Synthesis of a Lubricant to Mimic the Biorheological Behavior of Osteoarthritic and Revision Synovial Fluid
Abstract
1. Introduction
- How does the composition of the SF differ among patients undergoing primary implantation due to OA and revision surgery?
- How is the flow behavior related to changes in the chemical composition regarding protein and HA concentration?
- How can the flow behavior of human SF be mimicked by synthetic fluids?
2. Materials and Methods
2.1. Test Fluids
2.2. Chemical Composition
2.2.1. Total Protein Concentration of SF
2.2.2. Hyaluronic Acid Concentration in SF
2.3. Rheological Investigations
2.4. Mathematical Modelling of Flow Behavior
- error function, which should be minimized
- gradient tolerance of
- variable bounds and
2.5. Friction Measurements
2.6. Statistical Analysis
3. Results
3.1. Patient Data of Human Samples
3.2. Concentration of Proteins and HA
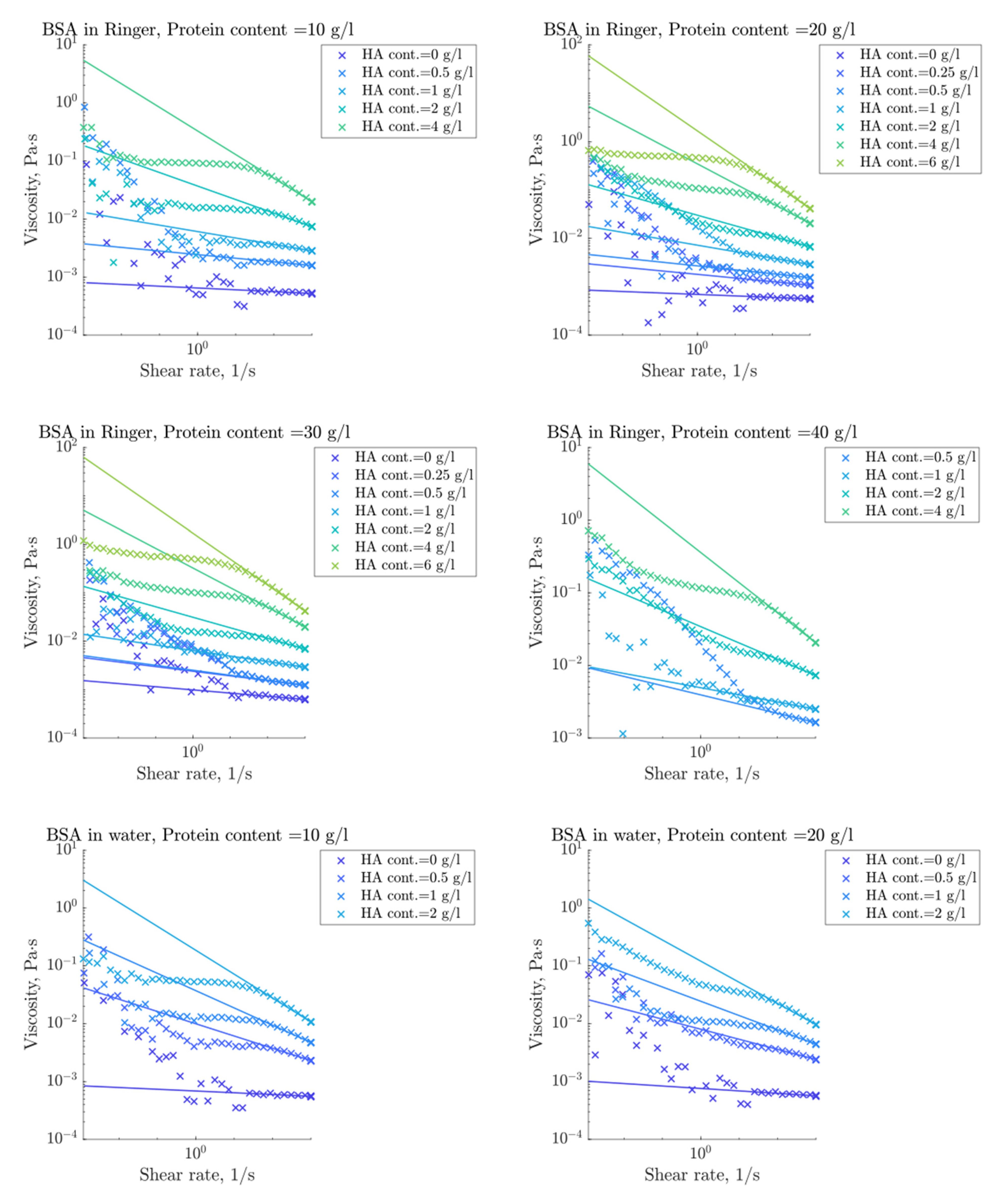
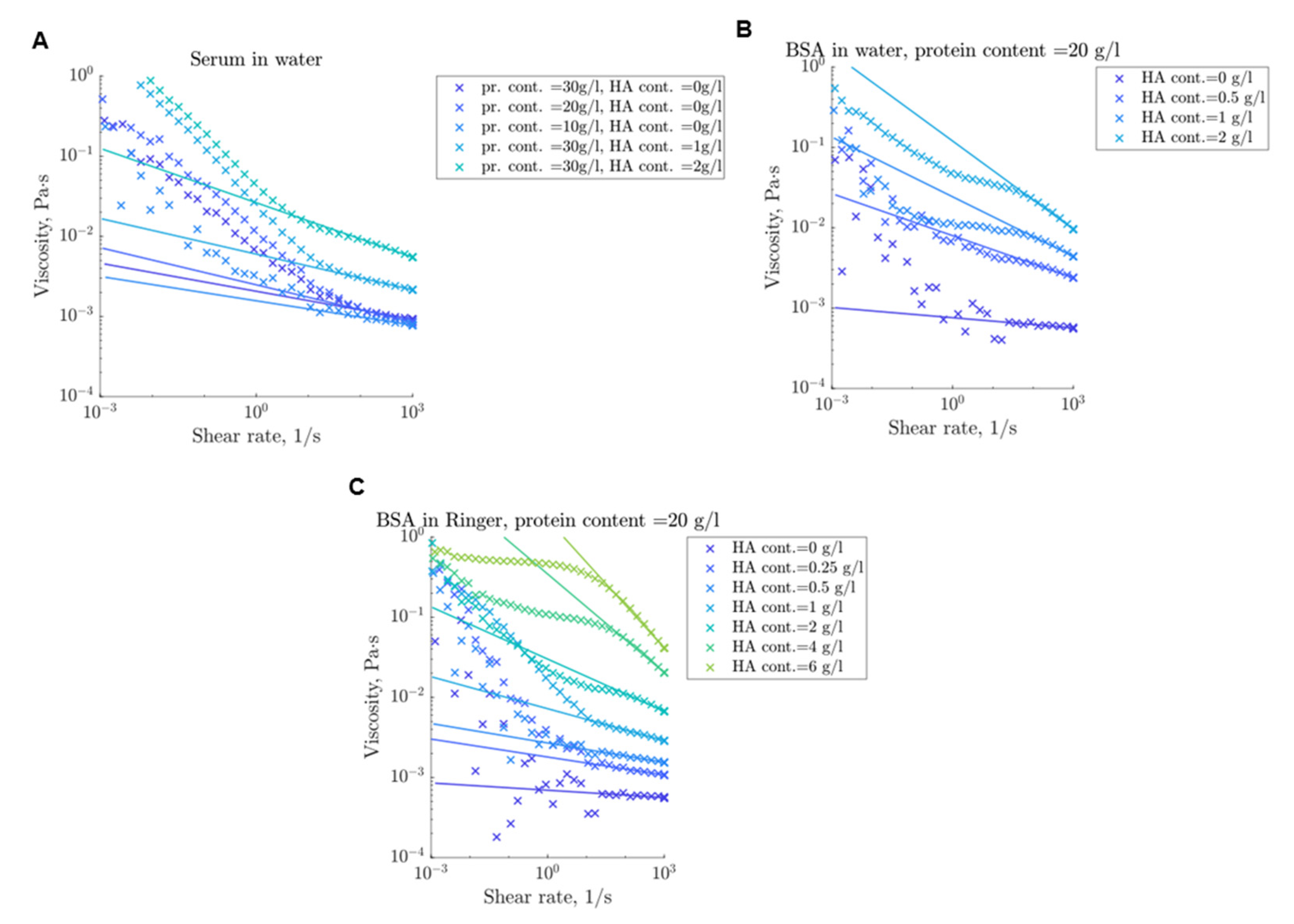
3.3. Rheological Properties
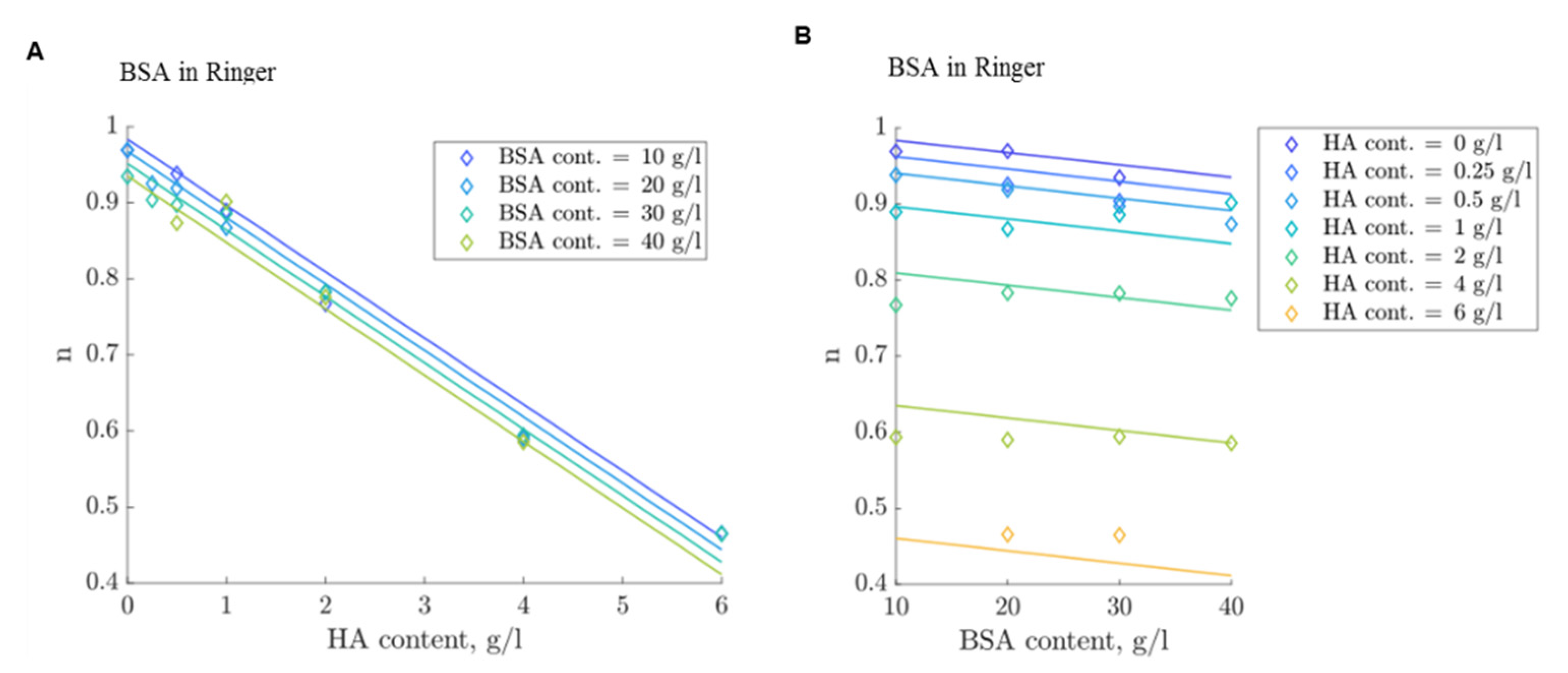
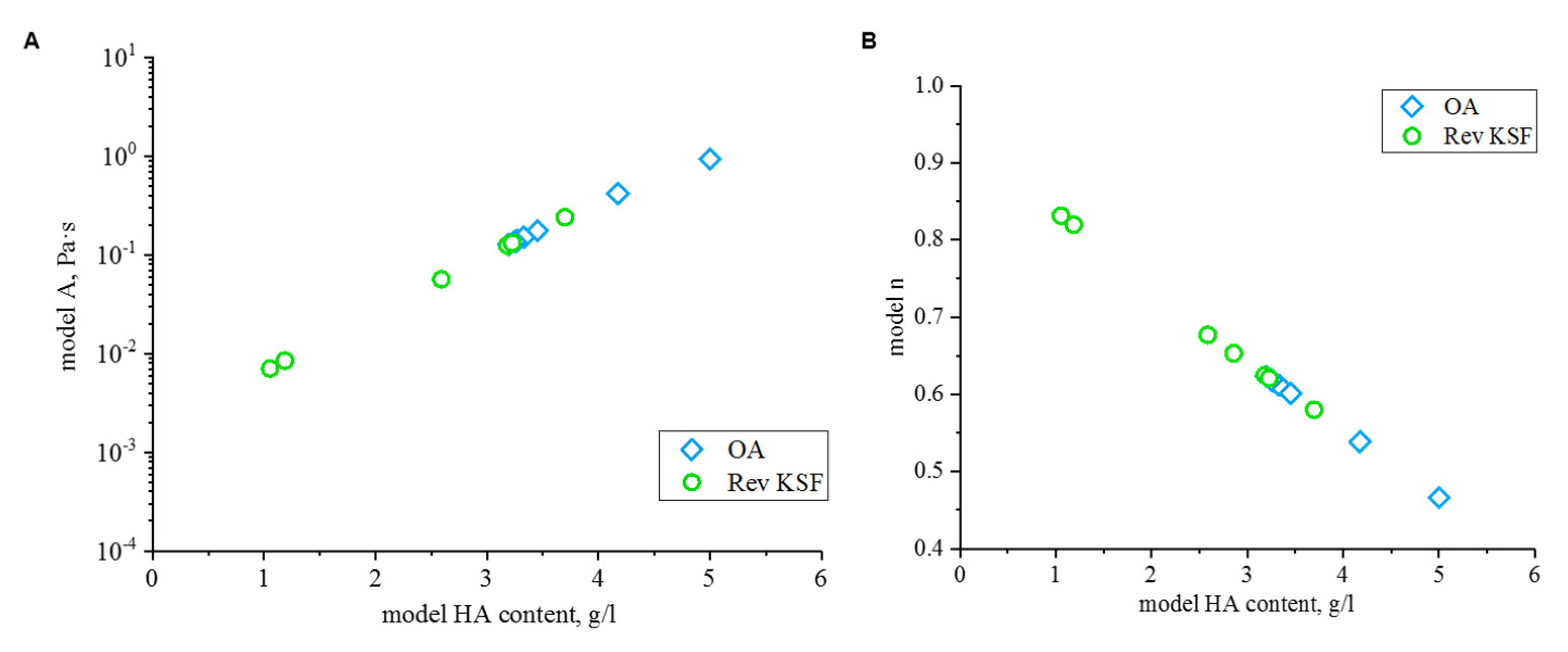
3.4. Modelling of Flow Behavior
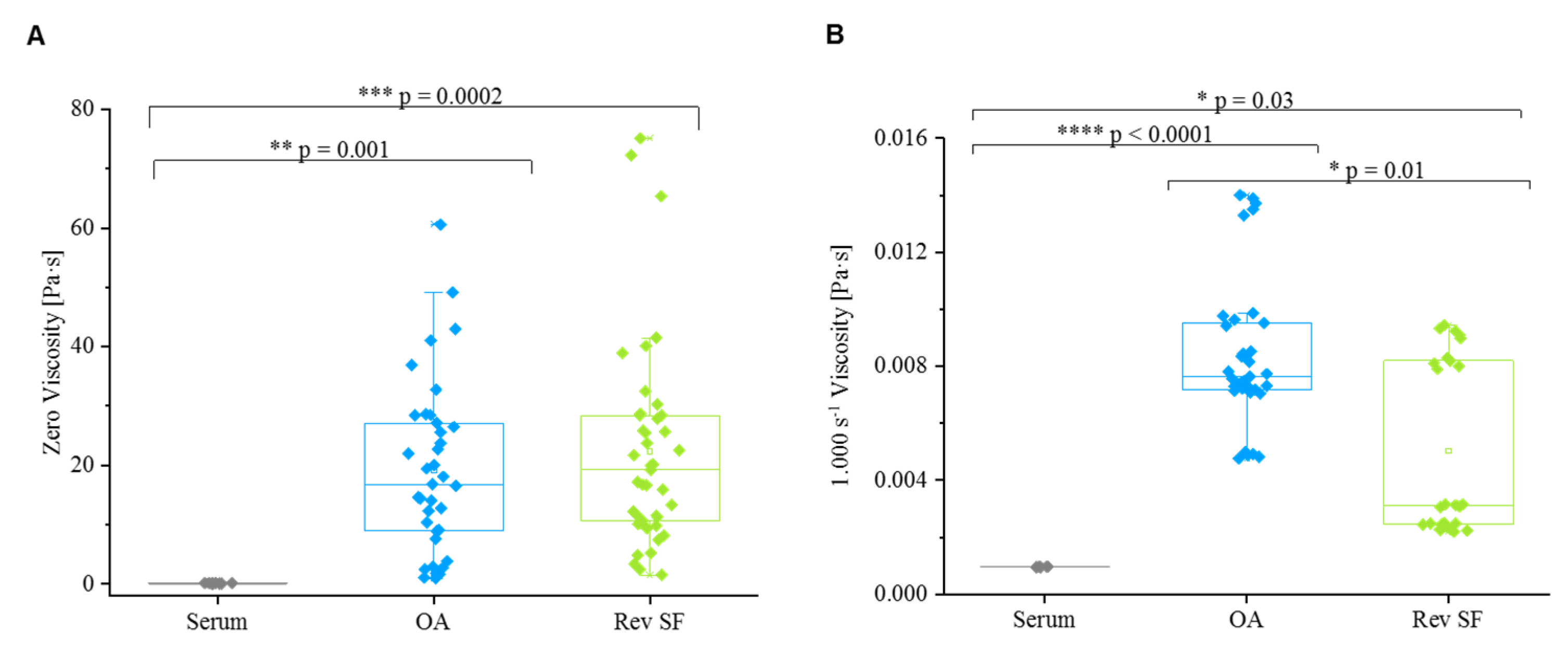
3.5. Friction Behavior
4. Discussion
4.1. Chemical Composition
4.2. Rheological Properties
4.3. Flow Curve Simulation Model
4.4. Friction Behavior
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

| No. | Serum (g/L) | BSA (g/L) | HA (g/L) | Deionised Water | Ringer Solution | Volume (mL) |
|---|---|---|---|---|---|---|
| 1 | 30 | 0.25 | balance | 4 | ||
| 2 | 30 | 0.5 | balance | 4 | ||
| 3 | 30 | 1 | balance | 4 | ||
| 4 | 30 | 2 | balance | 4 | ||
| 5 | 30 | 4 | balance | 4 | ||
| 6 | 30 | 6 | balance | 4 | ||
| 7 | 20 | 0.25 | balance | 4 | ||
| 8 | 20 | 0.5 | balance | 4 | ||
| 9 | 20 | 1 | balance | 4 | ||
| 10 | 20 | 2 | balance | 4 | ||
| 11 | 20 | 4 | balance | 4 | ||
| 12 | 20 | 6 | balance | 4 | ||
| 13 | 10 | 0.5 | balance | 4 | ||
| 14 | 10 | 1 | balance | 4 | ||
| 15 | 10 | 2 | balance | 4 | ||
| 16 | 10 | 4 | balance | 4 | ||
| 17 | 40 | 0.5 | balance | 4 | ||
| 18 | 40 | 1 | balance | 4 | ||
| 19 | 40 | 2 | balance | 4 | ||
