Insecticidal Effects of Fumigants (EF, MB, and PH3) towards Phosphine-Susceptible and -Resistant Sitophilus oryzae (Coleoptera: Curculionidae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Fumigants
2.3. Fumigation System
2.4. Measurement of Fumigant Concentrations
2.5. Determination of the Concentration × Time (Ct) of the Fumigants
2.6. Statistical Analysis
3. Results
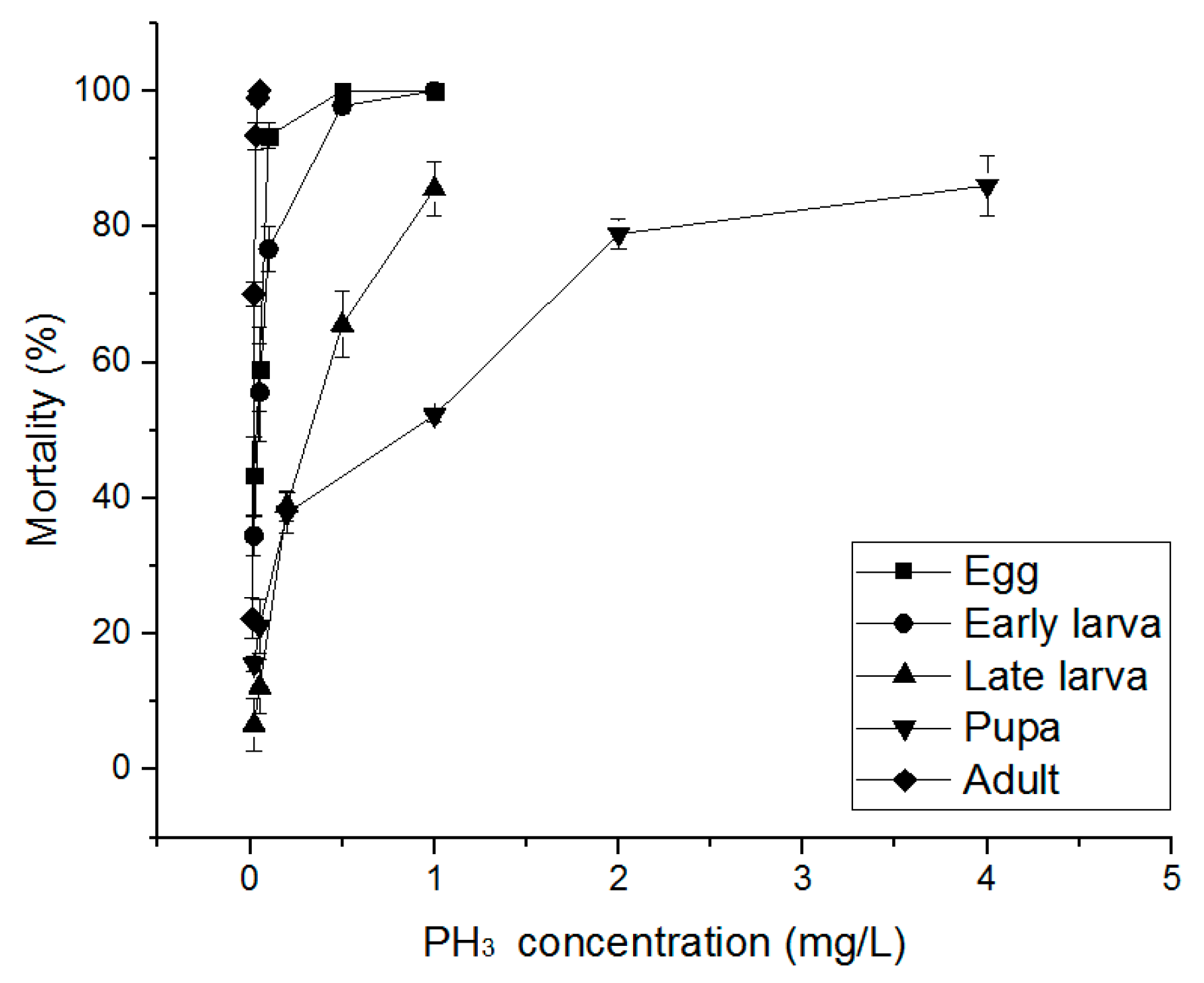
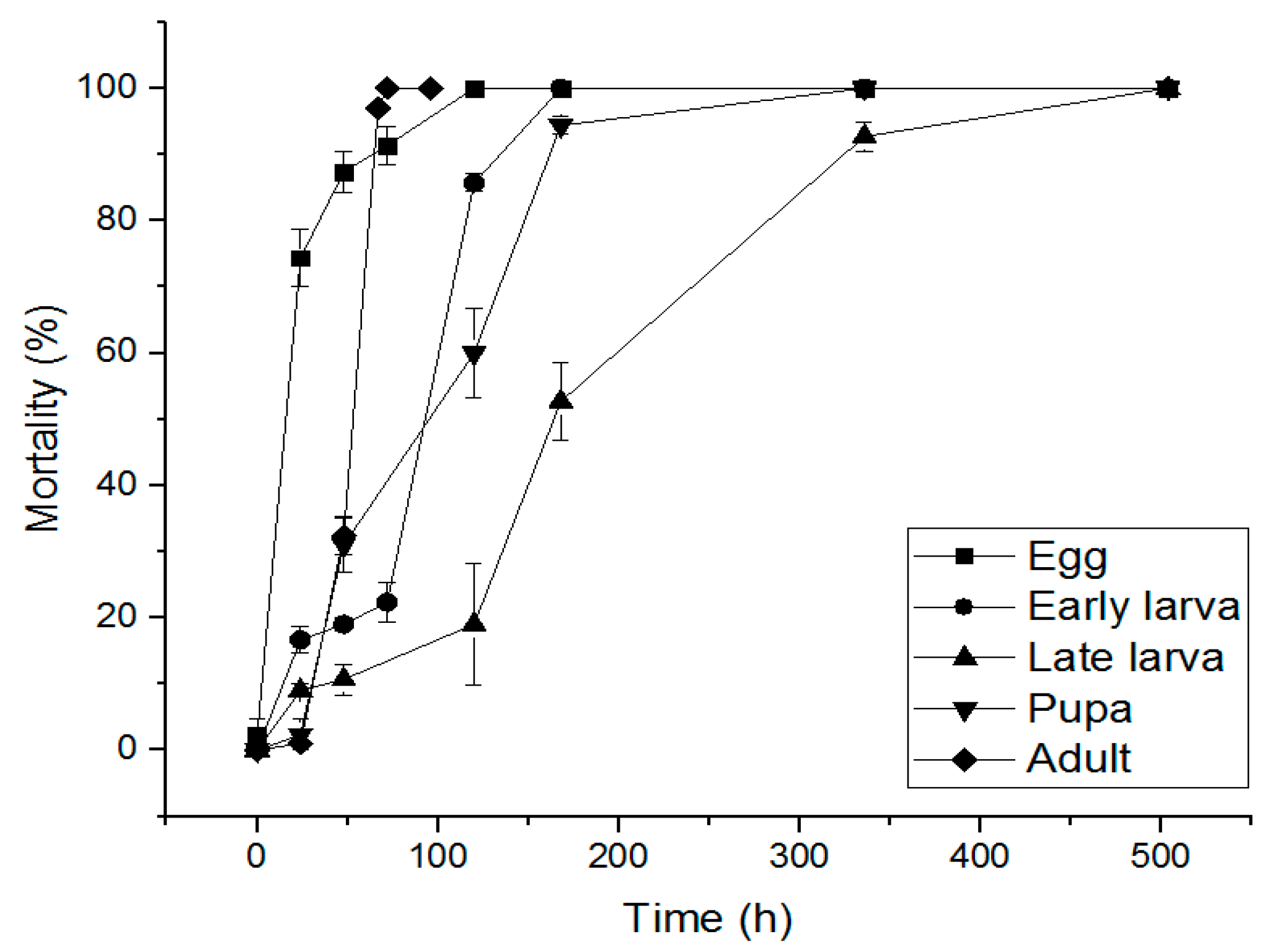
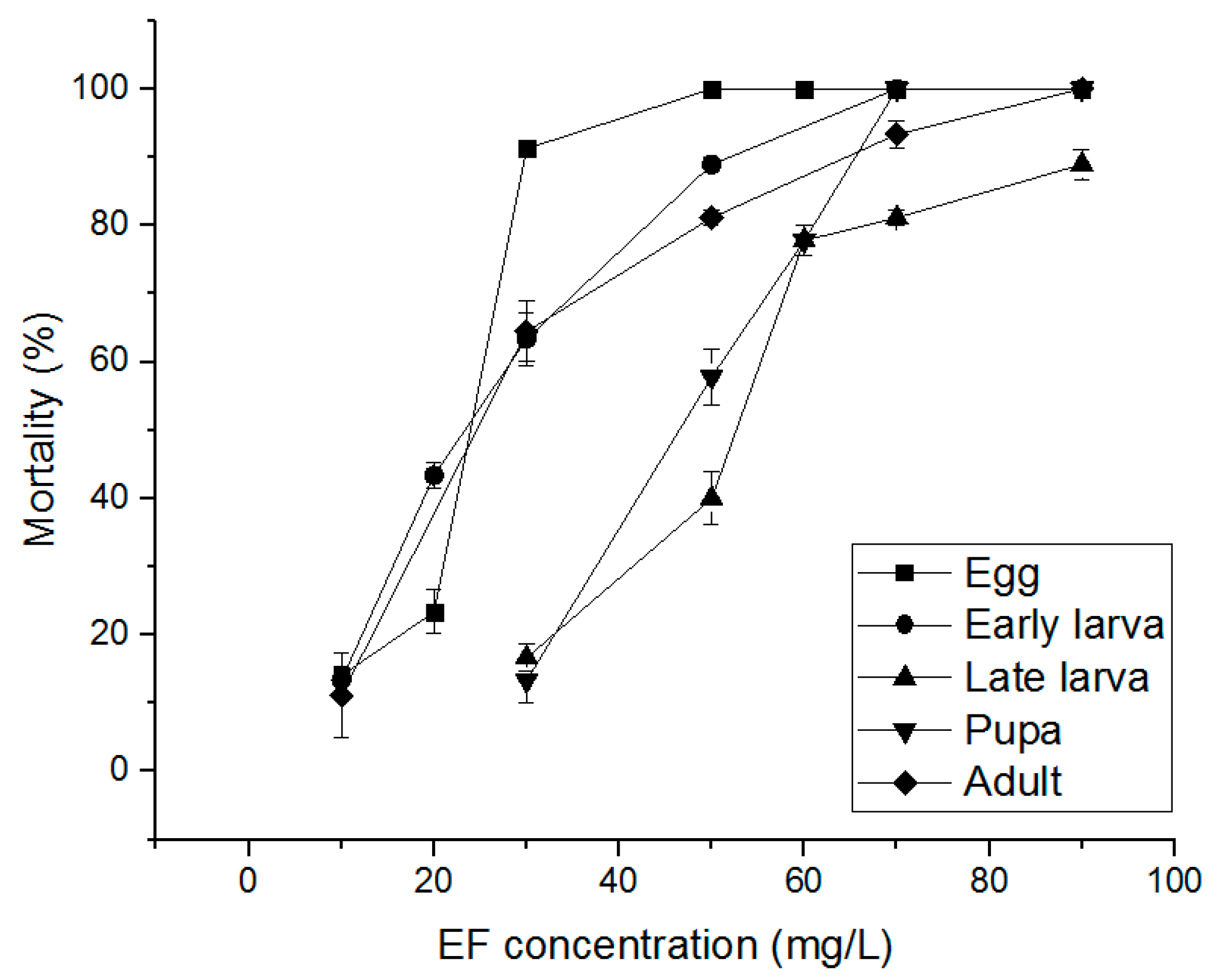
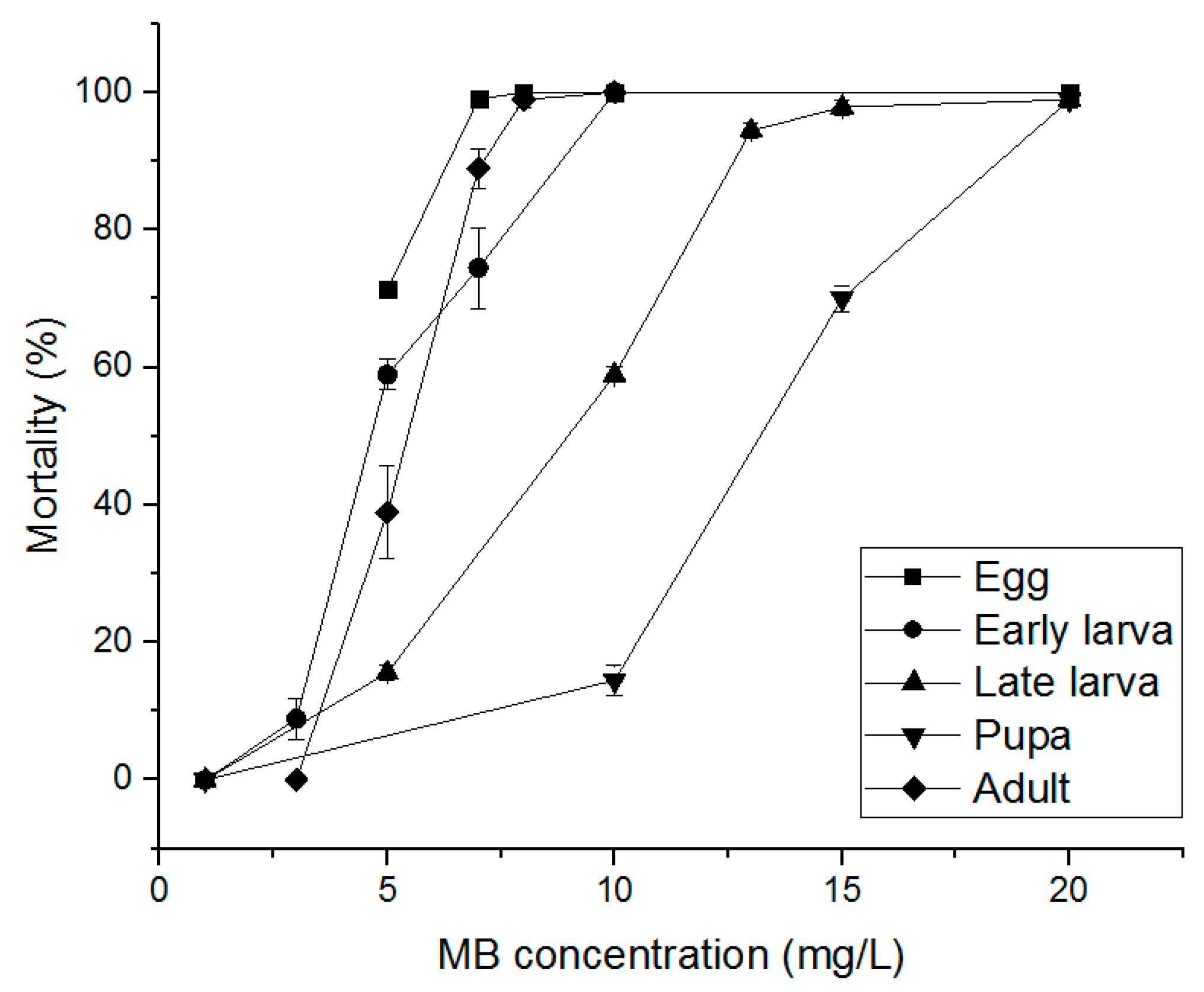
3.1. Fumigation of Phosphine-Susceptible S. oryzae
3.2. Evaluation of Phosphine-RESISTANT S. oryzae by Fumigation Time of Phosphine
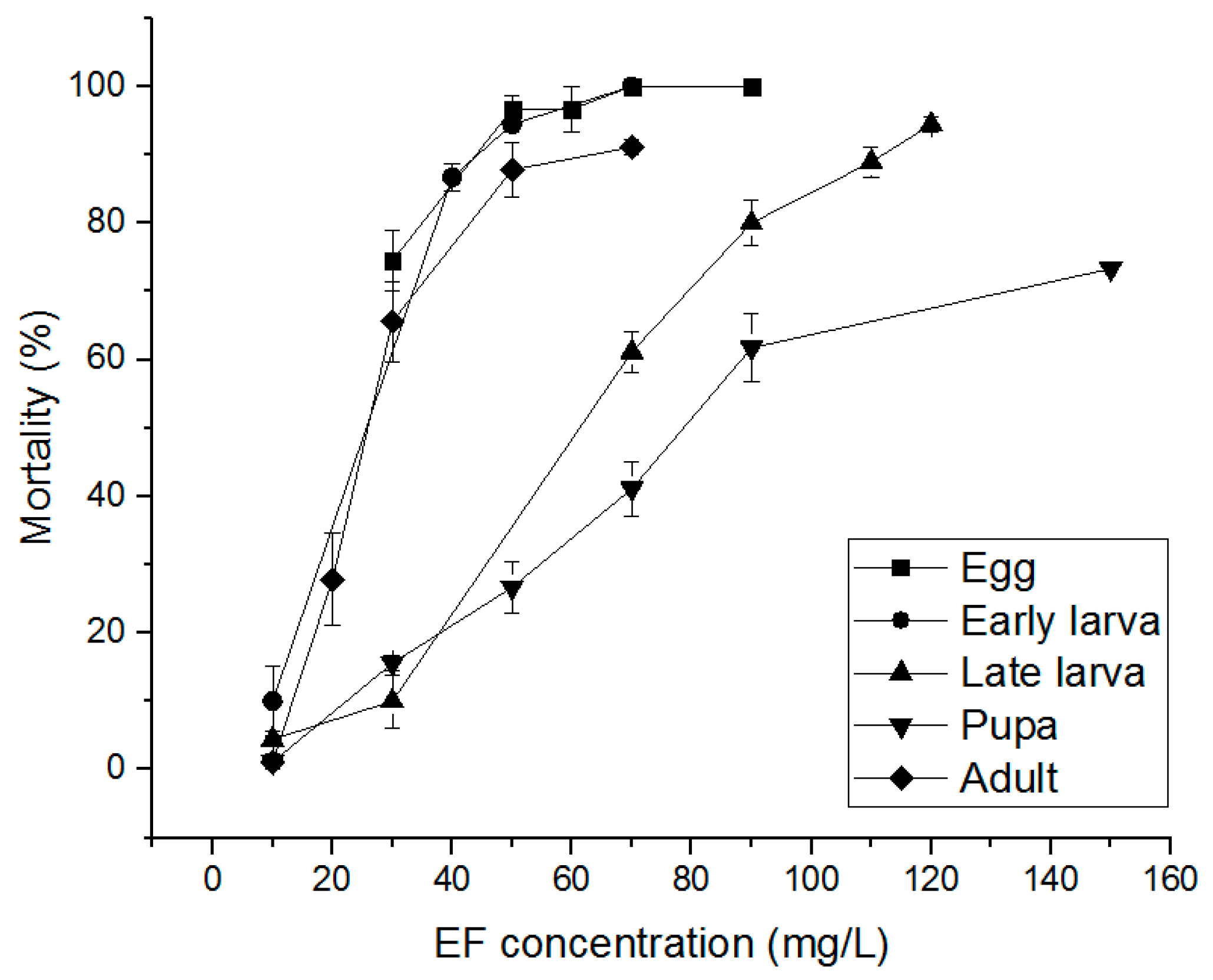
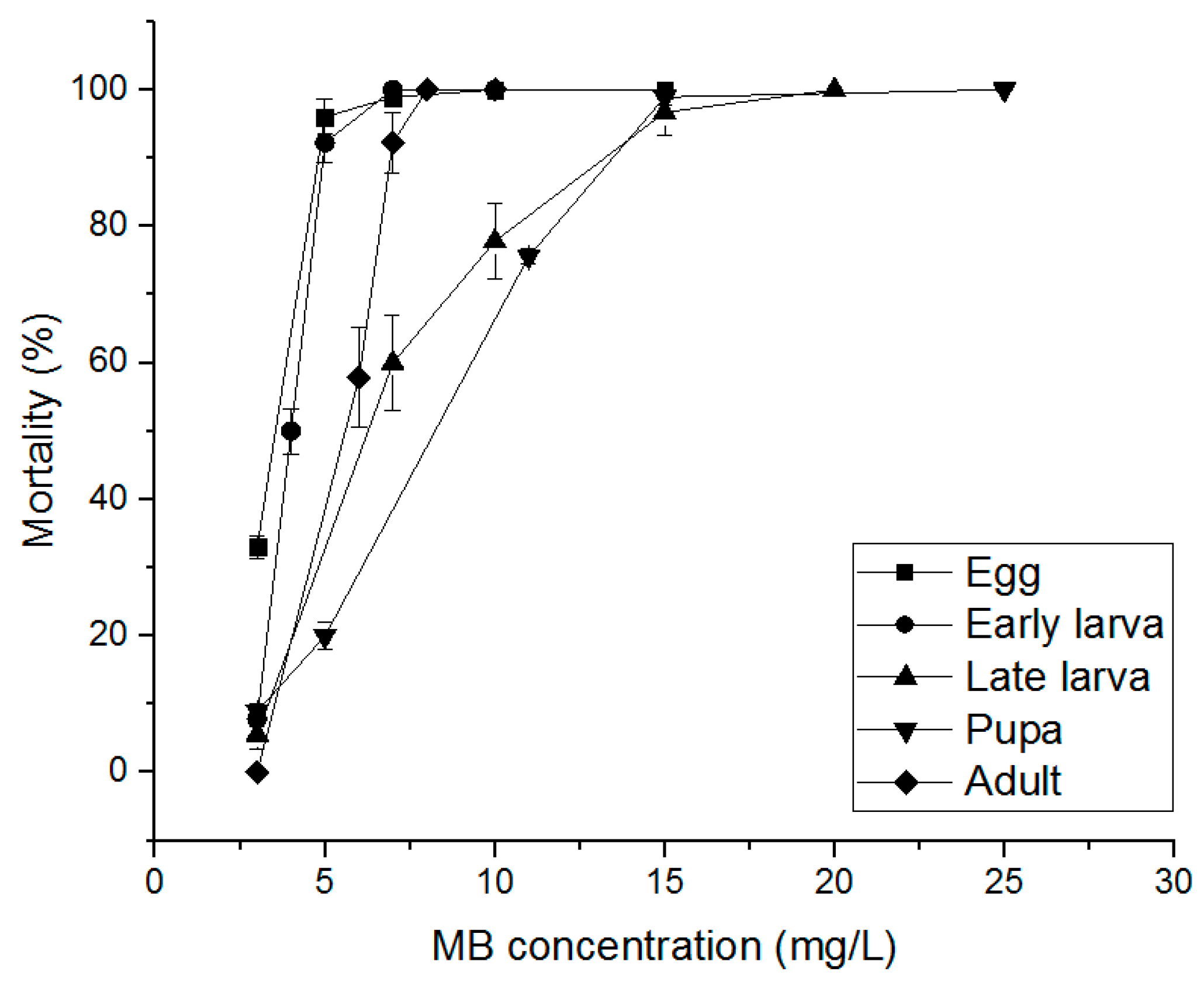
3.3. Alternative Fumigants for PH3-Resistant S. oryzae
3.4. LCt Analysis of the PH3-Susceptible and PH3-Resistant Rice Weevils
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dal Bello, G.; Padin, S.; Lastra, C.L.; Fabrizio, M. Laboratory evaluation of chemical-biological control of the rice weevil (Sitophilus oryzae L.) in stored grains. J. Stored Prod. Res. 2000, 37, 77–84. [Google Scholar] [CrossRef]
- Khan, A.R.; Selman, B.J. On the mortality of Tribolium castaneum adults treated sublethally as larvae with pirimiphos methyl, Nosema whitei and pirimiphos methyl—N. whitei doses. Entomophaga 1988, 33, 377–380. [Google Scholar] [CrossRef]
- Marsans, G.J. Manejo y Conservacion de Granos; Hemisferio Sur Editorial: Buenos Aires, Argentina, 1987; p. 266. [Google Scholar]
- Pinto, A.R., Jr.; Furiatti, R.S.; Pereira, P.R.V.S.; Lazzari, F.A. Evaluation of insecticides for control of Sitophilus oryzae (L.) (Coleoptera: Curculionidae), and Rhyzopertha dominica (Fab.) (Coleoptera: Bostrichidae) in stored rice. An. Soc. Entomol. Bras. 1997, 26, 285–290. [Google Scholar] [CrossRef]
