Competitive Adsorption of Ionic Liquids Versus Friction Modifier and Anti-Wear Additive at Solid/Lubricant Interface—Speciation with Vibrational Sum Frequency Generation Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Attenuated Total Reflectance Infrared (ATR-IR) Spectroscopy
2.3. Sum Frequency Generation (SFG) Spectroscopy
3. Results and Discussion
3.1. ATR-IR Spectra
3.2. SFG Spectra of Individual Compounds at Silica/Liquid Interface
3.3. Speciation of Silica/Lubricant Interface for PAO4 Containing Single Additive
3.3.1. PAO4 + OFM Mixture
3.3.2. PAO4 + PIBSI Mixture
3.3.3. PAO4 + sec-ZDDP Mixture
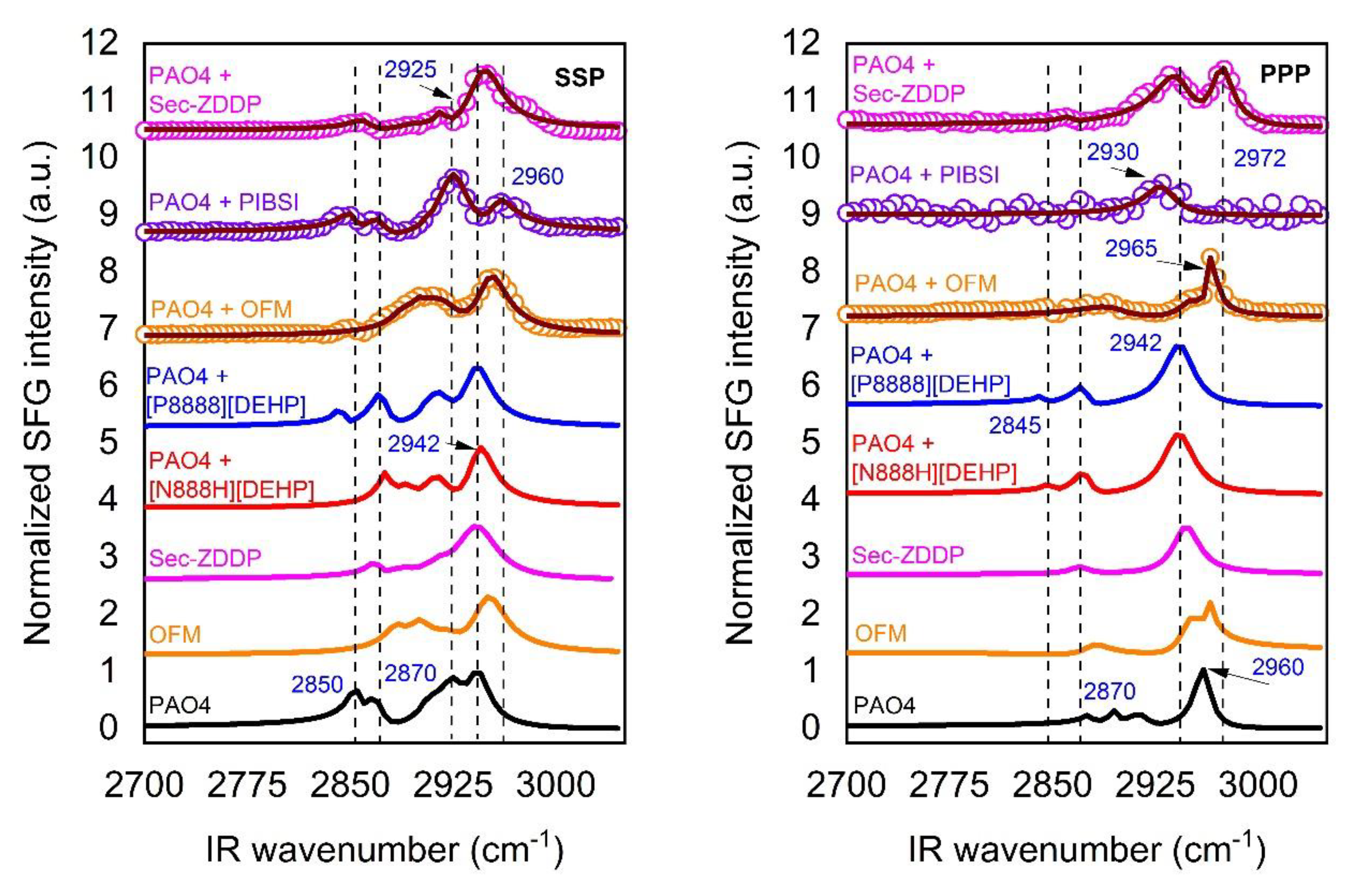
3.4. Speciation of Silica/Lubricant Interface for PAO4 Containing Two Additives
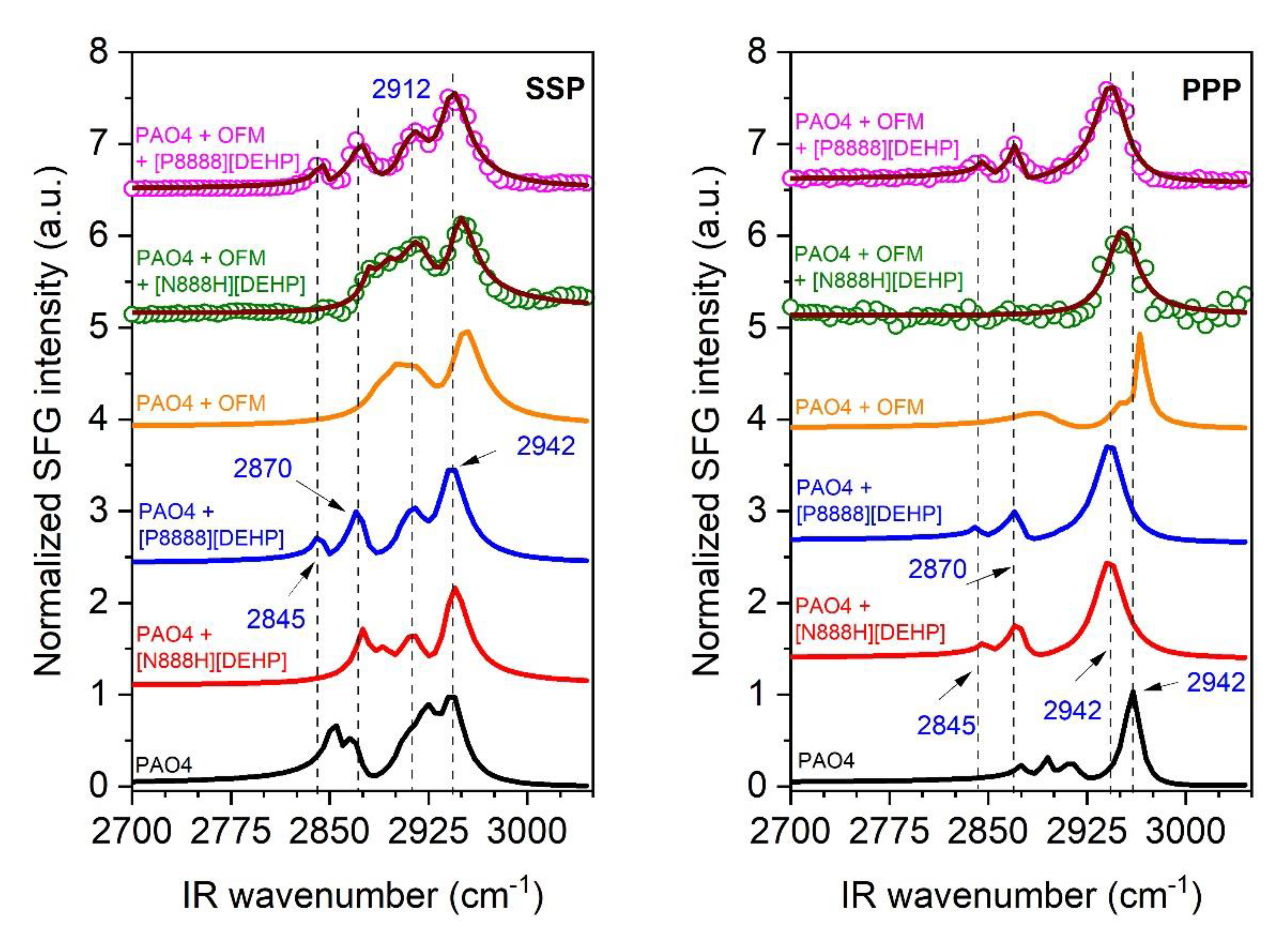
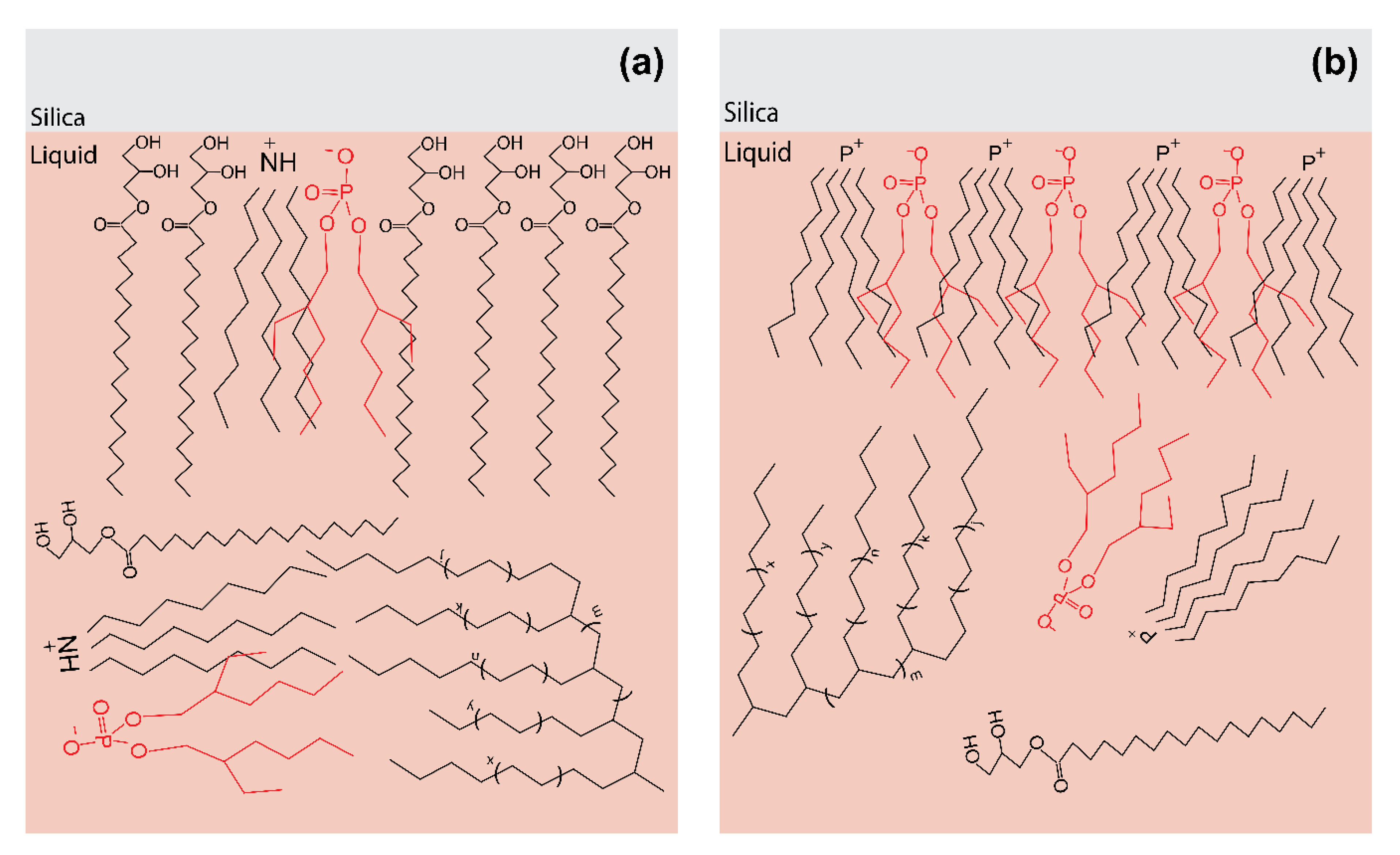
3.4.1. Mixtures of PAO4 + OFM + IL
3.4.2. Mixtures of PAO4 + sec-ZDDP + IL
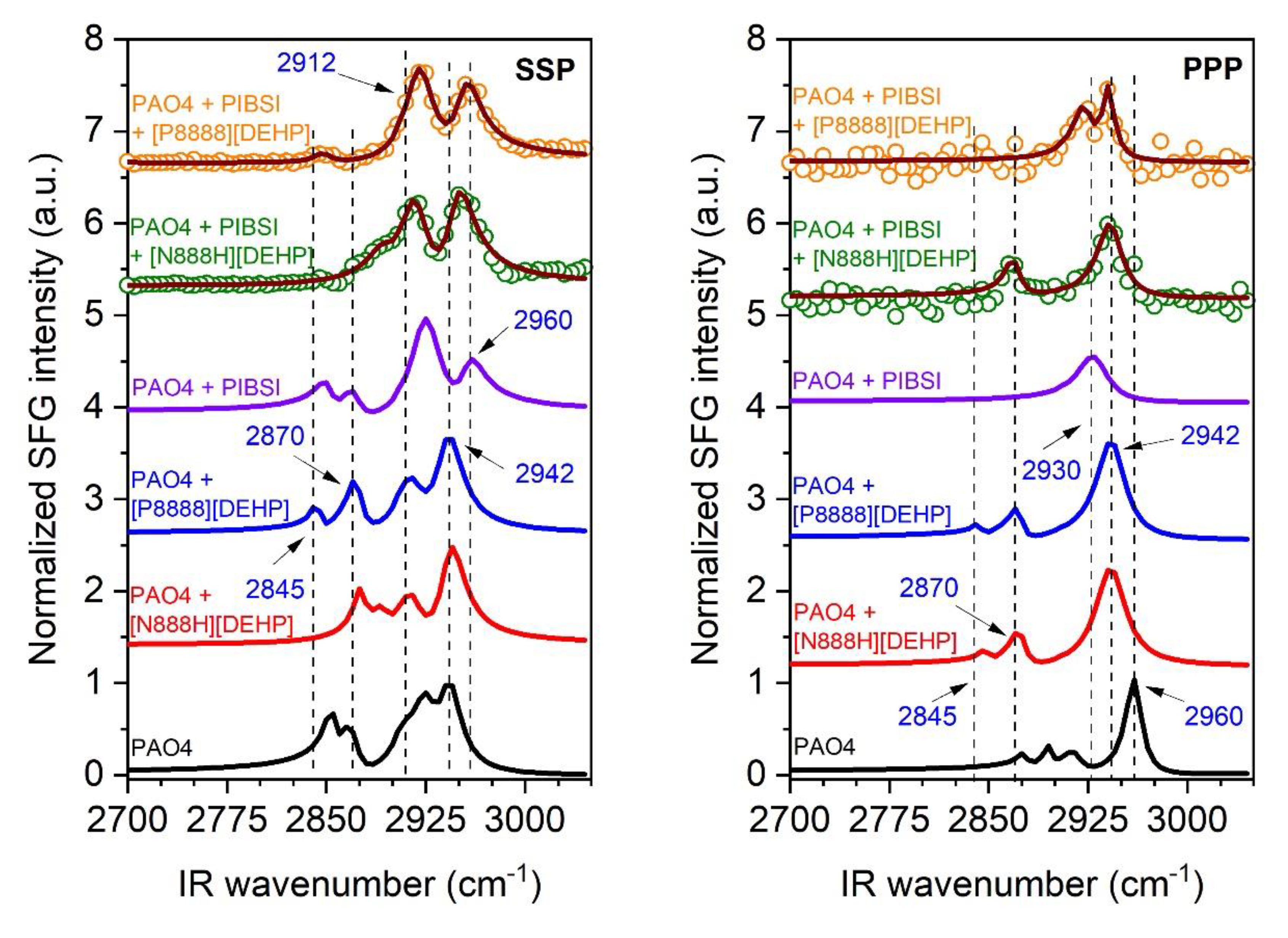
3.4.3. Mixtures of PAO4 + PIBSI + IL
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Seymour, B.T.; Fu, W.; Wright, R.A.E.; Luo, H.; Qu, J.; Dai, S.; Zhao, B. Improved Lubricating Performance by Combining Oil-Soluble Hairy Silica Nanoparticles and an Ionic Liquid as an Additive for a Synthetic Base Oil. ACS Appl. Mater. Interfaces 2018, 10, 15129–15139. [Google Scholar] [CrossRef]
- Cai, M.; Guo, R.; Zhou, F.; Liu, W. Lubricating a bright future: Lubrication contribution to energy saving and low carbon emission. Sci. China Technol. Sci. 2013, 56, 2888–2913. [Google Scholar] [CrossRef]
- Holmberg, K.; Andersson, P.; Erdemir, A. Global energy consumption due to friction in passenger cars. Tribol. Int. 2012, 47, 221–234. [Google Scholar] [CrossRef]