| 20 | 40 | 4 | balance | 4 | ||
| 21 | 30 | balance | 4 | |||
| 22 | 20 | balance | 4 | |||
| 23 | 10 | balance | 4 | |||
| 24 | 40 | 0.5 | balance | 4 | ||
| 25 | 40 | 1 | balance | 4 | ||
| 26 | 40 | 2 | balance | 4 | ||
| 27 | 30 | 0.5 | balance | 4 | ||
| 28 | 30 | 1 | balance | 4 | ||
| 29 | 30 | 2 | balance | 4 | ||
| 30 | 20 | 0.5 | balance | 4 | ||
| 31 | 20 | 1 | balance | 4 | ||
| 32 | 20 | 2 | balance | 4 | ||
| 33 | 10 | 0.5 | balance | 4 | ||
| 34 | 10 | 1 | balance | 4 | ||
| 35 | 10 | 2 | balance | 4 | ||
| 36 | 30 | balance | 4 | |||
| 37 | 20 | balance | 4 | |||
| 38 | 10 | balance | 4 | |||
| 39 | 30 | balance | 4 | |||
| 40 | 20 | balance | 4 | |||
| 41 | 10 | balance | 4 | |||
| 42 | 30 | 0.5 | balance | 4 | ||
| 43 | 30 | 1 | balance | 4 | ||
| 44 | 30 | 2 | balance | 4 |
| Sample | E | EA | En | ||
|---|---|---|---|---|---|
| OA 1 | 3.2 | 60 | 0.0096183 | 0.0002375 | 0.0093808 |
| OA 2 | 3.27 | 60 | 0.0469458 | 0.0008402 | 0.0461056 |
| OA 3 | 5 | 60 | 0.0073376 | 0.0002346 | 0.0071031 |
| OA 4 | 3.33 | 60 | 0.0109873 | 0.0002571 | 0.0107302 |
| OA 5 | 3.25 | 60 | 0.0052849 | 0.0001364 | 0.0051485 |
| OA 6 | 3.45 | 60 | 0.0032126 | 8.18 × 10−5 | 0.0031307 |
| OA 7 | 4.17 | 60 | 0.0053263 | 0.0001243 | 0.005202 |
| Rev KS 1 | 1.05 | 47.45 | 8.58 × 10−11 | 9.00 × 10−12 | 7.68 × 10−11 |
| Rev KS 2 | 3.23 | 60 | 0.0038182 | 0.0001019 | 0.0037163 |
| Rev KS 3 | 3.7 | 60 | 0.0134239 | 0.0002845 | 0.0131395 |
| Rev KS 4 | 2.86 | 60 | 0.0056988 | 0.000167 | 0.0055317 |
| Rev KS 5 | 2.09 | 59.99 | 4.40 × 10−5 | 2.18 × 10−6 | 4.19 × 10−5 |
| Rev KS 6 | 2.59 | 60 | 0.0585443 | 0.0012587 | 0.0572856 |
| Rev KS 7 | 3.18 | 60 | 0.0091594 | 0.0002284 | 0.008931 |
References
- Damm, P.; Bender, A.; Bergmann, G. Postoperative changes in in vivo measured friction in total hip joint prosthesis during walking. PLoS ONE 2015, 10, e0120438. [Google Scholar] [CrossRef]
- Ghosh, S.; Choudhury, D.; Roy, T.; Moradi, A.; Masjuki, H.H.; Pingguan-Murphy, B. Tribological performance of the biological components of synovial fluid in artificial joint implants. Sci. Technol. Adv. Mater. 2015, 16, 45002. [Google Scholar] [CrossRef]
