Diverging Effects of Landscape Factors and Inter-Row Management on the Abundance of Beneficial and Herbivorous Arthropods in Andalusian Vineyards (Spain)
Abstract
1. Introduction
2. Materials and Methods
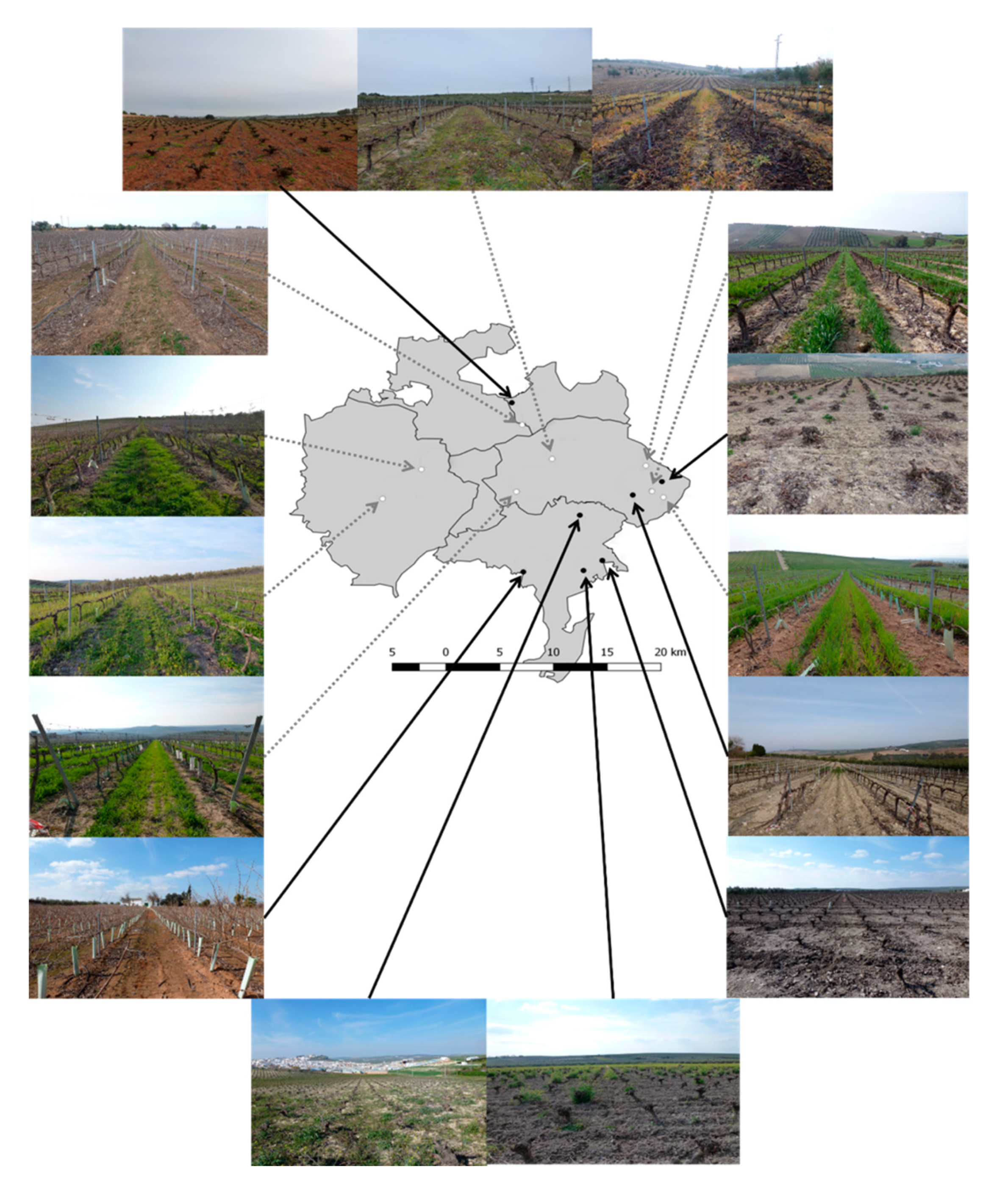
2.1. Study Area
2.2. Experimental Design and Sampling
2.2.1. Vineyards
2.2.2. Arthropod Sampling
2.3. Landscape Analysis
2.4. Data Analysis
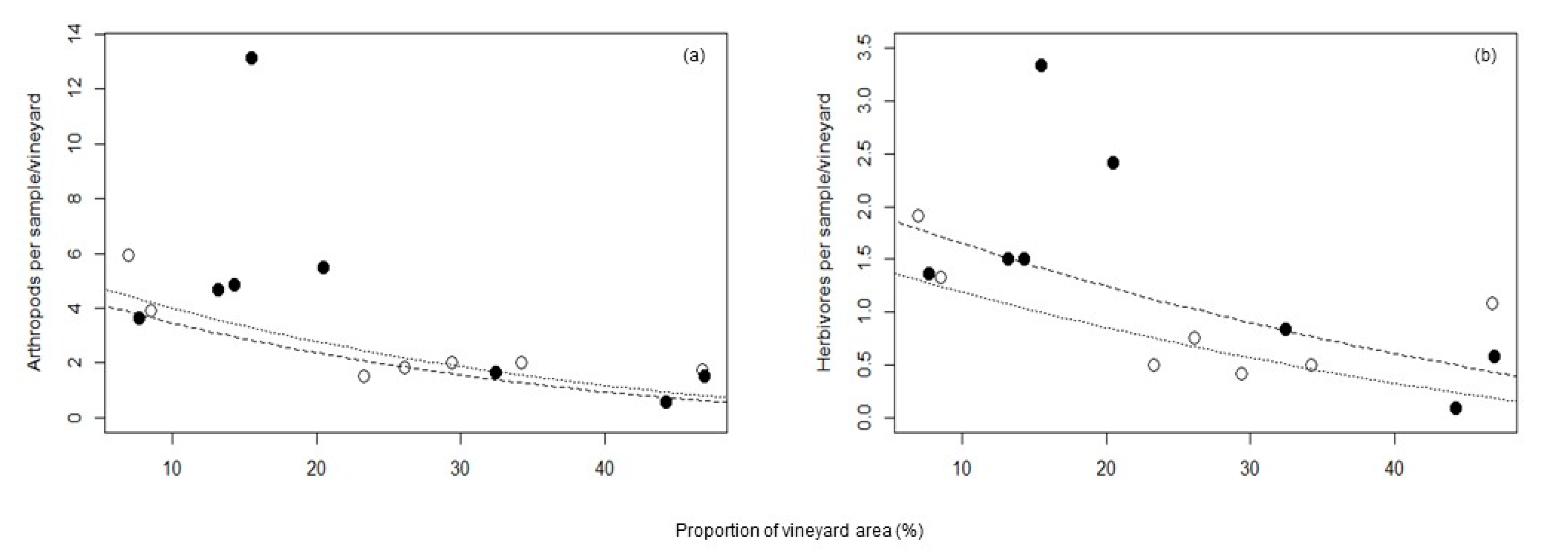
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Isenring, R. Pesticides and the Loss of Biodiversity; Pesticide Action Network Europe: London, UK, 2010; Volume 26. [Google Scholar]
- Bianchi, F.J.J.A.; Booij, C.J.H.; Tscharntke, T. Sustainable pest regulation in agricultural landscapes: A review on landscape composition, biodiversity and natural pest control. Proc. R. Soc. B Biol. Sci. 2006, 273, 1715–1727. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Wyckhuys, K.A.G. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]
- Wilby, A.; Thomas, M.B. Natural enemy diversity and pest control: Patterns of pest emergence with agricultural intensification. Ecol. Lett. 2002, 5, 353–360. [Google Scholar] [CrossRef]
- Geiger, F.; Bengtsson, J.; Berendse, F.; Weisser, W.W.; Emmerson, M.; Morales, M.B.; Ceryngier, P.; Liira, J.; Tscharntke, T.; Winqvist, C.; et al. Persistent negative effects of pesticides on biodiversity and biological control potential on European farmland. Basic Appl. Ecol. 2010, 11, 97–105. [Google Scholar] [CrossRef]
- Muneret, L.; Mitchell, M.; Seufert, V.; Aviron, S.; Djoudi, E.A.; Pétillon, J.; Plantegenest, M.; Thiéry, D.; Rusch, A. Evidence that organic farming promotes pest control. Nat. Sustain. 2018, 1, 361–368. [Google Scholar] [CrossRef]
- Convention on Biological Diversity. Available online: https://www.cbd.int/history/ (accessed on 22 May 2018).