- Rudnick, L.R. Lubricant Additives: Chemistry and Applications, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Guegan, J.; Southby, M.; Spikes, H. Friction Modifier Additives, Synergies and Antagonisms. Tribol. Lett. 2019, 67, 83. [Google Scholar] [CrossRef]
- Qu, J.; Barnhill, W.C.; Luo, H.; Meyer, H.M., III; Leonard, D.N.; Landauer, A.K.; Kheireddin, B.; Gao, H.; Papke, B.L.; Dai, S. Synergistic Effects Between Phosphonium-Alkylphosphate Ionic Liquids and Zinc Dialkyldithiophosphate (ZDDP) as Lubricant Additives. Adv. Mater. 2015, 27, 4767–4774. [Google Scholar] [CrossRef] [PubMed]
- Bec, S.; Tonck, A.; Georges, J.M.; Roper, G.W. Synergistic Effects of MoDTC and ZDTP on Frictional Behaviour of Tribofilms at the Nanometer Scale. Tribol. Lett. 2004, 17, 797–809. [Google Scholar] [CrossRef]
- Martin, J.M.; Grossiord, C.; Varlot, K.; Vacher, B.; Igarashi, J. Synergistic effects in binary systems of lubricant additives: A chemical hardness approach. Tribol. Lett. 2000, 8, 193–201. [Google Scholar] [CrossRef]
- Li, W.; Kumara, C.; Meyer, H.M.; Luo, H.; Qu, J. Compatibility between Various Ionic Liquids and an Organic Friction Modifier as Lubricant Additives. Langmuir 2018, 34, 10711–10720. [Google Scholar] [CrossRef]
- Li, W.; Kumara, C.; Luo, H.; Meyer, H.M.; He, X.; Ngo, D.; Kim, S.H.; Qu, J. Ultralow Boundary Lubrication Friction by Three-Way Synergistic Interactions among Ionic Liquid, Friction Modifier, and Dispersant. ACS Appl. Mater. Interfaces 2020, 12, 17077–17090. [Google Scholar] [CrossRef]
- Ngo, D.; He, X.; Luo, H.; Qu, J.; Kim, S.H. Competitive Adsorption of Lubricant Base Oil and Ionic Liquid Additives at Air/Liquid and Solid/Liquid Interfaces. Langmuir 2020, 36, 7582–7592. [Google Scholar] [CrossRef]
