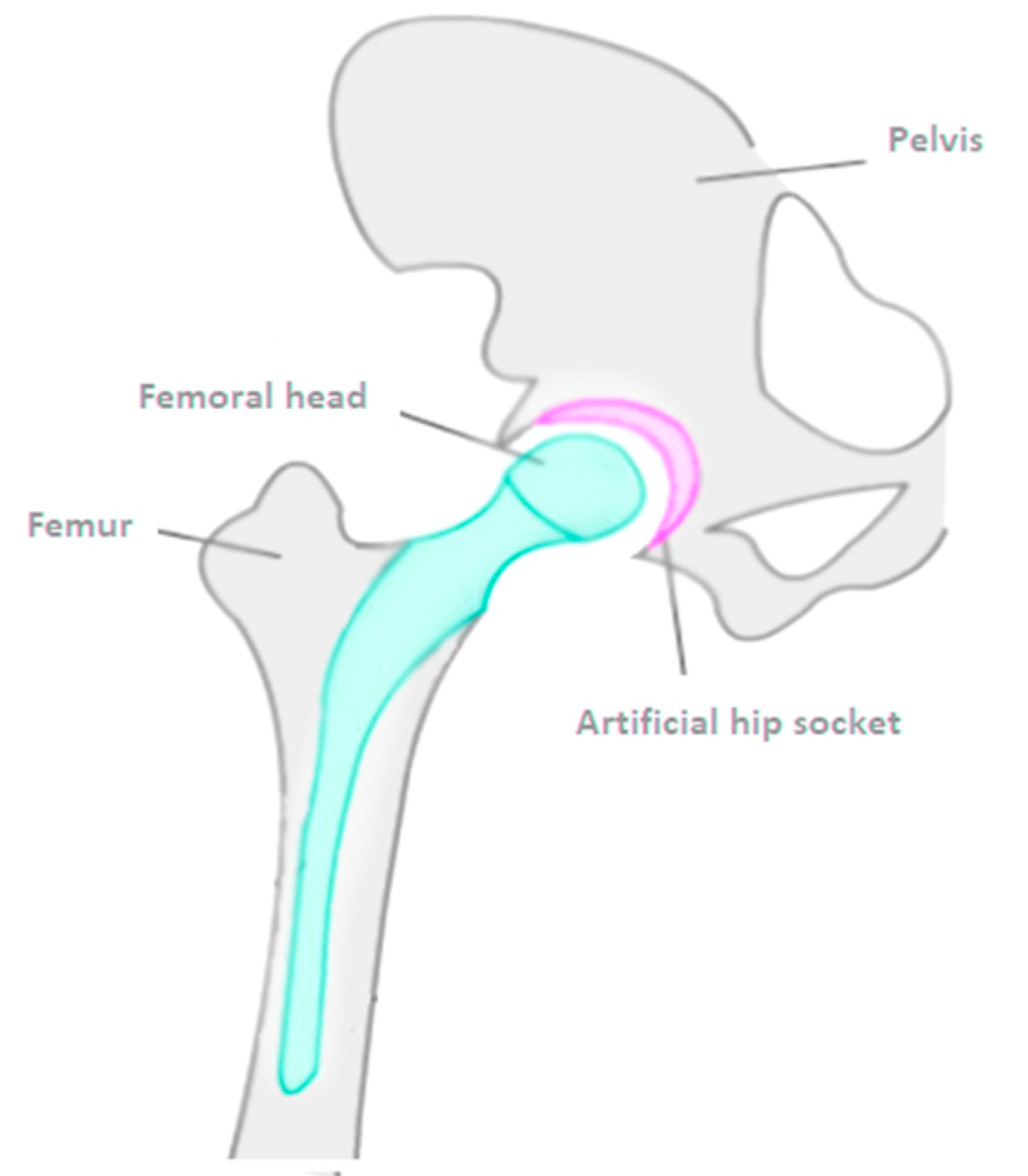
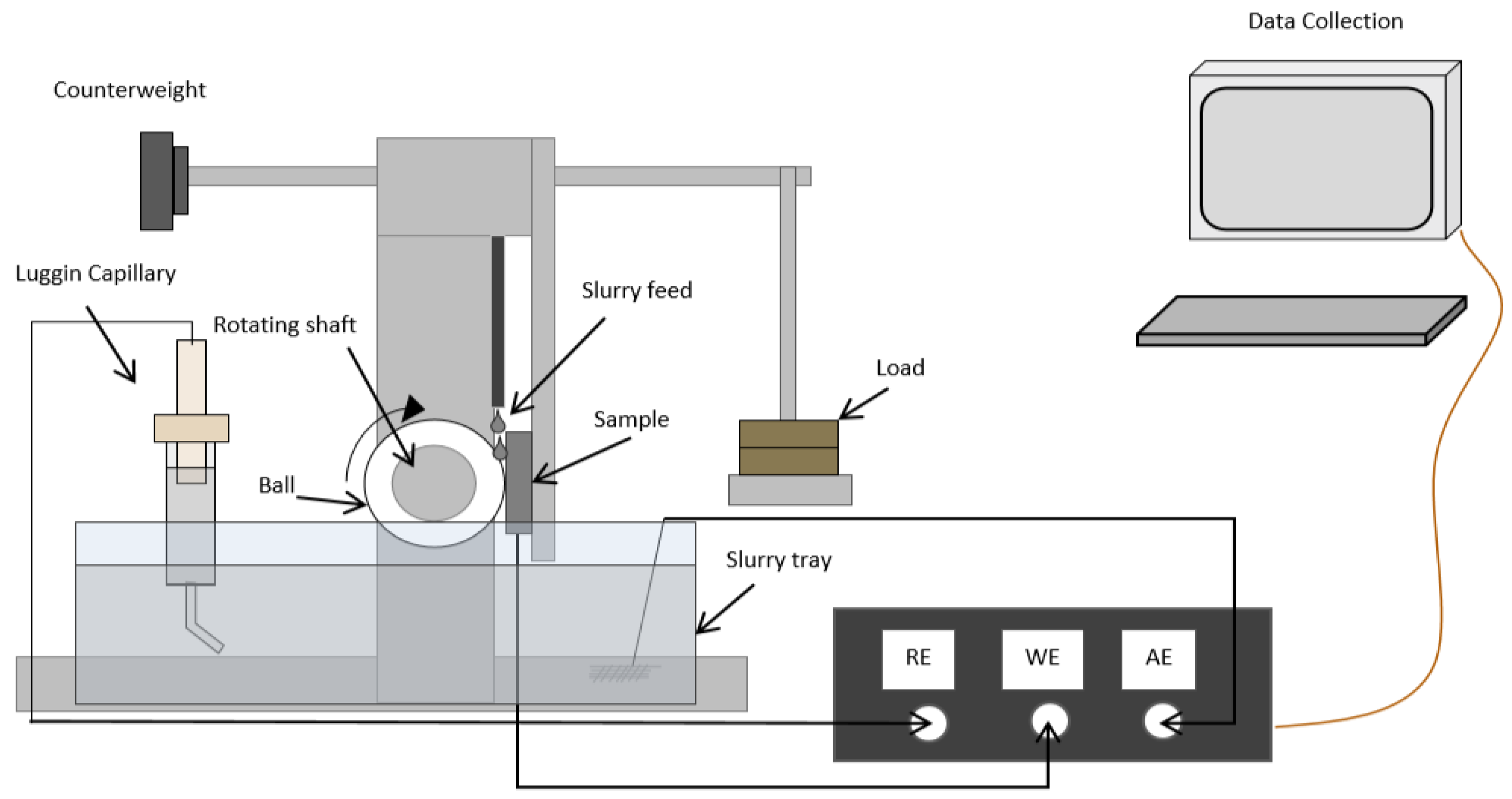
3.1. Polarisation Curves
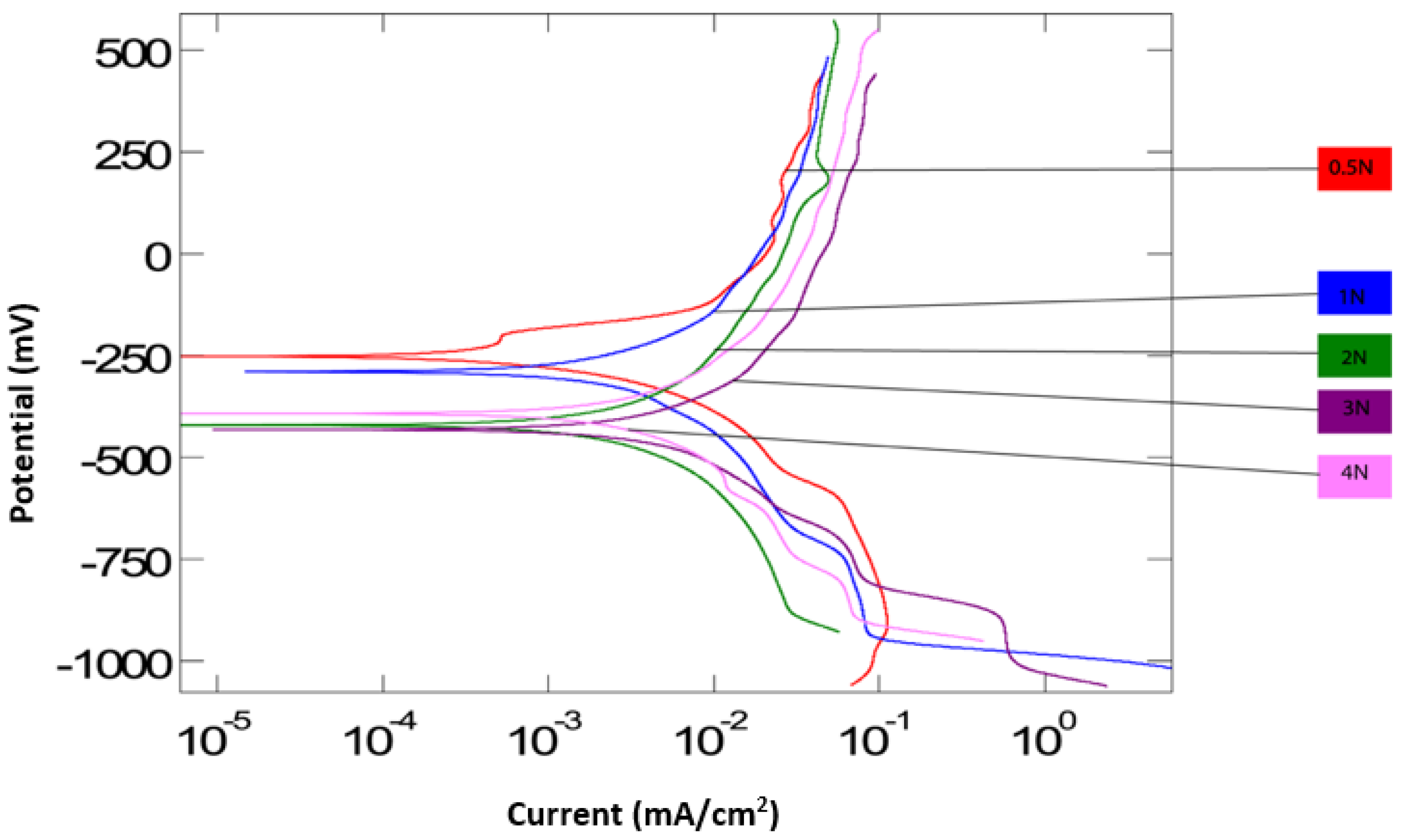
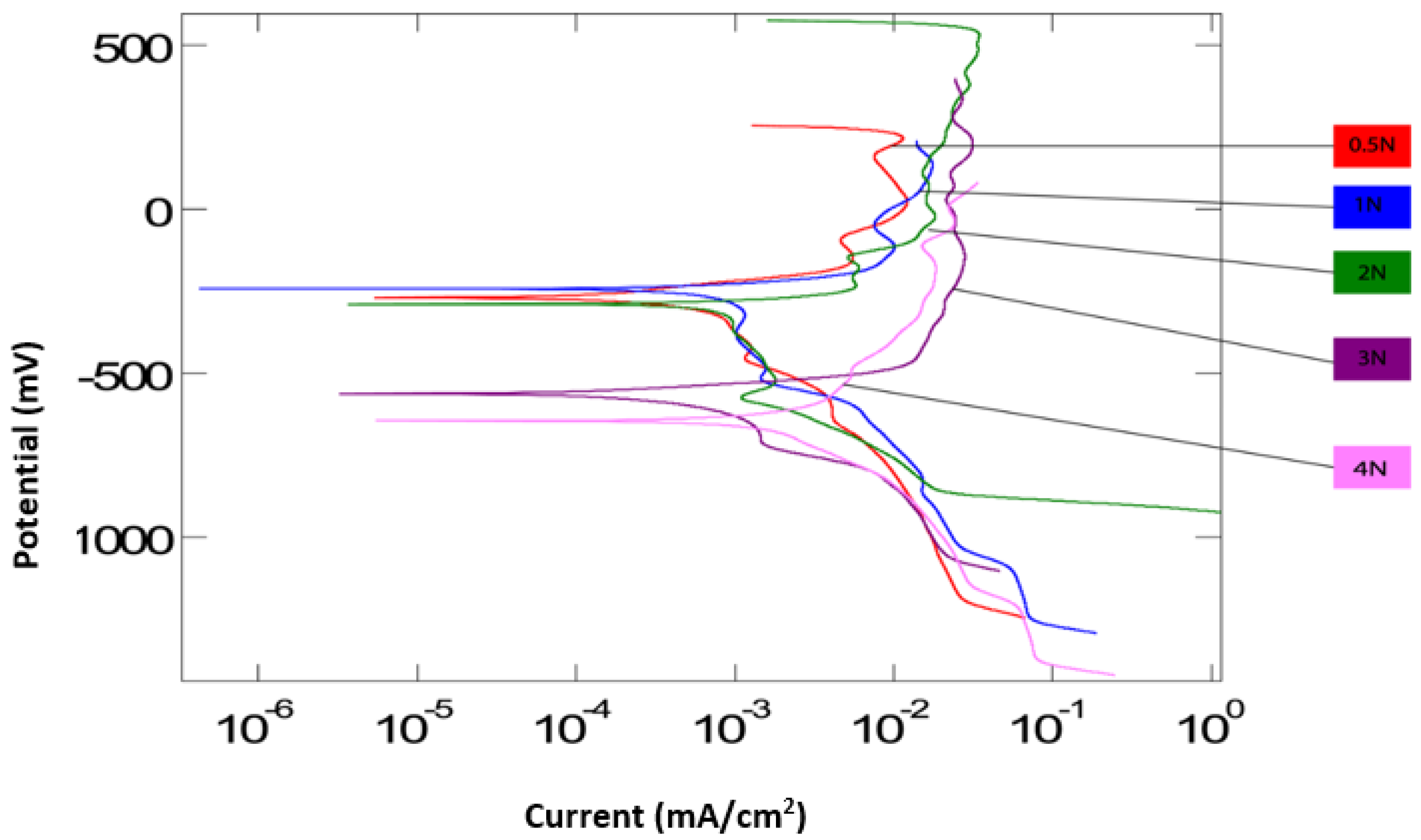
Figure 3 and
Figure 4 are the polarization curves generated from the potentiodynamic tests for all applied loads, both without and with particles. On average, the corrosion potential (
Ecorr) for the tests with particles is lower than the tests without particles. This is supported by both tests at 3 N and 4 N, having values below −500 mV. This suggests that corrosion begins to take place at lower potentials when with an increase in load in the presence of abrasive particles. This could be due to abrasive particles accelerating the removal of a protective oxide layer from the surface of the Ti-6AL-4V, hence the corrosion process begins earlier, at 3 N and 4 N. Both curves suggest that the surface electrochemical activity is reduced at lower applied loads. The polarization curve at 0.5 N applied load for both conditions is observed to remain towards a corrosion potential of around −250 mV, indicating the lowest electrochemical activity.