- Wilson, H.; Daane, K.M. Review of ecologically-based pest management in California Vineyards. Insects 2017, 8, 108. [Google Scholar] [CrossRef] [PubMed]
- Tscharntke, T.; Klein, A.M.; Kruess, A.; Steffan-Dewenter, I.; Thies, C. Landscape perspectives on agricultural intensification and biodiversity—Ecosystem service management. Ecol. Lett. 2005, 8, 857–874. [Google Scholar] [CrossRef]
- Pfiffner, L.; Cahenzli, F.; Steinemann, B.; Jamar, L.; Bjørn, M.C.; Porcel, M.; Tasin, M.; Telfser, J.; Kelderer, M.; Lisek, J.; et al. Design, implementation and management of perennial flower strips to promote functional agrobiodiversity in organic apple orchards: A pan-European study. Agric. Ecosyst. Environ. 2019, 278, 61–71. [Google Scholar] [CrossRef]
- Landis, D.A.; Wratten, S.D.; Gurr, G.M. Habitat management to conserve natural enemies of arthropod pests in agriculture. Annu. Rev. Entomol. 2000, 45, 175–201. [Google Scholar] [CrossRef]
- Losey, J.E.; Vaughan, M. The economic value of ecological services provided by insects. BioScience 2006, 56, 311–323. [Google Scholar]
- Schmidt, M.H.; Thies, C.; Nentwig, W.; Tscharntke, T. Contrasting responses of arable spiders to the landscape matrix at different spatial scales. J. Biogeogr. 2008, 35, 157–166. [Google Scholar] [CrossRef]
- Schäckermann, J.; Pufal, G.; Mandelik, Y.; Klein, A.-M. Agro-ecosystem services and dis-services in almond orchards are differentially influenced by the surrounding landscape: Services, dis-services, and landscapes. Ecol. Entomol. 2015, 40, 12–21. [Google Scholar] [CrossRef]
- Rusch, A.; Delbac, L.; Muneret, L.; Thiéry, D. Organic farming and host density affect parasitism rates of tortricid moths in vineyards. Agric. Ecosyst. Environ. 2015, 214, 46–53. [Google Scholar] [CrossRef]
- Tscharntke, T.; Karp, D.S.; Chaplin-Kramer, R.; Batáry, P.; DeClerck, F.; Gratton, C.; Hunt, L.; Ives, A.; Jonsson, M.; Larsen, A.; et al. When natural habitat fails to enhance biological pest control—Five hypotheses. Biol. Conserv. 2016, 204, 449–458. [Google Scholar] [CrossRef]
- Veres, A.; Petit, S.; Conord, C.; Lavigne, C. Does landscape composition affect pest abundance and their control by natural enemies? A review. Agric. Ecosyst. Environ. 2013, 166, 110–117. [Google Scholar] [CrossRef]
- Langellotto, G.A.; Denno, R.F. Responses of invertebrate natural enemies to complex-structured habitats: A meta-analytical synthesis. Oecologia 2004, 139, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gagic, V.; Paull, C.; Schellhorn, N.A. Ecosystem service of biological pest control in Australia: The role of non-crop habitats within landscapes: Native vegetation and biocontrol. Austral. Entomol. 2018, 57, 194–206. [Google Scholar] [CrossRef]
- González, E.; Salvo, A.; Valladares, G. Sharing enemies: Evidence of forest contribution to natural enemy communities in crops, at different spatial scales. Insect Conserv. Divers. 2015, 8, 359–366. [Google Scholar] [CrossRef]
- Rundlöf, M.; Smith, H.G. The effect of organic farming on butterfly diversity depends on landscape context: Organic farming, landscape and butterflies. J. Appl. Ecol. 2006, 43, 1121–1127. [Google Scholar] [CrossRef]
- Birkhofer, K.; Andersson, G.K.S.; Bengtsson, J.; Bommarco, R.; Dänhardt, J.; Ekbom, B.; Ekroos, J.; Hahn, T.; Hedlund, K.; Jönsson, A.M.; et al. Relationships between multiple biodiversity components and ecosystem services along a landscape complexity gradient. Biol. Conserv. 2018, 218, 247–253. [Google Scholar] [CrossRef]
- Karp, D.S.; Chaplin-Kramer, R.; Meehan, T.D.; Martin, E.A.; DeClerck, F.; Grab, H.; Gratton, C.; Hunt, L.; Larsen, A.E.; Martínez-Salinas, A.; et al. Crop pests and predators exhibit inconsistent responses to surrounding landscape composition. Proc. Natl. Acad. Sci. USA 2018, 115, E7863–E7870. [Google Scholar] [CrossRef] [PubMed]
- Djoudi, E.A.; Marie, A.; Mangenot, A.; Puech, C.; Aviron, S.; Plantegenest, M.; Pétillon, J. Farming system and landscape characteristics differentially affect two dominant taxa of predatory arthropods. Agric. Ecosyst. Environ. 2018, 259, 98–110. [Google Scholar] [CrossRef]