- Zhou, Y.; Qu, J. Ionic Liquids as Lubricant Additives: A Review. ACS Appl. Mater. Interfaces 2017, 9, 3209–3222. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.G.; Davies, P.B.; Neivandt, D.J. Implementing the Theory of Sum Frequency Generation Vibrational Spectroscopy: A Tutorial Review. Appl. Spectrosc. Rev. 2005, 40, 103–145. [Google Scholar] [CrossRef]
- Bain, C.D. Sum-frequency vibrational spectroscopy of the solid/liquid interface. J. Chem. Soc. Faraday Trans. 1995, 91, 1281–1296. [Google Scholar] [CrossRef]
- Chae, I.; Ngo, D.; Makarem, M.; Ounaies, Z.; Kim, S.H. Compression-Induced Topographic Corrugation of Air/Surfactant/Water Interface: Effect of Nanoparticles Adsorbed beneath the Interface. J. Phys. Chem. C 2019, 123, 25628–25634. [Google Scholar] [CrossRef]
- Romero, C.; Baldelli, S. Sum Frequency Generation Study of the Room-Temperature Ionic Liquids/Quartz Interface. J. Phys. Chem. B 2006, 110, 6213–6223. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Rubero, S.; Baldelli, S. Surface Spectroscopy of Room-temperature Ionic Liquids on a Platinum Electrode: A Sum Frequency Generation Study. J. Phys. Chem. B 2004, 108, 15133–15140. [Google Scholar] [CrossRef]
- Baldelli, S. Interfacial Structure of Room-Temperature Ionic Liquids at the Solid–Liquid Interface as Probed by Sum Frequency Generation Spectroscopy. J. Phys. Chem. Lett. 2013, 4, 244–252. [Google Scholar] [CrossRef]