- Rajendran, S. Postharvest pest losses. In Encyclopedia of Pest Management; Pimentel, D., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 2002; pp. 654–656. [Google Scholar]
- Follett, P.A.; Snook, K.; Janson, A.; Antonio, B.; Haruki, A.; Okamura, M.; Bisel, J. Irradiation quarantine treatment for control of Sitophilus oryzae (Coleoptera: Curculionidae) in rice. J. Stored Prod. Res. 2013, 52, 63–67. [Google Scholar] [CrossRef]
- Plarre, R. An attempt to reconstruct the natural and cultural history of the granary weevil, Sitophilus granarius (Coleoptera: Curculionidae). Eur. J. Entomol. 2010, 107, 1–11. [Google Scholar] [CrossRef]
- Zettler, J.L.; Arthur, F.H. Chemical control of stored product insects with fumigants and residual treatments. Crop Prot. 2000, 19, 577–582. [Google Scholar] [CrossRef]
- Ducom, P. Methyl bromide alternatives. In Proceedings of the 9th International Conference on Controlled Atmosphere and Fumigation in Stored Product, Antalya, Turkey, 15–19 October 2012; Volume 15, p. 19. [Google Scholar]
- Yagi, K.; Williams, J.; Wang, N.; Cicerone, R.J. Agricultural soil fumigation as a source of atmospheric methyl bromide. Proc. Natl. Acad. Sci. USA 1993, 90, 8420–8423. [Google Scholar] [CrossRef]
- Spurgeon, D. End agreed for Ozone-destroying pesticide. Nature 1997, 389, 319. [Google Scholar] [CrossRef]
- UNEP. Handbook for the Montreal Protocol on Substances that Deplete the Ozone Layer; UNEP/Earthprint: Nairobi, Kenya, 2006; pp. 10–11. [Google Scholar]
- Chaudhry, M.Q. A review of the mechanisms involved in the action of phosphine as an insecticide and phosphine resistance in stored-product insects. Pestic. Sci. 1997, 49, 213–228. [Google Scholar] [CrossRef]
- Chaudhry, M.Q. Phosphine resistance. Pestic. Outlook 2000, 11, 88–91. [Google Scholar] [CrossRef]
