Tribocorrosion Influenced Pitting of a Duplex Stainless Steel
Abstract
1. Introduction
2. Materials and Methods
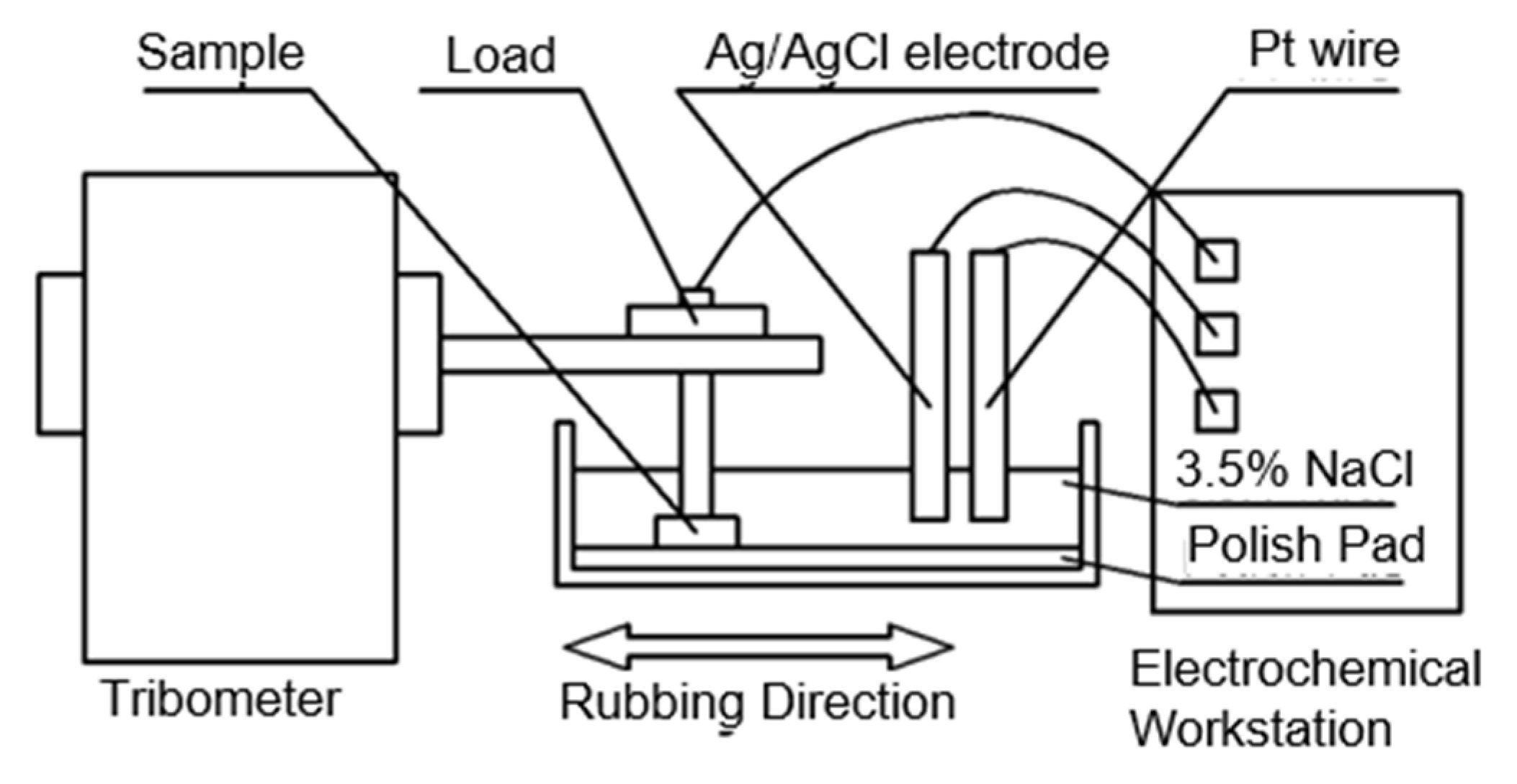
2.1. Experimental Procedure
2.2. Materials
3. Results and Discussion
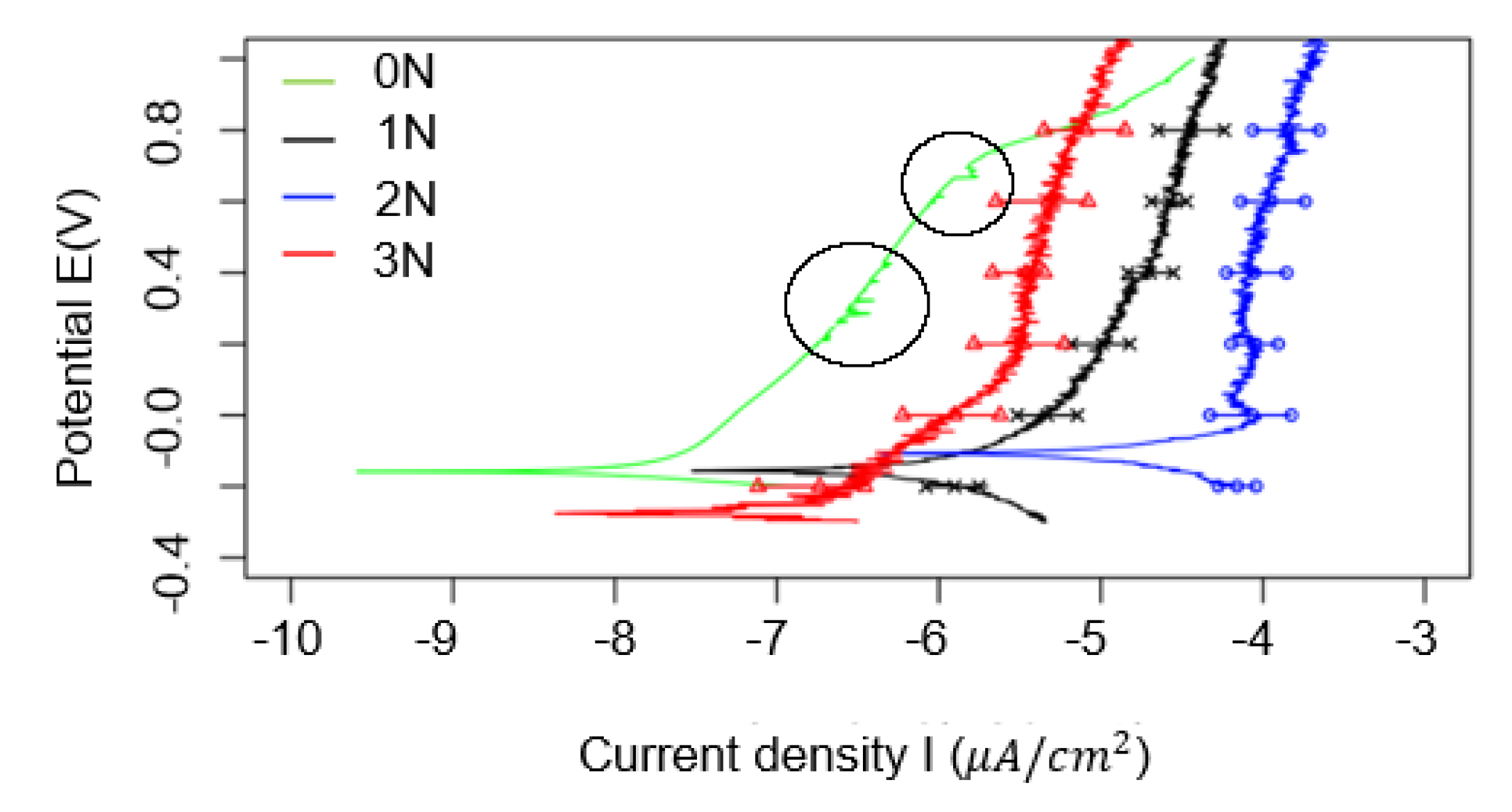
3.1. Passivation
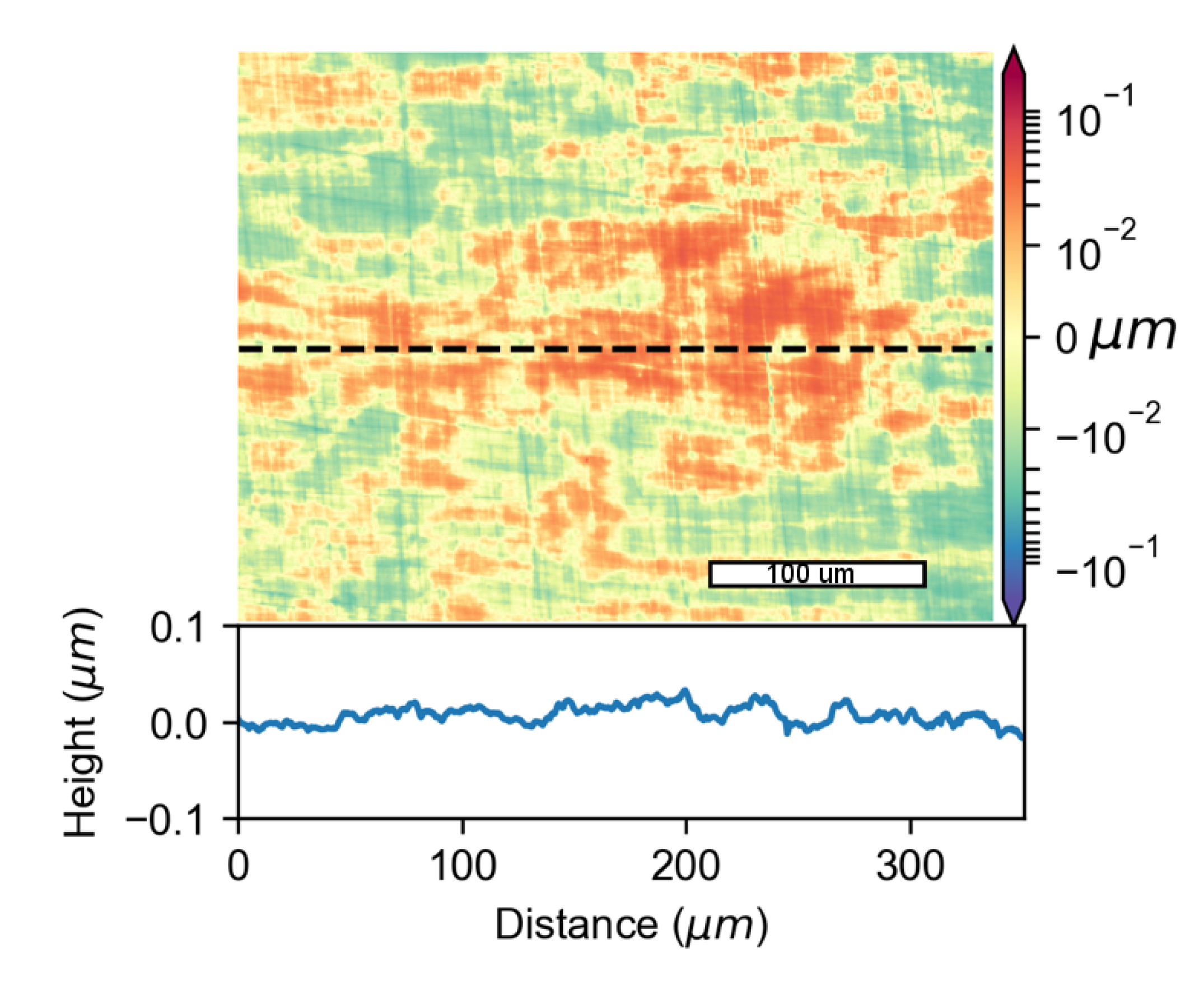
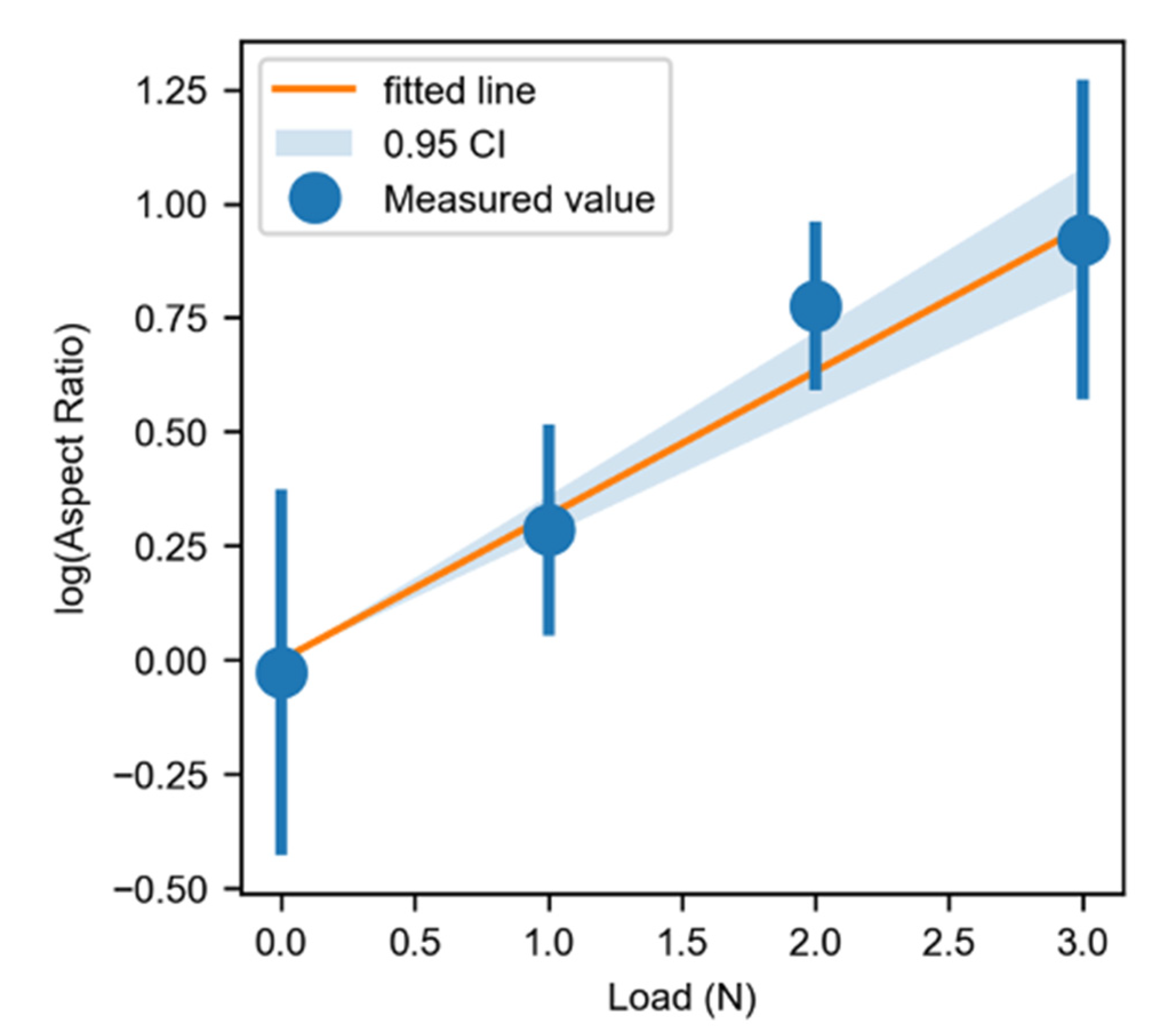
3.2. Formation of Pits
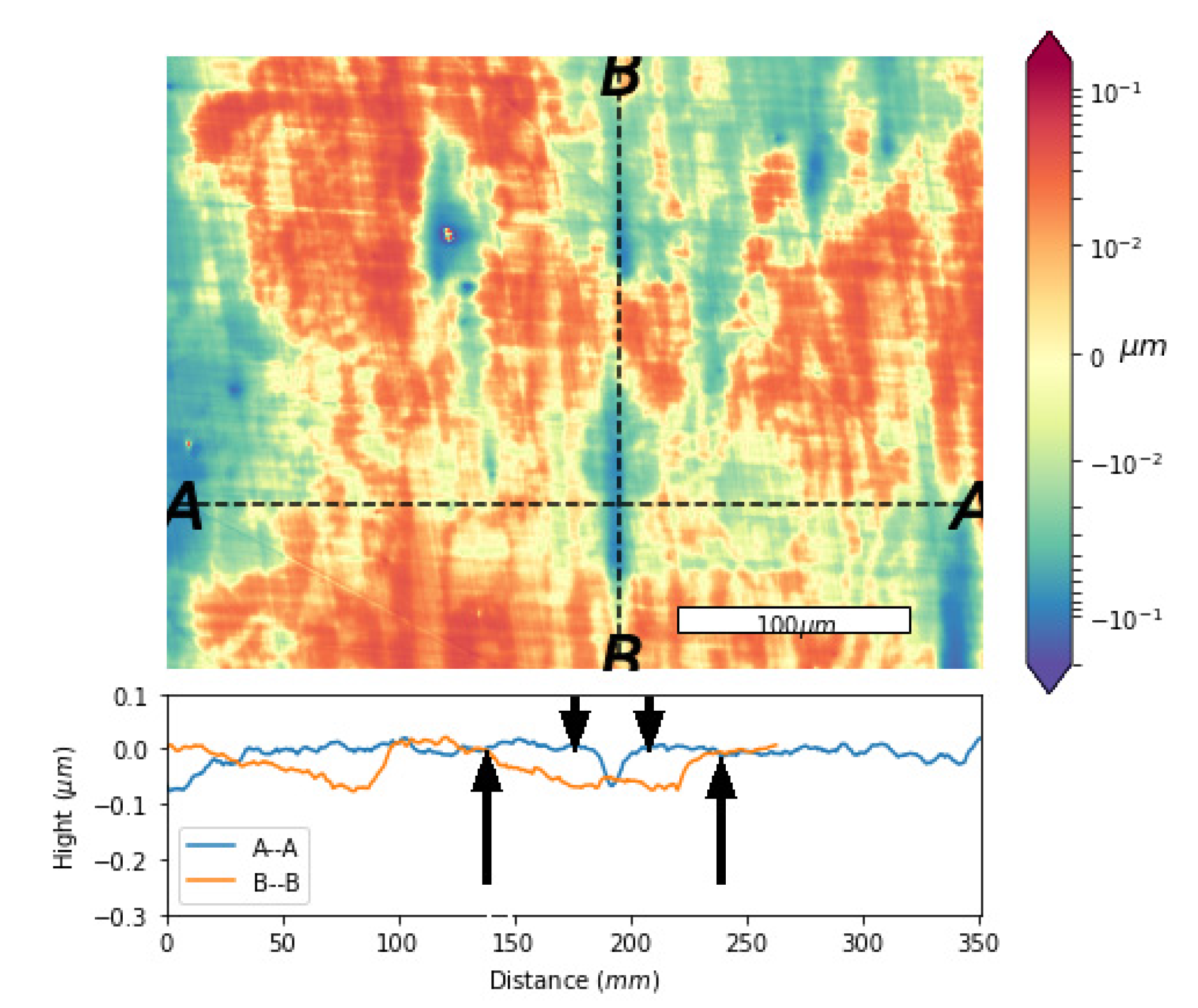
3.3. Pit Transformation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tuttle, R. Corrosion in Oil and Gas Production. J. Pet. Technol. 1987, 39, 756–762. [Google Scholar] [CrossRef]
- Kurtz, R.S. Oil Pipeline Regulation, Culture, and Integrity: The 2006 BP North Slope Spill. Public Integr. 2010, 13, 25–40. [Google Scholar] [CrossRef]
- Martínez, D.; Gonzalez, R.; Montemayor, K.; Juarez-Hernandez, A.; Fajardo, G.; Hernandez-Rodriguez, M. Amine Type Inhibitor Effect on Corrosion–Erosion Wear in Oil Gas Pipes. Wear 2009, 267, 255–258. [Google Scholar] [CrossRef]
- Barker, R.J.; Hu, X.; Neville, A.; Cushnaghan, S. Empirical Prediction of Carbon-Steel Degradation Rates on an Offshore Oil and Gas Facility: Predicting CO2 Erosion-Corrosion Pipeline Failures before They Occur. SPE J. 2014, 19, 425–436. [Google Scholar] [CrossRef]