- Barnhill, W.C.; Qu, J.; Luo, H.; Meyer, H.M.; Ma, C.; Chi, M.; Papke, B.L. Phosphonium-Organophosphate Ionic Liquids as Lubricant Additives: Effects of Cation Structure on Physicochemical and Tribological Characteristics. ACS Appl. Mater. Interfaces 2014, 6, 22585–22593. [Google Scholar] [CrossRef]
- Elsentriecy, H.H.; Qu, J.; Luo, H.; Meyer, H.M.; Ma, C.; Chi, M. Improving corrosion resistance of AZ31B magnesium alloy via a conversion coating produced by a protic ammonium-phosphate ionic liquid. Thin Solid Film. 2014, 568, 44–51. [Google Scholar] [CrossRef]
- Mendes Siqueira, A.L.; Beaumesnil, M.; Hubert-Roux, M.; Loutelier-Bourhis, C.; Afonso, C.; Pondaven, S.; Bai, Y.; Racaud, A. Characterization of polyalphaolefins using halogen anion attachment in atmospheric pressure photoionization coupled with ion mobility spectrometry-mass spectrometry. Analyst 2018, 143, 3934–3940. [Google Scholar] [CrossRef]
- Snyder, R.G.; Strauss, H.L.; Elliger, C.A. Carbon-hydrogen stretching modes and the structure of n-alkyl chains. 1. Long, disordered chains. J. Phys. Chem. 1982, 86, 5145–5150. [Google Scholar] [CrossRef]
- Snyder, R.G.; Hsu, S.L.; Krimm, S. Vibrational spectra in the C---H stretching region and the structure of the polymethylene chain. Spectrochim. Acta Part A Mol. Spectrosc. 1978, 34, 395–406. [Google Scholar] [CrossRef]
- Esenturk, O.; Walker, R.A. Surface Structure at Hexadecane and Halo-hexadecane Liquid/Vapor Interfaces. J. Phys. Chem. B 2004, 108, 10631–10635. [Google Scholar] [CrossRef]
- MacPhail, R.A.; Strauss, H.L.; Snyder, R.G.; Elliger, C.A. Carbon-hydrogen stretching modes and the structure of n-alkyl chains. 2. Long, all-trans chains. J. Phys. Chem. 1984, 88, 334–341. [Google Scholar] [CrossRef]
