Tribocorrosion Behaviour of Ti6Al4V Produced by Selective Laser Melting for Dental Implants
Abstract
1. Introduction
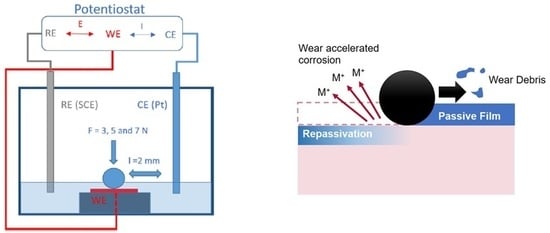
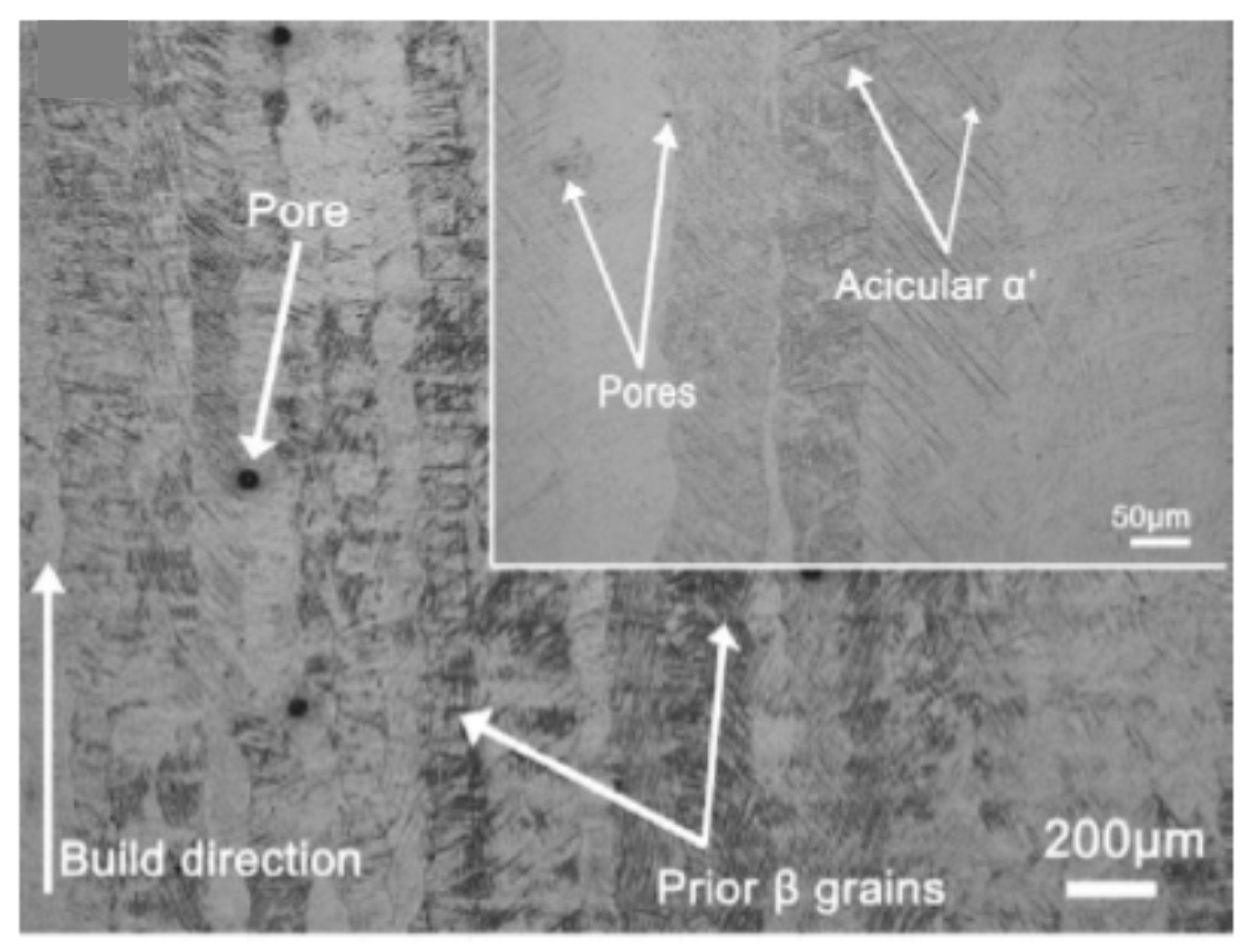
