Effects of Vegetation Strips, Fertilizer Levels and Varietal Resistance on the Integrated Management of Arthropod Biodiversity in a Tropical Rice Ecosystem
Abstract
1. Introduction
2. Materials and Methods
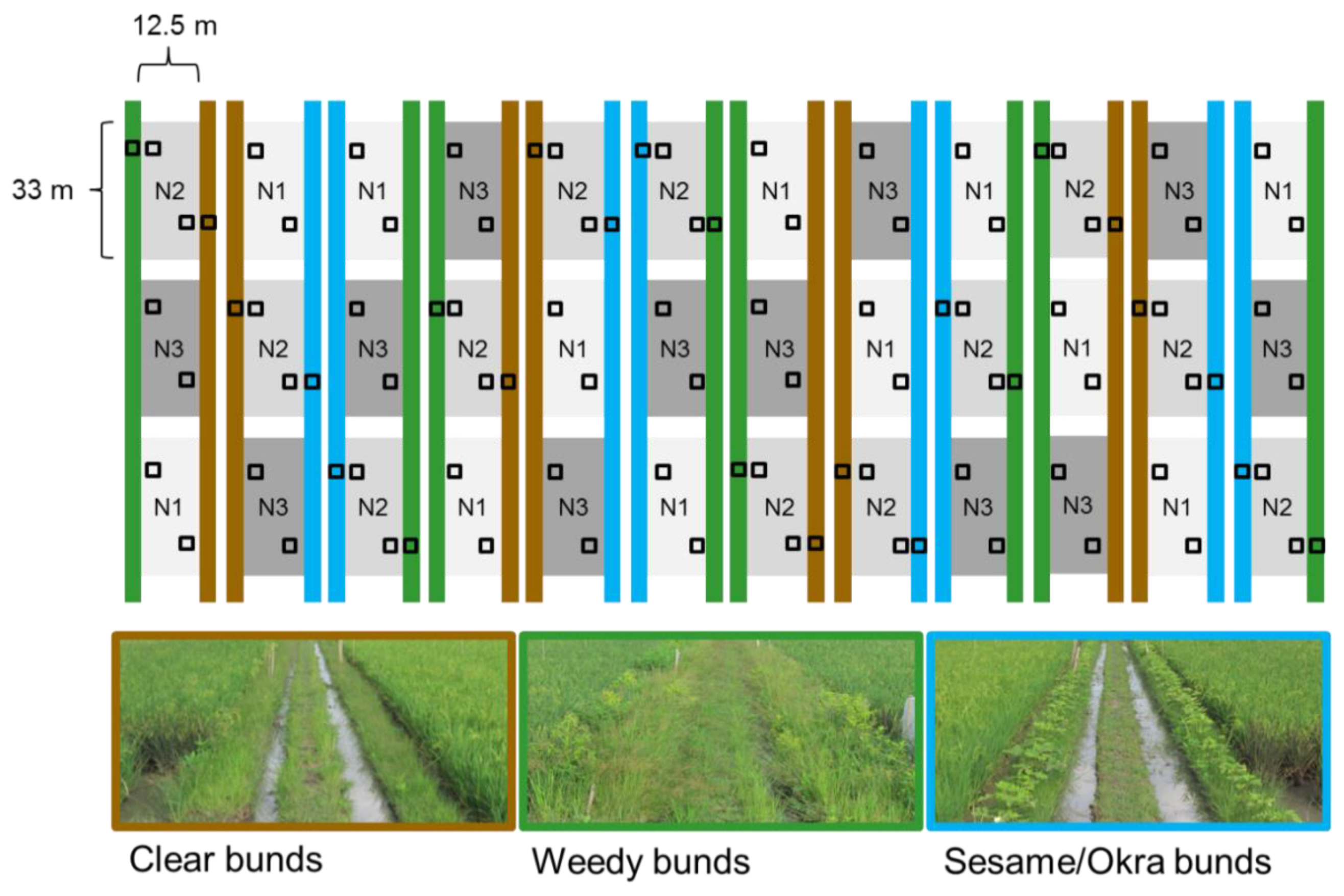
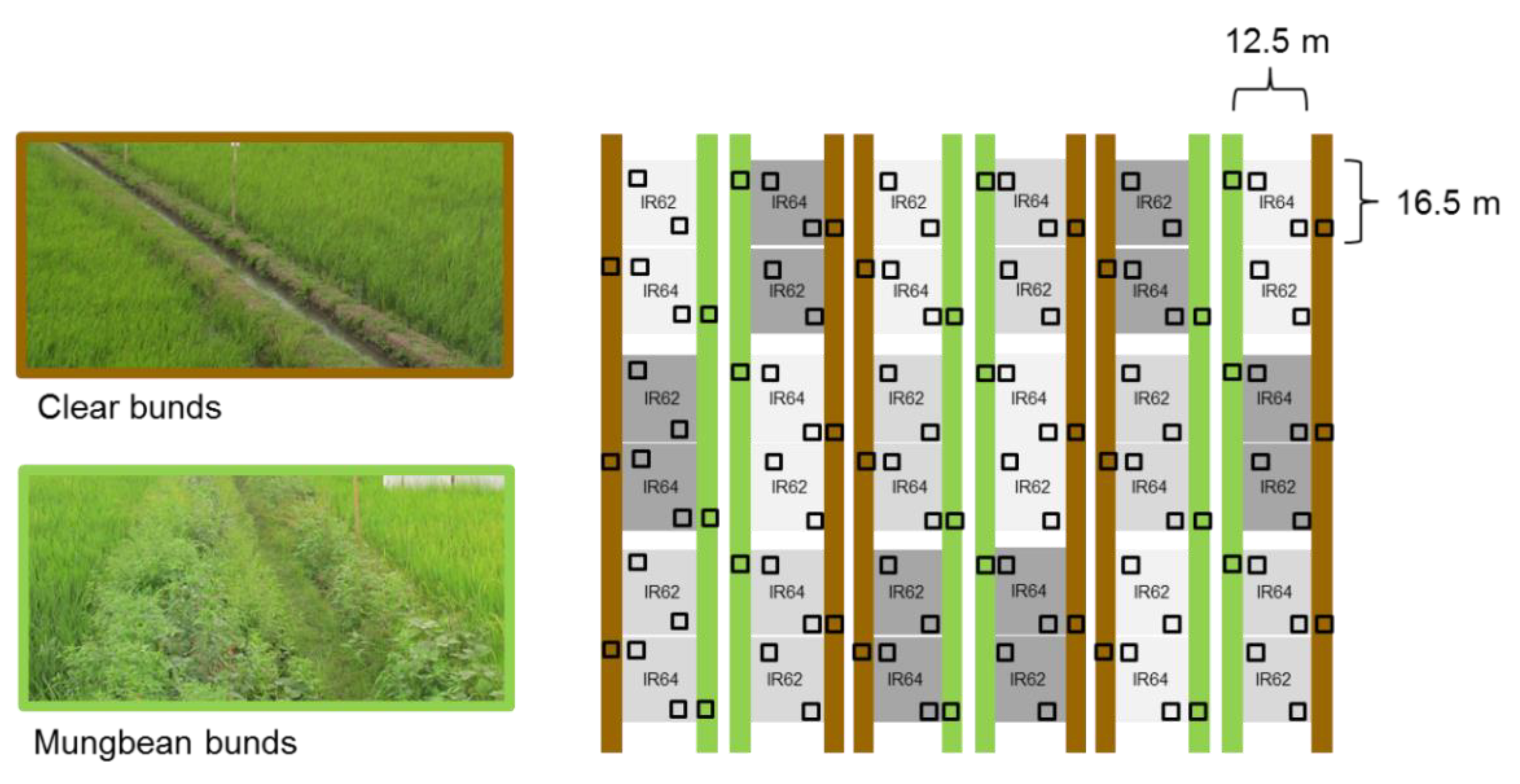
2.1. Experimental Platform
2.2. Bund Development and Plot Yields
2.3. Effects of Nitrogen and Bund Types on Arthropod Fauna