- Hu, X.; Neville, A. CO2 Erosion–Corrosion of Pipeline Steel (API X65) in Oil and Gas Conditions—A Systematic Approach. Wear 2009, 267, 2027–2032. [Google Scholar] [CrossRef]
- Farrell, A.J.; Norton, B.; Kennedy, D.M. Corrosive Effects of Salt Hydrate Phase Change Materials Used with Aluminium and Copper. J. Mater. Process. Technol. 2006, 175, 198–205. [Google Scholar] [CrossRef]
- Hernandez-Rodriguez, M.; Martinez-Delgado, D.; Gonzalez, R.; Unzueta, A.P.; Mercado-Solís, R.; Rodriguez, J. Corrosive Wear Failure Analysis in a Natural Gas Pipeline. Wear 2007, 263, 567–571. [Google Scholar] [CrossRef]
- Mercer, W.E.; Hillis, J.E. The Critical Contaminant Limits and Salt Water Corrosion Performance of Magnesium AE42 Alloy; SAE Technical Paper; SAE 1992 International Congress and Exposition: Detroit, MI, USA, 1992. [Google Scholar]
- Bello, J.; Wood, R.; Wharton, J. Synergistic Effects of Micro-Abrasion–Corrosion of UNS S30403, S31603 and S32760 Stainless Steels. Wear 2007, 263, 149–159. [Google Scholar] [CrossRef]
