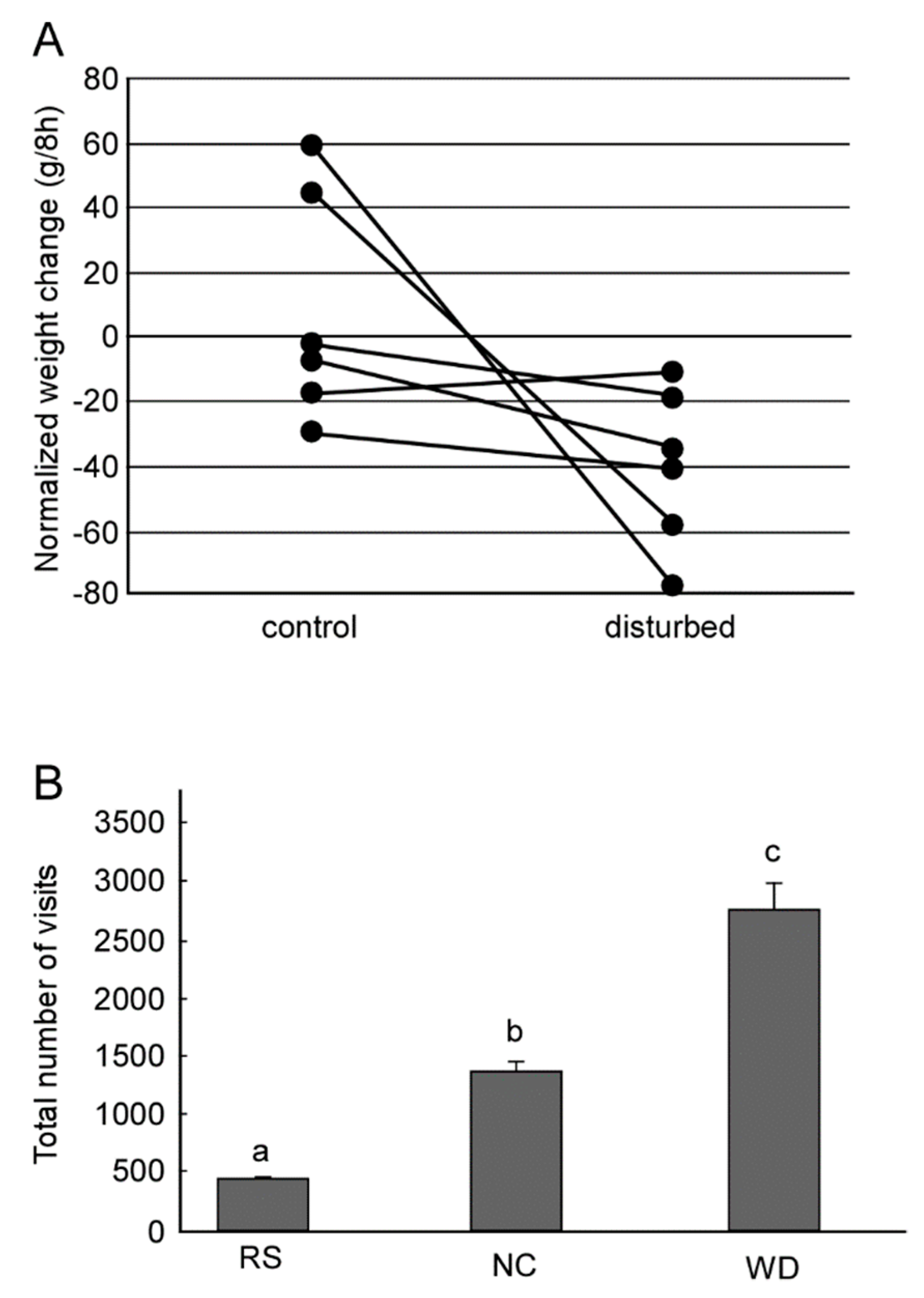
1. Behavioral Significance of the Waggle Dance
2. The Follower’s Behaviors Induced by Various Waggle Dance Signals and How These Signals Are Sensed
2.1. Distance
2.2. Direction
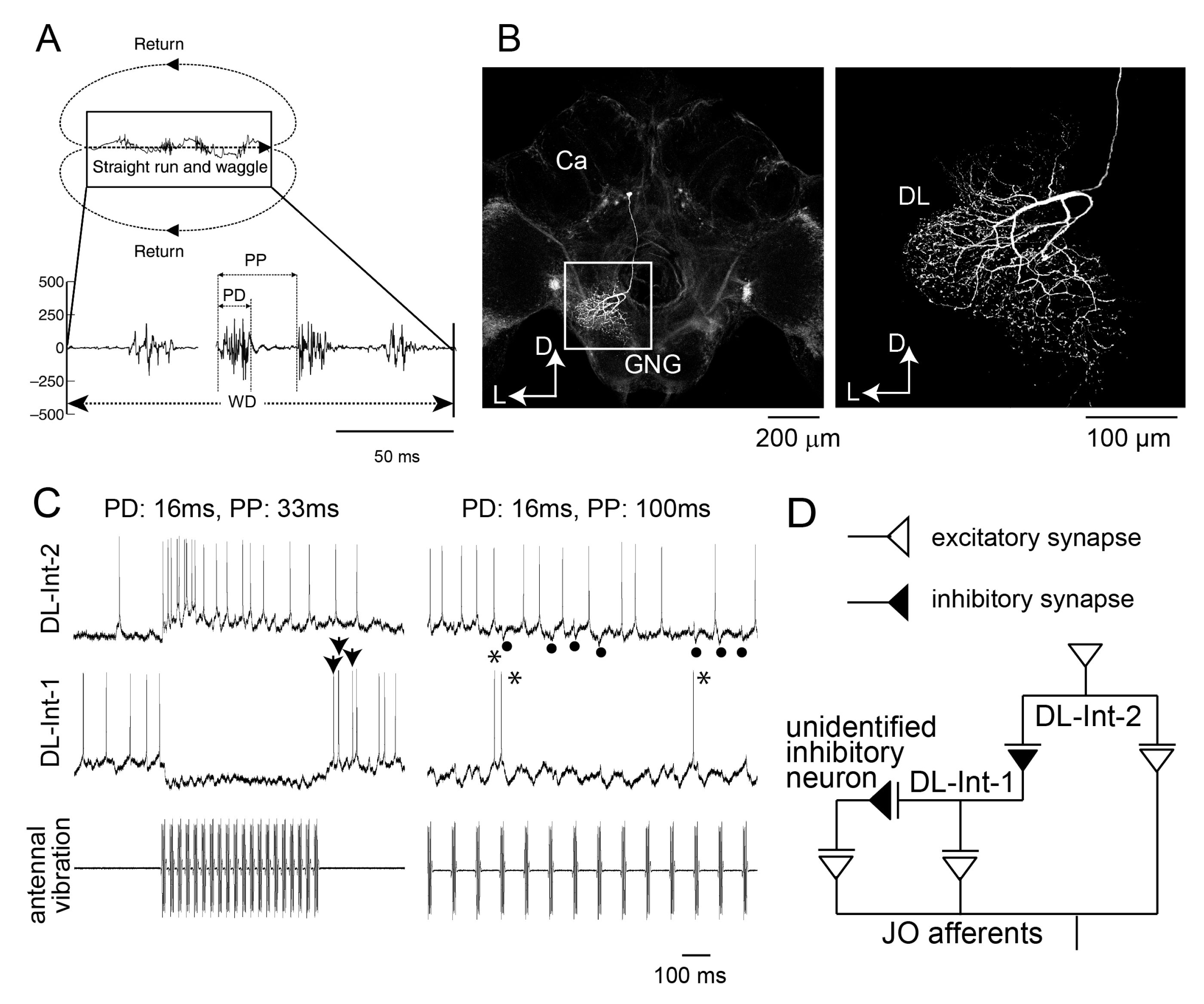
3. Neural Processing of Distance Information Encoded in Waggle Dance
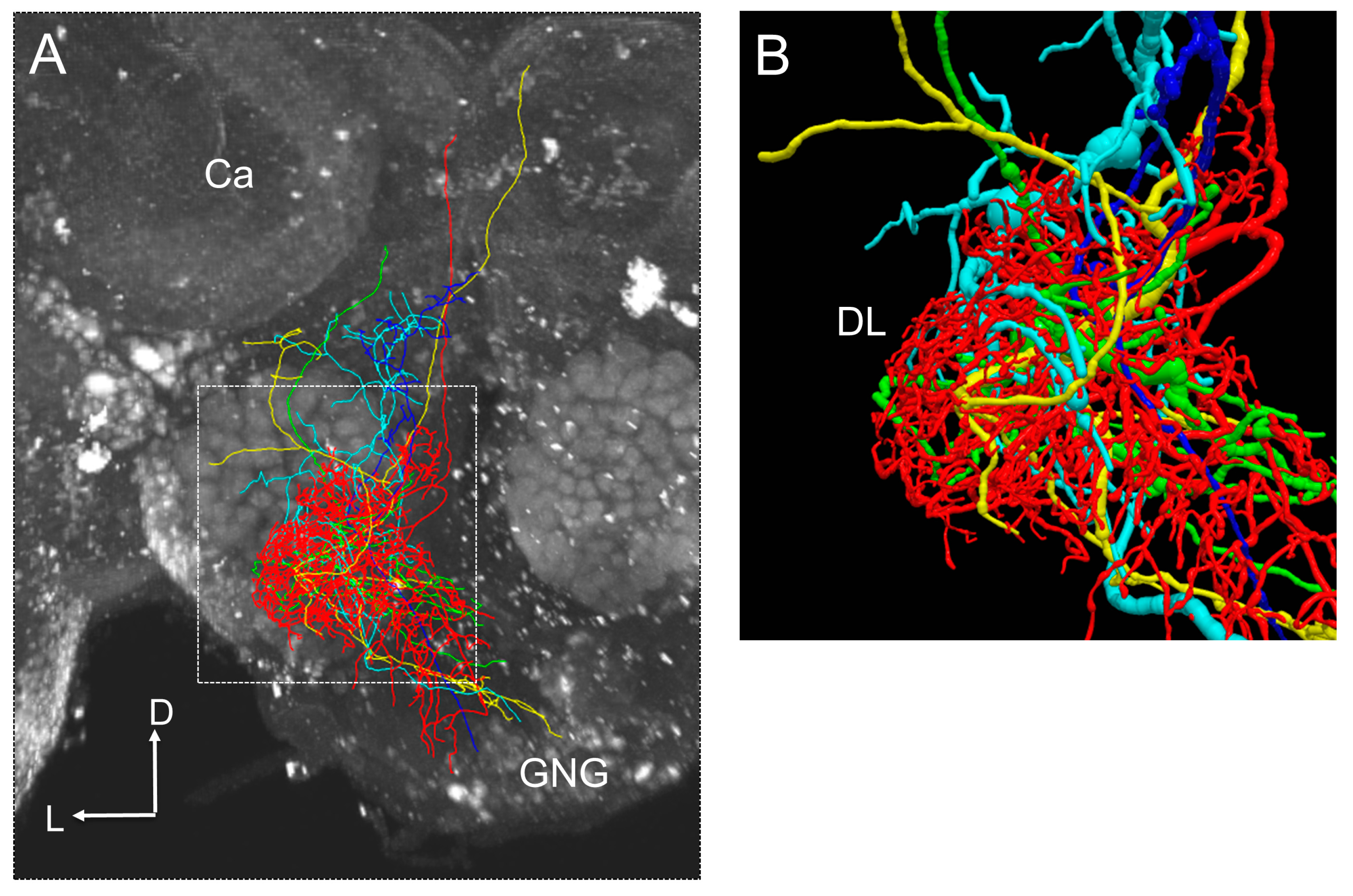
4. The Evaluation of the Neural Circuits for Processing Distance Information Encoded in the Waggle Dance Using the Honeybee Standard Brain (HSB)
5. Computational Analyses of the Neural Circuits Processing the Distance Information Encoded in the Waggle Dance
6. The Mechanism for Detecting the Azimuth and Distance toward the Feeding Site
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Von Frisch, K.; Lindauer, M. The “language” and orientation of the honey bee. Ann. Rev. Ent. 1956, 1, 45–58. [Google Scholar] [CrossRef]
- Seeley, T.D.; Visscher, P.K. Assessing the benefits of cooperation in honeybee foraging: Search costs, forage quality, and competitive ability. Behav. Ecol. Sociobiol. 1988, 22, 229–237. [Google Scholar] [CrossRef]
- Dyer, F.C. The biology of the dance language. Annu. Rev. Entomol. 2002, 47, 917–949. [Google Scholar] [CrossRef] [PubMed]
- Grüter, C.; Farina, W.M. The honeybee waggle dance: Can we follow the steps? Trend. Ecol. Evol. 2008, 24, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Couvillon, M.J. The dance legacy of Karl von Frisch. Insect. Soc. 2012, 59, 297–306. [Google Scholar] [CrossRef]
- Sherman, G.; Visscher, K.P. Honeybee colonies achieve fitness through dancing. Nature 2002, 419, 920–922. [Google Scholar] [CrossRef] [PubMed]
- Dornhaus, A.; Chittka, L. Why do honey bees dance? Behav. Ecol. Sociobiol. 2004, 55, 395–401. [Google Scholar] [CrossRef]
- Okada, R.; Akamatsu, T.; Iwata, K.; Ikeno, H.; Kimura, T.; Ohashi, M.; Aonuma, H.; Ito, E. Waggle dance effect: Dancing in autumn reduces the weight loss of a honeybee colony. J. Exp. Biol. 2012, 215, 1633–1641. [Google Scholar] [CrossRef][Green Version]