2.4. Effects of Nitrogen, Variety and Bund Type on Planthoppers, Leafhoppers and Their Natural Enemies
2.5. Herbivore Regulation
2.6. Data Analyses
3. Results
3.1. Development of Bund Vegetation
3.2. Rice Yields
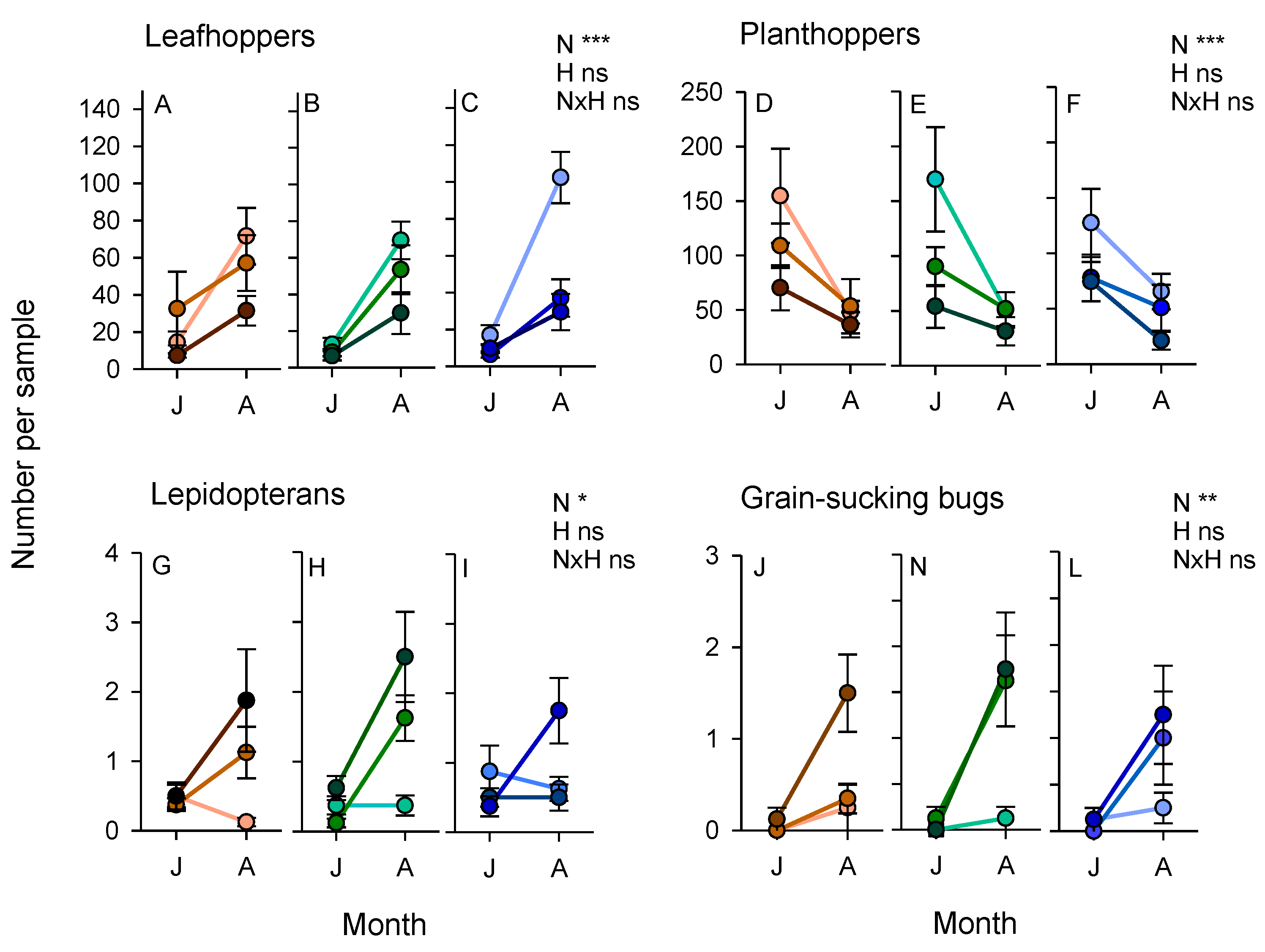
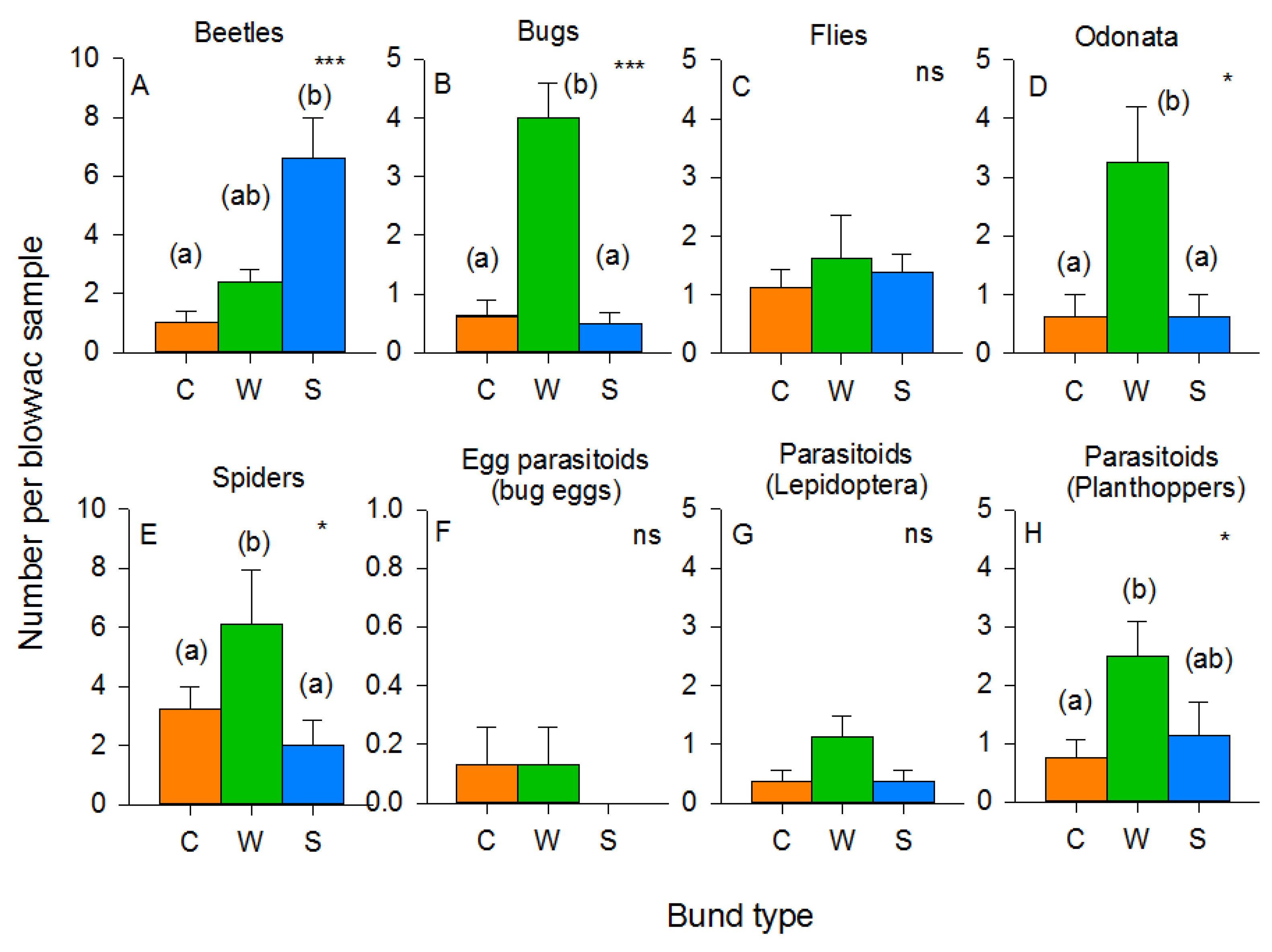
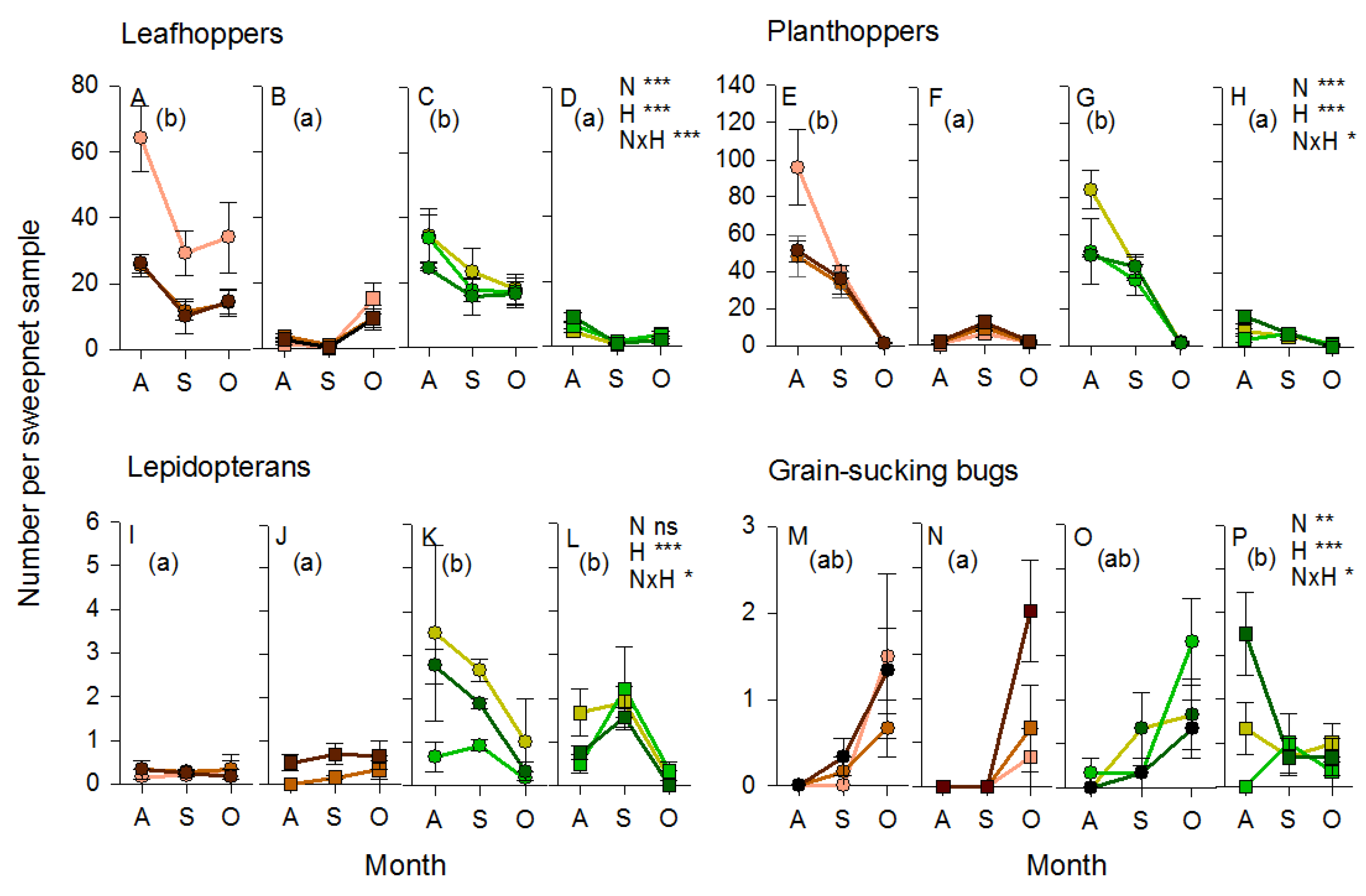
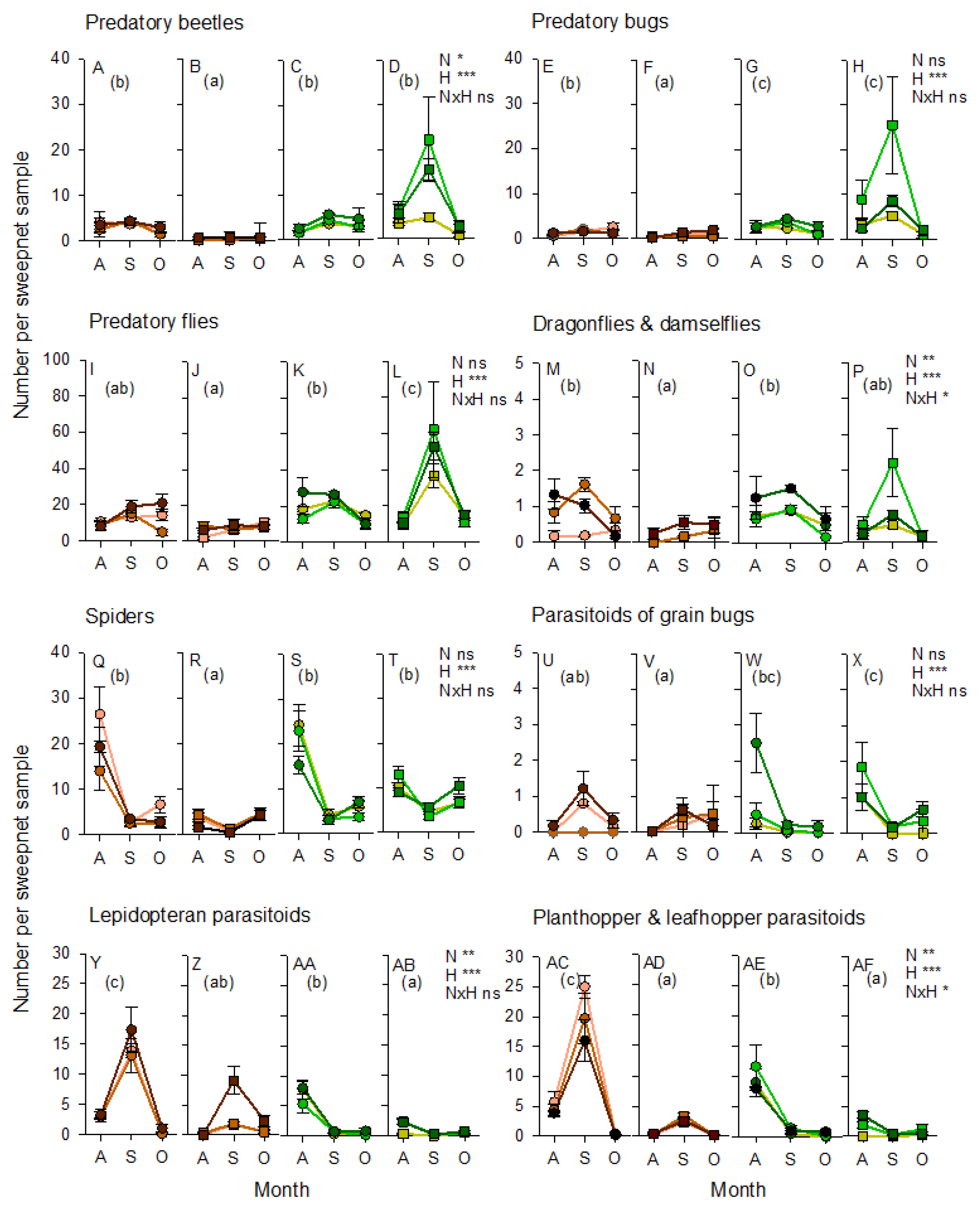
3.3. Arthropod Communities during the 2011 Wet Season Experiment