- Batáry, P.; Báldi, A.; Samu, F.; Szűts, T.; Erdős, S. Are spiders reacting to local or landscape scale effects in Hungarian pastures? Biol. Conserv. 2008, 141, 2062–2070. [Google Scholar] [CrossRef]
- Diehl, E.; Mader, V.L.; Wolters, V.; Birkhofer, K. Management intensity and vegetation complexity affect web-building spiders and their prey. Oecologia 2013, 173, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Drieu, R.; Rusch, A. Conserving species-rich predator assemblages strengthens natural pest control in a climate warming context: Natural pest control in a climate warming context. Agric. For. Entomol. 2017, 19, 52–59. [Google Scholar] [CrossRef]
- Chaplin-Kramer, R.; O’Rourke, M.E.; Blitzer, E.J.; Kremen, C. A meta-analysis of crop pest and natural enemy response to landscape complexity: Pest and natural enemy response to landscape complexity. Ecol. Lett. 2011, 14, 922–932. [Google Scholar] [CrossRef]
- Cotes, B.; Gonzales, M.; Benitez, E.; De Mas, E.; Clemente-Orta, G.; Campos, M.; Rodriguez, E. Spider communities and biological control in native habitats surrounding greenhouses. Insects 2018, 9, 33. [Google Scholar] [CrossRef]
- Zaller, J.G.; Moser, D.; Drapela, T.; Schmöger, C.; Frank, T. Parasitism of stem weevils and pollen beetles in winter oilseed rape is differentially affected by crop management and landscape characteristics. BioControl 2009, 54, 505–514. [Google Scholar] [CrossRef]
- Bosem Baillod, A.; Tscharntke, T.; Clough, Y.; Batáry, P. Landscape-scale interactions of spatial and temporal cropland heterogeneity drive biological control of cereal aphids. J. Appl. Ecol. 2017, 54, 1804–1813. [Google Scholar] [CrossRef]
- Schellhorn, N.A.; Parry, H.R.; Macfadyen, S.; Wang, Y.; Zalucki, M.P. Connecting scales: Achieving in-field pest control from areawide and landscape ecology studies: Connecting scales. Insect Sci. 2015, 22, 35–51. [Google Scholar] [CrossRef]
- Hanna, R.; Zalom, F.G.; Roltsch, W.J. Relative impact of spider predation and cover crop on population dynamics of Erythroneura variabilis in a raisin grape vineyard. Entomol. Exp. Appl. 2003, 107, 177–191. [Google Scholar] [CrossRef]
- Thomson, L.J.; Hoffmann, A.A. Vegetation increases the abundance of natural enemies in vineyards. Biol. Control 2009, 49, 259–269. [Google Scholar] [CrossRef]
- Pennington, T.; Kraus, C.; Alakina, E.; Entling, M.; Hoffmann, C. Minimal pruning and reduced plant protection promote predatory mites in grapevine. Insects 2017, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Colloff, M.J.; Lindsay, E.A.; Cook, D.C. Natural pest control in citrus as an ecosystem service: Integrating ecology, economics and management at the farm scale. Biol. Control 2013, 67, 170–177. [Google Scholar] [CrossRef]
- Daane, K.M.; Hogg, B.N.; Wilson, H.; Yokota, G.Y. Native grass ground covers provide multiple ecosystem services in Californian vineyards. J. Appl. Ecol. 2018, 55, 2473–2483. [Google Scholar] [CrossRef]
- Irvin, N.A.; Pinckard, T.R.; Perring, T.M.; Hoddle, M.S. Evaluating the potential of buckwheat and cahaba vetch as nectar producing cover crops for enhancing biological control of Homalodisca vitripennis in California vineyards. Biological Control 2014, 76, 10–18. [Google Scholar] [CrossRef]
- Vogelweith, F.; Thiéry, D. Cover crop differentially affects arthropods, but not diseases, occurring on grape leaves in vineyards: Cover crop effect on grapevine leaf communities. Aust. J. Grape Wine Res. 2017, 23, 426–431. [Google Scholar] [CrossRef]
- Kratschmer, S.; Pachinger, B.; Schwantzer, M.; Paredes, D.; Guzmán, G.; Goméz, J.A.; Entrenas, J.A.; Guernion, M.; Burel, F.; Nicolai, A.; et al. Response of wild bee diversity, abundance, and functional traits to vineyard inter-row management intensity and landscape diversity across Europe. Ecol. Evol. 2019, 9, 4103–4115. [Google Scholar] [CrossRef] [PubMed]
- Winter, S.; Bauer, T.; Strauss, P.; Kratschmer, S.; Paredes, D.; Popescu, D.; Landa, B.; Guzmán, G.; Gómez, J.A.; Guernion, M.; et al. Effects of vegetation management intensity on biodiversity and ecosystem services in vineyards: A meta-analysis. J. Appl. Ecol. 2018, 55, 2484–2495. [Google Scholar] [CrossRef] [PubMed]