- Gispert, M.P.; Serro, A.P.; Colaço, R.; Saramago, B. Friction and wear mechanisms in hip prosthesis: Comparison of joint materials behaviour in several lubricants. Wear 2006, 260, 149–158. [Google Scholar] [CrossRef]
- Yao, J.Q.; Laurent, M.P.; Johnson, T.S.; Blanchard, C.R.; Crowninshield, R.D. The influences of lubricant and material on polymer/CoCr sliding friction. Wear 2003, 255, 780–784. [Google Scholar] [CrossRef]
- ISO 14243-1. Implants for Surgery—Wear of Total Knee-Joint Prostheses: Part 1, Loading and Displacement Parameters for Wear-Testing Machines with Load Control and Corresponding Environmental Conditions for Test, 2nd ed.; Beuth Verlag GmbH: Berlin, Germany, 2009. [Google Scholar]
- F04 Committee. ASTM F1715-00e1 Standard Guide for Wear Assessment of Prosthetic Knee Designs in Simulator Devices; ASTM International: West Conshohocken, PA, USA, 2006. [Google Scholar] [CrossRef]
- Price, P.J.; Gregory, E.A. Relationship between in vitro growth promotion and biophysical and biochemical properties of the serum supplement. In Vitro 1982, 18, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Schwenke, T.; Kaddick, C.; Schneider, E.; Wimmer, M.A. Fluid composition impacts standardized testing protocols in ultrahigh molecular weight polyethylene knee wear testing. Proc. IMechE 2005, 219, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Trommer, R.M.; Maru, M.M. Importance of preclinical evaluation of wear in hip implant designs using simulator machines. Rev. Bras. Ortop. 2017, 52, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Bortel, E.; Charbonnier, B.; Heuberger, R. Development of a Synthetic Synovial Fluid for Tribological Testing. Lubricants 2015, 3, 664–686. [Google Scholar] [CrossRef]
- Murakami, T.; Yarimitsu, S.; Nakashima, K.; Sawae, Y.; Sakai, N. Influence of synovia constituents on tribological behaviors of articular cartilage. Friction 2013, 1, 150–162. [Google Scholar] [CrossRef]
- Mazzucco, D.; Scott, R.; Spector, M. Composition of joint fluid in patients undergoing total knee replacement and revision arthroplasty: Correlation with flow properties. Biomaterials 2004, 25, 4433–4445. [Google Scholar] [CrossRef] [PubMed]