3.4. Arthropod Communities during the 2013 Wet Season Experiment
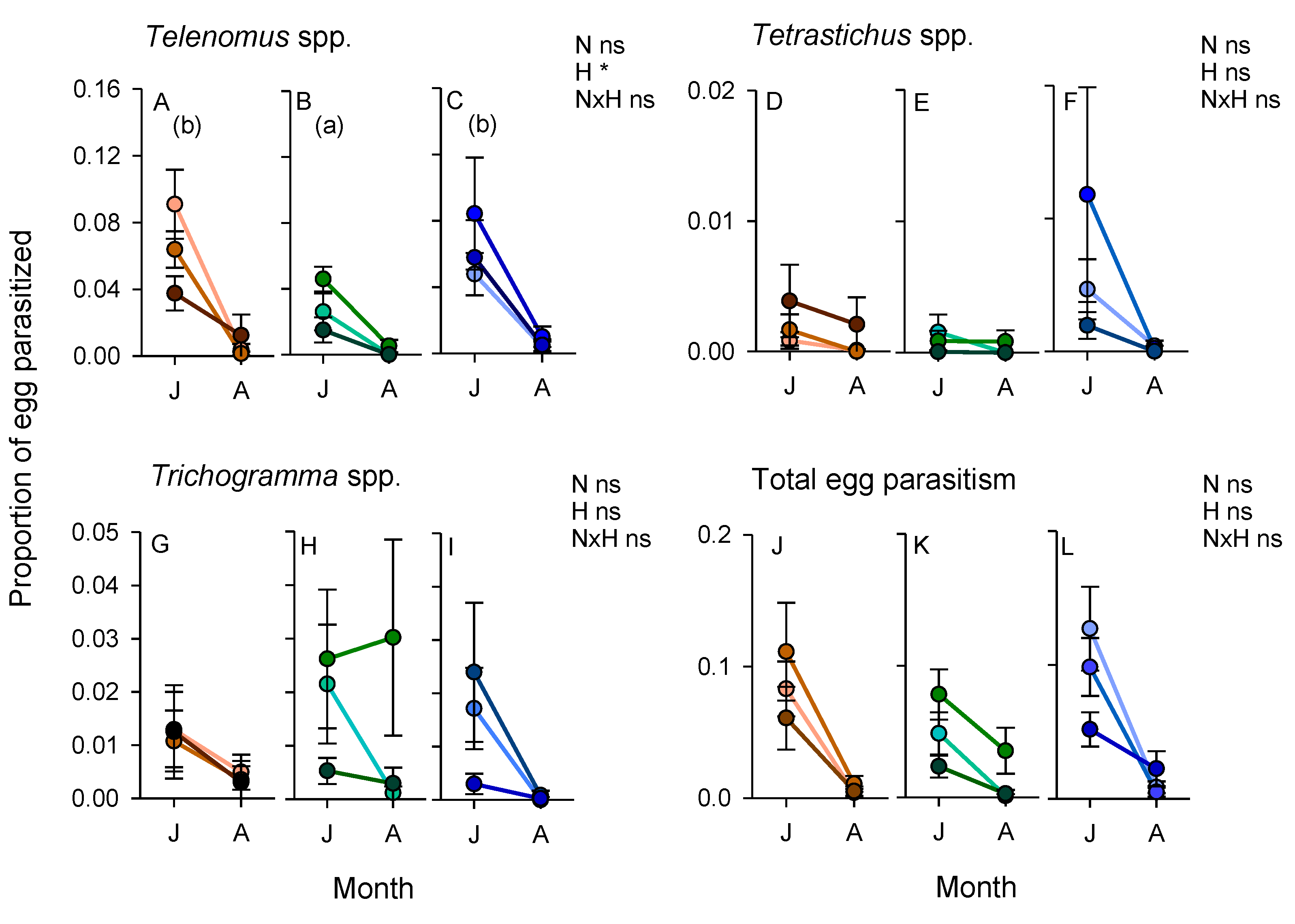
3.5. Parasitism of Planthopper and Stemborer Eggs (2011 Experiment)
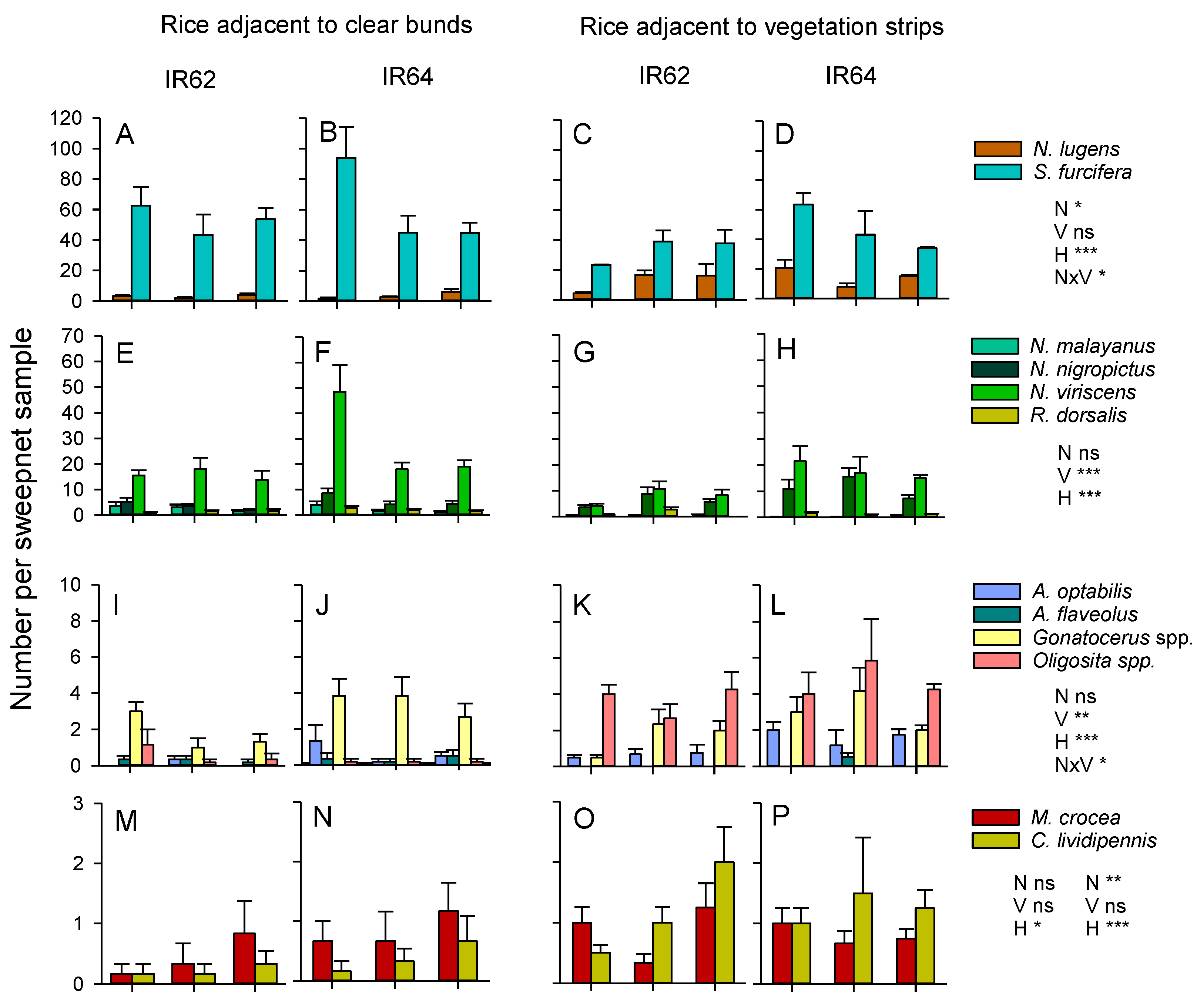
3.6. Combined Effects of Nitrogen, Variety and Bund Vegetation (2013 Experiment)
4. Discussion
4.1. Issues of Scale
4.2. Fertilizers
4.3. Fertilizers and Vegetation Bunds
4.4. Combining Host Plant Resistance with Vegetation Strips
4.5. Further Advantages of Vegetation Strips
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ellis, E.C.; Ramankutty, N. Putting people in the map: Anthropogenic biomes of the world. Front. Ecol. Environ. 2008, 6, 439–447. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. FAOSTAT. 2019. Available online: http://www.fao.org/faostat/en/#home (accessed on 15 July 2019).