- Bugg, R.L.; Waddington, C. Using cover crops to manage arthropod pests of orchards: A review. Agric. Ecosyst. Environ. 1994, 50, 11–28. [Google Scholar] [CrossRef]
- Danne, A.; Thomson, L.J.; Sharley, D.J.; Penfold, C.M.; Hoffmann, A.A. Effects of native grass cover crops on beneficial and pest invertebrates in Australian vineyards. Environ. Entomol. 2010, 39, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Paredes, D.; Cayuela, L.; Campos, M. Synergistic effects of ground cover and adjacent vegetation on natural enemies of olive insect pests. Agric. Ecosyst. Environ. 2013, 173, 72–80. [Google Scholar] [CrossRef]
- Saunders, M.E.; Luck, G.W. Interaction effects between local flower richness and distance to natural woodland on pest and beneficial insects in apple orchards: Local and landscape interaction effect on insects. Agric. For. Entomol. 2018, 20, 279–287. [Google Scholar] [CrossRef]
- Winkler, K.J.; Viers, J.H.; Nicholas, K.A. Assessing ecosystem services and multifunctionality for vineyard systems. Front. Environ. Sci. 2017, 5. [Google Scholar] [CrossRef]
- Hall, R.W.; Ehler, L.E. Rate of establishment of natural enemies in classical biological control. Bull. Entomol. Soc. Am. 1979, 25, 280–283. [Google Scholar] [CrossRef]
- Risch, S.J.; Andow, D.; Altieri, M.A. Agroecosystem diversity and pest control: Data, tentative conclusions, and new research directions. Environ. Entomol. 1983, 12, 625–629. [Google Scholar] [CrossRef]
- Lu, Z.-X.; Zhu, P.-Y.; Gurr, G.M.; Zheng, X.-S.; Read, D.M.Y.; Heong, K.-L.; Yang, Y.-J.; Xu, H.-X. Mechanisms for flowering plants to benefit arthropod natural enemies of insect pests: Prospects for enhanced use in agriculture: Flowering plants benefit natural enemies. Insect Sci. 2014, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, G.; Cabezas, J.M.; Sánchez-Cuesta, R.; Lora, Á.; Bauer, T.; Strauss, P.; Winter, S.; Zaller, J.G.; Gómez, J.A. A field evaluation of the impact of temporary cover crops on soil properties and vegetation communities in southern Spain vineyards. Agric. Ecosyst. Environ. 2019, 272, 135–145. [Google Scholar] [CrossRef]
- Viers, J.H.; Williams, J.N.; Nicholas, K.A.; Barbosa, O.; Kotzé, I.; Spence, L.; Webb, L.B.; Merenlender, A.; Reynolds, M. Vinecology: Pairing wine with nature: Vinecology. Conserv. Lett. 2013, 6, 287–299. [Google Scholar] [CrossRef]
- Agencia Estatal de Meteorología-AEMET. Gobierno de España. Available online: http://www.aemet.es/es/ (accessed on 24 April 2018).
- Álvarez, M.; Moreno, I.M.; Jos, Á.M.; Cameán, A.M.; González, A.G. Study of mineral profile of Montilla-Moriles “fino” wines using inductively coupled plasma atomic emission spectrometry methods. J. Food Compos. Anal. 2007, 20, 391–395. [Google Scholar] [CrossRef]
- Chinery, M. Pareys Buch der Insekten, 2nd ed.; Franckh-Kosmos Verlags-GmbH & Co.: Stuttgart, Germany, 2012; ISBN 978-3-440-13289-0. [Google Scholar]
- ESRI. ArcGIS Desktop: Release 10.2. (Environmental Systems Research Institute, 2013); ESRI: Redlands, CA, USA, 2013. [Google Scholar]
- QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation, 2015. Available online: http://qgis.osgeo.org (accessed on 23 August 2019).
- McGarigal, K.; Cushman, S.; Ene, E. FRAGSTATS v4: Spatial Pattern Analysis Program for Categorical and Continuous Maps. Computer Software Program Produced by the Authors at the University of Massachusetts. Amherst. 2012. Available online: http://www.umass.edu/landeco/research/fragstats/fragstats.html (accessed on 23 August 2019).
- Boussard, H.; Baudry, J. Documentation Utilisateur Pour le Logiciel Chloe2012; ISRA: Paris, France, 2014. [Google Scholar]
- Johnson, J.B.; Omland, K.S. Model selection in ecology and evolution. Trends Ecol. Evol. 2004, 19, 101–108. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2015. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Barton, K. Mu-MIn: Multi-Model Inference. R Package Version 1.9.13. 2013. Available online: https://cran.r-project.org/web/packages/MuMIn/index.html (accessed on 22 June 2017).