- Okada, R.; Ikeno, H.; Aonuma, H.; Ito, E. Biological insights into robotics: Honeybee foraging behavior by waggle dance. Adv. Robotics 2018, 22, 1665–1681. [Google Scholar] [CrossRef]
- Okada, R.; Ikeno, H.; Sasayama, N.; Aonuma, H.; Kurabayashi, D.; Ito, E. The dance of the honeybee: How do they dance to transfer the food information effectively? Acta Biol. Hung. 2018, 59, 157–162. [Google Scholar] [CrossRef]
- De Marco, R.; Gurevitz, J.M.; Menzel, R. Variability in the encoding of spatial information by dancing bees. J. Exp. Biol. 2008, 211, 1635–1644. [Google Scholar] [CrossRef] [PubMed]
- Seeley, T.D. Honey bee foragers as sensory units of their colonies. Behav. Ecol. Sociobiol. 1994, 34, 51–62. [Google Scholar] [CrossRef]
- De Marco, R.J.; Farian, W.M. Changes in food source profitability affect the trophallactic and dance behavior of forager honeybees (Apis mellifera L.). Behav. Ecol. Sociobiol. 2001, 50, 441–449. [Google Scholar] [CrossRef]
- George, E.A.; Brockmann, A. Social modulation of individual differences in dance communication in honey bees. Behav. Ecol. Sociobiol. 2019, 73, 41. [Google Scholar] [CrossRef]
- Seeley, T.D.; Camazine, S.; Sneyd, J. Collective decision-making in honey bees: How colonies choose among nectar sources. Behav. Ecol. Sociobiol. 1991, 28, 277–290. [Google Scholar] [CrossRef]
- Camazine, S.; Sneyd, J. A model of collective nectar source selection by honey-bees: Self-organization through simple rules. J. Theor. Biol. 1991, 149, 547–571. [Google Scholar] [CrossRef]
- Okada, R.; Ikeno, H.; Kimura, T.; Ohashi, M.; Aonuma, H.; Ito, E. Markov model of the honeybee social behavior. Information 2010, 13, 1115–1130. [Google Scholar]
- Okada, R.; Ikeno, H.; Kimura, T.; Ohashi, M.; Aonuma, H.; Ito, E. Error in the honeybee waggle dance improves foraging flexibility. Sci. Rep. 2014, 4, 4175. [Google Scholar] [CrossRef]
- Grüter, C.; Sol Balbuena, M.; Farina, W.M. Informational conflicts created by the waggle dance. Proc. R Soc. B 2008, 275, 1321–1327. [Google Scholar] [CrossRef]