- Greenland, D.J. The Sustainability of Rice Farming; Cab International: Wallingford, UK, 1997. [Google Scholar]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Horgan, F.G.; Ramal, A.F.; Bernal, C.C.; Villegas, J.M.; Stuart, A.M.; Almazan, M.L.P. Applying ecological engineering for sustainable and resilient rice production systems. Procedia Food Sci. 2016, 6, 7–15. [Google Scholar] [CrossRef]
- Seck, P.A.; Diagne, A.; Mohanty, S.; Wopereis, M.C. Crops that feed the world 7: Rice. Food Secur. 2012, 4, 7–24. [Google Scholar] [CrossRef]
- Spielman, D.J.; Hartwich, F.; Grebmer, K. Public–private partnerships and developing-country agriculture: Evidence from the international agricultural research system. Public Adm. Dev. 2010, 30, 261–276. [Google Scholar] [CrossRef]
- Horgan, F.G. Insect Herbivores of Rice: Their Natural Regulation and Ecologically Based Management. In Rice Production Worldwide; Chauhan, B.S., Jabran, K., Mahajan, G., Eds.; Springer Nature: Cham, Switzerland, 2017; pp. 279–302. [Google Scholar]
- Horgan, F.G. Integrating gene deployment and crop management for improved rice resistance to Asian planthoppers. Crop Prot. 2018, 110, 21–33. [Google Scholar] [CrossRef]
- Kenmore, P.E.; Perez, C.; Dyck, V.; Gutierrez, A. Population regulation of the rice brown planthopper (Nilaparvata lugens Stǻl) within rice fields in the Philippines. J. Plant Prot. Trop. 1984, 1, 19–37. [Google Scholar]
- Pingali, P.L.; Roger, P.A. Impact of Pesticides on Farmer Health and the Rice Environment; Springer Science and Business Media: New York, NY, USA, 1995; Volume 7. [Google Scholar]
- Tanaka, K.; Endo, S.; Kazano, H. Toxicity of insecticides to predators of rice planthoppers: Spiders, the mirid bug and the dryinid wasp. Appl. Entomol. Zool. 2000, 35, 177–187. [Google Scholar] [CrossRef]
- Horgan, F.G.; Crisol, E. Hybrid rice and insect herbivores in Asia. Entomol. Exp. Appl. 2013, 148, 1–19. [Google Scholar] [CrossRef]
- Gurr, G.M.; Lu, Z.; Zheng, X.; Xu, H.; Zhu, P.; Chen, G.; Yao, X.; Cheng, J.; Zhu, Z.; Catindig, J.L.; et al. Multi-country evidence that crop diversification promotes ecological intensification of agriculture. Nat. Plants 2016, 2, 16014. [Google Scholar] [CrossRef]
- Brotodjojo, R.R.R.; Arochman, T.; Solichah, C. Effect of flowering plants on population dynamics of rice stem borers and their natural enemies. IOP Conf. Ser. Earth Environ. Sci. 2019, 250, 012015. [Google Scholar] [CrossRef]
- Shanker, C.; Mohan, M.; Sampathkumar, M.; Lydia, C.; Katti, G. Selection of flowering forbs for conserving natural enemies in rice fields. Biocontrol. Sci. Technol. 2013, 23, 480–484. [Google Scholar] [CrossRef]
- Ali, M.P.; Bari, M.N.; Haque, S.S.; Kabir, M.M.M.; Afrin, S.; Nowrin, F.; Islam, M.S.; Landis, D.A. Establishing next-generation pest control services in rice fields: Eco-agriculture. Sci. Rep. 2019, 9, 10180. [Google Scholar] [CrossRef]
- Horgan, F.G.; Ramal, A.F.; Villegas, J.M.; Almazan, M.L.P.; Bernal, C.C.; Jamoralin, A.; Pasang, J.M.; Orboc, G.; Agreda, V.; Arroyo, C. Ecological engineering with high diversity vegetation patches enhances bird activity and ecosystem services in Philippine rice fields. Reg. Environ. Chang. 2017, 17, 1355–1367. [Google Scholar] [CrossRef]
- Vu, Q.; Ramal, A.F.; Villegas, J.M.; Jamoralin, A.; Bernal, C.C.; Pasang, J.M.; Almazan, M.L.P.; Ramp, D.; Settele, J.; Horgan, F.G. Enhancing the parasitism of insect herbivores through diversification of habitat in Philippine rice fields. Paddy Water Environ. 2018, 16, 379–390. [Google Scholar] [CrossRef]
- Lu, Z.; Zhu, P.; Gurr, G.M.; Zheng, X.; Chen, G.; Heong, K.L. Rice pest management by ecological engineering: A pioneering attempt in China. In Rice Planthoppers; Heong, K.L., Cheng, J., Escalada, M., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 161–178. [Google Scholar]
- Horgan, F.G.; Ramal, A.F.; Villegas, J.M.; Jamoralin, A.; Bernal, C.C.; Perez, M.O.; Pasang, J.M.; Naredo, A.I.; Almazan, M.L.P. Effects of bund crops and insecticide treatments on arthropod diversity and herbivore regulation in tropical rice fields. J. Appl. Entomol. 2017, 141, 587–599. [Google Scholar] [CrossRef]
- Zhu, P.; Zheng, X.; Zhang, F.; Xu, H.; Yang, Y.; Chen, G.; Lu, Z.; Johnson, A.C.; Gurr, G.M. Quantifying the respective and additive effects of nectar plant crop borders and withholding insecticides on biological control of pests in subtropical rice. J. Pest Sci. 2018, 91, 575–584. [Google Scholar] [CrossRef]
- De Kraker, J.; Rabbinge, R.; Van Huis, A.; Van Lenteren, J.; Heong, K.L. Impact of nitrogenous-fertilization on the population dynamics and natural control of rice leaffolders (Lep.: Pyralidae). Int. J. Pest Manag. 2000, 46, 225–235. [Google Scholar] [CrossRef]
- Rashid, M.M.; Ahmed, N.; Jahan, M.; Islam, K.S.; Nansen, C.; Willers, J.L.; Ali, M.P. Higher fertilizer inputs increase fitness traits of brown planthopper in rice. Sci. Rep. 2017, 7, 4719. [Google Scholar] [CrossRef]
- Horgan, F.G.; Vu, Q.; Bernal, C.C.; Ramal, A.F.; Villegas, J.M.; Almazan, M.L.P. Population development of rice black bug, Scotinophara latiuscula (Breddin), under varying nitrogen in a field experiment. Entomol. Gen. 2018, 37, 19–33. [Google Scholar] [CrossRef]
- Horgan, F.G.; Crisol-Martínez, E.; Almazan, M.L.P.; Romena, A.; Ramal, A.F.; Ferrater, J.B.; Bernal, C.C. Susceptibility and tolerance in hybrid and pure-line rice varieties to herbivore attack: Biomass partitioning and resource-based compensation in response to damage. Ann. Appl. Biol. 2016, 169, 200–213. [Google Scholar] [CrossRef]