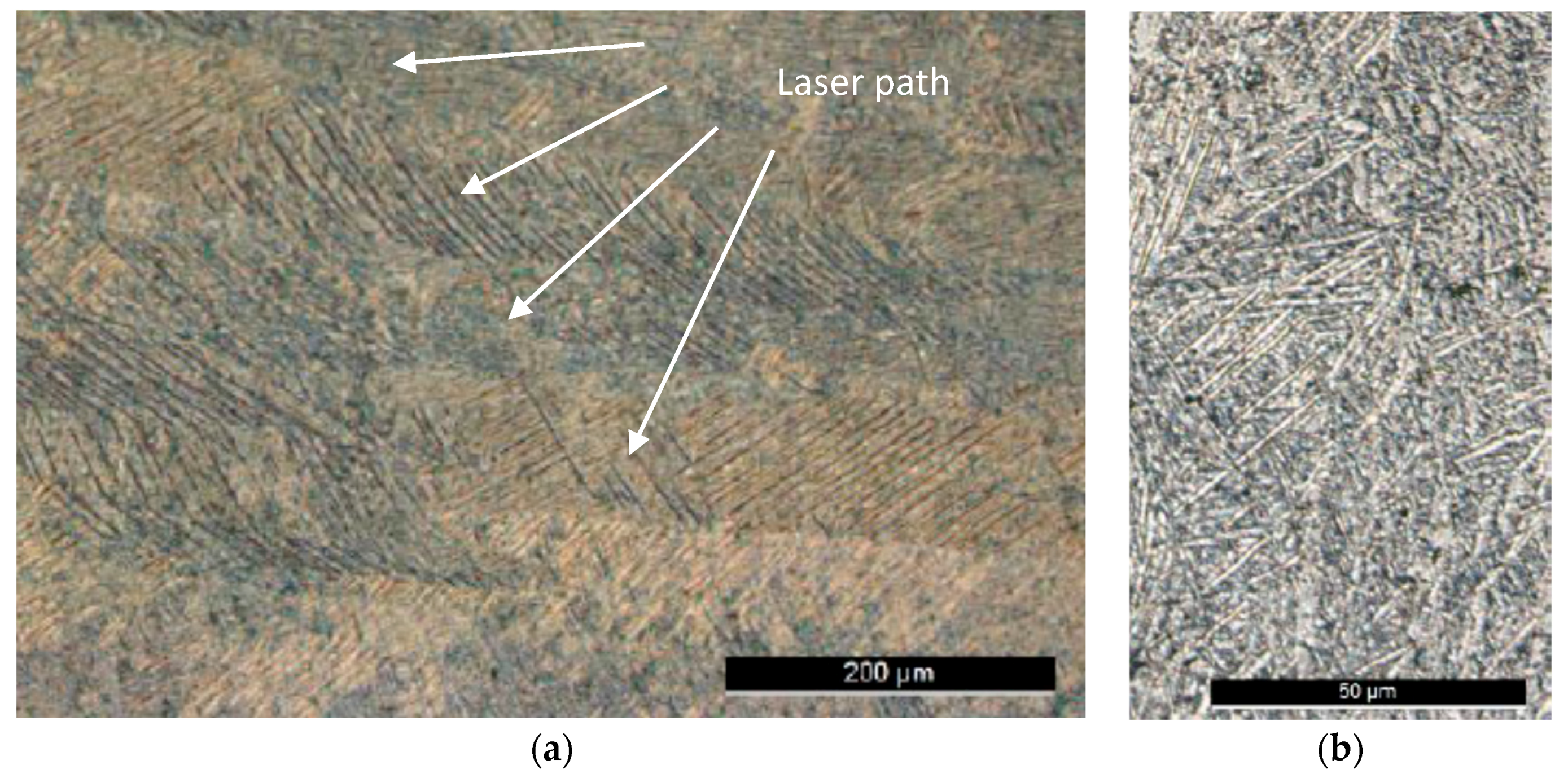
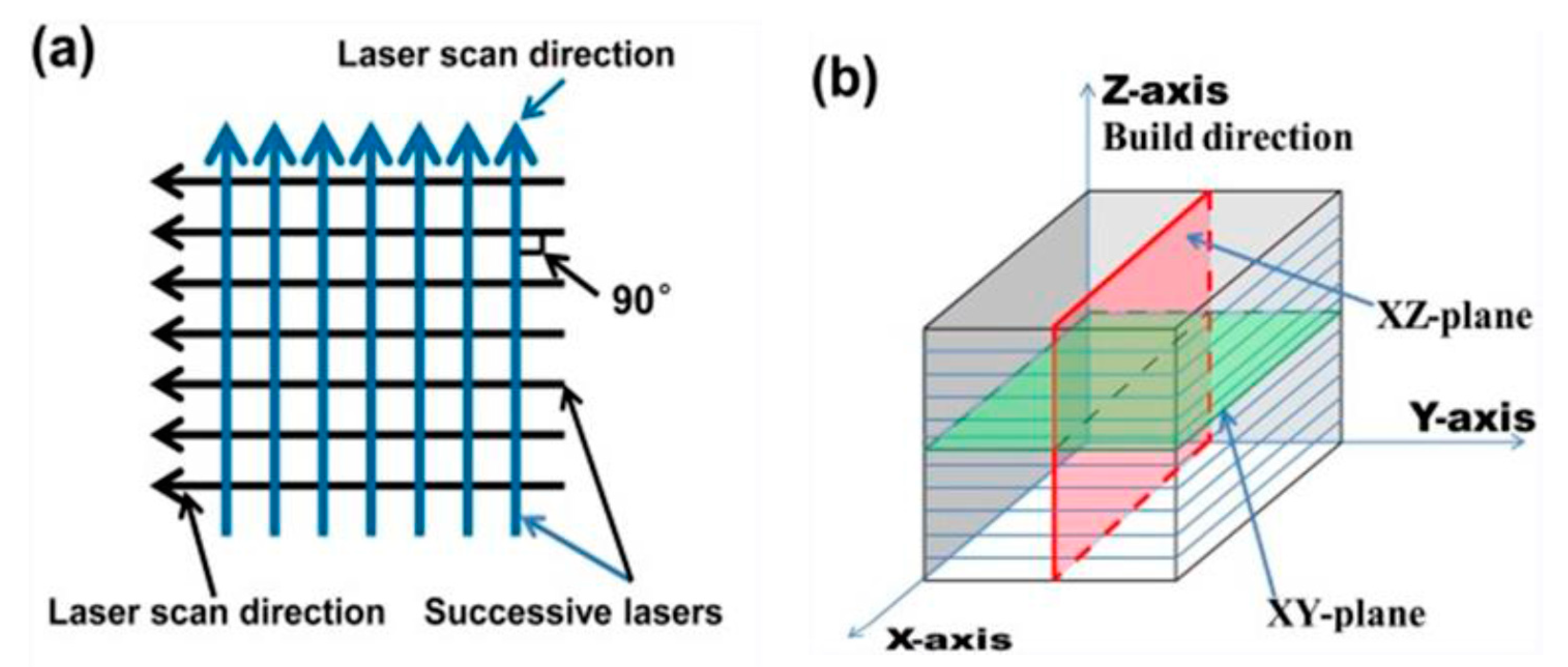
2. Materials and Methods
3. Results and Discussion
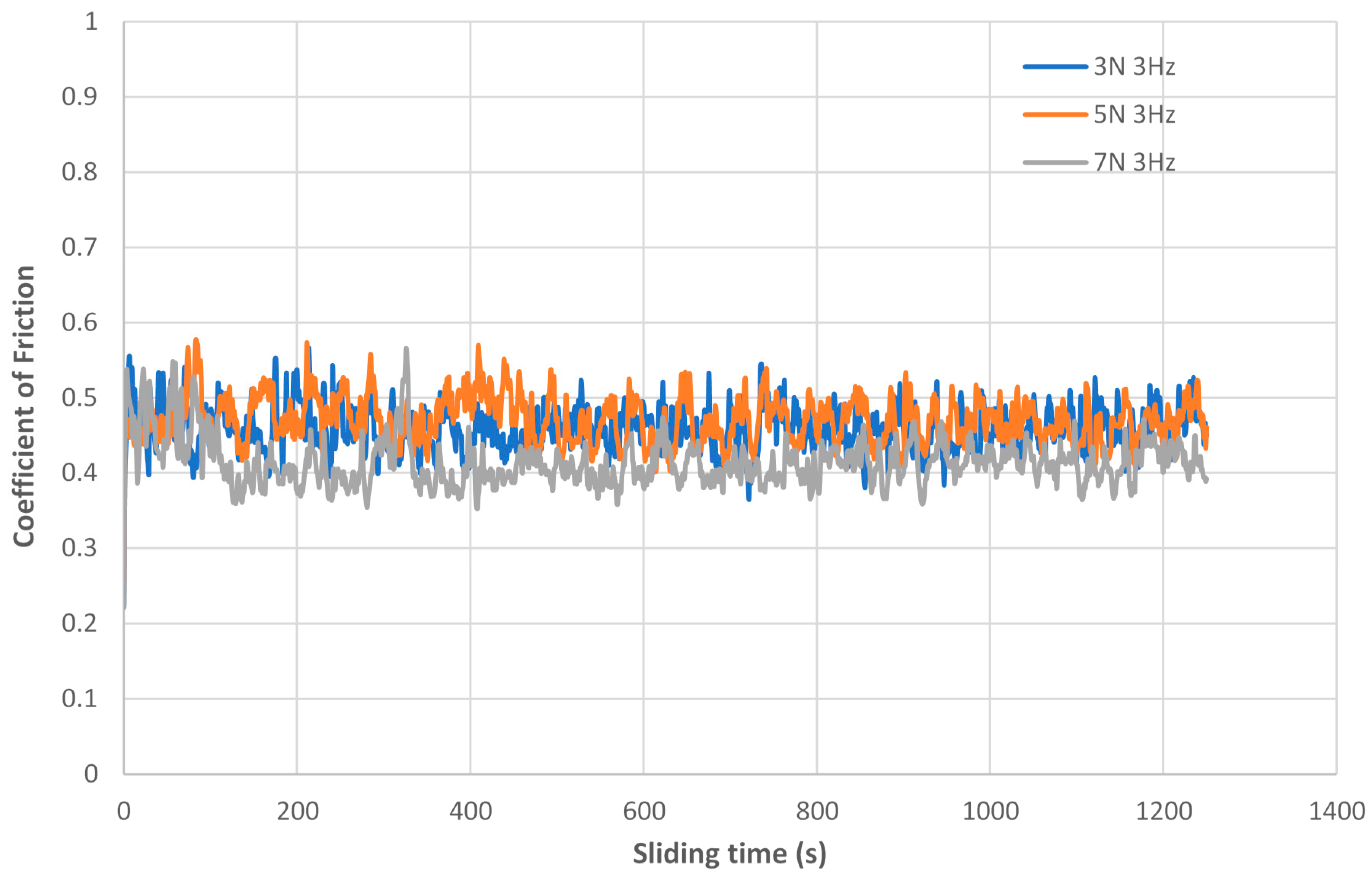