- Athié, I.; Gomes, R.A.; Bolonhezi, S.; Valentini, S.R.; De Castro, M.F.P.M. Effects of carbon dioxide and phosphine mixtures on resistant populations of stored-grain insects. J. Stored Prod. Res. 1998, 34, 27–32. [Google Scholar] [CrossRef]
- Rajendran, S. Phosphine resistance in stored insect pests in India. In Stored Products Protection, Proceedings of the 7th International Working Conference on Stored-product Protection, Beijing, China, 14–19 October 1998; Jin, Z., Liang, Q., Liang, Y., Tan, X., Guan, L., Eds.; Sichuan Publishing House of Science and Technology: Chengdu, China, 1999; pp. 635–641. [Google Scholar]
- Zeng, L. Development and countermeasures of phosphine resistance in stored grain insects in Guangdong of China. In Stored Products Protection, Proceedings of the 7th International Working Conference on Stored-product Protection, Beijing, China, 14–19 October 1998; Jin, Z., Liang, Q., Liang, Y., Tan, X., Guan, L., Eds.; Sichuan Publishing House of Science and Technology: Chengdu, China, 1999; pp. 642–647. [Google Scholar]
- Benhalima, H.; Chaudhry, M.Q.; Mills, K.A.; Price, N.R. Phosphine resistance in stored-product insects collected from various grain storage facilities in Morocco. J. Stored Prod. Res. 2004, 40, 241–249. [Google Scholar] [CrossRef]
- Pimentel, M.A.G.; Faroni, L.R.D.A.; da Silva, F.H.; Batista, M.D.; Guedes, R.N. Spread of phosphine resistance among Brazilian populations of three species of stored product insects. Neotrop. Entomol. 2010, 39, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Collins, P.J.; Ebert, P.R. Inheritance and characterization of strong resistance to phosphine in Sitophilus oryzae (L.). PLoS ONE 2015, 10, e0124335. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Collins, P.J.; Duong, T.M.; Schlipalius, D.I.; Ebert, P.R. Genetic conservation of phosphine resistance in the rice weevil Sitophilus oryzae (L.). J. Hered. 