Figure 3 displays more uniformity in
Ecorr values for all loads in the absence of abrasive particles. In the case of the solution without abrasives,
Ecorr appears to have remained relatively unchanged over the range of applied loads, unlike the solution with particles. This indicates that the corrosion potential is more unpredictable in the presence of particles.
The corrosion current densities are relatively similar in both, suggesting that the corrosion rate is the same in the presence of abrasive particles as it is in the absence of abrasive particles. However, as the load increased for the abrasive solution, the maximum currents tended to increase. The highest current densities were recorded for 2 and 4 N, suggesting an increasing corrosion at those loads. As the applied load reached 4 N in the case of abrasives, a cathodic shift is observed. Over the spectrum of applied loads, it appears that electrochemical activity at the contact surface may be enhanced at higher loading; it is likely that a transition in lubrication mode occurs as a result of changing the contact pressure, which may explain the cathodic shift at 4 N.
3.2. Mass Loss
Mass loss data was produced following an established method [
21,
22]. The total mass of material lost due to micro-abrasion-corrosion,
Kac (g), is equal to the sum of mass lost due to micro-abrasion,
Ka (g), and the mass loss due to corrosion,
Kc (g).
The total mass loss (
Kac) was calculated by multiplying the volume of the wear scar (
V) by the density of the material. The mass loss volumes were estimated using a standard technique for measuring wear scar of spherical geometry. It is assumed that the shape of the crater conforms to the shape of the ball [
23]. Wear volume (
V) may be calculated by the measurement of the crater diameter (
b) and
R the ball radius, as shown below:
Mass loss due to corrosion (
Kc) was estimated using Faraday’s Law, as follows:
Equation (3) may also be written as:
where
Q is the charge passed (C); M is the atomic mass of the test material,
Icorr is the corrosion current density (mA/cm
2),
t is the test duration,
Z is the number of valence electrons in the test and
F is Faraday’s constant (96,500 C·mol
−1).