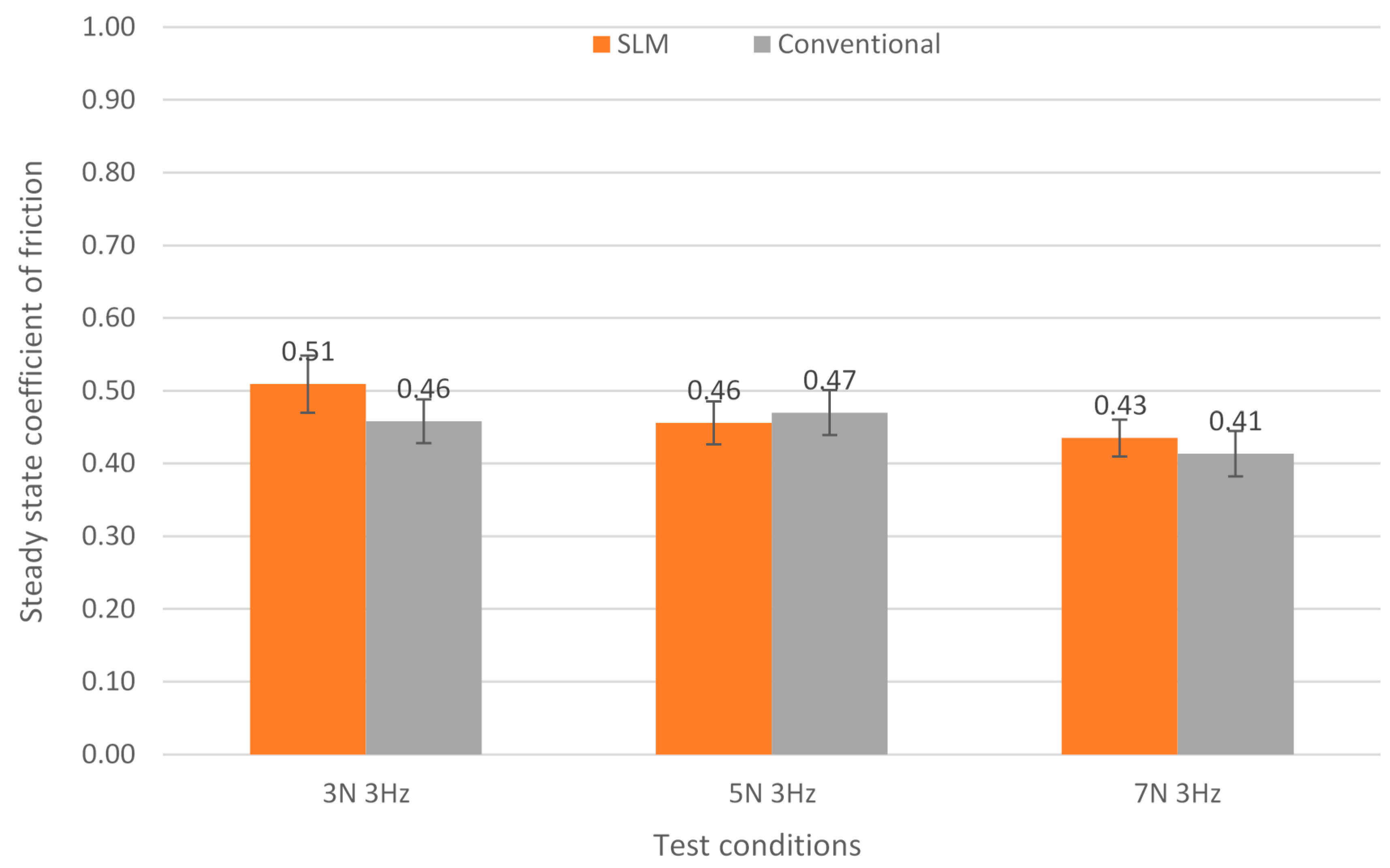
3.1. Friction Behaviour
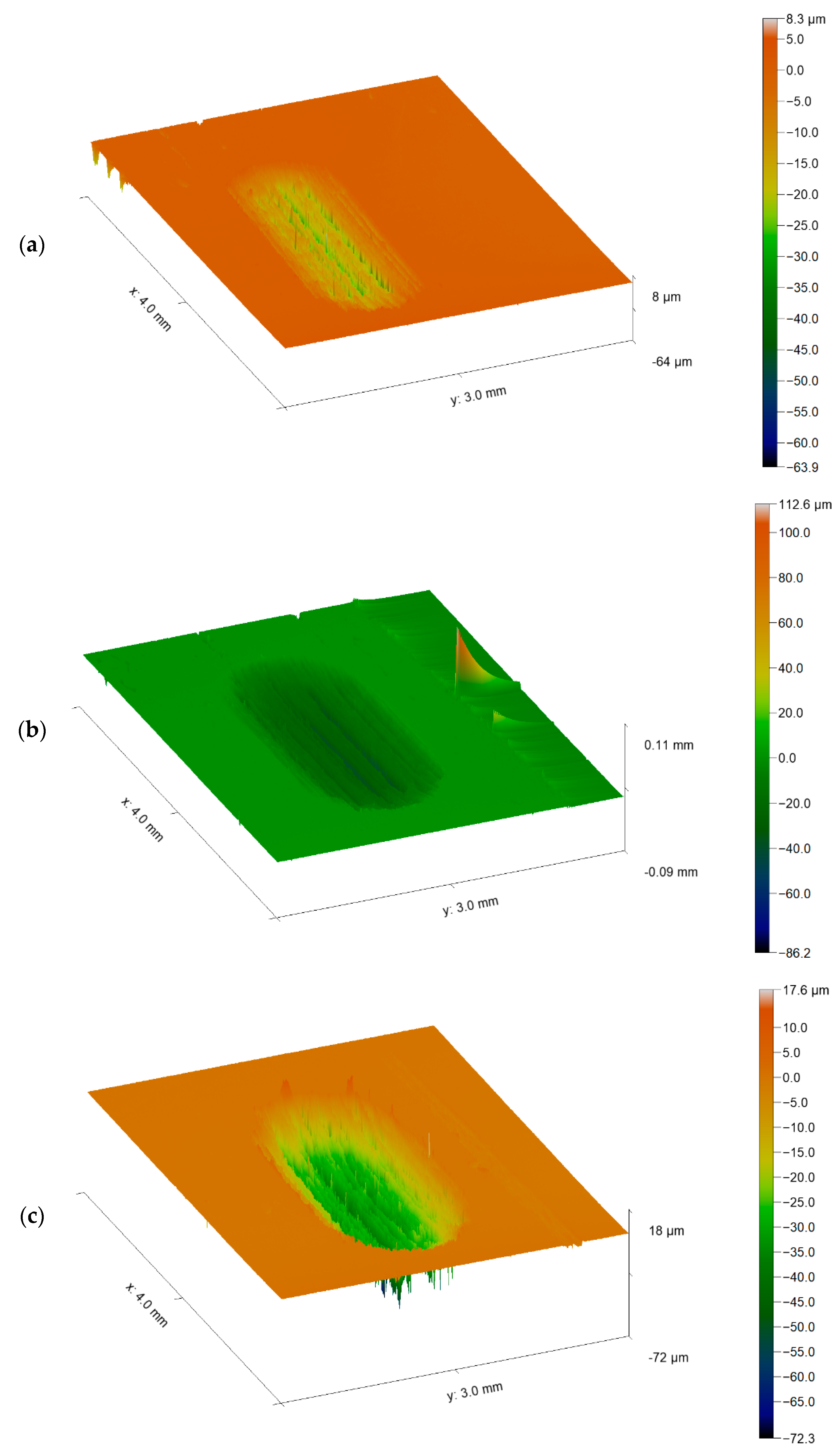
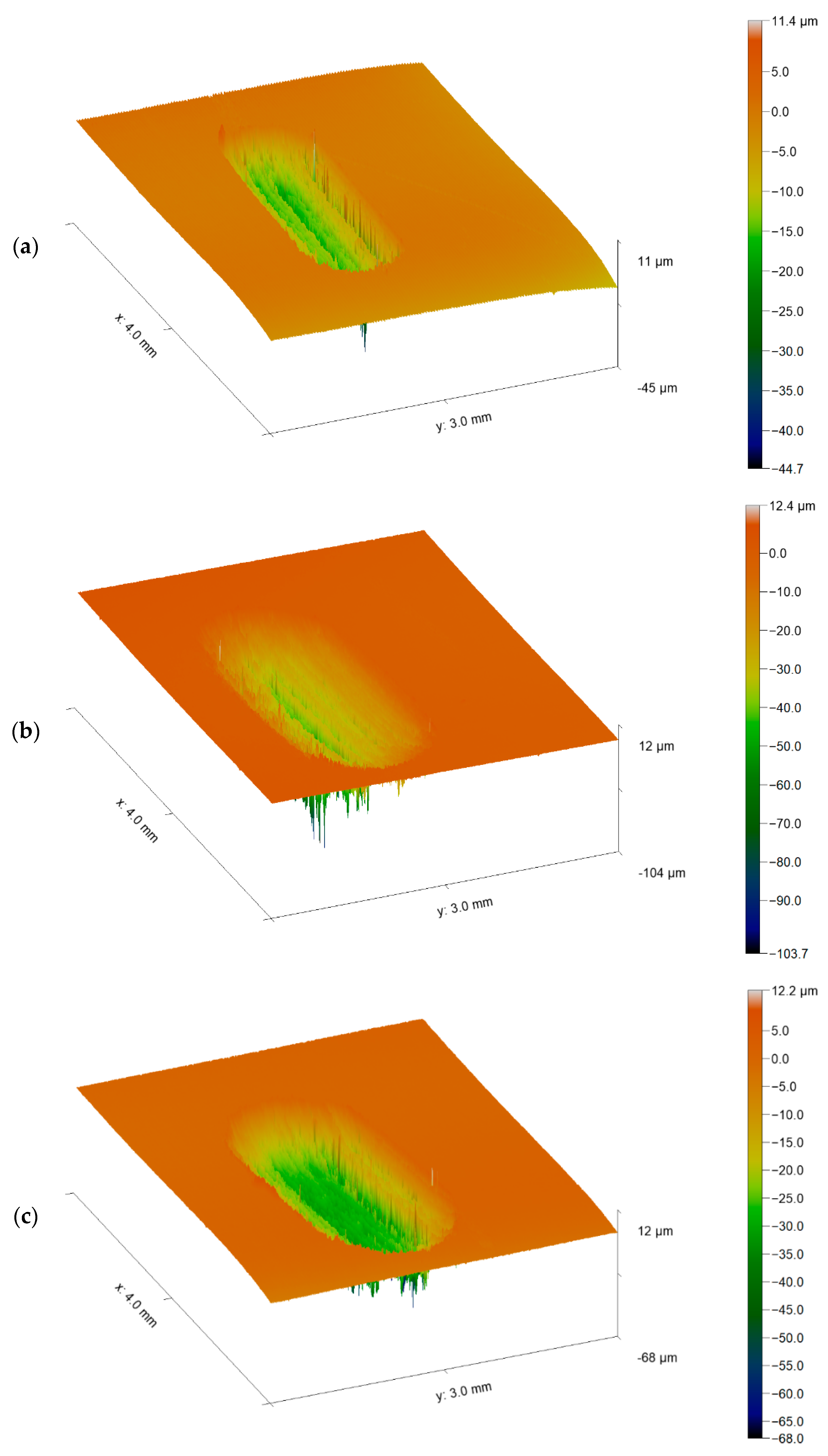
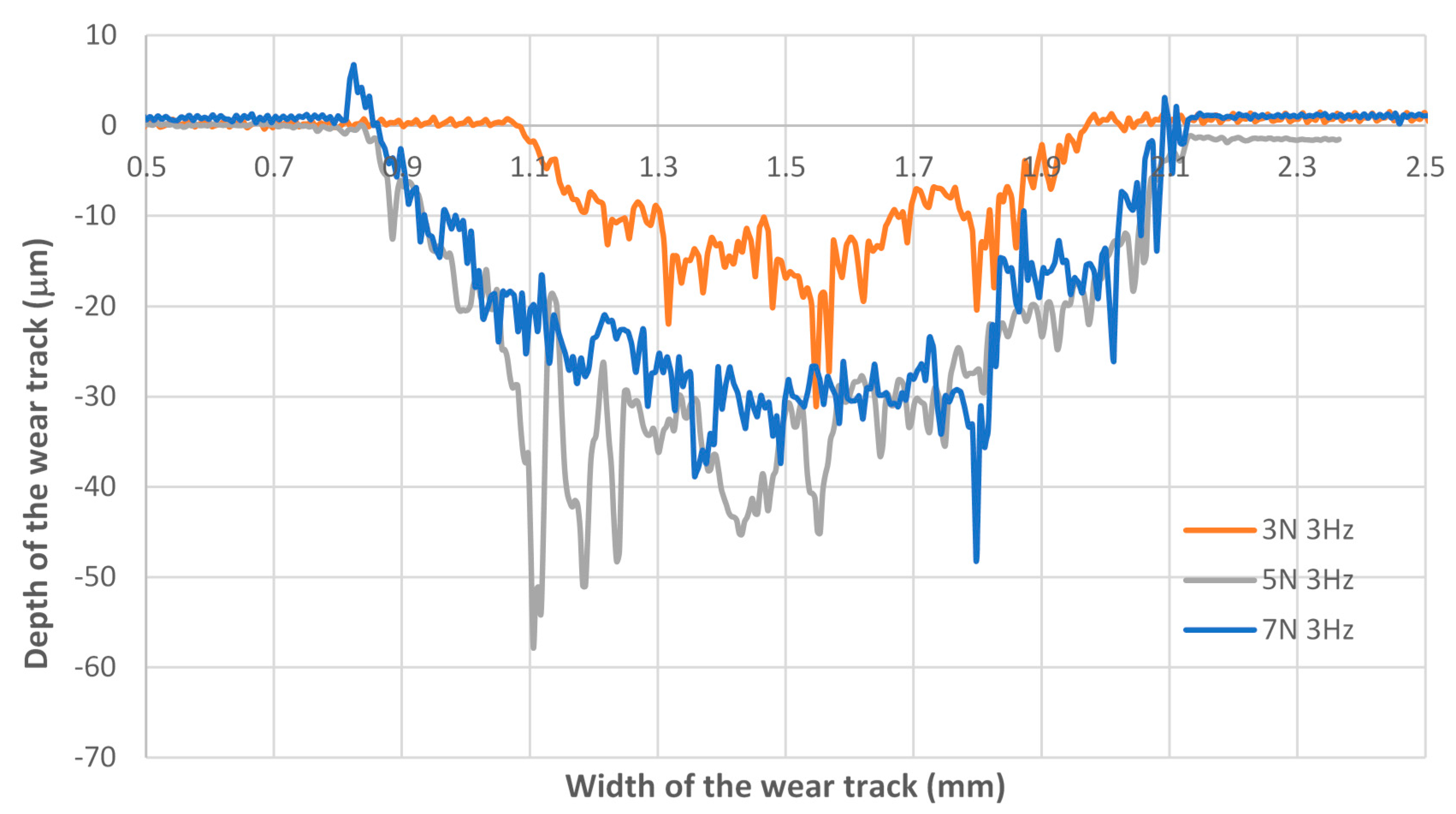
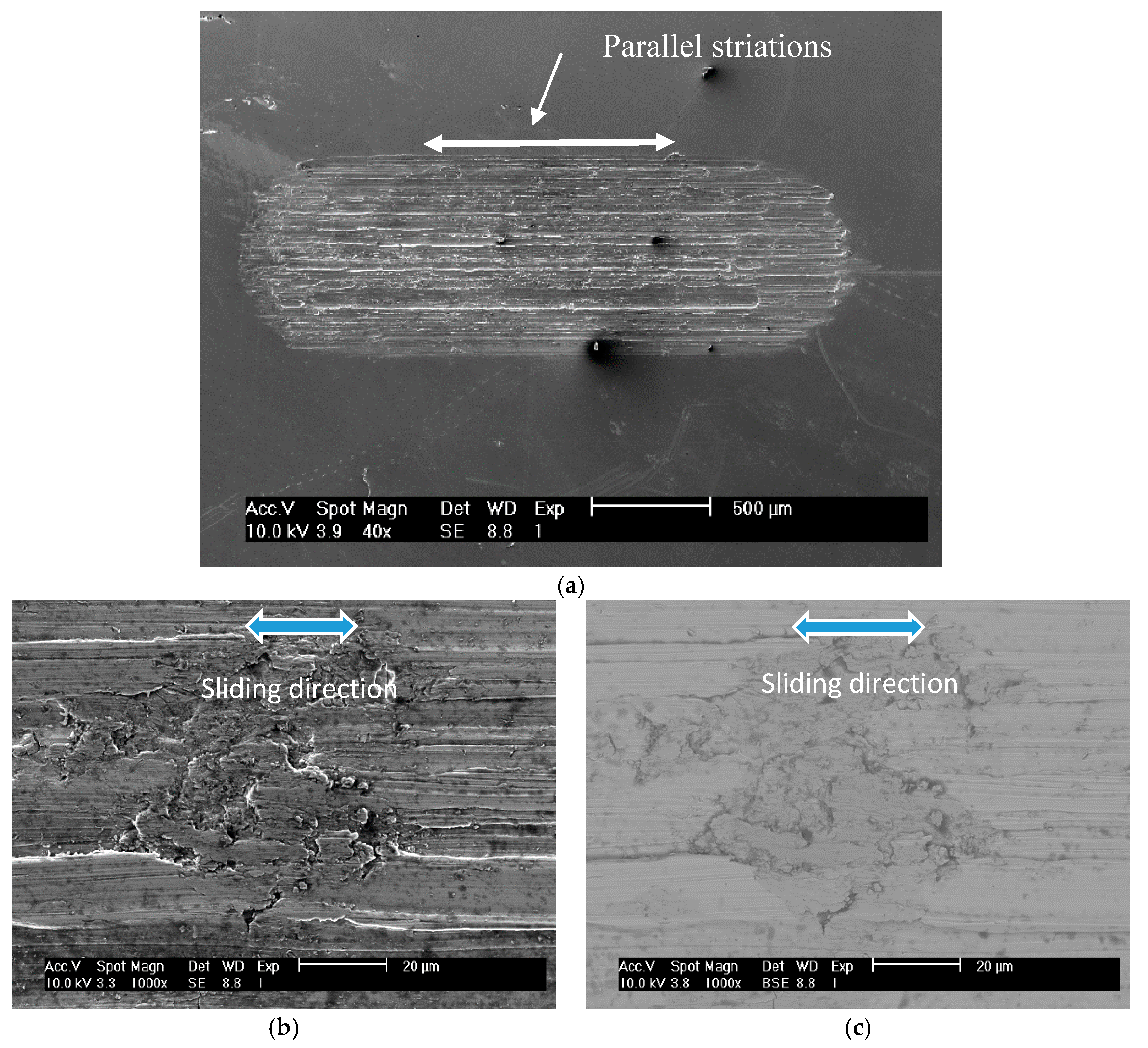