- Thom, C.; Gilley, D.C.; Hooper, J.; Esch, H.E. The scent of the waggle dance. PLoS Biol. 2007, 5, e228. [Google Scholar] [CrossRef]
- I’Anson Price, R.; Dulex, N.; Vial, N.; Vincent, C.; Grüter, C. Honeybees forage more successfully without the “dance language” in challenging environments. Sci. Adv. 2019, 5, eaat0450. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.V. Honeybee communication: A signal for danger. Curr. Biol. 2010, 20, R366–R368. [Google Scholar] [CrossRef] [PubMed]
- Farina, W.H. The interplay between dancing and trophallactic behavior in the honey bee Apis mellifera. J. Comp. Physiol. A 2000, 186, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Cholé, H.; Carcaud, J.; Mazeau, H.; Famie, S.; Arnold, G.; Sandoz, J.C. Social contact acts as appetitive reinforcement and supports associative learning in honeybees. Curr. Biol. 2019, 29, 1407–1413. [Google Scholar]
- Frasnelli, E.; Vallortigara, G. Distribution of antennal olfactory and non-olfactory sensilla in different species of bees. Symmetry 2017, 9, 135. [Google Scholar] [CrossRef]
- Galizia, C.G.; Saches, S.; Rappert, A.; Menzel, R. The glomerular code for odor representation is species specific in the honeybee Apis mellifera. Nat. Neurosci. 1999, 2, 473–478. [Google Scholar] [CrossRef]
- Faber, T.; Joerges, J.; Menzel, R. Associative learning modifies neural representations of odors in the insect brain. Nat. Neurosci. 1999, 2, 74–78. [Google Scholar] [CrossRef]
- Yamashita, T.; Haupt, S.S.; Ikeno, H.; Ai, H. Walking patterns induced by learned odors in the honeybee, Apis mellifera L. J. Exp. Biol. 2017, 219, 12–16. [Google Scholar] [CrossRef]
- Nieh, J.C.; Tautz, J. Behaviour-locked signal analysis reveals weak 200–300 Hz comb vibrations during the honeybee waggle dance. J. Exp. Biol. 2000, 203, 1573–1579. [Google Scholar]
- Tautz, J. Honeybee waggle dance: Recruitment success depends on the dance floor. J. Exp. Biol. 1996, 199, 1375–1381. [Google Scholar]