- Chen, Y.H.; Bernal, C.C. Arthropod diversity and community composition on wild and cultivated rice. Agric. For. Entomol. 2011, 13, 181–189. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, G.; Zeng, L. Effects of rice-planting neighboring with vegetable crops on the population dynamics of Cnaphalocrocis medinalis, plant hopper, and their predatory enemies. Chin. J. Ecol. 2011, 30, 281–289. [Google Scholar]
- Zheng, X.; Lu, Y.; Zhu, P.; Zhang, F.; Tian, J.; Xu, H.; Chen, G.; Nansen, C.; Lu, Z. Use of banker plant system for sustainable management of the most important insect pest in rice fields in China. Sci. Rep. 2017, 7, 45581. [Google Scholar] [CrossRef]
- Horgan, F.G. Integrated pest management for sustainable rice cultivation: A holistic approach. In Achieving Sustainable Cultivation of Rice; Sasaki, T., Ed.; Burleigh-Dodds: Cambridge, UK, 2017; Volume 2, pp. 309–342. [Google Scholar]
- Cuong, N.L.; Ben, P.T.; Phuong, L.T.; Chau, L.M.; Cohen, M.B. Effect of host plant resistance and insecticide on brown planthopper Nilaparvata lugens (Stål) and predator population development in the Mekong Delta, Vietnam. Crop Prot. 1997, 16, 707–715. [Google Scholar] [CrossRef]
- Horgan, F.G.; Peñalver Cruz, A.; Bernal, C.C.; Ramal, A.F.; Almazan, M.L.P.; Wilby, A. Resistance and tolerance to the brown planthopper, Nilaparvata lugens (Stål), in rice infested at different growth stages across a gradient of nitrogen applications. Field Crops Res. 2018, 217, 53–65. [Google Scholar] [CrossRef]
- Jiang, M.; Cheng, J. Effects of fertilization levels on the whitebacked planthopper (Hemiptera: Delphacidae) population in rice. Chin. J. Rice Sci. 2003, 17, 270–274. [Google Scholar]
- Zhu, Z.-R.; Cheng, J.; Jiang, M.-X.; Zhang, X.-X. Complex influence of rice variety, fertilization timing, and insecticide on population dynamics of Sogatella furcifera (Horváth), Nilaparvata lugens (Stål) (Homoptera: Delphacidae) and their natural enemies in rice in Hangzhou, China. J. Pest Sci. 2004, 77, 65–74. [Google Scholar] [CrossRef]
- Heinrichs, E.; Reissig, W.; Valencia, S.; Chelliah, S. Rates and effect of resurgence-inducing insecticides on populations of Nilaparvata lugens (Homoptera: Delphacidae) and its predators. Environ. Entomol. 1982, 11, 1269–1273. [Google Scholar] [CrossRef]
- Visarto, P.; Zalucki, M.P.; Nesbitt, H.J.; Jahn, G.C. Effect of fertilizer, pesticide treatment, and plant variety on the realized fecundity and survival rates of brown planthopper, Nilaparvata lugens (Stål) (Homoptera: Delphacidae)—generating outbreaks in Cambodia. J. Asia-Pac. Entomol. 2001, 4, 75–84. [Google Scholar] [CrossRef]
- Woodward, G.; Benstead, J.P.; Beveridge, O.S.; Blanchard, J.; Brey, T.; Brown, L.E.; Cross, W.F.; Friberg, N.; Ings, T.C.; Jacob, U.; et al. Ecological Networks in a Changing Climate. In Advances in Ecological Research; Woodward, G., Ed.; Academic Press: Cambridge, MA, USA, 2010; pp. 71–138. [Google Scholar]
- Thébault, E.; Fontaine, C. Stability of Ecological Communities and the Architecture of Mutualistic and Trophic Networks. Science 2010, 329, 853. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.E.; Schoenly, K.; Heong, K.L.; Justo, H.; Arida, G.; Barrion, A.T.; Litsinger, J.A. A Food Web Approach to Evaluating the Effect of Insecticide Spraying on Insect Pest Population Dynamics in a Philippine Irrigated Rice Ecosystem. J. Appl. Ecol. 1994, 31, 747–763. [Google Scholar] [CrossRef]
- Bottrell, D.G.; Schoenly, K.G. Resurrecting the ghost of green revolutions past: The brown planthopper as a recurring threat to high-yielding rice production in tropical Asia. J. Asia-Pac. Entomol. 2012, 15, 122–140. [Google Scholar] [CrossRef]
- Khush, G.S.; Virk, P.S. IR Varieties and Their Impact; International Rice Research Institute: Manila, Philippines, 2005. [Google Scholar]
- Peñalver Cruz, A.; Arida, A.; Heong, K.L.; Horgan, F.G. Aspects of brown planthopper adaptation to resistant rice varieties with the Bph3 gene. Entomol. Exp. Appl. 2011, 141, 245–257. [Google Scholar] [CrossRef]
- Zhu, P.; Gurr, G.M.; Lu, Z.; Heong, K.L.; Chen, G.; Zheng, X.; Xu, H.; Yang, Y. Laboratory screening supports the selection of sesame (Sesamum indicum) to enhance Anagrus spp. parasitoids (Hymenoptera: Mymaridae) of rice planthoppers. Biol. Control. 2013, 64, 83–89. [Google Scholar] [CrossRef]
- Zhu, P.; Wang, G.; Zheng, X.; Tian, J.; Lu, Z.; Heong, K.L.; Xu, H.; Chen, G.; Yang, Y.; Gurr, G.M. Selective enhancement of parasitoids of rice Lepidoptera pests by sesame (Sesamum indicum) flowers. BioControl 2015, 60, 157–167. [Google Scholar] [CrossRef]
- Horgan, F.G.; Srinivasan, T.S.; Bentur, J.S.; Kumar, R.; Bhanu, K.V.; Sarao, P.S.; van Chien, H.; Almazan, M.L.P.; Bernal, C.C.; Ramal, A.F.; et al. Geographic and research origins of rice resistance to Asian planthoppers and leafhoppers: Implications for rice breeding and gene deployment. Agronomy 2017, 7, 62. [Google Scholar] [CrossRef]
- Alam, S.N.; Cohen, M.B. Detection and analysis of QTLs for resistance to the brown planthopper, Nilaparvata lugens, in a doubled-haploid rice population. Theor. Appl. Genet. 1998, 97, 1370–1379. [Google Scholar] [CrossRef]
- Jearakongman, S.; Immark, S.; Noenplub, A.; Fukai, S.; Cooper, M. Effect of plot size on accuracy of yield estimation of rainfed lowland rice genotypes with different plant heights and grown under different soil fertility conditions. Plant Prod. Sci. 2003, 6, 95–102. [Google Scholar] [CrossRef][Green Version]
- Rebetzke, G.J.; Fischer, R.T.A.; Van Herwaarden, A.F.; Bonnett, D.G.; Chenu, K.; Rattey, A.R.; Fettell, N. Plot size matters: Interference from intergenotypic competition in plant phenotyping studies. Funct. Plant Biol. 2014, 41, 107–118. [Google Scholar] [CrossRef]