- Yan, Y.; Neville, A.; Dowson, D.; Williams, S. Tribocorrosion in Implants—Assessing High Carbon and Low Carbon Co–Cr–Mo Alloys by in Situ Electrochemical Measurements. Tribol. Int. 2006, 39, 1509–1517. [Google Scholar] [CrossRef]
- Galliano, F.; Galvanetto, E.; Mischler, S.; Landolt, D. Tribocorrosion Behavior of Plasma Nitrided Ti–6Al–4V Alloy in Neutral NaCl Solution. Surf. Coat. Technol. 2001, 145, 121–131. [Google Scholar] [CrossRef]
- Benea, L.; Wenger, F.; Ponthiaux, P.; Celis, J.-P. Tribocorrosion Behaviour of Ni–SiC Nano-Structured Composite Coatings Obtained by Electrodeposition. Wear 2009, 266, 398–405. [Google Scholar] [CrossRef]
- Gao, F.; Liang, H. Effects of Potential and Mechanical Stimulation on Oxidation of Tantalum during Electrochemical Mechanical Polishing. J. Electron. Mater. 2012, 41, 624–631. [Google Scholar] [CrossRef]
- Gao, F.; Liang, H. Transformable Oxidation of Tantalum in Electrochemical Mechanical Polishing (ECMP). J. Electron. Mater. 2011, 40, 134–140. [Google Scholar] [CrossRef]