- Furey, M.J.; Burkhardt, B.M. Biotribology: Friction, wear, and lubrication of natural synovial joints. Lubr. Sci. 1997, 9, 255–271. [Google Scholar] [CrossRef]
- Larsen, N.E.; Balazs, E.A. Drug delivery systems using hyaluronan and its derivatives. Adv. Drug Deliv. Rev. 1991, 7, 279–293. [Google Scholar] [CrossRef]
- Bennike, T.; Ayturk, U.; Haslauer, C.M.; Froehlich, J.W.; Proffen, B.L.; Barnaby, O.; Birkelund, S.; Murray, M.M.; Warman, M.L.; Stensballe, A.; et al. A normative study of the synovial fluid proteome from healthy porcine knee joints. J. Proteome Res. 2014, 13, 4377–4387. [Google Scholar] [CrossRef] [PubMed]
- Schurz, J.; Ribitsch, V. Rheology of synovial fluid. Biorheology 1987, 24, 385–399. [Google Scholar] [CrossRef]
- Hui, A.Y.; McCarty, W.J.; Masuda, K.; Firestein, G.S.; Sah, R.L. A systems biology approach to synovial joint lubrication in health, injury, and disease. Wiley Interdiscip. Rev. Syst. Biol. Med. 2012, 4, 15–37. [Google Scholar] [CrossRef]
- Dumbleton, J.H. Tribology of Natural and Artificial Joints; Elsevier: Amsterdam, The Netherlands, 1981. [Google Scholar]
- Fam, H.; Bryant, J.T.; Kontopoulou, M. Rheological properties of synovial fluids. Biorheology 2007, 44, 59–74. [Google Scholar]
- Rainer, F.; Ribitsch, V. Viskoelastische Eigenschaften der intakten Human-Synovia und ihr Bezug zur Biomechanik. Z Rheumatol. 1985, 44, 114–119. [Google Scholar] [PubMed]
- Cowman, M.K.; Schmidt, T.A.; Raghavan, P.; Stecco, A. Viscoelastic Properties of Hyaluronan in Physiological Conditions. F1000Research 2015, 4, 622. [Google Scholar] [CrossRef] [PubMed]
- Cook, R.B.; Bolland, B.J.R.F.; Wharton, J.A.; Tilley, S.; Latham, J.M.; Wood, R.J.K. Pseudotumour formation due to tribocorrosion at the taper interface of large diameter metal on polymer modular total hip replacements. J. Arthroplast. 2013, 28, 1430–1436. [Google Scholar] [CrossRef]
- Simon, L.S. Viscosupplementation therapy with intra-articular hyaluronic acid. Fact or fantasy? Rheum. Dis. Clin. N. Am. 1999, 25, 345–357. [Google Scholar] [CrossRef]