- Michelsen, A. Signals and flexibility in the dance communication of honeybees. J. Comp. Physiol. A 2003, 189, 165–174. [Google Scholar]
- Landgraf, T.; Bierbach, D.; Kirbach, A.; Cusing, R.; Oertel, M.; Lehmann, K.; Greggers, U.; Menzel, R.; Rojas, R. Dancing honey bee robot elicits dance-following and recruits foragers. arXiv 2018, arXiv:1803.07126v1. [Google Scholar]
- Michelsen, A.; Anderson, B.B.; Kirchner, W.H.; Lindauer, M. Honeybees can be recruited by a mechanical model of a dancing bee. Naturwissehschaften 1989, 76, 277–280. [Google Scholar] [CrossRef]
- Hrncir, M.; Maia-Silva, C.; Mc Cabe, S.I.; Farina, W.M. The recruiter’s excitement-features of thoracic vibrations during the honey bee’s waggle dance related to food source profitability. J. Exp. Biol. 2011, 214, 4055–4064. [Google Scholar] [CrossRef] [PubMed]
- Towne, W.F.; Kirchner, W.H. Hearing in honey bees: Detection of air-particle oscillations. Science 1989, 244, 686–688. [Google Scholar] [CrossRef] [PubMed]
- Kirchner, W.H.; Dreller, C.; Towne, W.F. Hearing in honeybees: Operant conditioning and spontaneous reactions to airborne sound. J. Comp. Physiol. A 1991, 168, 85–89. [Google Scholar] [CrossRef]
- Wenner, A.M. Sound production during the waggle dance of the honey bee. Anim. Behav. 1962, 10, 79–95. [Google Scholar] [CrossRef]
- Lopuch, S.; Tofilski, A. Direct visual observation of wing movements during the honey bee waggle dance. J. Insect Behav. 2017, 30, 199–210. [Google Scholar] [CrossRef]
- Dreller, C.; Kirchner, W.H. Hearing in honeybees: Localization of the auditory sense organ. J. Comp. Physiol. A 1993, 173, 275–279. [Google Scholar] [CrossRef]
- Dreller, C.; Kirchner, W.H. How honeybees perceive the information of the dance language. Naturwissenschaften 1993, 80, 319–321. [Google Scholar] [CrossRef]
- Tsujiuchi, S.; Sivan-Loukianova, E.; Eberl, D.F.; Kitagawa, Y.; Kadowaki, T. Dynamic range compression in the honey bee auditory system toward waggle dance sounds. PLoS ONE 2007, 2, e234. [Google Scholar] [CrossRef] [PubMed]