Micro-abrasion weight loss (
Ka) can be further divided up into pure micro-abrasion weight loss (
Kao) and the synergistic effect of corrosion on the micro-abrasion (Δ
Ka):
The pure micro-abrasion mass loss (
Kao) was calculated using Equation (2); the material density and the wear scars from the pure micro-abrasion tests (cathodic conditions at −960 mV) for each applied load. Corrosion mass loss (
Kc) can similarly be divided into pure corrosion mass loss (
Kco) and the additive effect of micro-abrasion on the corrosive mass loss (Δ
Kc):
Approximate values of pure corrosion weight loss (Kco) were calculated using Equation (3) and the current density values from the polarization curves with and without particles for every applied load.
When the synergy components have a negative value, it is termed “antagonistic”, as the two wear mechanisms work against each other instead of together. Therefore, the total micro-abrasion-corrosion mass loss can be represented by the following equation:
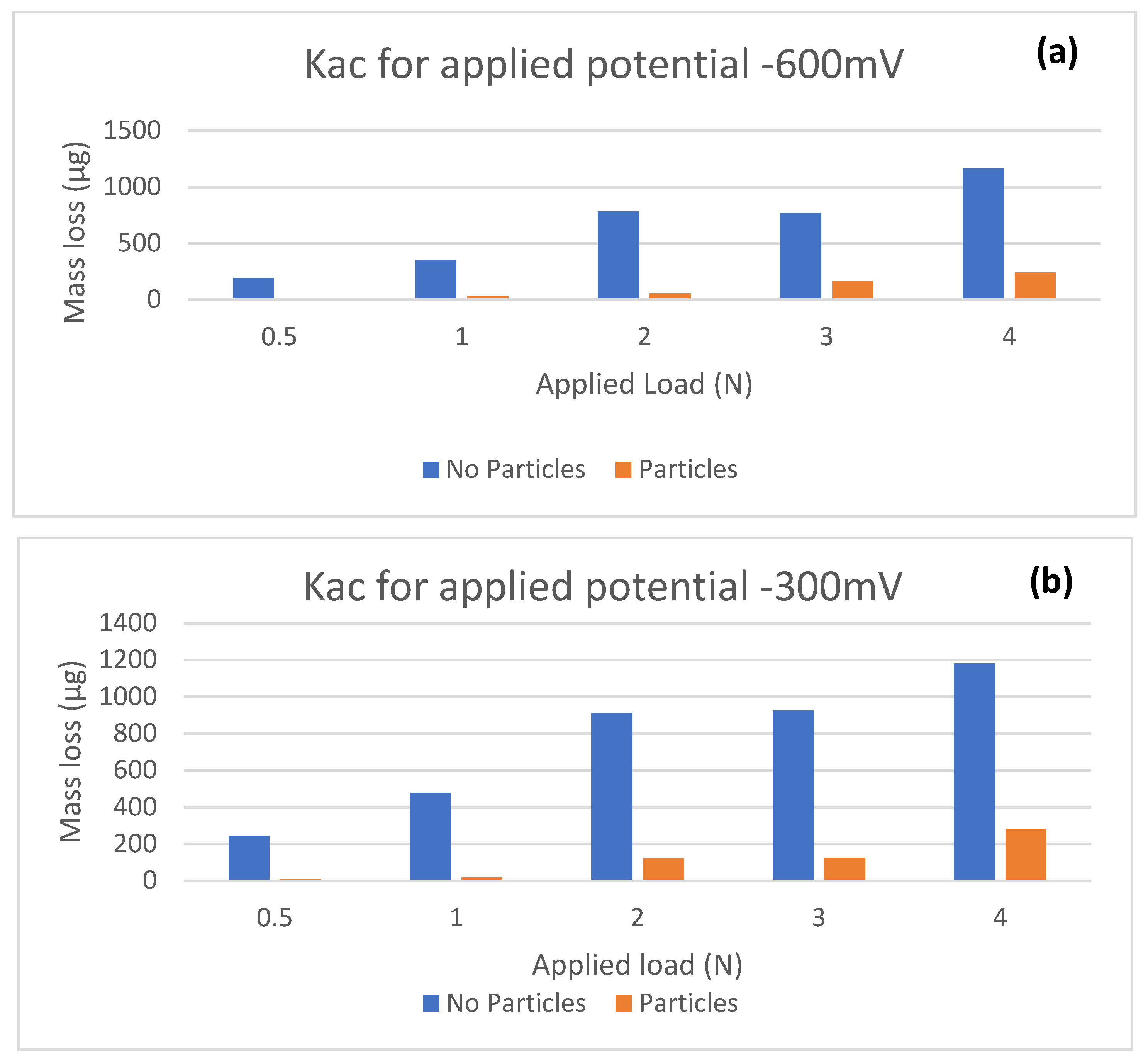
The mass loss results using this analysis are shown in
Table 5 and
Table 6. Total mass losses, with and without particles in Ringer’s solution, are shown in
Figure 5.
Table 5 and
Table 6 show that, for every test condition, the total and micro-abrasion mass loss values are very higher in magnitude for tests without solid particles, whilst the corrosion mass loss is significantly lower for both, without and with solid particles. The corrosion contribution is small, but has been shown to play a critical role in the wear synergism. It can be seen clearly that the electrochemical mass loss (
Kc) remains a consistent fraction of the total mass loss contribution (
Kac). This is consistent with previous papers [
12,
20,
24] and should be expected, since Ti-6Al-4V is a highly corrosion resistant material. The majority of
Kc/
Ka values are less than 0.1, which indicates that micro-abrasion is the dominant wear regime in both conditions. At an applied potential of −600 mV with particles present, there appears to be a relationship between the mass loss due to abrasion, and mass loss due to corrosion. As the rate of micro-abrasion increases, the rate of corrosion also appears to intensify. This would indicate that, as the rate of micro-abrasion increases, the rate at which a protective passive layer is removed also increases, thus allowing for increased corrosive wear. However, this trend is not visible across all potentials, suggesting that the material is perhaps more vulnerable to corrosive wear at this specific corrosion potential.