- Link, J.M.; Salinas, E.Y.; Hu, J.C.; Athanasiou, K.A. The tribology of cartilage: Mechanisms, experimental techniques, and relevance to translational tissue engineering. Clin. Biomech. 2020, 79, 104880. [Google Scholar] [CrossRef] [PubMed]
- Waller, K.A.; Zhang, L.X.; Fleming, B.C.; Jay, G.D. Preventing friction-induced chondrocyte apoptosis: Comparison of human synovial fluid and hylan G-F 20. J. Rheumatol. 2012, 39, 1473–1480. [Google Scholar] [CrossRef]
- Jay, G.D.; Lane, B.P.; Sokoloff, L. Characterization of a bovine synovial fluid lubricating factor III. The interaction with hyaluronic acid. Connect. Tissue Res. 1992, 28, 245–255. [Google Scholar] [CrossRef]
- Lee, D.W.; Banquy, X.; Das, S.; Cadirov, N.; Jay, G.; Israelachvili, J. Effects of molecular weight of grafted hyaluronic acid on wear initiation. Acta Biomater. 2014, 10, 1817–1823. [Google Scholar] [CrossRef]
- Bonnevie, E.D.; Galesso, D.; Secchieri, C.; Bonassar, L.J. Frictional characterization of injectable hyaluronic acids is more predictive of clinical outcomes than traditional rheological or viscoelastic characterization. PLoS ONE 2019, 14, e0216702. [Google Scholar] [CrossRef]
- Kwiecinski, J.J.; Dorosz, S.G.; Ludwig, T.E.; Abubacker, S.; Cowman, M.K.; Schmidt, T.A. The effect of molecular weight on hyaluronan’s cartilage boundary lubricating ability--alone and in combination with proteoglycan 4. Osteoarthr. Cart. 2011, 19, 1356–1362. [Google Scholar] [CrossRef]
- McNulty, D.; Swope, S.; Liao, Y.S.; McKellop, H.; Shen, F.W.; Galvin, A.; Fisher, J. Multi-lab evaluation of hip simulator test conditions on relative wear rates of crosslinked polyethylenes. In Proceedings of the 52nd Annual Meeting of the Orthopaedic Research Society, Chicago, IL, USA, 19–22 March 2006; p. 649. [Google Scholar]
- Wang, A.; Essner, A.; Schmidig, G. The effects of lubricant composition on in vitro wear testing of polymeric acetabular components. J. Biomed. Mater. Res. 2004, 68, 45–52. [Google Scholar] [CrossRef]