2016, 107, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Muthu, M.; Rajendran, S.; Krishnamurthy, T.S.; Narasimhan, K.S.; Rangaswarmy, J.R.; Jayaram, M.; Majumder, S.K. Ethyl formate as a safe general fumigant. In Proceedings of the International Conference on Practical Aspects of CAF in Stored Products, Perth, Australia, 11–22 April 1983; Ripp, B.E., Ed.; Elsevier: New York, NY, USA, 1984; pp. 369–393. [Google Scholar]
- Hilton, S.J.; Banks, H.J. Ethyl formate as a fumigant of sultanas; sorption and efficacy against six insect pests species. In Proceedings of the International Conference on Controlled Atmosphere and Fumigation in Stored Products, Nicosia, Cyprus, 21–26 April 1996; Donahaya, E.J., Navarro, S., Varnava, A., Eds.; Printco: Nicosia, Cyprus, 1997; pp. 409–422. [Google Scholar]
- Damcevski, K.; Dojchinov, G.; Haritos, V.S. VAPORMATETM, a formulation of ethyl formate with CO2, for disinfestation of grain. In Stored Grain in Australia 2003, Proceedings of the Australian Postharvest Technical Conference, Canberra, Australia, 25–27 June 2003; Wright, E.J., Webb, M.C., Highley, E., Eds.; CSIRO: Canberra, Australia, 2003; pp. 199–204. [Google Scholar]
- Desmarchelier, J.M.; Ren, Y.L. Analysis of fumigant residues-a critical review. J. AOAC Int. 1999, 82, 1261–1280. [Google Scholar]
- Desmarchelier, J.M. Ethyl formate and formic acid: Occurrence and environmental fate. Postharvest News Inf. 1999, 10, 7–12. [Google Scholar]
- Simmons, P.; Fisher, C.K. Ethyl formate and isopropyl formate as fumigants for packages of dried fruits. J. Econ. Entomol. 1946, 38, 715–716. [Google Scholar] [CrossRef]
- Budavari, S.; O’Neill, M.; Smith, A.; Heckleman, P. The Merck Index. An Encyclopedia of Chemicals, Drugs, and Biologicals, 11th ed.; Merck & Co. Rahway: Rahway, NJ, USA, 1989. [Google Scholar]