- Wratten, S.D.; van Emden, H.F. Habitat management for enhanced activity of natural enemies of insect pests. In Ecology and Integrated Farming Systems; John Wiley and Sons: Chichester, UK, 1995; pp. 117–145. [Google Scholar]
- Hoffmann, C.; Köckerling, J.; Biancu, S.; Gramm, T.; Michl, G.; Entling, M. Can flowering greencover crops promote biological control in german vineyards? Insects 2017, 8, 121. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, L.; Rusch, A.; Muiruri, E.W.; Gravellier, B.; Thiery, D.; Castagneyrol, B. Avian pest control in vineyards is driven by interactions between bird functional diversity and landscape heterogeneity. J. Appl. Ecol. 2017, 54, 500–508. [Google Scholar] [CrossRef]
- Gurr, G.M.; Lu, Z.; Zheng, X.; Xu, H.; Zhu, P.; Chen, G.; Yao, X.; Cheng, J.; Zhu, Z.; Catindig, J.L.; et al. Multi-country evidence that crop diversification promotes ecological intensification of agriculture. Nat. Plants 2016, 2, 16014. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.J.J.A.; Schellhorn, N.A.; Cunningham, S.A. Habitat functionality for the ecosystem service of pest control: Reproduction and feeding sites of pests and natural enemies. Agric. For. Entomol. 2013, 15, 12–23. [Google Scholar] [CrossRef]
- Macfadyen, S.; Davies, A.P.; Zalucki, M.P. Assessing the impact of arthropod natural enemies on crop pests at the field scale: Impact of arthropod natural enemies. Insect Sci. 2015, 22, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Rusch, A.; Chaplin-Kramer, R.; Gardiner, M.M.; Hawro, V.; Holland, J.; Landis, D.; Thies, C.; Tscharntke, T.; Weisser, W.W.; Winqvist, C.; et al. Agricultural landscape simplification reduces natural pest control: A quantitative synthesis. Agric. Ecosyst. Environ. 2016, 221, 198–204. [Google Scholar] [CrossRef]
- Kratschmer, S.; Pachinger, B.; Schwantzer, M.; Paredes, D.; Guernion, M.; Burel, F.; Nicolai, A.; Strauss, P.; Bauer, T.; Kriechbaum, M.; et al. Tillage intensity or landscape features: What matters most for wild bee diversity in vineyards? Agric. Ecosyst. Environ. 2018, 266, 142–152. [Google Scholar] [CrossRef]
- Drapela, T.; Moser, D.; Zaller, J.G.; Frank, T. Spider assemblages in winter oilseed rape affected by landscape and site factors. Ecography 2008, 31, 254–262. [Google Scholar] [CrossRef]
- Jonsson, M.; Buckley, H.L.; Case, B.S.; Wratten, S.D.; Hale, R.J.; Didham, R.K. Agricultural intensification drives landscape-context effects on host-parasitoid interactions in agroecosystems: Land-use intensity decreases parasitism rates. J. Appl. Ecol. 2012, 706–714. [Google Scholar] [CrossRef]
- Pfingstmann, A.; Paredes, D.; Buchholz, J.; Querner, P.; Bauer, T.; Strauss, P.; Kratschmer, S.; Winter, S.; Zaller, J.G. Contrasting effects of tillage and landscape structure on spiders and springtails in vineyards. Sustainability 2019, 11, 2095. [Google Scholar] [CrossRef]
- Bruggisser, O.T.; Schmidt-Entling, M.H.; Bacher, S. Effects of vineyard management on biodiversity at three trophic levels. Biol. Conserv. 2010, 143, 1521–1528. [Google Scholar] [CrossRef]
- Schmidt, M.H.; Roschewitz, I.; Thies, C.; Tscharntke, T. Differential effects of landscape and management on diversity and density of ground-dwelling farmland spiders: Landscape vs. management effects on spiders. J. Appl. Ecol. 2005, 42, 281–287. [Google Scholar] [CrossRef]
- Dunning, J.B.; Danielson, B.J.; Pulliam, H.R. Ecological processes that affect populations in complex landscapes. Oikos 1992, 65, 169–175. [Google Scholar] [CrossRef]
- Wood, J.R.; Holdaway, R.J.; Orwin, K.H.; Morse, C.; Bonner, K.I.; Davis, C.; Bolstridge, N.; Dickie, I.A. No single driver of biodiversity: Divergent responses of multiple taxa across land use types. Ecosphere 2017, 8, e01997. [Google Scholar] [CrossRef]