- Gao, F.; Liang, H. Material Removal Mechanisms in Electrochemical–Mechanical Polishing of Tantalum. Electrochim. Acta 2009, 54, 6808–6815. [Google Scholar] [CrossRef]
- Ng, D.; Sen, T.; Gao, F.; Liang, H. Friction and Wear-Mode Comparison in Copper Electrochemical Mechanical Polishing. J. Electrochem. Soc. 2008, 155, H520. [Google Scholar] [CrossRef]
- Ng, D.; Kulkarni, M.; Johnson, J.; Zinovev, A.; Yang, D.; Liang, H. Oxidation and Removal Mechanisms during Chemical–Mechanical Planarization. Wear 2007, 263, 1477–1483. [Google Scholar] [CrossRef]
- Kulkarni, M.; Greisen, D.; Ng, D.; Liang, H. New Approaches in Investigation of Removal Mechanisms during Copper Chemical-Mechanical Polishing. J. ASTM Int. 2006, 3, 1–7. [Google Scholar]
- Xu, G.H.; Liang, H. Effects of Electric Potential on Chemical-Mechanical Polishing of Copper. J. Electron. Mater. 2002, 31, 272–277. [Google Scholar] [CrossRef]
- Kar, P.; Asthana, P.; Liang, H. Formation and Characterization of Tribofilms. J. Tribol. 2008, 130. [Google Scholar] [CrossRef]
- Kar, P.; Wang, K.; Liang, H. Force-Dominated Non-Equilibrium Oxidation Kinetics of Tantalum. Electrochim. Acta 2008, 53, 5084–5091. [Google Scholar] [CrossRef]