- Brockmann, A.; Robinson, G.E. Central projections of sensory systems involved in honey bee dance language communication. Brain Behav. Evol. 2007, 70, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Ai, H.; Nishino, H.; Itoh, T. Topographic organization of sensory afferents of Johnston’s organ in the honeybee brain. J. Comp. Neurol. 2007, 502, 1030–1046. [Google Scholar] [CrossRef] [PubMed]
- Kamikouchi, A.; Shimada, T.; Ito, K. Comprehensive classification of the auditory sensory projections in the brain of the fruit fly Drosophila melanogaster. J. Comp. Neurol. 2006, 499, 317–356. [Google Scholar] [CrossRef]
- Gil, M.; de Marco, R.J. Decoding information in the honeybee dance: Revisiting the tactile hypothesis. Anim. Behav. 2010, 80, 887–894. [Google Scholar] [CrossRef]
- Greggers, U.; Koch, G.; Schmidt, V.; Fuer, A.; Floriou-Servou, A.; Pipenbrock, D.; Goefert, M.C.; Menzel, R. Reception and learning of electric fields in bees. Proc. Royal Soc. B 2013, 290, 20130528. [Google Scholar] [CrossRef]
- Ai, H.; Kai, K.; Kumaraswamy, A.; Ikeno, H.; Wachtler, T. Interneurons in the honeybee primary auditory center responding to waggle dance-like vibration pulses. J. Neurosci. 2017, 37, 10624–10635. [Google Scholar] [CrossRef]
- Ai, H.; Kumaraswamy, A.; Kohashi, T.; Ikeno, H.; Wachtler, T. Inhibitory pathways for processing the temporal structure of sensory signals in the insect brain. Front. Psychol. 2018, 9, 1517. [Google Scholar] [CrossRef]
- Ikeno, H.; Kumaraswamy, A.; Kai, K.; Wachtler, T.; Ai, H. A Segmentation Scheme for Complex Neuronal Arbors and Application to Vibration Sensitive Neurons in the Honeybee Brain. Front. Neuroinform. 2018, 12, 61. [Google Scholar] [CrossRef]
- Ascoli, G.; Donohue, D.; Halavi, M. NeuroMorpho.Org: A Central Resource for Neuronal Morphologies. J. Neurosci. 2007, 27. [Google Scholar] [CrossRef]
- NeuroMopho.org. Available online: http://neuromorpho.org/ (accessed on 10 October 2019).