The highest recorded value for corrosion occurs with particles present, indicating that mechanical abrasion as a result of the presence of particles may disrupt and remove this film, leading to increased corrosion. The values for abrasive wear loss are considerably higher for this study; approximately ten times more than previously recorded for Ti-6Al-4V in a bio-simulated environment [
13,
25]. This is likely due to the ceramic counterpart. The Al
2O
3 ball has a significantly higher Vickers hardness value in comparison to the polyethene counterpart (UHMWPE) used in both these previous studies. This can be assumed that abrasive wear could be accelerated in a ceramic/metal pairing, potentially reducing the hip implant lifetime. However, in this case, tests results show a different scenario, i.e., micro-abrasion wear significantly reduced in the presence of abrasive
Table 4 and
Table 5.
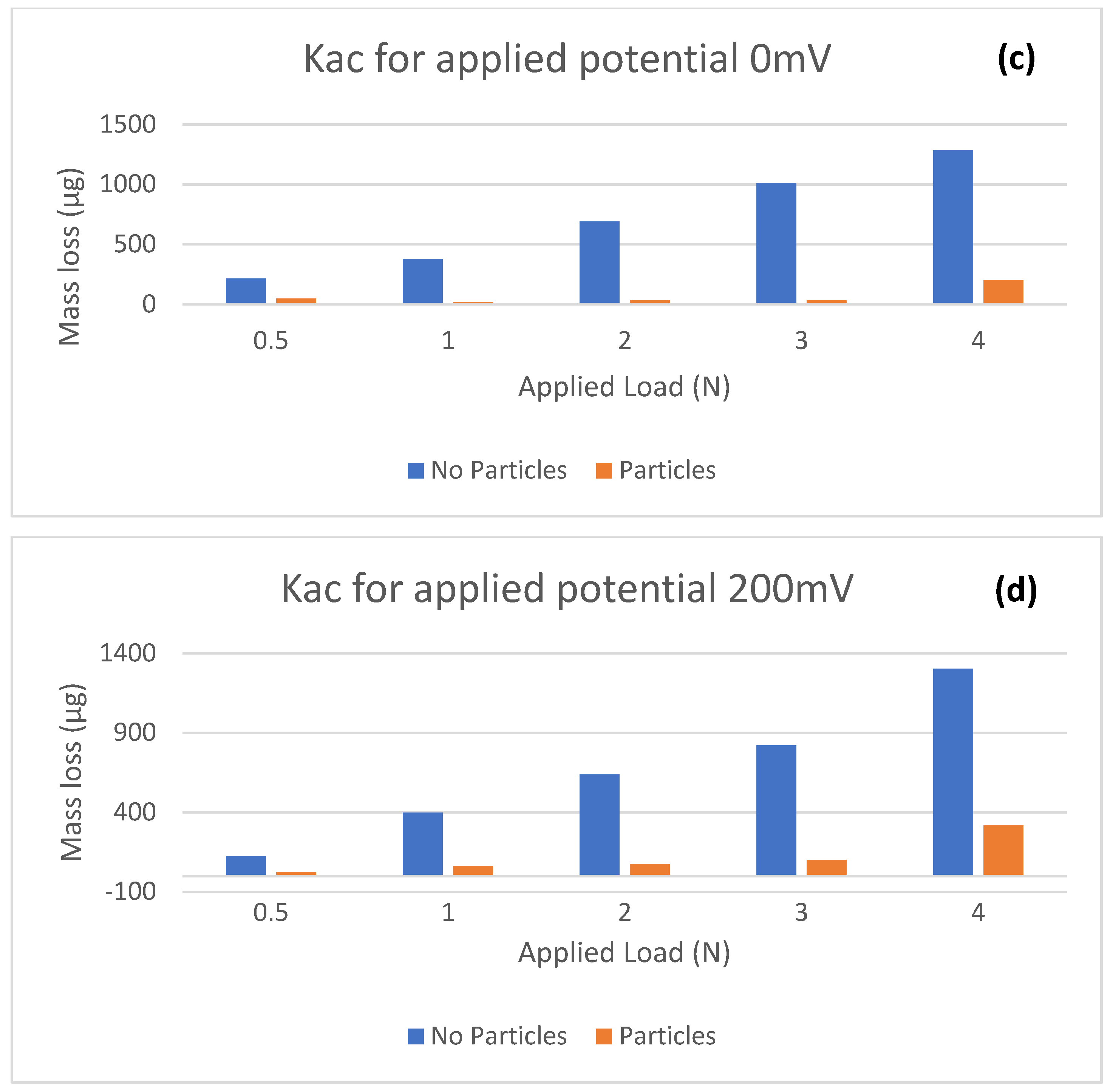
According to
Figure 5, the mass loss (
Kac) is considerably higher for the solution without abrasives across all applied potentials. This suggests that the two-body abrasion is the dominant wear mode in the case without particles. This reinforces that two-body abrasion is the more severe mode of wear. In the presence of SiC, hard solid particles were embedded in the contact area of the specimens to form a composite layer, thus reducing the total mass loss. It can also be seen that mass losses in both solutions tended to increase as applied load was increased; consistent with Archard’s wear model, which states that the wear rate of the material is proportional to the applied load and sliding distance.