- Kar, P.; Wang, K.; Liang, H. Oxidation of Tantalum with Mechanical Force. Electrochem. Solid State Lett. 2007, 11, C13. [Google Scholar] [CrossRef]
- Kulkarni, M.; Gao, F.; Liang, H. Chemical-mechanical polishing (CMP): A controlled tribocorrosion process. In Tribocorrosion of Passive Metals and Coatings; Elsevier: Amsterdam, The Netherlands, 2011; pp. 498–516, 517e–518e. [Google Scholar]
- Zhou, Y.; Engelberg, D.L. Fast Testing of Ambient Temperature Pitting Corrosion in Type 2205 Duplex Stainless Steel by Bipolar Electrochemistry Experiments. Electrochem. Commun. 2020, 117, 106779. [Google Scholar] [CrossRef]
- Chen, L.; Tan, H.; Wang, Z.; Li, J.; Jiang, Y. Influence of Cooling Rate on Microstructure Evolution and Pitting Corrosion Resistance in the Simulated Heat-Affected Zone of 2304 Duplex Stainless Steels. Corros. Sci. 2012, 58, 168–174. [Google Scholar] [CrossRef]
- Tan, H.; Jiang, Y.; Deng, B.; Sun, T.; Xu, J.; Li, J. Effect of Annealing Temperature on the Pitting Corrosion Resistance of Super Duplex Stainless Steel UNS S32750. Mater. Charact. 2009, 60, 1049–1054. [Google Scholar] [CrossRef]