- Simpson, T.; Bikoba, V.; Tipping, C.; Mitcham, E.J. Ethyl formate as a postharvest fumigant for selected pests of table grapes. J. Econ. Entomol. 2007, 100, 1084–1090. [Google Scholar] [CrossRef]
- Ren, Y.; Lee, B.; Mahon, D.; Xin, N.; Head, M.; Reid, R. Fumigation of wheat using liquid ethyl formate plus methyl isothiocyanate in 50-tonne farm bins. J. Econ. Entomol. 2008, 101, 623–630. [Google Scholar] [CrossRef]
- Bond, E.J.; Monro, H.A.U. Manual of Fumigation for Insect Control; FAO Plant production and Protection Paper No 54; Food and Agriculture Organization of the United Nations: Rome, Italy, 1984; p. 432. [Google Scholar]
- Bell, C.H. Fumigation in the 21st century. Crop Prot. 2000, 19, 563–569. [Google Scholar] [CrossRef]
- Monro, H.A.U. Manual of fumigation for insect control. FAO Agric. Stud. 1969, 79, 381. [Google Scholar] [CrossRef]
- Finney, D.J. Probit Analysis, 3rd ed.; Cambridge University Press: London, UK, 1971. [Google Scholar]
- Hole, B.D.; Bell, C.H.; Mills, K.A.; Goodship, G. The toxicity of phosphine to all developmental stages of thirteen species of stored product beetles. J. Stored Prod. Res. 1976, 12, 235–244. [Google Scholar] [CrossRef]
- Kim, H.K.; Lee, S.W.; Kim, J.I.; Yang, J.O.; Koo, H.N.; Kim, G.H. Synergistic effects of oxygen on phosphine and ethyl formate for the control of Phthorimaea operculella (Lepidoptera: Gelechiidae). J. Econ. Entomol. 2015, 108, 2572–2580. [Google Scholar] [CrossRef] [PubMed]
- Damcevski, K.A.; Annis, P.C. The response of three stored product insect species to ethyl formate vapour at different temperatures. In Stored Grain in Australia 2000, Proceedings of the Australian Postharvest Technical Conference, Adelaide, Australia, 1–4 August 2000; Wright, E.J., Banks, H.J., Highley, E., Eds.; CSIRO: Canberra, Australia, 2002; pp. 78–81. [Google Scholar]
- Anon. Fumigation Standard No. 12. Bull. OEPP EPPO Bull. 1984, 23, 212. [Google Scholar]