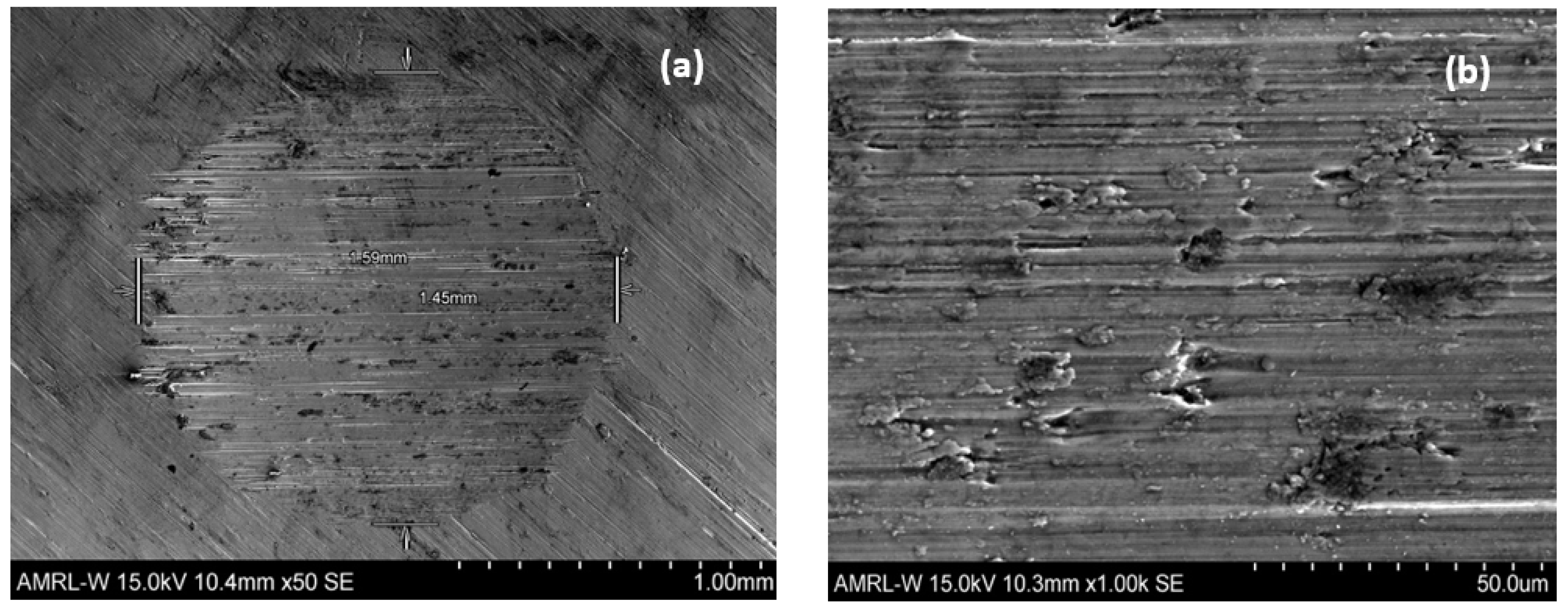
3.3. SEM Images of Wear Scars
Figure 6 and
Figure 7 allow for a comparison of different applied loads, with and without abrasive particles. In
Figure 6a,b, there is clear evidence of directional grooving, indicating a uniform dominant two-body abrasion. However, in the presence of particles
Figure 7a,b, there is no evidence of this phenomenon. Wear scars are showing embedded abrasive particles and plastic deformation, suggesting the formation of a composite layer, which results in a reduction in abrasive mass loss due to the presence of solid particles wear scars. This is consistent with the conclusions reached from the mass loss data in
Figure 5. The diameter is also almost double in the absence of abrasive particles, indicating that two-body abrasive wear is more severe than that in the presence of solid particles.
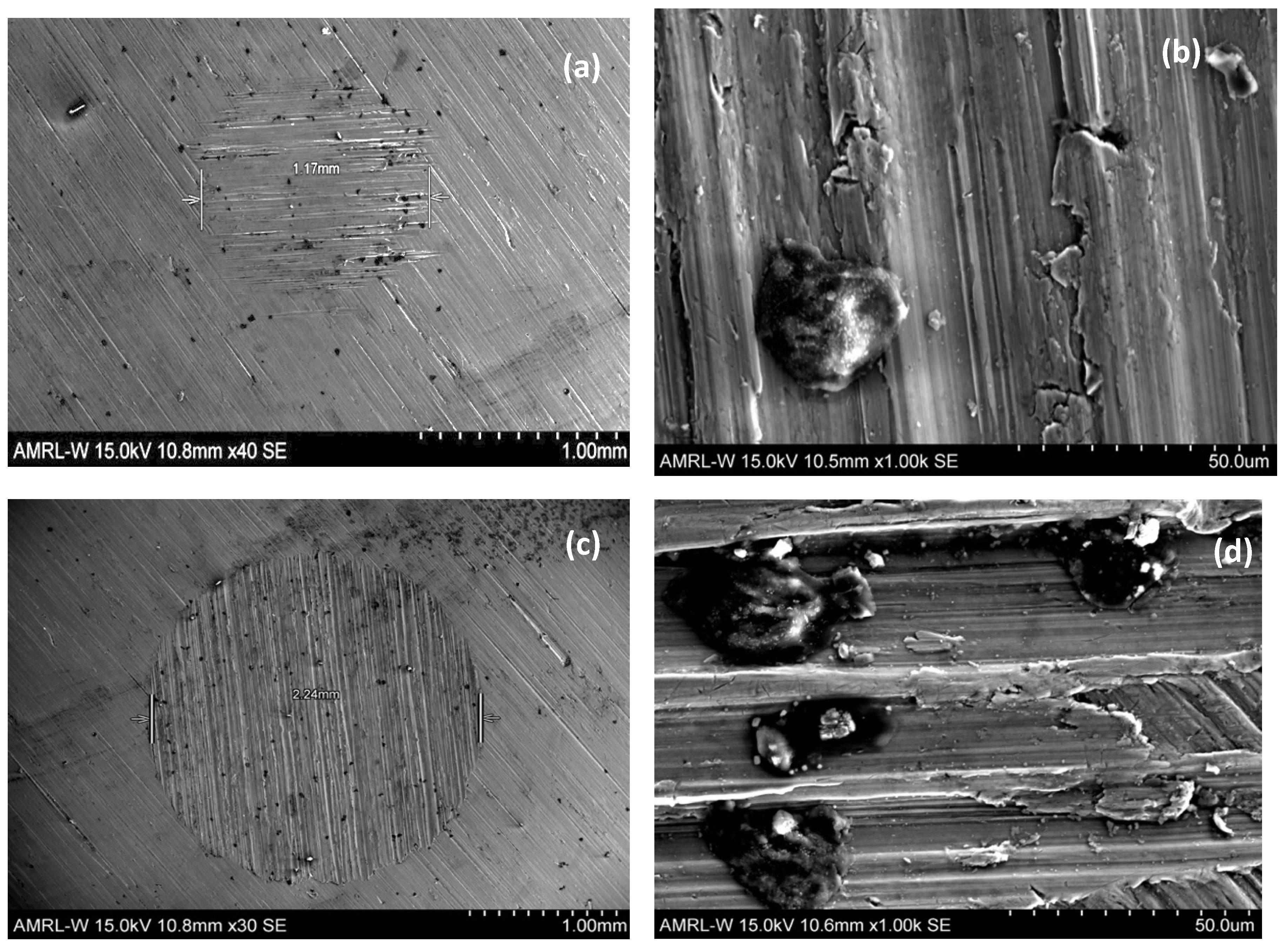
Figure 6c,d show how the change in applied load can affect the type of wear that is produced and how, at some intermediate load, a transition must take place between the wear mechanisms. In these figures, the exaggeration of the parallel grooves as the applied load is increased is visible. This suggests a transition to a two-body dominated regime as the particles become entrained in the cratering ball, resulting in a grooving. Additionally, the reduction of surface ridges could contribute to this transition. In
Figure 7a–d, fine SiC particles appear embedded across wear surface, including larger agglomerated particles, signifying that a deposition effect occurs across a range of applied loads. An increased number of agglomerated particles are present at the higher load, suggesting that there is a positive relationship between organic deposition and applied load. From these microscopy results, it can also be noted that the substrate material did not exhibit any form of uninform corrosion or pitting, again demonstrating the corrosion resistance of grade 23 Ti-6Al-4V alloy.