- INSECT BRAIN DATABASE. Available online: https://insectbraindb.org (accessed on 10 October 2019).
- FlyCircuit 1.2. Available online: http://www.flycircuit.tw/ (accessed on 10 October 2019).
- Peng, H.; Hawrylycz, M.; Roskams, H.; Hill, S.; Spruston, N.; Meijering, E.; Ascoli, G. BigNeuron: Large-Scale 3D NeuronReconstruction from Optical Microscopy Images. Neuron 2015, 87. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Bria, A.; Zhou, Z.; Iannello, G.; Long, F. Extensible visualization and analysis for multidimensional images using Vaa3D. Nature Protocols 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Zhao, T.; Kim, J. neuTube 1.0: A New Design for Efficient Neuron Reconstruction Software Based on the SWC Format. eNeuro 2015, 2. [Google Scholar] [CrossRef] [PubMed]
- Rybak, J.; Kuß, A.; Holler, W.; Brandt, R.; Hege, H.; Nawrot, M.; Menzel, R. The HoneyBee Standard Brain (HSB)—A versatile atlas tool for integrating data and data exchange in the neuroscience community. BMC Neurosci. 2009, 10. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Scmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9. [Google Scholar] [CrossRef] [PubMed]
- Yoo, T.S.; Ackerman, M.J.; Lorensen, W.E.; Schroeder, W.; Chalana, V.; Aylward, S.; Metaxas, D.; Whitaker, R. Engineering and algorithm design for an image processing Api: A technical report on ITK - the Insight Toolkit. Stud. Health Technol. Inform. 2002, 85, 586–592. [Google Scholar] [PubMed]
- Franconville, R.; Beron, C.; Jayaraman, V. Building a functional connectome of the Drosophila central complex. eLIFE 2018, 7. [Google Scholar] [CrossRef]
- Felix, R.A.; Fridberger, A.; Leijon, S.; Berrebi, A.S.; Magnusson, A.K. Sound rhythms are encoded by postinhibitory rebound spiking in the superior paraolivary nucleus. J. Neurosci. 2011, 31, 12566–12578. [Google Scholar] [CrossRef]
- Beiderbeck, B.; Myoga, M.H.; Müller, N.I.C.; Callan, A.R.; Friauf, E.; Grothe, B.; Pecka, M. Precisely timed inhibition facilitates action potential firing for spatial coding in the auditory brainstem. Nat. Commun. 2018, 9, 1771. [Google Scholar] [CrossRef]
- Kumaraswamy, A.; Maksutov, A.; Kai, K.; Ai, H.; Ikeno, H.; Wachtler, T. Network simulations of interneuron circuits in the honeybee primary auditory center. bioRxiv 2017, 159533. [Google Scholar] [CrossRef]
- Kumaraswamy, A.; Ai, H.; Kai, K.; Ikeno, H.; Wachtler, T. Adaptations during maturation in an identified honeybee interneuron responsive to waggle dance vibration signals. eNeuro 2019, 6. [Google Scholar] [CrossRef] [PubMed]
- Seeley, T.D. The Wisdom of the Hive: The Social Physiology of Honey Bee Colonies; Harvard University Press: Cambridge, MS, USA, 1995. [Google Scholar]
- Kumaraswamy, A.; Kai, K.; Ai, H.; Ikeno, H.; Wachtler, T. Spatial registration of neuron morphologies based on maximization of volume overlap. BMC Bioinform. 2018, 19, 143. [Google Scholar] [CrossRef] [PubMed]
- Devaud, J.M.; Masson, C. Dendritic pattern development of the honeybee antennal lobe neurons: A laser scanning confocal microscopic study. J. Neurobiol. 1999, 39, 461–474. [Google Scholar] [CrossRef]
- Farris, S.M.; Abrams, A.I.; Strausfeld, N.J. Development and morphology of Class II Kenyon cells in the mushroom bodies of the honey bee, Apis mellifera. J. Comp. Neurol. 2004, 474, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Strutt, J. (Lord Rayleigh) On the light from the sky, its polarization and colour. Phil. Mag. 1871, 41, 107–120. [Google Scholar] [CrossRef]