- Walker, R.A.; Conboy, J.C.; Richmond, G.L. Molecular Structure and Ordering of Phospholipids at a Liquid−Liquid Interface. Langmuir 1997, 13, 3070–3073. [Google Scholar] [CrossRef]
- Conboy, J.C.; Messmer, M.C.; Richmond, G.L. Dependence of Alkyl Chain Conformation of Simple Ionic Surfactants on Head Group Functionality As Studied by Vibrational Sum-Frequency Spectroscopy. J. Phys. Chem. B 1997, 101, 6724–6733. [Google Scholar] [CrossRef]
- Tyrode, E.; Hedberg, J. A Comparative Study of the CD and CH Stretching Spectral Regions of Typical Surfactants Systems Using VSFS: Orientation Analysis of the Terminal CH3 and CD3 Groups. J. Phys. Chem. C 2012, 116, 1080–1091. [Google Scholar] [CrossRef]
- Lu, R.; Gan, W.; Wu, B.-H.; Chen, H.; Wang, H.-F. Vibrational Polarization Spectroscopy of CH Stretching Modes of the Methylene Group at the Vapor/Liquid Interfaces with Sum Frequency Generation. J. Phys. Chem. B 2004, 108, 7297–7306. [Google Scholar] [CrossRef]
- Wang, H.-F.; Gan, W.; Lu, R.; Rao, Y.; Wu, B.-H. Quantitative spectral and orientational analysis in surface sum frequency generation vibrational spectroscopy (SFG-VS). Int. Rev. Phys. Chem. 2005, 24, 191–256. [Google Scholar] [CrossRef]
- Peñalber-Johnstone, C.; Adamová, G.; Plechkova, N.V.; Bahrami, M.; Ghaed-Sharaf, T.; Ghatee, M.H.; Seddon, K.R.; Baldelli, S. Sum frequency generation spectroscopy of tetraalkylphosphonium ionic liquids at the air–liquid interface. J. Chem. Phys. 2018, 148, 193841. [Google Scholar] [CrossRef]
- Bell, G.R.; Manning-Benson, S.; Bain, C.D. Effect of Chain Length on the Structure of Monolayers of Alkyltrimethylammonium Bromides (CnTABs) at the Air−Water Interface. J. Phys. Chem. B 1998, 102, 218–222. [Google Scholar] [CrossRef]