3.2. Wear Behaviour
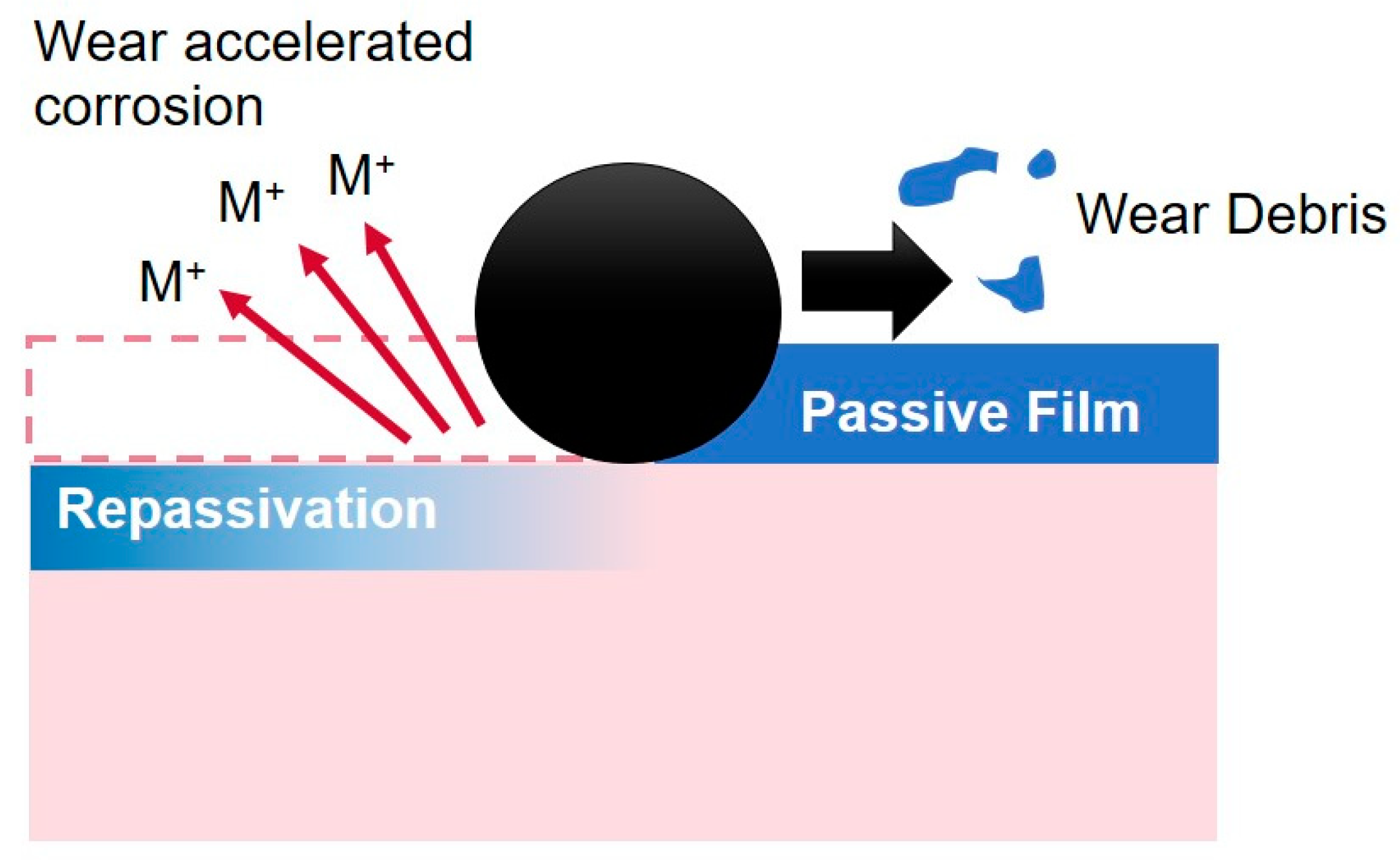
3.3. Electrochemical Behaviour
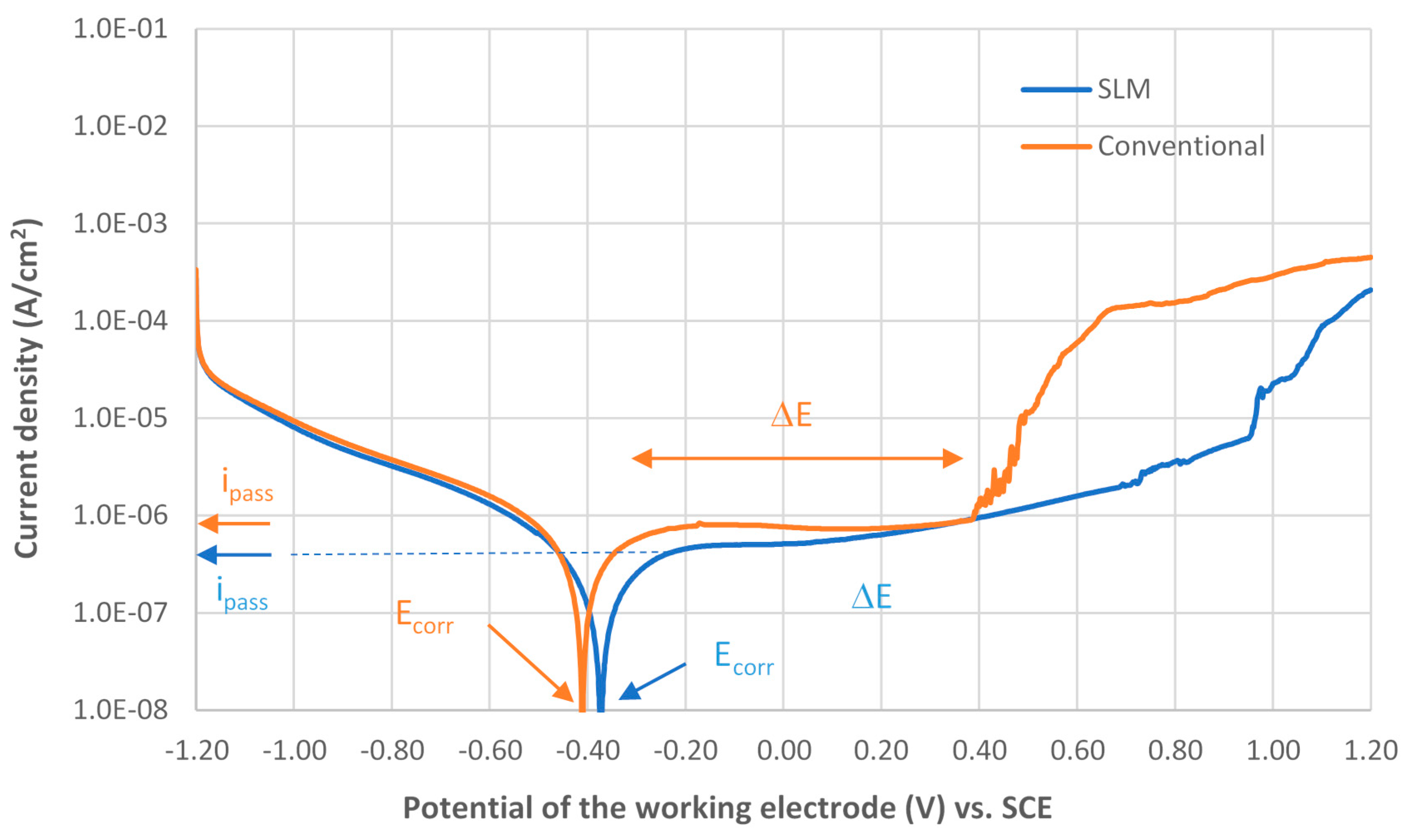
3.3.1. Potentiodynamic Polarization Curves