- Saikko, V. Effect of Lubricant Protein Concentration on the Wear of Ultra-High Molecular Weight Polyethylene Sliding Against a CoCr Counterface. J. Tribol. 2003, 125, 638–642. [Google Scholar] [CrossRef]
- Liao, Y.-S.; McKellop, H.; Lu, Z.; Campbell, P.; Benya, P. The effect of frictional heating and forced cooling on the serum lubricant and wear of UHMW polyethylene cups against cobalt–chromium and zirconia balls. Biomaterials 2003, 24, 3047–3059. [Google Scholar] [CrossRef]
- Roba, M.; Naka, M.; Gautier, E.; Spencer, N.D.; Crockett, R. The adsorption and lubrication behavior of synovial fluid proteins and glycoproteins on the bearing-surface materials of hip replacements. Biomaterials 2009, 30, 2072–2078. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Fleming, L.; Wudebwe, U.; Bowen, J.; Grover, L.M. Development of a synovial fluid analogue with bio-relevant rheology for wear testing of orthopaedic implants. J. Mech. Behav. Biomed. Mater. 2014, 32, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Sava, M.-M.; Boulocher, C.; Matei, C.I.; Munteanu, B.; Schramme, M.; Viguier, E.; Roger, T.; Berthier, Y.; Blanchin, M.G.; Trunfio-Sfarghiu, A.M. Structural and tribological study of healthy and biomimetic SF. Comput. Methods Biomech. Biomed. Eng. 2013, 16, 216–218. [Google Scholar] [CrossRef]
- Deutsches Institut für Normung, e.V. Viscosity—Part 3; Non-Newtonian Liquids 2003-11; Beuth Verlag GmbH: Berlin, Germany, 2003.
- Poliakov, A.; Pakhaliuk, V.; Popov, V.L. Current Trends in Improving of Artificial Joints Design and Technologies for Their Arthroplasty. Front. Mech. Eng. 2020, 6, 4. [Google Scholar] [CrossRef]
- Afoke, N.Y.; Byers, P.D.; Hutton, W.C. Contact pressures in the human hip joint. J. Bone Jt. Surg. Br. 1987, 69, 536–541. [Google Scholar] [CrossRef]
- Yoshida, H.; Faust, A.; Wilckens, J.; Kitagawa, M.; Fetto, J.; Chao, E.Y.-S. Three-dimensional dynamic hip contact area and pressure distribution during activities of daily living. J. Biomech. 2006, 39, 1996–2004. [Google Scholar] [CrossRef] [PubMed]