- Price, L.A.; Mills, K.A. The toxicity of phosphine to the immature stages of resistant and susceptible strains of some common stored product beetles, and implications for their control. J. Stored Prod. Res. 1988, 24, 51–59. [Google Scholar] [CrossRef]
- Lee, B.H.; Huh, W.; Ren, Y.L.; Mahon, D.; Choi, W.S. New Formulations of Ethyl Formate to Control Internal Stages of Sitophilus oryzae. J. Asia Pac. Entomol. 2007, 10, 369–374. [Google Scholar] [CrossRef]

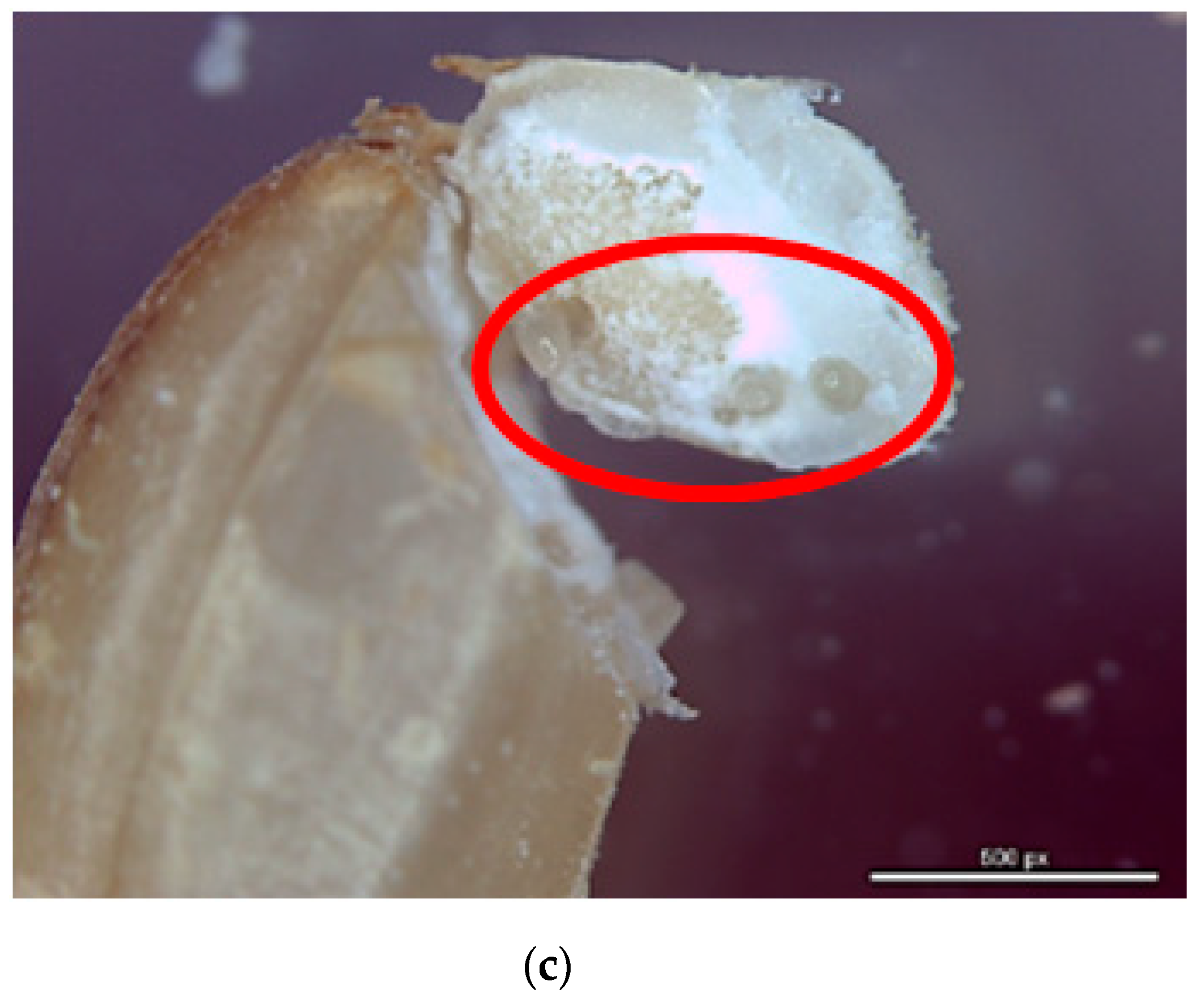
| Strain a | Stages | n | LCT50 (mg h/L) (95% CL b) | RR50 c | LCT99 (mg h/L) (95% CL) | RR99 | Slope ± SE | df | χ2 |
|---|---|---|---|---|---|---|---|---|---|
| S | Egg | 542 | 75.795 (35.294–99.852) | 1.00 | 186.623 (162.962–233.473) | 1.00 | 5.946 ± 0.921 | 5 | 0.00 |
| Early larva | 450 | 60.110 (40.398–78.077) | 1.00 | 213.792 (160.004–346.215) | 1.00 | 4.222 ± 0.332 | 4 | 0.04 | |
| Late larva | 600 | 160.491 (97.945–265.422) | 1.00 | 775.478 (389.069–14622.164) | 1.00 | 3.401 ± 0.789 | 6 | 8.13 | |
| Pupa | 600 | 255.797 (209.902–340.130) | 1.00 | 2373.015 (1289.504–6737.892) | 1.00 | 2.405 ± 0.233 | 6 | 0.80 | |
| Adult | 540 | 77.711 (58.090–100.392) | 1.00 | 316.190 (204.109–837.332) | 1.00 | 3.818 ± 0.494 | 5 | 1.97 | |
| R | Egg | 760 | 60.034 (2.415–98.658) | 0.79 | 217.692 (127.285–2673.091) | 1.17 | 4.159 ± 1.378 | 7 | 40.65 |
| Early larva | 630 | 64.450 (45.514–81.298) | 1.07 | 311.913 (224.910–557.809) | 1.46 | 3.398 ± 0.396 | 6 | 5.58 | |
| Late larva | 540 | 149.028 (85.417–191.420) | 0.93 | 449.200 (289.551–5031.511) | 0.58 | 4.856 ± 1.089 | 5 | 17.00 | |
| Pupa | 540 | 140.408 (53.470–187.556) | 0.55 | 312.447 (217.735–10214.042) | 0.13 | 6.698 ± 1.307 | 5 | 7.38 | |
| Adult | 540 | 66.043 (43.299–87.325) | 0.85 | 394.584 (266.764–807.344) | 1.25 | 2.997 ± 0.32 | 5 | 3.27 |