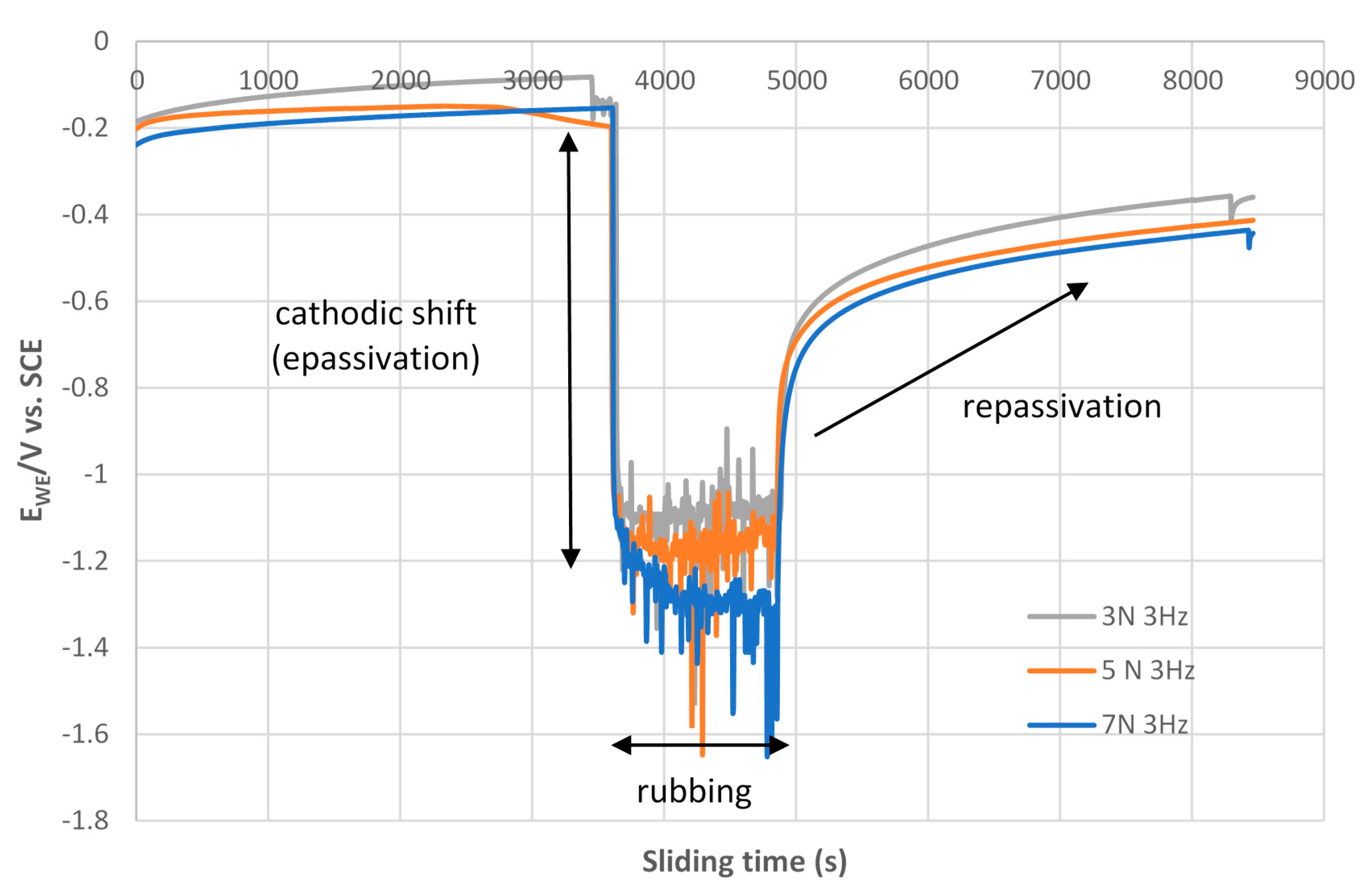
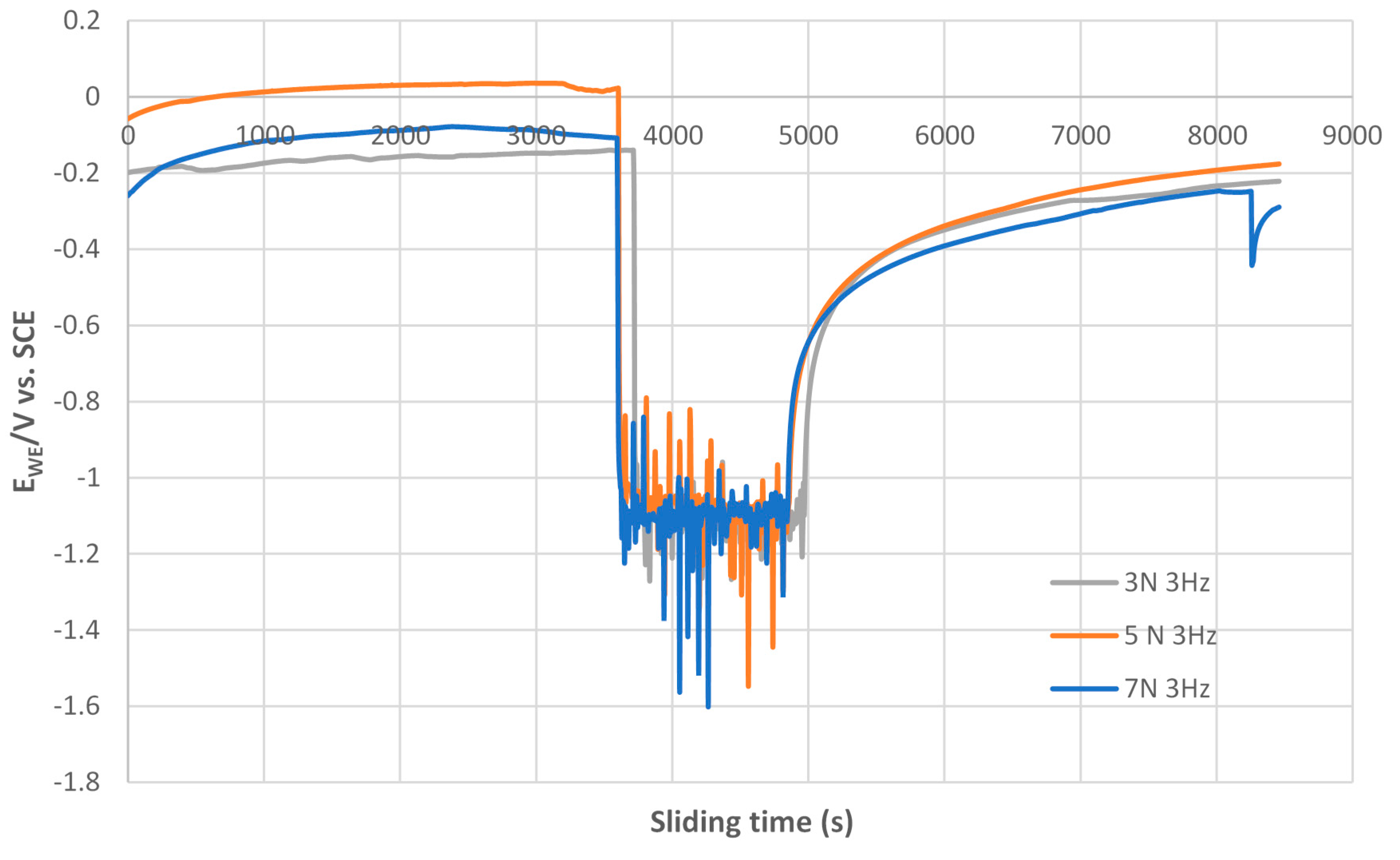
3.3.2. Open Circuit Potential (OCP) Method
4. Conclusions
- The mechanical and tribocorrosion behaviour of Ti6Al4V produced by SLM is significantly dependent on the scan optimization variables since the process is anisotropic. However, we have only tested the surface that is normal to the building direction.