- Wootton, J.T. Effects of disturbance on species diversity: A multitrophic perspective. Am. Nat. 1998, 152, 803–825. [Google Scholar] [CrossRef] [PubMed]
- Mackey, R.L.; Currie, D.J. The diversity-disturbance relationship: Is it generally strong and peaked? Ecology 2001, 82, 3479–3492. [Google Scholar]
- Wilby, A.; Villareal, S.C.; Lan, L.P.; Heong, K.L.; Thomas, M.B. Functional benefits of predator species diversity depend on prey identity. Ecol. Entomol. 2005, 30, 497–501. [Google Scholar] [CrossRef]
- Kuusk, A.-K.; Ekbom, B. Lycosid spiders and alternative food: Feeding behavior and implications for biological control. Biol. Control 2010, 55, 20–26. [Google Scholar] [CrossRef]
- Buchholz, J.; Querner, P.; Paredes, D.; Bauer, T.; Strauss, P.; Guernion, M.; Scimia, J.; Cluzeau, D.; Burel, F.; Kratschmer, S.; et al. Soil biota in vineyards are more influenced by plants and soil quality than by tillage intensity or the surrounding landscape. Sci. Rep. 2017, 7, 17445. [Google Scholar] [CrossRef] [PubMed]
- Powell, W. Enhancing parasitoid activity in crops. In Insect Parasitoids; Academic Press: London, UK, 1986; pp. 319–340. [Google Scholar]
- Gurr, G.M.; Wratten, S.D.; Altieri, M.A. Ecological Engineering for Pest. Management: Habitat Manipulation for Arthropods; CSIRO Publishing: Collingwood, Australia, 2004; ISBN 0 643 099022 3. [Google Scholar]
- Nicholls, C.I.; Parrella, M.P.; Altieri, M.A. Reducing the abundance of leafhoppers and thrips in a northern California organic vineyard through maintenance of full season floral diversity with summer cover crops. Agric. For. Entomol. 2000, 2, 107–113. [Google Scholar] [CrossRef]
- English-Loeb, G.; Rhainds, M.; Martinson, T.; Ugine, T. Influence of flowering cover crops on Anagrus parasitoids (Hymenoptera: Mymaridae) and Erythroneura leafhoppers (Homoptera: Cicadellidae) in New York vineyards. Agric. For. Entomol. 2003, 5, 173–181. [Google Scholar] [CrossRef]
- Smith, I.M.; Hoffmann, A.A.; Thomson, L.J. Ground cover and floral resources in shelterbelts increase the abundance of beneficial hymenopteran families: Shelterbelts increase wasp abundance. Agric. For. Entomol. 2015, 17, 120–128. [Google Scholar] [CrossRef]
- Van Rijn, P.C.J.; Wäckers, F.L. Nectar accessibility determines fitness, flower choice and abundance of hoverflies that provide natural pest control. J. Appl. Ecol. 2016, 53, 925–933. [Google Scholar] [CrossRef]
- Costello, M.J.; Daane, K.M. Influence of ground cover on spider populations in a table grape vineyard. Ecol. Entomol. 1998, 23, 33–40. [Google Scholar] [CrossRef]
- Costello, M.J.; Daane, K.M. Spider and Leafhopper (Erythroneura spp.) Response to Vineyard Ground Cover. Environ. Entom. 2003, 32, 1085–1098. [Google Scholar] [CrossRef]
- Benz, G.; Nyffeler, M. Spiders in natural pest control: A review. J. Appl. Entomol. 1987, 103, 321–339. [Google Scholar]
- Isaia, M.; Bona, F.; Badino, G. Influence of Landscape Diversity and Agricultural Practices on Spider Assemblage in Italian Vineyards of Langa Astigiana (Northwest Italy). Environ. Entomol. 2006, 35, 297–307. [Google Scholar] [CrossRef]
- Hogg, B.N.; Daane, K.M. The role of dispersal from natural habitat in determining spider abundance and diversity in California vineyards. Agric. Ecosyst. Environ. 2010, 135, 260–267. [Google Scholar] [CrossRef]
- Sunderland, K.; Samu, F. Effects of agricultural diversification on the abundance, distribution, and pest control potential of spiders: A review. Entomol. Exp. et Appl. 2000, 95, 1–13. [Google Scholar] [CrossRef]

| Landscape Structure | Inter-Row Management | |
|---|---|---|
| Bare Soil (in %) | Vegetation Cover (in %) | |
| Semi-natural elements 1 | 7.9 ± 3.3 | 9.0 ± 3.1 |