- Walker, R.A.; Gruetzmacher, J.A.; Richmond, G.L. Phosphatidylcholine Monolayer Structure at a Liquid−Liquid Interface. J. Am. Chem. Soc. 1998, 120, 6991–7003. [Google Scholar] [CrossRef]
- Bain, C.D.; Davies, P.B.; Ong, T.H.; Ward, R.N.; Brown, M.A. Quantitative analysis of monolayer composition by sum-frequency vibrational spectroscopy. Langmuir 1991, 7, 1563–1566. [Google Scholar] [CrossRef]
- Dreesen, L.; Humbert, C.; Hollander, P.; Mani, A.A.; Ataka, K.; Thiry, P.A.; Peremans, A. Study of the water/poly(ethylene glycol) interface by IR-visible sum-frequency generation spectroscopy. Chem. Phys. Lett. 2001, 333, 327–331. [Google Scholar] [CrossRef]
- Hommel, E.L.; Merle, J.K.; Ma, G.; Hadad, C.M.; Allen, H.C. Spectroscopic and Computational Studies of Aqueous Ethylene Glycol Solution Surfaces. J. Phys. Chem. B 2005, 109, 811–818. [Google Scholar] [CrossRef]
- Matsuura, H.; Miyazawa, T. Infrared spectra and molecular vibrations of ethylene glycol and deuterated derivatives. Bull. Chem. Soc. Jpn. 1967, 40, 85–94. [Google Scholar] [CrossRef]
- Koshima, H.; Kamano, H.; Hisaeda, Y.; Liu, H.; Ye, S. Analyses of the Adsorption Structures of Friction Modifiers by Means of Quantitative Structure-Property Relationship Method and Sum Frequency Generation Spectroscopy. Tribol. Online 2010, 5, 165–172. [Google Scholar] [CrossRef]
- Koshima, H.; Iyotani, Y.; Peng, Q.; Ye, S. Study of Friction-Reduction Properties of Fatty Acids and Adsorption Structures of their Langmuir-Blodgett Monolayers using Sum-Frequency Generation Spectroscopy and Atomic Force Microscopy. Tribol. Lett. 2016, 64, 34. [Google Scholar] [CrossRef]