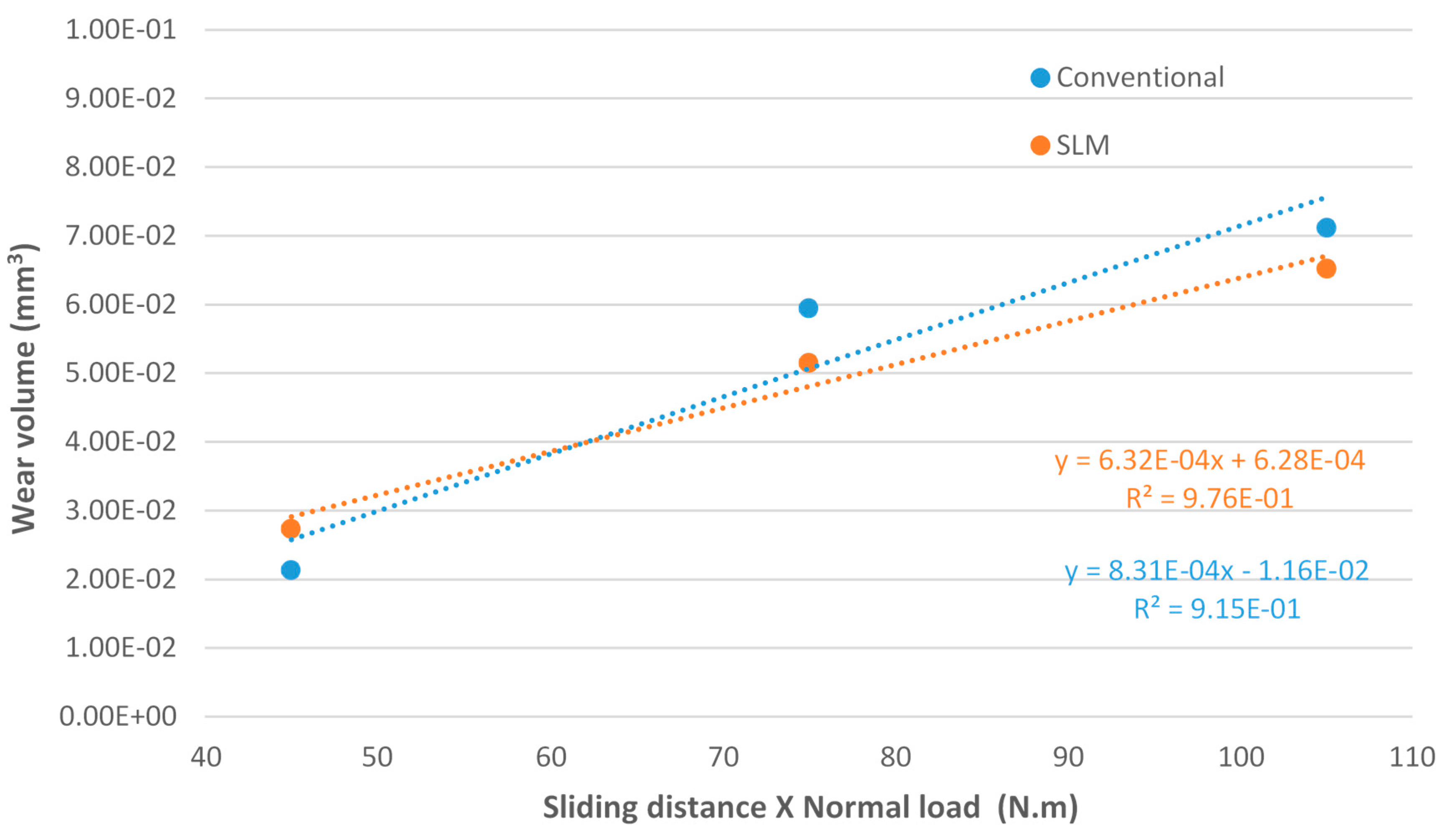
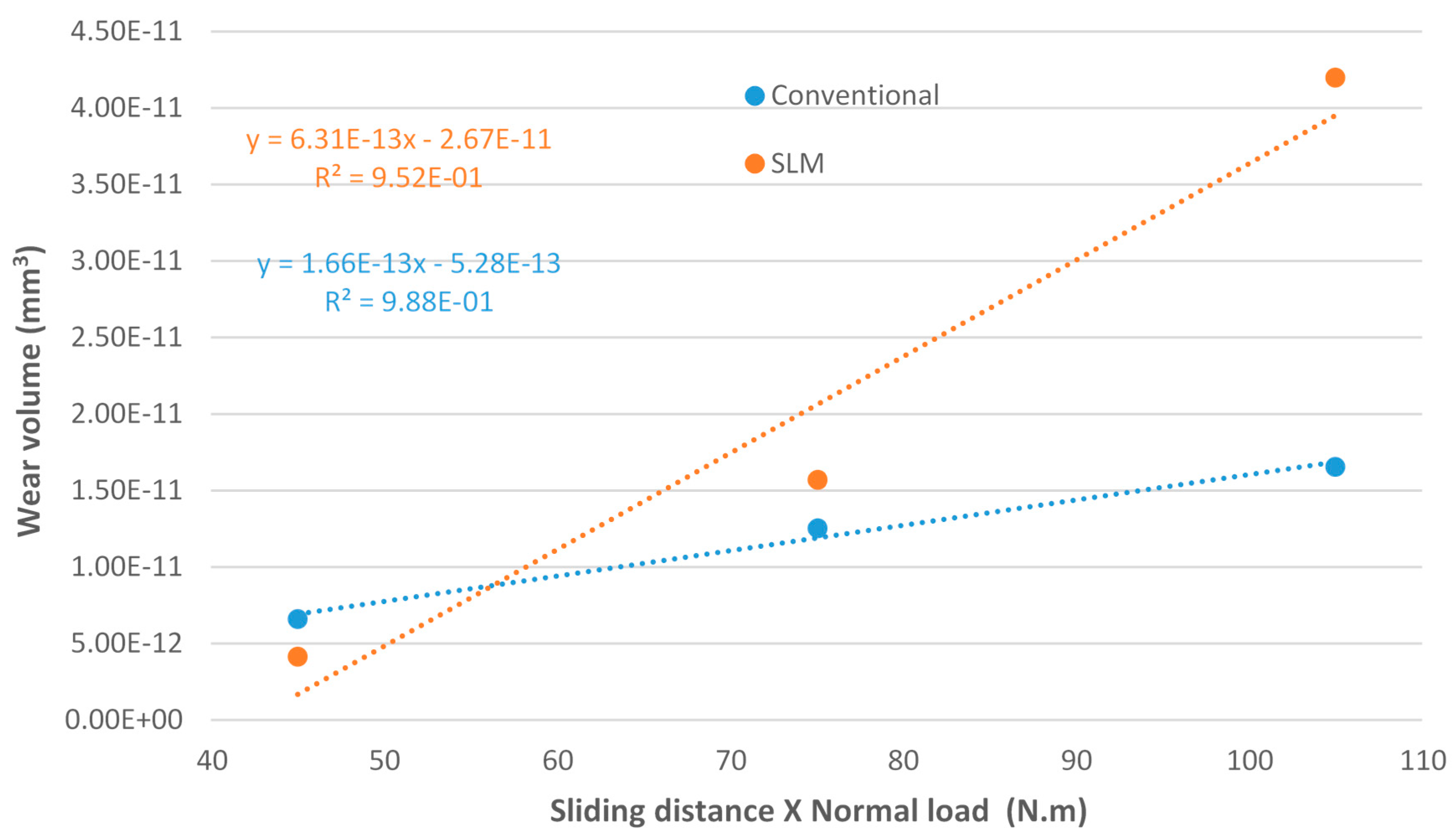
- Regarding the tribological behaviour of the Ti6Al4V specimens obtained by SLM and conventional method, they exhibited similar COF in the order of 0.41–0.51. The wear rate coefficients (k) for Ti6Al4V obtained by SLM and conventional method are of the same order showing the following values: k (SLM) = 6.3 × 10−4 mm3/N.m and k (Conventional) = 8.3 × 10−4 mm3/N.m. The wear mechanism is mainly abrasive wear with grooves aligned in the direction of sliding.
- The corrosion resistance of Ti6Al4V obtained by SLM is slightly higher than the corrosion resistance of Ti6Al4V obtained by conventional method. The potential range of passive film ΔE (SLM) > ΔE (conventional) that implies higher stability of the passive film; the passive current density: ipass (SLM) < ipass (conventional) that implies easy passivation of the alloy; the corrosion potential: Ecorr (SLM) > Ecorr (conventional) that implies a noble alloy.
- Ti6Al4V can be produced by additive manufacturing methods to at least comparable mechanical and tribological properties then those obtained by conventional produced methods. Even when properties between SLM and conventional produced samples are comparable, SLM can still be a vastly superior choice simply due to its design freedom and manufacturing-flexibility advantages, provided designs are sufficiently intricate, require better quality control or/and cater to the low to medium volume production. The implant industry fits all these requirements as the demands are custom tailored to individuals. Thus, requires the utmost quality, complex osseointegration to the bone with a suitable tailored/fine-tuned porosity, and don’t need mass production.