- Wehner, R. The ant’s celestial compass system: Spectral and polarization channels. In Orientation and Communication in Arthropods; Lehrer, M., Ed.; Birkhäuser Verlag: Basel, Switzerland, 1997; pp. 145–185. [Google Scholar]
- Wehner, R.; Labhart, T. Polarisation vision. In Invertebrate Vision; Warrant, E., Nilsson, D.-E., Eds.; Cambridge University Press: Cambridge, UK, 2006; pp. 291–348. [Google Scholar]
- Heinze, S. Polarized-light processing in insect brains: Recent insights from the desert locust, the monarch butterfly. In Polarized Light and Polarization Vision in Animal Sciences; Horváth, G., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 61–111. [Google Scholar]
- Rossel, S.; Wehner, R. The bee’s e-vector compass. In Neurobiology and Behavior of Honeybees; Menzel, R., Mercer, A., Eds.; Springer: Berlin, Germany, 1987; pp. 76–93. [Google Scholar]
- Evangelista, C.; Kraft, P.; Dacke, M.; Labhart, T.; Srinivasan, M.V. Honeybee navigation: Critically examining the role of the polarization compass. Phil. Trans. R. Soc. B 2014, 369, 20130037. [Google Scholar] [CrossRef]
- Kraft, P.; Evangelista, C.; Dacke, M.; Labhart, T.; Srinivasan, M.V. Honeybee navigation: Following routes using polarized-light cues. Phil. Trans. R. Soc. B 2011, 366, 703–708. [Google Scholar] [CrossRef]
- Schinz, R.H. Structural specialization in the dorsal retina of the Bee, Apis mellifera. Cell Tissue Res. 1975, 162, 23–34. [Google Scholar] [CrossRef]
- Wehner, R.; Bernard, G.D.; Geiger, E. Twisted and non-twisted rhabdoms and their significance for polarization detection in the bee. J. Comp. Physiol. 1975, 104, 225–245. [Google Scholar] [CrossRef]
- Meyer, E.P.; Labhart, T. Pore canals in the cornea of a functionally specialized area of the honey bee’s compound eye. Cell Tissue Res. 1981, 216, 491–501. [Google Scholar] [CrossRef]
- Labhart, T. Specialized photoreceptors at the dorsal rim of the honeybee’s compound eye: Polarizational and angular sensitivity. J. Comp. Physiol. A 1980, 141, 19–30. [Google Scholar] [CrossRef]
- Vitzthum, H.; Müller, M.; Homberg, U. Neurons of the central complex of the locust Schistocerca gregaria are sensitive to polarized light. J. Neurosci. 2002, 22, 1114–1125. [Google Scholar] [CrossRef] [PubMed]
- Heinze, S.; Homberg, U. Maplike representation of celestial e-vector orientations in the brain of an insect. Science 2007, 315, 995–997. [Google Scholar] [CrossRef] [PubMed]
- Heinze, S.; Gotthardt, S.; Homberg, U. Transformation of polarized light information in the central complex of the locust. J. Neurosci. 2009, 29, 11783–11793. [Google Scholar] [CrossRef] [PubMed]
- Labhart, T. Polarization-opponent interneurons in the insect visual system. Nature 1988, 331, 435–437. [Google Scholar] [CrossRef]
- Sakura, M.; Lambrinos, D.; Labhart, T. Polarized skylight navigation in insects: Model and electrophysiology of e-vector coding by neurons in the central complex. J. Neurophysiol. 2008, 99, 667–682. [Google Scholar] [CrossRef] [PubMed]
- El Jundi, B.; Warrant, E.; Byrne, M.J.; Khaldy, L.; Baird, E.; Smolka, J.; Dacke, M. Neural coding underlying the cue preference for celestial orientation. Proc. Natl. Acad. Sci. USA 2015, 112, 11395–11400. [Google Scholar] [CrossRef]
- Heinze, S.; Reppert, S.M. Sun compass integration of skylight cues in migratory monach butterflies. Neuron 2011, 69, 345–358. [Google Scholar] [CrossRef]