| Orchards 2 | 50.8 ± 7.8 | 49.6 ± 17.2 |
| Vineyards | 26.5 ± 12.5 | 23.1 ± 15.9 |
| Other agriculture 3 | 5.5 ± 6.9 | 14.1 ± 17.0 |
| Arthropod Taxa | Counts | % of Total Catch |
|---|---|---|
| Predators | 314 | 48.3 |
| Aeolothrips | 130 | 20.0 |
| Ants | 97 | 14.9 |
| Spiders | 75 | 11.5 |
| Chrysoperla carnea larvae | 6 | 0.9 |
| Cocinellidae | 2 | 0.3 |
| Neuroptera | 2 | 0.3 |
| Raphidioptera | 1 | 0.15 |
| Anthocorids | 1 | 0.15 |
| Parasitoids | 120 | 18.5 |
| Herbivores | 216 | 33.2 |
| Thrips | 72 | 11.1 |
| Cicada | 50 | 7.7 |
| Grasshoppers | 48 | 7.3 |
| Aphids | 27 | 4.2 |
| Psyllids | 19 | 2.9 |
| Total arthropods | 650 | 100 |
| Model Parameter | Total | Predators | Herbivores | Aeolothrips | Parasitoids | Spiders | Cicadas |
|---|---|---|---|---|---|---|---|
| Basic models | |||||||
| Null | 29.0 | 26.0 | 16.6 | 11.0 | 11.9 | 7.3 | 4.6 |
| SNE | 32.2 | 29.1 | 19.8 | 14.1 | 15.1 | 9.6 | 7.6 |
| Other agric. | 26.3 | 22.8 | 15.8 | 13.7 | 9.8 | −10.3 | 4.5 |
| Viticulture | 20.2 | 21.5 | 11.4 | 7.1 | 2.1 | 6.6 | 1.5 |
| Orchards | 31.3 | 28.6 | 18.9 | 10.4 | 14.9 | 9.5 | 7.4 |
| Management models | |||||||
| Null | 29.0 | 27.9 | 14.4 | 13.7 | 12.4 | 10.4 | 7.1 |
| SNE + management | 32.7 | 31.7 | 17.8 | 17.5 | 16.2 | 13.1 | 10.6 |
| SNE × management | 37.1 | 35.5 | 22.3 | 22.1 | 20.1 | 15.8 | 15.2 |
| Other agric. + management | 28.4 | 26.5 | 15.8 | 17.3 | 12.3 | −9.2 | 8.2 |
| Other agric. × management | 33.1 | 31.1 | 20.2 | 21.6 | 16.8 | −4.6 | 11.8 |
| Viticulture + management | 19.4 | 24.3 | 7.8 | 10.7 | 2.0 | 10.4 | 4.9 |
| Viticulture × management | 24.0 | 27.8 | 11.6 | 15.1 | 4.3 | 15.0 | 9.4 |
| Orchards + management | 31.4 | 31.0 | 16.5 | 13.4 | 15.9 | 13.2 | 10.4 |
| Orchards × management | 35.7 | 35.7 | 20.8 | 17.9 | 19.4 | 17.8 | 14.5 |
| Multiple R2 | 0.67 | - | 0.65 | 0.38 | 0.58 | 0.79 | 0.34 |
| Adjusted R2 | 0.62 | - | 0.59 | 0.33 | 0.55 | 0.76 | 0.29 |
| Taxa | Estimates | |||
|---|---|---|---|---|
| Intercept | Vineyard as % of Surrounding Area | Agric. Land as % of Surrounding Area | Presence of Vegetation Cover | |
| Total arthropods | 1.86 | −0.028 | - | 0.38 |
| Herbivores | 0.93 | −0.01 | - | 0.34 |
| Aeolothrips | 0.83 | −0.02 | - | - |
| Parasitoids | 0.83 | −0.02 | - | 0.19 |
| Spiders | 0.15 | - | 0.02 | −0.11 |
| Cicada | 0.47 | −0.01 | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Judt, C.; Guzmán, G.; Gómez, J.A.; Cabezas, J.M.; Entrenas, J.A.; Winter, S.; Zaller, J.G.; Paredes, D. Diverging Effects of Landscape Factors and Inter-Row Management on the Abundance of Beneficial and Herbivorous Arthropods in Andalusian Vineyards (Spain). Insects 2019, 10, 320. https://doi.org/10.3390/insects10100320
Judt C, Guzmán G, Gómez JA, Cabezas JM, Entrenas JA, Winter S, Zaller JG, Paredes D. Diverging Effects of Landscape Factors and Inter-Row Management on the Abundance of Beneficial and Herbivorous Arthropods in Andalusian Vineyards (Spain). Insects. 2019; 10(10):320. https://doi.org/10.3390/insects10100320
Chicago/Turabian StyleJudt, Christine, Gema Guzmán, José A. Gómez, José M. Cabezas, José A. Entrenas, Silvia Winter, Johann G. Zaller, and Daniel Paredes. 2019. "Diverging Effects of Landscape Factors and Inter-Row Management on the Abundance of Beneficial and Herbivorous Arthropods in Andalusian Vineyards (Spain)" Insects 10, no. 10: 320. https://doi.org/10.3390/insects10100320
APA StyleJudt, C., Guzmán, G., Gómez, J. A., Cabezas, J. M., Entrenas, J. A., Winter, S., Zaller, J. G., & Paredes, D. (2019). Diverging Effects of Landscape Factors and Inter-Row Management on the Abundance of Beneficial and Herbivorous Arthropods in Andalusian Vineyards (Spain). Insects, 10(10), 320. https://doi.org/10.3390/insects10100320