- Dominik, C.; Seppelt, R.; Horgan, F.G.; Marquez, L.; Settele, J.; Václavík, T. Regional-scale effects override the influence of fine-scale landscape heterogeneity on rice arthropod communities. Agric. Ecosyst. Environ. 2017, 246, 269–278. [Google Scholar] [CrossRef]
- Dominik, C.; Seppelt, R.; Horgan, F.G.; Settele, J.; Václavík, T. Landscape composition, configuration, and trophic interactions shape arthropod communities in rice agroecosystems. J. Appl. Ecol. 2018, 55, 2461–2472. [Google Scholar] [CrossRef]
- Schoenly, K.G.; Justo, H.D., Jr.; Barrion, A.T.; Harris, M.K.; Bottrell, D.G. Analysis of Invertebrate Biodiversity in a Philippine Farmer’s Irrigated Rice Field. Environ. Entomol. 1998, 27, 1125–1136. [Google Scholar] [CrossRef]
- Domingo, I.; Schoenly, K.G. An improved suction apparatus for sampling invertebrate communities in flooded rice. Int. Rice Res. Notes 1998, 23, 1. [Google Scholar]
- Heinrichs, E.A. Biology and Management of Rice Insects; International Rice Research Institute: Manila, Philippines, 1994. [Google Scholar]
- Pathak, M.D.; Khan, Z.R. Insect Pests of Rice; International Rice Research Institute: Manila, Philippines, 1994. [Google Scholar]
- Shepard, B.M.; Barrion, A.T.; Litsinger, J.A. Friends of the Rice Farmer: Helpful Insects, Spiders, and Pathogens; International Rice Research Institute: Manila, Philippines, 1987. [Google Scholar]
- Gurr, G.M.; Liu, J.; Read, D.M.Y.; Catindig, J.L.A.; Cheng, J.A.; Lan, L.P.; Heong, K.L. Parasitoids of Asian rice planthopper (Hemiptera: Delphacidae) pests and prospects for enhancing biological control by ecological engineering. Ann. Appl. Biol. 2011, 158, 149–176. [Google Scholar] [CrossRef]
- Gurr, G.M.; Read, D.M.Y.; Catindig, J.L.A.; Cheng, J.A.; Liu, J.; Lan, L.P.; Heong, K.L. Parasitoids of the rice leaffolder Cnaphalocrocis medinalis and prospects for enhancing biological control with nectar plants. Agric. Forest Entomol. 2012, 14, 1–12. [Google Scholar] [CrossRef]
- Westphal, C.; Vidal, S.; Horgan, F.G.; Gurr, G.M.; Escalada, M.; Van Chien, H.; Tscharntke, T.; Heong, K.L.; Settele, J. Promoting multiple ecosystem services with flower strips and participatory approaches in rice production landscapes. Basic Appl. Ecol. 2015, 16, 681–689. [Google Scholar] [CrossRef]
- Zhu, P.; Lu, Z.; Heong, K.L.; Chen, G.; Zheng, X.; Xu, H.; Yang, Y.; Nicol, H.I.; Gurr, G.M. Selection of nectar plants for use in ecological engineering to promote biological control of rice pests by the predatory bug, Cyrtorhinus lividipennis, (Heteroptera: Miridae). PLoS ONE 2014, 9, e108669. [Google Scholar] [CrossRef]
- De Datta, S.K. Principles and Practices of Rice Production; International Rice Research Institute: Manila, Philippines, 1981. [Google Scholar]
- Reissig, W.; Heinrichs, E.A.; Valencia, S. Insecticide-induced resurgence of the brown planthopper, Nilaparvata lugens, on rice varieties with different levels of resistance. Environ. Entomol. 1982, 11, 165–168. [Google Scholar] [CrossRef]
- Choudhury, A.; Kennedy, I.R. Nitrogen fertilizer losses from rice soils and control of environmental pollution problems. Commun. Soil Sci. Plan. 2005, 36, 1625–1639. [Google Scholar] [CrossRef]
- Peñalver Cruz, A. Interactions Between Crop Management and Rice Resistance to the Brown Planthopper, Nilaparvata lugens (Stål.): Fertilizer Levels, Insecticides, Biocontrol and the Integrity of Resistance. Ph.D. Thesis, Lancaster University, Lancaster, UK, 2014. [Google Scholar]
- Horgan, F.G.; Srinivasan, T.S.; Naik, B.S.; Ramal, A.F.; Bernal, C.C.; Almazan, M.L.P. Effects of nitrogen on egg-laying inhibition and ovicidal response in planthopper-resistant rice varieties. Crop Prot. 2016, 89, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Rubia, E.G.; Shepard, B.M.; Yambao, E.B.; Ingram, K.T.; Arida, G.S.; de Penning Vries, F. Stem borer damage and grain yield of flooded rice. J. Plant Prot. Trop. 1989, 6, 205–211. [Google Scholar]
- Rubia, E.; Heong, K.L.; Zalucki, M.; Gonzales, B.; Norton, G. Mechanisms of compensation of rice plants to yellow stem borer Scirpophaga incertulas (Walker) injury. Crop Prot. 1996, 15, 335–340. [Google Scholar] [CrossRef]
- Rubia-Sanchez, E.; Suzuki, Y.; Miyamoto, K.; Watanabe, T. The potential for compensation of the effects of the brown planthopper Nilaparvata lugens Stål (Homoptera: Delphacidae) feeding on rice. Crop Prot. 1999, 18, 39–45. [Google Scholar] [CrossRef]
- Lu, Z.-X.; Zhu, P.-Y.; Gurr, G.M.; Zheng, X.-S.; Read, D.M.Y.; Heong, K.L.; Yang, Y.-J.; Xu, H.-X. Mechanisms for flowering plants to benefit arthropod natural enemies of insect pests: Prospects for enhanced use in agriculture. Insect Sci. 2014, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- MacLean, R.H.; Litsinger, J.A.; Moody, K.; Watson, A.K.; Libetario, E.M. Impact of Gliricidia sepium and Cassia spectabilis hedgerows on weeds and insect pests of upland rice. Agric. Ecosyst. Environ. 2003, 94, 275–288. [Google Scholar] [CrossRef]
- Khan, Z.R.; Midega, C.A.; Wadhams, L.J.; Pickett, J.A.; Mumuni, A. Evaluation of Napier grass (Pennisetum purpureum) varieties for use as trap plants for the management of African stemborer (Busseola fusca) in a push–pull strategy. Entomol. Exp. Appl. 2007, 124, 201–211. [Google Scholar] [CrossRef]
- Van den Berg, J. Vetiver grass (Vetiveria zizanioides (L.) Nash) as trap plant for Chilo partellus (Swinhoe) (Lepidoptera: Pyralidae) and Busseola fusca (Fuller) (Lepidoptera: Noctuidae). Ann. Soc. Entomol. Fr. 2006, 42, 449–454. [Google Scholar] [CrossRef]