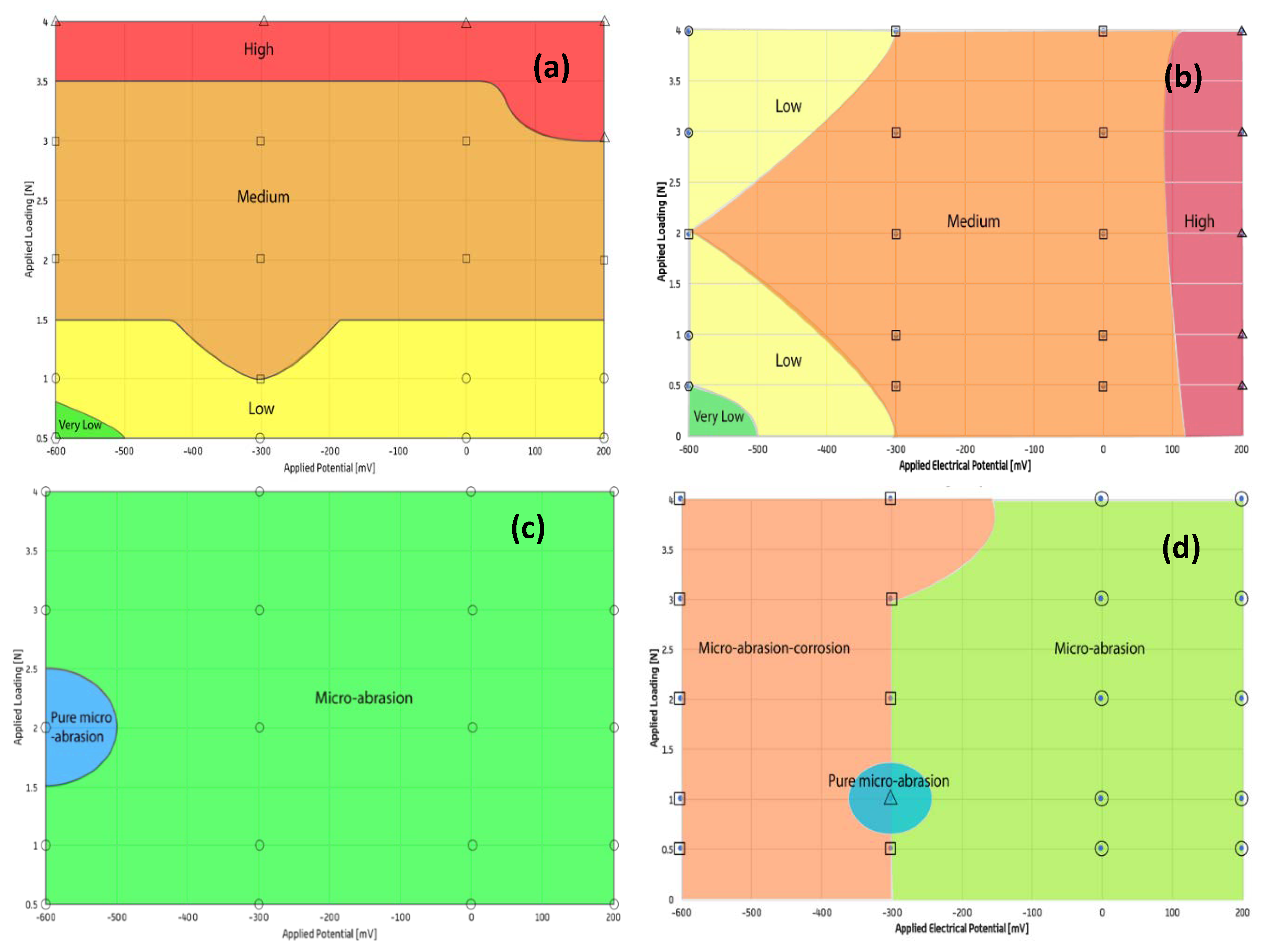
3.4. Wear Maps
A series of tribo-corrosion maps have been constructed for Ti-6Al-4V in Ringer’s solution. Three categories of maps were constructed: wastage, mechanism and synergy maps. The maps were drawn by plotting the results from potentiostatic tests (in the presence and absence of abrasive particles) on a chart and interpolating between the points to determine the boundary lines. It was assumed that the wear results varied linearly between each condition. Previously developed mapping techniques and their defined categories [
20,
24,
26] were adapted as follows (
Table 7):
The wastage map in the absence of abrasive particles
Figure 8a demonstrates that the applied electrical potential clearly has no significant effect on the total wear compared to the applied load. This is due to the fact that the amount of material loss caused by corrosion was much lower than micro-abrasion.