- Potgieter, J. Influence of σ Phase on General and Pitting Corrosion Resistance of SAF 2205 Duplex Stainless Steel. Br. Corros. J. 1992, 27, 219–223. [Google Scholar] [CrossRef]
- Sriram, R.; Tromans, D. Pitting Corrosion of Duplex Stainless Steels. Corrosion 1989, 45, 804–810. [Google Scholar] [CrossRef]
- Fargas, G.; Mestra, A.; Mateo, A. Effect of Sigma Phase on the Wear Behavior of a Super Duplex Stainless Steel. Wear 2013, 303, 584–590. [Google Scholar] [CrossRef]
- Paro, J.; Hänninen, H.; Kauppinen, V. Tool Wear and Machinability of HIPed P/M and Conventional Cast Duplex Stainless Steels. Wear 2001, 249, 279–284. [Google Scholar] [CrossRef]
- Pereira Neto, J.O.; da Silva, R.O.; da Silva, E.H.; Moreto, J.A.; Bandeira, R.M.; Manfrinato, M.D.; Rossino, L.S. Wear and Corrosion Study of Plasma Nitriding F53 Super Duplex Stainless Steel. Mater. Res. 2016, 19, 1241–1252. [Google Scholar] [CrossRef]
- Hunter, J.D. Matplotlib: A 2D Graphics Environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Seabold, S.; Perktold, J. Statsmodels: Econometric and Statistical Modeling with Python. In Proceedings of the 9th Python in Science Conference (SciPy), Austin, TX, USA, 28–30 July 2010; p. 61. [Google Scholar]