Author Contributions
Funding
Conflicts of Interest
References
- Oliveira, M.N.; Schunemann, W.V.H.; Mathew, M.T.; Henriques, B.; de Magini, R.S.; Teughels, W.; Souza, J.C.M. Can degradation products released from dental implants affect peri-implant tissues? J. Periodontal Res. 2018, 53, 1–11. [Google Scholar] [CrossRef]
- Smith, D.C. Dental implants: Materials and design considerations. Int. J. Prosthodont. 1993, 6, 106–117. [Google Scholar]
- Triplett, R.G.; Frohberg, U.; Sykaras, N.; Woody, R.D. Implant materials, design, and surface topographies: Their influence on Osseointegration of dental implants. J. Long Term Eff. Med. Implants 2003, 13, 485–501. [Google Scholar] [CrossRef]
- Anusavice, J.K.; Shen, C.; Rawls, H.R. (Eds.) Dental implants. In Phillips’ Science of Dental Materials, 12th ed.; Elsevier: Philadelphia, PA, USA, 2013; pp. 715–736. [Google Scholar]
- Vilhena, L.M.; Boakye, G.O.; Ramalho, A. Tribocorrosion of different biomaterials under reciprocating sliding conditions in artificial saliva. Lubr. Sci. 2019, 31, 364–380. [Google Scholar] [CrossRef]
- Fuentes, E.; Alves, S.; López-Ortega, A.; Mendizabal, L.; de Viteri, V.S. Advanced Surface Treatments on Titanium and Titanium Alloys Focused on Electrochemical and Physical Technologies for Biomedical Applications; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Mathew, M.T.; Pai, P.S.; Pourzal, R.; Fischer, A.; Wimmer, M.A. Significance of Tribocorrosion in Biomedical Applications: Overview and Current Status. Adv. Tribol. 2009, 2009, 250986. [Google Scholar] [CrossRef]
- Landolt, D.; Mischler, S. Tribocorrosion of Passive Metals and Coatings; Woodhead Publishing: Cambridge, UK, 2011; ISBN 978-1-84569-966-6. [Google Scholar]
- Watson, S.W.; Friedersdorf, F.J.; Madsen, B.W.; Cramer, S.D. Methods of measuring wear-corrosion synergism. Wear 1995, 181–183, 476–484. [Google Scholar] [CrossRef]
- Mischler, S.; Rosset, E.A.; Landolt, D. Effect ofCorrosion on theWear Behavior of PassivatingMetals in Aqueous Solutions. Tribol. Interface Eng. Ser. 1993, 25, 245–253. [Google Scholar]
- G119-09 ASTM. Standard Guide for Determining Synergism Between Wear and Corrosio; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- Landolt, D. Electrochemical and materials aspects of tribocorrosion systems. J. Phys. D Appl. Phys. 2006, 39, 3121–3127. [Google Scholar] [CrossRef]
- Landolt, D.; Mischler, S.; Stemp, M. Electrochemical methods in tribocorrosion: A critical appraisal. Electrochim. Acta 2001, 46, 3913–3929. [Google Scholar] [CrossRef]
- López-Ortega, A.; Arana, J.L.; Bayón, R. Tribocorrosion of Passive Materials: A Review on Test Procedures and Standards. Int. J. Corros. 2018, 2018, 7345346. [Google Scholar] [CrossRef]
- Cassar, J.; Mallia, B.; Mazzonello, A.; Karl, A.; Buhagiar, J. Improved Tribocorrosion Resistance of a CoCrMo Implant Material by Carburising. Lubricants 2018, 6, 76. [Google Scholar] [CrossRef]
- Wysocki, B.; Maj, P.; Sitek, R.; Buhagiar, J.; Kurzydłowski, K.; Święszkowski, W. Laser and electron beam additive manufacturing methods of fabricating titanium bone implants. Appl. Sci. 2017, 7, 657. [Google Scholar] [CrossRef]
- Liu, S.; Shin, Y.C. Additive manufacturing of Ti6Al4V alloy: A review. Mater. Des. 2019, 164, 107552. [Google Scholar] [CrossRef]
- Dai, N.; Zhang, L.-C.; Zhang, J.; Zhang, X.; Ni, Q.; Chen, Y.; Wu, M.; Yang, C. Distinction in corrosion resistance of selective laser melted Ti-6Al-4V alloy on different planes. Corros. Sci. 2016, 111, 703–710. [Google Scholar] [CrossRef]
- Mullen, L.; Stamp, R.C.; Brooks, W.K.; Jones, E.; Sutcliffe, C.J. Selective laser melting: A regular unit cell approach for the manufacture of porous, titanium, bone in-growth constructs, suitable for orthopaedic applications. J. Biomed. Mater. Res. B 2009, 89, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.C.; Klemm, D.; Eckert, J.; Hao, Y.L.; Sercombe, T.B. Manufacture by selective laser melting and mechanical behavior of a biomedical Ti-24Nb-4Zr-8Sn alloy. Scripta Mater. 2011, 65, 21–24. [Google Scholar] [CrossRef]
- Attar, H.; Prashanth, K.G.; Chaubey, A.K.; Calin, M.; Zhang, L.C.; Scudino, S.; Eckert, J. Comparison of wear properties of commercially pure titanium prepared by selective laser melting and casting processes. Mater. Lett. 2015, 142, 38–41. [Google Scholar] [CrossRef]
- Attar, H.; Bönisch, M.; Calin, M.; Zhang, L.C.; Scudino, S.; Eckert, J. Selective laser melting of in-situ titanium-titanium boride composites: Processing, microstructure and mechanical properties. Acta Mater. 2014, 76, 13–22. [Google Scholar] [CrossRef]
- Liu, Y.J.; Li, X.P.; Zhang, L.C.; Sercombe, T.B. Processing and properties of topologically optimised biomedical Ti-24Nb-4Zr-8Sn scaffolds manufactured by selective laser melting. Mater. Sci. Eng. A 2015, 642, 268–278. [Google Scholar] [CrossRef]
- Thijs, L.; Verhaeghe, F.; Craeghs, T.; Humbeeck, J.V.; Kruth, J.P. A study of the microstructural evolution during selective laser melting of Ti-6Al-4V. Acta Mater. 2010, 58, 3303–3312. [Google Scholar] [CrossRef]
- Sallica-Leva, E.; Jardini, A.L.; Fogagnolo, J.B. Microstructure and mechanical behavior of porous Ti-6Al-4V obtained by selective laser melting. J. Mech. Behav. Biomed. Mater. 2013, 26, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Tamilselvi, S.; Raman, V.; Rajendran, N. Corrosion behaviour of Ti-6Al-7Nb and Ti-6Al-4V ELI alloys in the simulated body fluid solution by electrochemical impedance spectroscopy. Electrochim. Acta 2006, 52, 839–846. [Google Scholar] [CrossRef]
- Branquinho, A.N. Análise da Propagação de Fendas por Fadiga em Provetes de Titânio Obtidos por Fusão Seletiva por Laser. Master’s Thesis, University of Coimbra, Coimbra, Portugal, 2018. Available online: http://hdl.handle.net/10316/86066 (accessed on 17 December 2019).
- Madyira, D.M.; Laubscher, R.F.; van Rensburg, N.J.; Henning, P.F.J. High speed machining induced residual stresses in Grade 5 titanium alloy. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2013, 227, 208–215. [Google Scholar] [CrossRef]
- Walczak, M.; Drozd, K. Tribological characteristics of dental metal biomaterials. Curr. Issues Pharm. Med. Sci. 2016, 29, 158–162. [Google Scholar] [CrossRef]
- Papageorgiou, N.; Mischler, S. Electrochemical Simulation of the Current and Potential Response in Sliding Tribocorrosion. Tribol. Lett. 2012, 48, 271–283. Available online: https://link.springer.com/article/10.1007/s11249-012-0022-9 (accessed on 17 December 2019). [CrossRef]




| Chemical Element | Al | V | Fe | C | O | N | H | Ti |
|---|---|---|---|---|---|---|---|---|
| Ti6Al4V SLM | 6.2 | 4.1 | 0.17 | 0.06 | 0.14 | 0.03 | 0.009 | Remainder |
| Ti6Al4V Conventional | 6 | 4 | 0.2 | 0.1 | 0.2 | 0.03 | 0.015 | 89.45 |
| Hardness [HV] Kgf/mm2 | Young’s Modulus [GPa] | Yield Strength [MPa] | Tensile Strength [MPa] |
|---|---|---|---|
| 358 | 120 | 1000 | 1147 |
| 349 | 115 | 939 | 1027 |
| Compound | Content (g/L) |
|---|---|
| NaCl | 0.600 |
| KCl | 0.720 |
| CaCl2.2H2O | 0.220 |
| KH2PO4 | 0.680 |
| Na2HPO4.12H2O | 0.856 |
| KSCN | 0.060 |
| NaHCO3 | 1.500 |
| Citric acid | 0.030 |
| Step | Description | Time (s) |
|---|---|---|
| 1 | Open circuit potential (OCP) | 3600 |
| 2 | Open circuit potential (OCP) with tribological experiment (reciprocating sliding) | 1250 (3 Hz) |
| 3 | Restabilization of the system | 3600 |
| Step | Description | Time (s) |
|---|---|---|
| 1 | Stabilization of the system under open circuit potential (OCP) | 3600 |
| 2 | Potentiodynamic polarization (−1200 mV to +1200 mV vs. SCE) (scan rate = 1 mV/s) | 2400 |
| Specimen | Ecorr (V vs SCE) | ipass (µA/cm2) | ΔE (V vs SCE) | Corrosion Rate (mm/year) |
|---|---|---|---|---|
| SLM | −0.37 | 0.46 | −0.25 to +0.72 | 1.86 × 10−3 |
| Conventional | −0.40 | 0.74 | −0.29 to + 0.38 | 3.37 × 10−3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilhena, L.M.; Shumayal, A.; Ramalho, A.; Ferreira, J.A.M. Tribocorrosion Behaviour of Ti6Al4V Produced by Selective Laser Melting for Dental Implants. Lubricants 2020, 8, 22. https://doi.org/10.3390/lubricants8020022
Vilhena LM, Shumayal A, Ramalho A, Ferreira JAM. Tribocorrosion Behaviour of Ti6Al4V Produced by Selective Laser Melting for Dental Implants. Lubricants. 2020; 8(2):22. https://doi.org/10.3390/lubricants8020022
Chicago/Turabian StyleVilhena, Luís M., Ahmad Shumayal, Amílcar Ramalho, and José António Martins Ferreira. 2020. "Tribocorrosion Behaviour of Ti6Al4V Produced by Selective Laser Melting for Dental Implants" Lubricants 8, no. 2: 22. https://doi.org/10.3390/lubricants8020022
APA StyleVilhena, L. M., Shumayal, A., Ramalho, A., & Ferreira, J. A. M. (2020). Tribocorrosion Behaviour of Ti6Al4V Produced by Selective Laser Melting for Dental Implants. Lubricants, 8(2), 22. https://doi.org/10.3390/lubricants8020022