The wastage map in the presence of abrasive particles
Figure 8b shows that, over the cathodic range of potentials, the wastage regimes remain low despite increasing load. In the cathodic region at loads below 1 N, no significant corrosive effects appear to take place. In comparison to an earlier study [
12], there is some similarity, where a high wastage regime dominates for increasing loads with anodic potentials. The map demonstrates that the low-medium wastage regime is dominant. As a result, any variation in material loss due to corrosion had no significant effect on total wear in both cases (with and without particles). This is further evidenced by the highest wastage occurring at 4 N and the lowest wastage occurring at a load of 0.5 N
Figure 5.
The mechanism map of Ti-6Al-4V without particles,
Figure 8c, is entirely micro-abrasion dominated, reinforcing the titanium alloys strong corrosion resistance. The mechanism map in the presence of particles
Figure 8d is also micro-abrasion dominated, but displays different behaviour at cathodic potentials. At an electrical potential of -600 mV and for the range of applied loads, a micro-abrasion-corrosion region is present. This could be due to a deposition effect on the surface, as the system is cathodic, and the fact that oxide reduction occurred at low electrical potentials and low applied loads.
Moreover, from the results in
Table 6, at −600 mV, despite the highest ratio of corrosion to micro-abrasion at 0.5 N, micro abrasion is still significantly greater than corrosion for the range of applied loads. The presence of a micro-abrasion corrosion regime at lower potentials is inconsistent with previous papers [
12]. During tests, a constant stream of solution could not always be achieved and may have been a source of inconsistency among the tests, as the variation in forces on the sample could have affected the wear mechanisms.
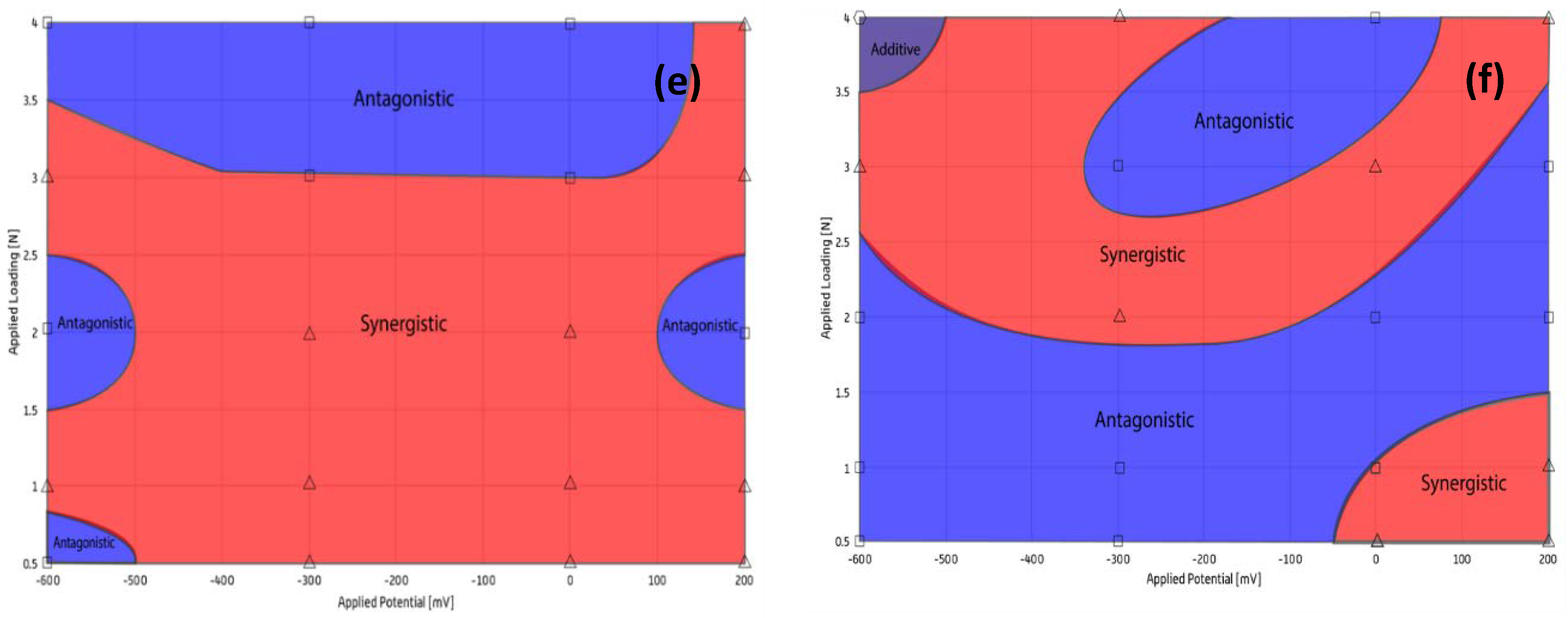
In the non-abrasive synergy map
Figure 8e generally, as the applied load is increased, the wear tends to change from a synergistic regime to an antagonistic regime. This could be the result of oxide layers forming a protective barrier to wear, which is then mechanically removed at higher loads, which has been observed in previous corrosion studies [
21,
25,
27]. The synergy map for the abrasive solution
Figure 8f has a significant antagonistic coverage in comparison to
Figure 8e, where abrasive particles are not present. Possible reasons for this could be increased solution viscosity, improving the lubrication regime and reducing the effect of corrosion–wear due to additional particles. In addition, hard SiC particles are likely to have been embedded on the surface of the softer specimen; therefore, underlying a transition to a two-body abrasion regime towards the center of the contact zone [
21]. In Tribo-Corrosion, when the wear mechanism is in the antagonistic wear regime, i.e., micro-abrasion and corrosion in this case, work against each other, instead of together. Synergistic maps,
Figure 8e,f, suggest that the antagonistic behaviour of micro-abrasion and corrosion is dominated in both cases, i.e., with and without abrasive particles.