- Liu, Z.; Dong, C.; Li, X.; Zhi, Q.; Cheng, Y. Stress Corrosion Cracking of 2205 Duplex Stainless Steel in H2S–CO2 Environment. J. Mater. Sci. 2009, 44, 4228–4234. [Google Scholar] [CrossRef]
- Park, C.; Rao, V.S.; Kwon, H.-S. Effects of Sigma Phase on the Initiation and Propagation of Pitting Corrosion of Duplex Stainless Steel. Corrosion 2005, 61, 76–83. [Google Scholar] [CrossRef]
- Moura, V.; Lima, L.; Pardal, J.; Kina, A.; Corte, R.; Tavares, S. Influence of Microstructure on the Corrosion Resistance of the Duplex Stainless Steel UNS S31803. Mater. Charact. 2008, 59, 1127–1132. [Google Scholar] [CrossRef]
- Nilsson, J.-O.; Karlsson, L.; Andersson, J.-O. Secondary Austenite for Mation and Its Relation to Pitting Corrosion in Duplex Stainless Steel Weld Metal. Mater. Sci. Technol. 1995, 11, 276–283. [Google Scholar] [CrossRef]
- Garfias-Mesias, L.; Sykes, J.; Tuck, C. The Effect of Phase Compositions on the Pitting Corrosion of 25 Cr Duplex Stainless Steel in Chloride Solutions. Corros. Sci. 1996, 38, 1319–1330. [Google Scholar] [CrossRef]

| Parameter | Value |