| Strain a | Stages | n | LCT50 (mg h/L) (95% CL b) | RR50 c | LCT99 (mg h/L) (95% CL) | RR99 | Slope ± SE | df | χ2 |
|---|---|---|---|---|---|---|---|---|---|
| S | Egg | 576 | 9.997 (7.125–12.255) | 1.00 | 24.111 (21.131–28.229) | 1.00 | 6.086 ± 0.554 | 5 | 0.041 |
| Early larva | 540 | 12.113 (7.018–14.187) | 1.00 | 19.929 (17.331–30.090) | 1.00 | 10.762 ± 2.137 | 5 | 10.61 | |
| Late larva | 540 | 18.952 (15.578–21.901) | 1.00 | 60.351 (49.342–82.503) | 1.00 | 4.626 ± 0.373 | 5 | 0.53 | |
| Pupa | 540 | 21.104 (11.907–29.393) | 1.00 | 67.795 (44.055–255.928) | 1.00 | 4.591 ± 0.826 | 5 | 30.65 | |
| Adult | 540 | 17.824 (16.772–18.433) | 1.00 | 22.297 (20.853–26.931) | 1.00 | 23.929 ± 3.761 | 5 | 0.04 | |
| R | Egg | 545 | 17.842 (6.726–22.183) | 1.78 | 24.683 (16.463–27.220) | 1.02 | 16.509 ± 3.323 | 5 | 0.00 |
| Early larva | 540 | 14.900 (13.461–17.159) | 1.23 | 34.098 (26.534–53.390) | 1.71 | 6.471 ± 0.601 | 5 | 0.96 | |
| Late larva | 630 | 25.840 (21.031–32.636) | 1.36 | 68.905 (48.205–161.691) | 1.14 | 5.462 ± 0.863 | 6 | 17.13 | |
| Pupa | 450 | 43.520 (35.203–73.578) | 2.06 | 73.072 (50.790–186.850) | 1.08 | 10.339 ± 1.080 | 4 | 0.43 | |
| Adult | 540 | 16.397 (14.842–17.388) | 0.92 | 23.792 (21.992–27.719) | 1.07 | 14.392 ± 1.655 | 5 | 0.00 |
| Strain a | Stages | n | LCT50 (mg h/L) (95% CL b) | RR50 c | LCT99 (mg h/L) (95% CL) | RR99 | Slope ± SE | df | χ2 |
|---|---|---|---|---|---|---|---|---|---|
| S | Egg | 540 | 0.440 (0.042–0.861) | 1.00 | 4.625 (2.911–17.448) | 1.00 | 2.277 ± 0.425 | 5 | 7.73 |
| Early larva | 540 | 0.602 (0.307–0.907) | 1.00 | 12.243 (7.436–29.192) | 1.00 | 1.779 ± 0.173 | 5 | 0.55 | |
| Late larva | 540 | 3.901 (2.799–5.862) | 1.00 | 119.032 (49.421–529.324) | 1.00 | 1.567 ± 0.133 | 5 | 2.33 | |
| Pupa | 632 | 6.171 (3.462–11.297) | 1.00 | 2450.358 (536.688–9983.186) | 1.00 | 0.895 ± 0.099 | 6 | 6.90 | |