- Stone, T.; Webb, B.; Adden, A.; Weddig, N.B.; Honkanen, A.; Templin, R.; Wcislo, W.; Scimeca, L.; Warrant, E.; Heinze, S. An anatomically constrained model for path integration in the bee brain. Curr. Biol. 2017, 27, 3069–3085. [Google Scholar] [CrossRef]
- Zeller, M.; Held, M.; Bender, J.; Berz, A.; Heinloth, T.; Hellfritz, T.; Pfeiffer, K. Transmedulla neurons in the sky compass network of the honeybee (Apis mellifera) are a possible site of circadian input. PLoS ONE 2015, 10, e0143244. [Google Scholar] [CrossRef][Green Version]
- Held, M.; Berz, A.; Hensgen, R.; Muenz, T.S.; Scholl, C.; Rössler, W.; Homberg, U.; Pfeiffer, K. Microglomerular synaptic complexes in the sky-compass network of the honeybee connect parallel pathways from the anterior optic tubercle to the central complex. Front. Behav. Neurosci. 2016, 10, 186. [Google Scholar] [CrossRef] [PubMed]
- Rossel, S.; Wehner, R. Celestial orientation in bees: The use of spectral cues. J. Comp. Physiol. 1984, 155, 605–613. [Google Scholar] [CrossRef]
- Coemans, M.A.J.M.; Vos Hzn, J.J.; Nuboer, J.F.W. The relation between celestial colour gradients and the position of the sun, with regard to the sun compass. Vision Res. 1994, 34, 1461–1470. [Google Scholar] [CrossRef]
- Pfeiffer, K.; Homberg, U. Coding of azimuthal directions via time-compensated combination of celestial compass cues. Curr. Biol. 2007, 17, 960–965. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.V.; Zhang, S.; Altwein, M.; Tautz, J. Honeybee navigation: Nature and calibration of the “Odometer”. Science 2000, 287, 851–953. [Google Scholar] [CrossRef] [PubMed]
- Esch, H.E.; Zhang, S.; Srinivasan, M.V.; Tautz, J. Honeybee dances communicate distances measured by optic flow. Nature 2001, 411, 581–583. [Google Scholar] [CrossRef]
- Chittka, L.; Tautz, J. The spectral input to honeybee visual odometry. J. Exp. Biol. 2003, 206, 2393–2397. [Google Scholar] [CrossRef]
- Heinze, S. Unraveling the neural basis of insect navigation. Curr. Opin. Insect. Sci. 2017, 24, 58–67. [Google Scholar] [CrossRef]
- Barron, A.B.; Plath, J.A. The evolution of honey bee dance communication: A mechanistic perspective. J. Exp. Biol. 2017, 220, 4339–4346. [Google Scholar] [CrossRef]
- Chatterjee, A.; George, E.A.; Prabhudev, M.V.; Basu, P.; Brockmann, A. Honey bees flexibly use two navigational memories when updating dance distance information. J. Exp. Biol. 2019, 222, jeb195099. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ai, H.; Okada, R.; Sakura, M.; Wachtler, T.; Ikeno, H. Neuroethology of the Waggle Dance: How Followers Interact with the Waggle Dancer and Detect Spatial Information. Insects 2019, 10, 336. https://doi.org/10.3390/insects10100336
Ai H, Okada R, Sakura M, Wachtler T, Ikeno H. Neuroethology of the Waggle Dance: How Followers Interact with the Waggle Dancer and Detect Spatial Information. Insects. 2019; 10(10):336. https://doi.org/10.3390/insects10100336
Chicago/Turabian StyleAi, Hiroyuki, Ryuichi Okada, Midori Sakura, Thomas Wachtler, and Hidetoshi Ikeno. 2019. "Neuroethology of the Waggle Dance: How Followers Interact with the Waggle Dancer and Detect Spatial Information" Insects 10, no. 10: 336. https://doi.org/10.3390/insects10100336
APA StyleAi, H., Okada, R., Sakura, M., Wachtler, T., & Ikeno, H. (2019). Neuroethology of the Waggle Dance: How Followers Interact with the Waggle Dancer and Detect Spatial Information. Insects, 10(10), 336. https://doi.org/10.3390/insects10100336