- Stukenborg-Colsman, C.; Ostermeier, S.; Hurschler, C.; Wirth, C.J. Tibiofemoral contact stress after total knee arthroplasty: Comparison of fixed and mobile-bearing inlay designs. Acta Orthop. Scand. 2002, 73, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Moewis, P.; Checa, S.; Kutzner, I.; Hommel, H.; Duda, G.N. Physiological joint line total knee arthroplasty designs are especially sensitive to rotational placement—A finite element analysis. PLoS ONE 2018, 13, e0192225. [Google Scholar] [CrossRef]
- Holm, S. A Simple Sequentially Rejective Multiple Test Procedure. Scand. J. Stat. 1979, 6, 65–70. [Google Scholar]
- Kruskal, W.H.; Wallis, W.A. Use of Ranks in One-Criterion Variance Analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Dunn, O.J. Multiple Comparisons Using Rank Sums. Technometrics 1964, 6, 241. [Google Scholar] [CrossRef]
- Kellgreen, J.H.; Lawrence, J.S. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Dunn, S.; Kolomytkin, O.V.; Marino, A.A. Pathophysiology of osteoarthritis: Evidence against the viscoelastic theory. Pathobiology 2009, 76, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, H.H.; Erdil, M.; Polat, G.; Kara, D.; Kilic, E.; Kocyigit, A.; Tuncay, I. Does intra-articular fracture change the lubricant content of synovial fluid? J. Orthop. Surg. Res. 2015, 10, 89. [Google Scholar] [CrossRef][Green Version]
- Kung, M.; Markantonis, J.; Nelson, S.; Campbell, P. The Synovial Lining and Synovial Fluid Properties after Joint Arthroplasty. Lubricants 2015, 3, 394–412. [Google Scholar] [CrossRef]
- Burkandt, A.; Katzer, A.; Thaler, K.; Baehr, V.; von Friedrich, R.E.; Rüther, W.; Amling, M.; Zustin, J. Proliferation of the Synovial Lining Cell Layer in Suggested Metal Hypersensitivity. In Vivo 2011, 25, 679–686. [Google Scholar]
- DesJardins, J.; Aurora, A.; Tanner, S.L.; Pace, T.B.; Acampora, K.B.; LaBerge, M. Increased Total Knee Arthroplasty Ultra-High Molecular Weight Polyethylene Wear Using a Clinically Relevant Hyaluronic Acid Simulator Lubricant. Proc. Inst. Mech. Eng. H 2006, 220, 609–623. [Google Scholar] [CrossRef]
- Sawae, Y.; Murakami, T.; Chen, J. Effect of synovia constituents on friction and wear of ultra-high molecular weight polyethylene sliding against prosthetic joint materials. Wear 1998, 216, 213–219. [Google Scholar] [CrossRef]