- Leal, W.S.; Ueda, Y.; Ono, M. Attractant pheromone for male rice bug, Leptocorisa chinensis: Semiochemicals produced by both male and female. J. Chem. Ecol. 1996, 22, 1429. [Google Scholar] [CrossRef]
- Watanabe, T.; Takeuchi, H.; Ishizaki, M.; Yasuda, T.; Tachibana, S.-I.; Sasaki, R.; Nagano, K.; Okutani-Akamatsu, Y.; Matsuki, N. Seasonal attraction of the rice bug, Leptocorisa chinensis Dallas (Heteroptera: Alydidae), to synthetic attractant. Appl. Entomol. Zool. 2009, 44, 155–164. [Google Scholar] [CrossRef]
- Babendreier, D.; Wan, M.; Tang, R.; Gu, R.; Tambo, J.; Liu, Z.; Grossrieder, M.; Kansiime, M.; Wood, A.; Zhang, F.; et al. Impact Assessment of Biological Control-Based Integrated Pest Management in Rice and Maize in the Greater Mekong Subregion. Insects 2019, 10, 226. [Google Scholar] [CrossRef]
- Fleming, R.A.; Candau, J.-N. Influences of climatic change on some ecological processes of an insect outbreak system in Canada’s boreal forests and the implications for biodiversity. Environ. Monit. Assess. 1998, 49, 235–249. [Google Scholar] [CrossRef]
- Pan, H.; Preisser, E.L.; Chu, D.; Wang, S.; Wu, Q.; Carriere, Y.; Zhou, X.; Zhang, Y. Insecticides promote viral outbreaks by altering herbivore competition. Ecol. Appl. 2015, 25, 1585–1595. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, T.S.; Almazan, M.L.P.; Bernal, C.C.; Ramal, A.F.; Subbarayalu, M.K.; Horgan, F.G. Interactions between nymphs of Nilaparvata lugens and Sogatella furcifera (Hemiptera: Delphacidae) on resistant and susceptible rice varieties. Appl. Entomol. Zool. 2016, 51, 81–90. [Google Scholar] [CrossRef]
- Lou, Y.-G.; Du, M.-H.; Turlings, T.C.; Cheng, J.-A.; Shan, W.-F. Exogenous application of jasmonic acid induces volatile emissions in rice and enhances parasitism of Nilaparvata lugens eggs by the parasitoid Anagrus nilaparvatae. J. Chem. Ecol. 2005, 31, 1985–2002. [Google Scholar] [CrossRef]
- Lou, Y.-G.; Ma, B.; Cheng, J.A. Attraction of the parasitoid Anagrus nilaparvatae to rice volatiles induced by the rice brown planthopper. Nilaparvata lugens. J. Chem. Ecol. 2005, 31, 2357–2372. [Google Scholar] [CrossRef]
- Sokame, B.M.; Rebaudo, F.; Musyoka, B.; Obonyo, J.; Mailafiya, D.D.; Le Ru, B.P.; Kilalo, D.C.; Juma, G.; Calatayud, P.-A. Carry-over niches for lepidopteran maize stemborers and associated parasitoids during non-cropping season. Insects 2019, 10, 191. [Google Scholar] [CrossRef]


| Bund Type | Nitrogen Level 1 | Bund Vegetation | Rice Yields (Kg m−2) | |
|---|---|---|---|---|
| Vegetation Biomass (g Dry Weight m−1) | Weed Species Richness | |||
| Clear | N1 | 19.39 (3.12) A | 4.50 (0.46) AB | 0.40 (0.03) a |
| N2 | 20.15 (6.71) | 4.75 (0.83) | 0.55 (0.04) b | |
| N3 | 17.88 (3.20) | 4.50 (0.38 | 0.68 (0.05) c | |
| Sesame/okra | N1 | 32.28 (5.95) A | 3.63 (0.53) A | 0.41 (0.02) a |
| N2 | 29.15 (5.65) | 4.88 (0.58) | 0.53 (0.02) b | |
| N3 | 27.28 (5.54) | 4.13 (0.61) | 0.69 (0.03) c | |
| Weedy | N1 | 163.46 (15.60) B | 6.13 (0.58) B | 0.40 (0.02) a |
| N2 | 179.05 (22.29) | 5.13 (0.23) | 0.53 (0.03) b | |
| N3 | 215.81 (32.60) | 5.75 (0.70) | 0.64 (0.03) c | |
| F-Nitrogen 2 | 0.849 ns | 0.069 ns | 65.034 *** | |
| F-Bund 2 | 120.131 *** | 5.275 ** | 0.607 ns | |
| Bund Type and Nitrogen Level 1 | Weed Biomass (g Dry Weight m−1) | Mung Bean Height (cm) | Mung Bean Biomass (g Dry Weight m−1) | Pod Weight (g Dry Weight m−1) | Mung Bean Seeds (g Dry Weight m−1) | Rice Yields IR64 (Kg m−2) | Rice Yields IR62 (Kg m−2) | |
|---|---|---|---|---|---|---|---|---|
| Clear | N1 | 6.43 (1.34) | - | - | - | - | 0.40 (0.02) a | 0.46 (0.03) |
| N2 | 8.49 (1.93) | - | - | - | - | 0.49 (0.06) b | 0.62 (0.04) | |
| N3 | 6.14 (0.64) | - | - | - | - | 0.50 (0.04) ab | 0.55 (0.03) | |
| Mung bean | N1 | 133.72 (9.50) | 46.33 (4.69) a | 21.52 (6.89) | 11.42 (2.86) | 3.58 (0.73) a | 0.48 (0.02) | 0.56 (0.04) |
| N2 | 127.38 (19.12) | 57.17 (7.79) ab | 28.12 (3.58) | 15.75 (5.01) | 2.09 (0.59) a | 0.56 (0.07) | 0.57 (0.04) | |
| N3 | 164.01 (21.24) | 77.60 (7.43) b | 26.79 (10.74) | 29.64 (8.33) | 6.16 (0.73) b | 0.53 (0.06) | 0.58 (0.05) | |
| F-nitrogen | 1.147 ns 2 | 5.486 *,3 | 0.208 ns 3 | 2.647 ns 3 | 8.922 ***,3 | 4.252 *,4 | ||
| F-bund | 178.894 ***,2 | 3.251 ns 4 | ||||||
| F-variety | 6.401 **,4 | |||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horgan, F.G.; Crisol Martínez, E.; Stuart, A.M.; Bernal, C.C.; de Cima Martín, E.; Almazan, M.L.P.; Ramal, A.F. Effects of Vegetation Strips, Fertilizer Levels and Varietal Resistance on the Integrated Management of Arthropod Biodiversity in a Tropical Rice Ecosystem. Insects 2019, 10, 328. https://doi.org/10.3390/insects10100328
Horgan FG, Crisol Martínez E, Stuart AM, Bernal CC, de Cima Martín E, Almazan MLP, Ramal AF. Effects of Vegetation Strips, Fertilizer Levels and Varietal Resistance on the Integrated Management of Arthropod Biodiversity in a Tropical Rice Ecosystem. Insects. 2019; 10(10):328. https://doi.org/10.3390/insects10100328
Chicago/Turabian StyleHorgan, Finbarr G., Eduardo Crisol Martínez, Alexander M. Stuart, Carmencita C. Bernal, Elena de Cima Martín, Maria Liberty P. Almazan, and Angelee Fame Ramal. 2019. "Effects of Vegetation Strips, Fertilizer Levels and Varietal Resistance on the Integrated Management of Arthropod Biodiversity in a Tropical Rice Ecosystem" Insects 10, no. 10: 328. https://doi.org/10.3390/insects10100328
APA StyleHorgan, F. G., Crisol Martínez, E., Stuart, A. M., Bernal, C. C., de Cima Martín, E., Almazan, M. L. P., & Ramal, A. F. (2019). Effects of Vegetation Strips, Fertilizer Levels and Varietal Resistance on the Integrated Management of Arthropod Biodiversity in a Tropical Rice Ecosystem. Insects, 10(10), 328. https://doi.org/10.3390/insects10100328