- Watanabe, S.; Nakano, M.; Miyake, K.; Sasaki, S. Analysis of the Interfacial Molecular Behavior of a Lubrication Film of n-Dodecane Containing Stearic Acid under Lubricating Conditions by Sum Frequency Generation Spectroscopy. Langmuir 2016, 32, 13649–13656. [Google Scholar] [CrossRef]
- Fry, B.M.; Moody, G.; Spikes, H.A.; Wong, J.S.S. Adsorption of Organic Friction Modifier Additives. Langmuir 2020, 36, 1147–1155. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, X.; Rafailovich, M.H.; Sokolov, J.; Composto, R.J.; Smith, S.D.; Russell, T.P.; Dozier, W.D.; Mansfield, T.; Satkowski, M. Segregation of chain ends to polymer melt surfaces and interfaces. Macromolecules 1993, 26, 561–562. [Google Scholar] [CrossRef]
- Stein, G.E.; Laws, T.S.; Verduzco, R. Tailoring the Attraction of Polymers toward Surfaces. Macromolecules 2019, 52, 4787–4802. [Google Scholar] [CrossRef]
- Liu, J.; Conboy, J.C. Direct Measurement of the Transbilayer Movement of Phospholipids by Sum-Frequency Vibrational Spectroscopy. J. Am. Chem. Soc. 2004, 126, 8376–8377. [Google Scholar] [CrossRef] [PubMed]
- Spikes, H. Friction Modifier Additives. Tribol. Lett. 2015, 60, 5. [Google Scholar] [CrossRef]
- Wood, M.H.; Casford, M.T.; Steitz, R.; Zarbakhsh, A.; Welbourn, R.J.L.; Clarke, S.M. Comparative Adsorption of Saturated and Unsaturated Fatty Acids at the Iron Oxide/Oil Interface. Langmuir 2016, 32, 534–540. [Google Scholar] [CrossRef]
- Qu, J.; Bansal, D.G.; Yu, B.; Howe, J.Y.; Luo, H.; Dai, S.; Li, H.; Blau, P.J.; Bunting, B.G.; Mordukhovich, G.; et al. Antiwear Performance and Mechanism of an Oil-Miscible Ionic Liquid as a Lubricant Additive. ACS Appl. Mater. Interfaces 2012, 4, 997–1002. [Google Scholar] [CrossRef]