| Parameter | OA | Rev SF |
|---|---|---|
| Number of patients | 18 | 18 |
| Age (years) | 59 ± 13 (26–80) | 72 ± 9 (56–84) |
| Gender | ||
| Female | 6 (33 %) | 9 (50 %) |
| Male | 12 (67 %) | 9 (50 %) |
| KLS/Implant material of each component | Grade 2 (N = 7) Grade 3 (N = 7) Grade 4 (N = 4) | Femur: Co-28Cr-6Mo (N = 18) Tibia: Ti-6Al-4V (N = 10), Co-28Cr-6Mo (N = 8) Inlay: UHMWPE (N = 18) |
| Implantation time (years) | n.a. | 8.5 ± 6.4 (1–17) |
| Side of the joint | ||
| Left | 10 (56%) | 12 (67%) |
| Right | 8 (44%) | 6 (33%) |
| BMI (kg/m2) | 32.3 ± 6.6 (24.5–50.3) | 30.7 ± 5.2 (22.4–38.7) |
| Protein [g/L] | HA [g/L] | Number of Samples | |
|---|---|---|---|
| OA | 38.1 ± 8.9 (21.5–55.3) | 2.8 ± 1.1 (0.4–5.9) | N = 18 |
| Rev SF | 40.5 ± 9.8 (24.1–59.3) | 1.7 ± 1.5 (0.2–5.2) | N = 18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herbster, M.; Nizinkovskyi, R.; Bollmann, M.; Bartel, D.; Lohmann, C.H.; Krüger, M.; Halle, T.; Bertrand, J. Synthesis of a Lubricant to Mimic the Biorheological Behavior of Osteoarthritic and Revision Synovial Fluid. Lubricants 2021, 9, 87. https://doi.org/10.3390/lubricants9090087
Herbster M, Nizinkovskyi R, Bollmann M, Bartel D, Lohmann CH, Krüger M, Halle T, Bertrand J. Synthesis of a Lubricant to Mimic the Biorheological Behavior of Osteoarthritic and Revision Synovial Fluid. Lubricants. 2021; 9(9):87. https://doi.org/10.3390/lubricants9090087
Chicago/Turabian StyleHerbster, Maria, Rostyslav Nizinkovskyi, Miriam Bollmann, Dirk Bartel, Christoph H. Lohmann, Manja Krüger, Thorsten Halle, and Jessica Bertrand. 2021. "Synthesis of a Lubricant to Mimic the Biorheological Behavior of Osteoarthritic and Revision Synovial Fluid" Lubricants 9, no. 9: 87. https://doi.org/10.3390/lubricants9090087
APA StyleHerbster, M., Nizinkovskyi, R., Bollmann, M., Bartel, D., Lohmann, C. H., Krüger, M., Halle, T., & Bertrand, J. (2021). Synthesis of a Lubricant to Mimic the Biorheological Behavior of Osteoarthritic and Revision Synovial Fluid. Lubricants, 9(9), 87. https://doi.org/10.3390/lubricants9090087