|---|---|
| Load | 0, 1, 2, 3N |
| Speed | 0.5 cm/s (max) |
| Distance | 3 cm |
| Contact area | 1 cm2 |
| Temperature | 24 °C |
| Parameter | Value |
|---|---|
| Potential (vs. Vref) | −0.2 V to 1.2 V |
| Scanning rate | 2 mV/s |
| Sample period | 700 s |
| Conditioning time | 60 s |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renner, P.; Chen, Y.; Huang, Z.; Raut, A.; Liang, H. Tribocorrosion Influenced Pitting of a Duplex Stainless Steel. Lubricants 2021, 9, 52. https://doi.org/10.3390/lubricants9050052
Renner P, Chen Y, Huang Z, Raut A, Liang H. Tribocorrosion Influenced Pitting of a Duplex Stainless Steel. Lubricants. 2021; 9(5):52. https://doi.org/10.3390/lubricants9050052
Chicago/Turabian StyleRenner, Peter, Yan Chen, Zhihao Huang, Ajinkya Raut, and Hong Liang. 2021. "Tribocorrosion Influenced Pitting of a Duplex Stainless Steel" Lubricants 9, no. 5: 52. https://doi.org/10.3390/lubricants9050052
APA StyleRenner, P., Chen, Y., Huang, Z., Raut, A., & Liang, H. (2021). Tribocorrosion Influenced Pitting of a Duplex Stainless Steel. Lubricants, 9(5), 52. https://doi.org/10.3390/lubricants9050052