| Adult | 540 | 0.295 (0.225–0.342) | 1.00 | 0.634 (0.555–0.797) | 1.00 | 6.996 ± 0.776 | 5 | 1.37 | |
| R | Egg | 717 | 6.595 (0.773–13.142) | 14.99 | 57.206 (42.018–99.369) | 12.37 | 2.480 ± 0.512 | 7 | 1.03 |
| Early larva | 725 | 28.456 (0.943–45.574) | 47.27 | 107.914 (60.927–276614.079) | 8.81 | 4.019 ± 1.390 | 7 | 67.32 | |
| Late larva | 633 | 48.170 (26.561–105.326) | 12.35 | 241.311 (108.523–5887.895) | 2.03 | 3.325 ± 0.845 | 6 | 25.57 | |
| Pupa | 630 | 29.106 (24.576–33.114) | 4.72 | 91.760 (77.518–116.869) | 0.04 | 4.666 ± 0.364 | 6 | 2.71 | |
| Adult | 540 | 16.550 (12.113–19.290) | 56.10 | 25.938 (22.889–30.448) | 40.91 | 11.924 ± 1.581 | 5 | 0.26 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, B.; Song, J.-E.; Park, J.S.; Park, Y.; Shin, E.-M.; Yang, J. Insecticidal Effects of Fumigants (EF, MB, and PH3) towards Phosphine-Susceptible and -Resistant Sitophilus oryzae (Coleoptera: Curculionidae). Insects 2019, 10, 327. https://doi.org/10.3390/insects10100327
Kim B, Song J-E, Park JS, Park Y, Shin E-M, Yang J. Insecticidal Effects of Fumigants (EF, MB, and PH3) towards Phosphine-Susceptible and -Resistant Sitophilus oryzae (Coleoptera: Curculionidae). Insects. 2019; 10(10):327. https://doi.org/10.3390/insects10100327
Chicago/Turabian StyleKim, BongSu, Ja-Eun Song, Jeong Sun Park, YoungJu Park, Eun-Mi Shin, and JeongOh Yang. 2019. "Insecticidal Effects of Fumigants (EF, MB, and PH3) towards Phosphine-Susceptible and -Resistant Sitophilus oryzae (Coleoptera: Curculionidae)" Insects 10, no. 10: 327. https://doi.org/10.3390/insects10100327
APA StyleKim, B., Song, J.-E., Park, J. S., Park, Y., Shin, E.-M., & Yang, J. (2019). Insecticidal Effects of Fumigants (EF, MB, and PH3) towards Phosphine-Susceptible and -Resistant Sitophilus oryzae (Coleoptera: Curculionidae). Insects, 10(10), 327. https://doi.org/10.3390/insects10100327