- Yu, B.; Bansal, D.G.; Qu, J.; Sun, X.; Luo, H.; Dai, S.; Blau, P.J.; Bunting, B.G.; Mordukhovich, G.; Smolenski, D.J. Oil-miscible and non-corrosive phosphonium-based ionic liquids as candidate lubricant additives. Wear 2012, 289, 58–64. [Google Scholar] [CrossRef]
- Barnhill, W.C.; Luo, H.; Meyer, H.M.; Ma, C.; Chi, M.; Papke, B.L.; Qu, J. Tertiary and Quaternary Ammonium-Phosphate Ionic Liquids as Lubricant Additives. Tribol. Lett. 2016, 63, 22. [Google Scholar] [CrossRef]
- Wright, R.A.; Wang, K.; Qu, J.; Zhao, B. Oil-soluble polymer brush grafted nanoparticles as effective lubricant additives for friction and wear reduction. Angew. Chem. 2016, 128, 8798–8802. [Google Scholar] [CrossRef]
- Spikes, H. The History and Mechanisms of ZDDP. Tribol. Lett. 2004, 17, 469–489. [Google Scholar] [CrossRef]
- Zhang, J.; Ewen, J.P.; Ueda, M.; Wong, J.S.S.; Spikes, H.A. Mechanochemistry of Zinc Dialkyldithiophosphate on Steel Surfaces under Elastohydrodynamic Lubrication Conditions. ACS Appl. Mater. Interfaces 2020, 12, 6662–6676. [Google Scholar] [CrossRef] [PubMed]
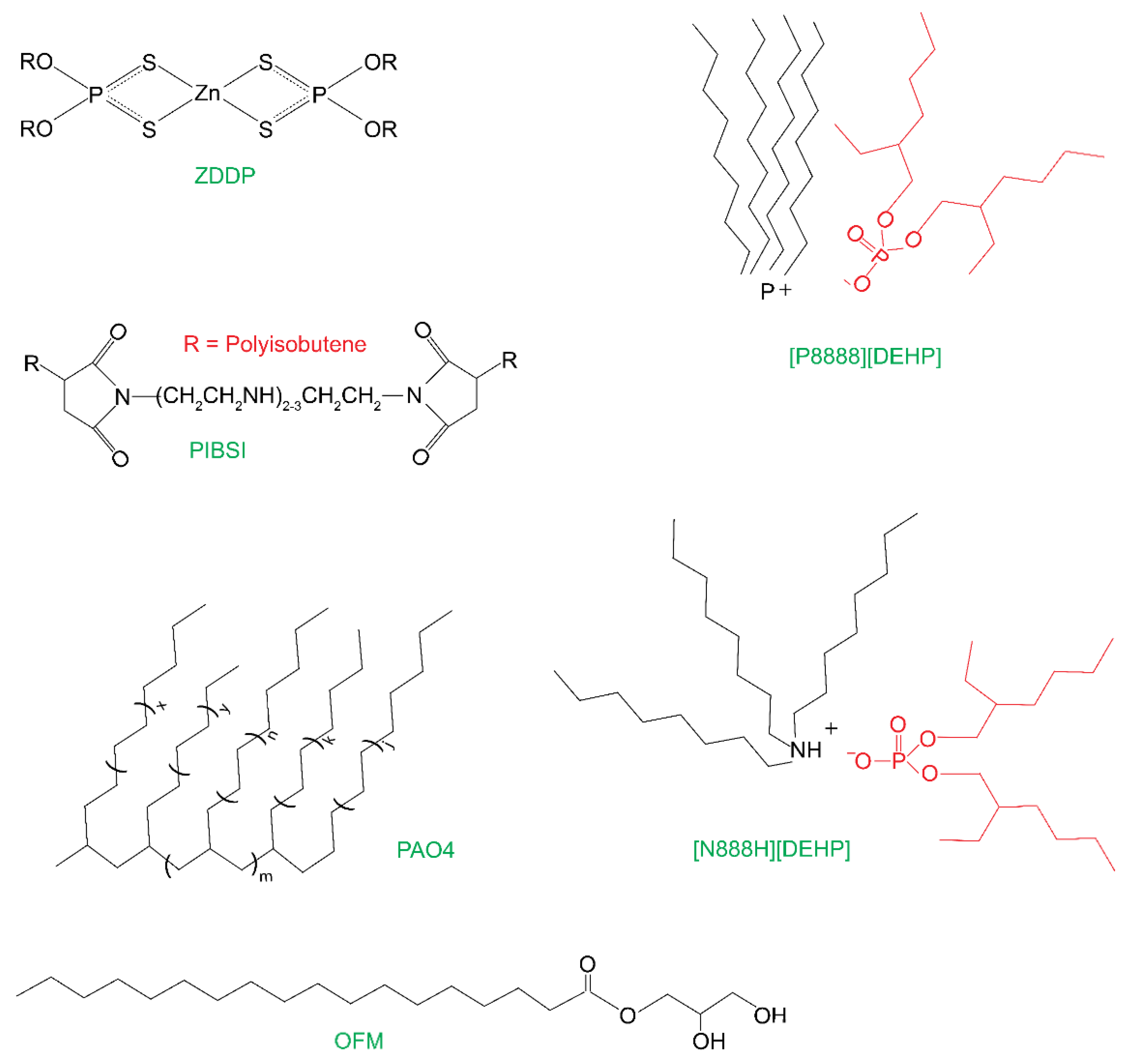
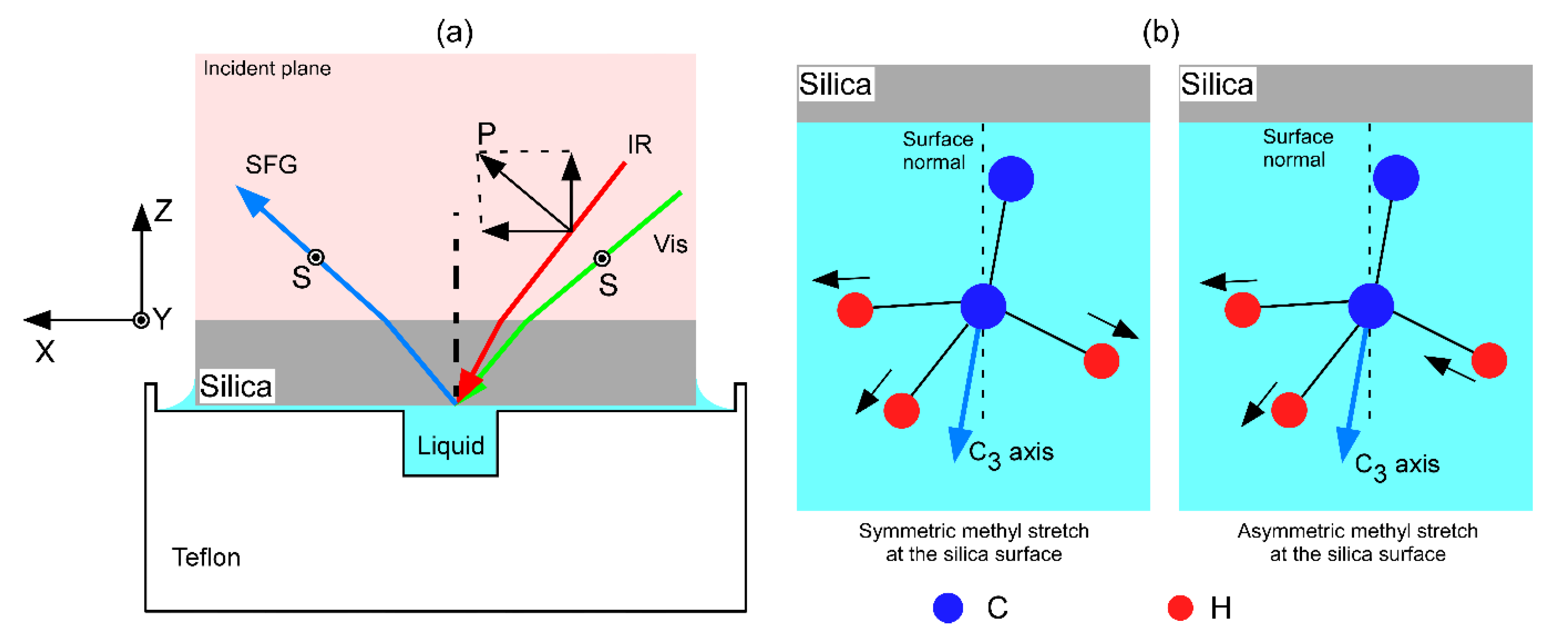

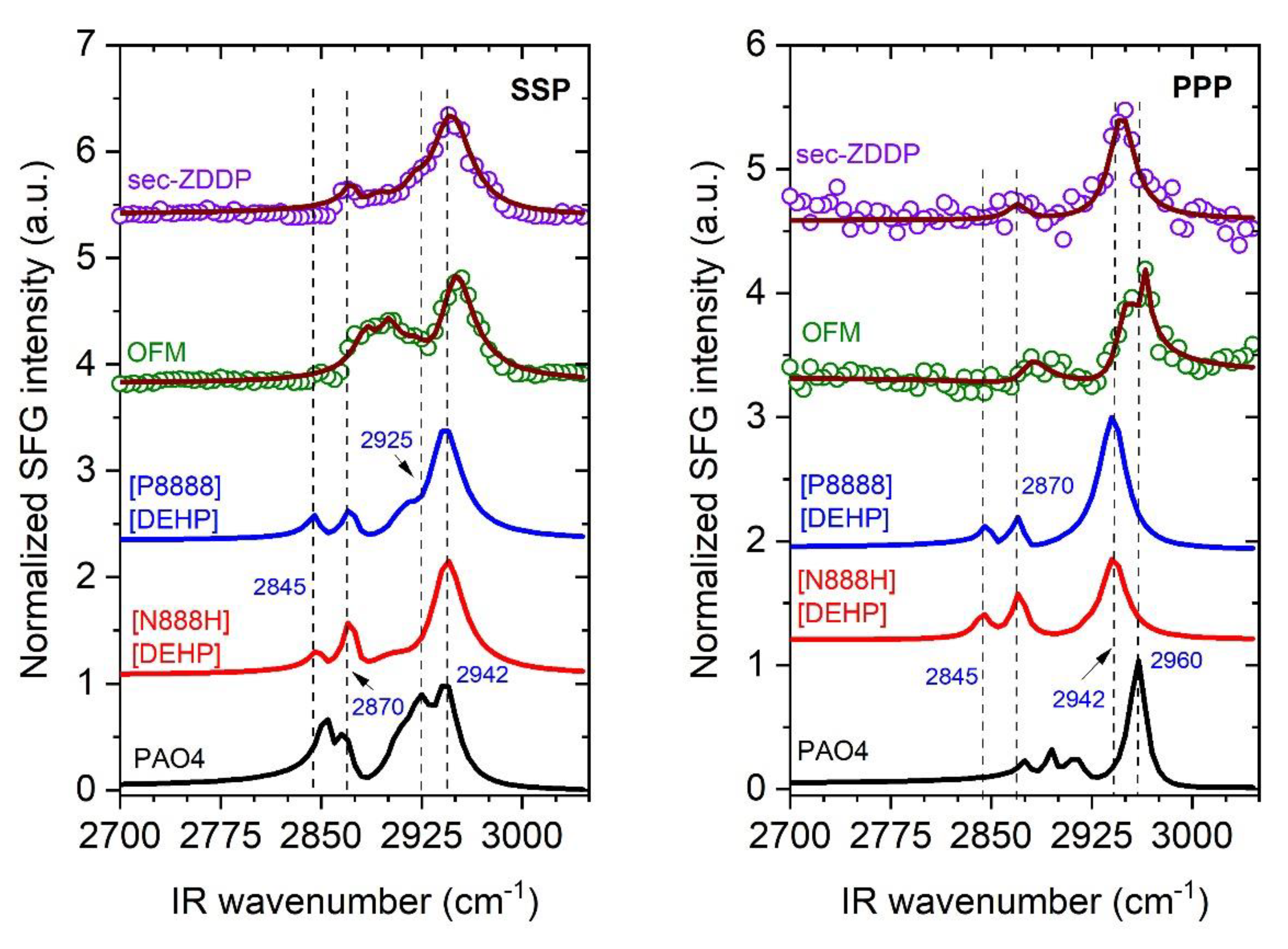
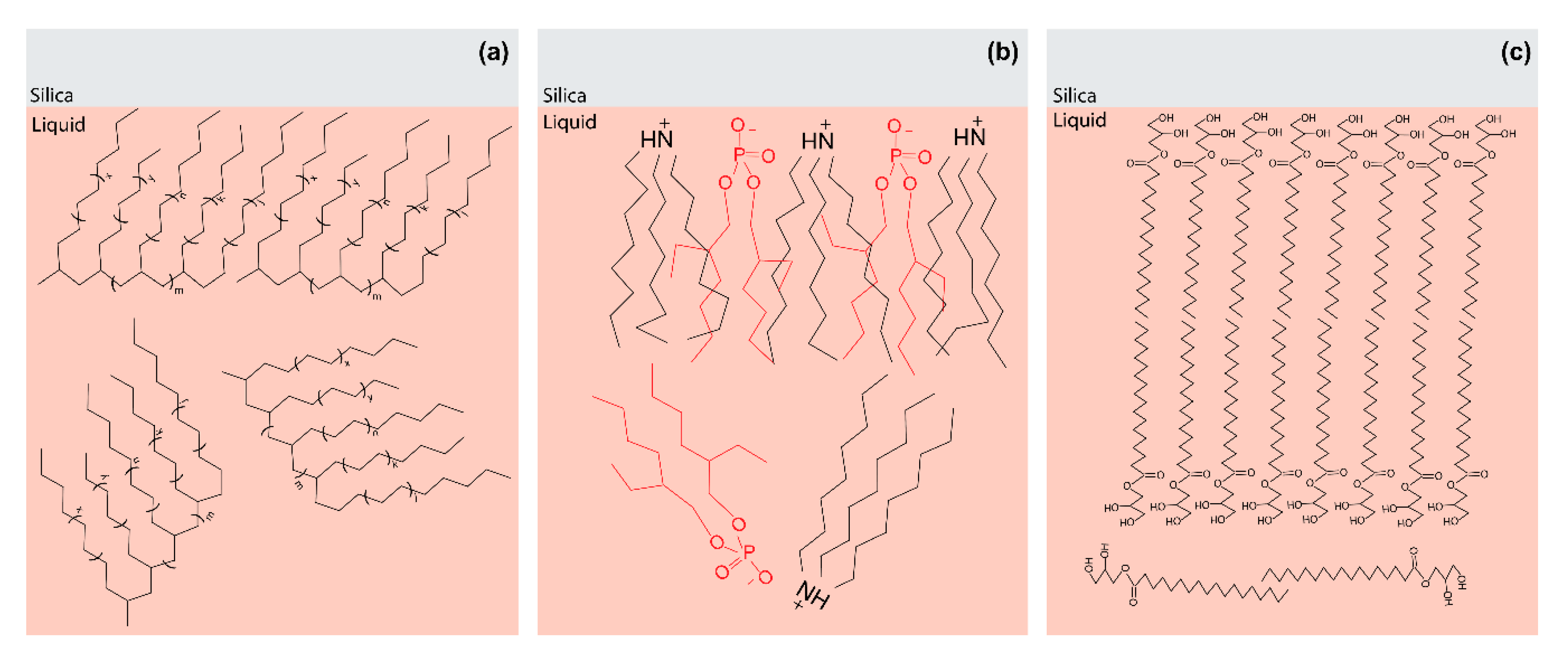

| Sample | Weight Percent Ratio |
|---|---|
| PAO4 + [N888H][DEHP] | 99.13:0.87 |
| PAO4 + [P8888][DEHP] | 98.96:1.04 |
| PAO4 + OFM | 99.20:0.80 |
| PAO4 + PIBSI | 98.00:2.00 |
| PAO4 + sec-ZDDP | 99.20:0.80 |
| PAO4 + OFM + [N888H][DEHP] | 98.33:0.80:0.87 |
| PAO4 + OFM + [P8888][DEHP] | 98.16:0.80:1.04 |
| PAO4 + PIBSI + [N888H][DEHP] | 97.13:2.00:0.87 |
| PAO4 + PIBSI + [P8888][DEHP] | 96.96:2.00:1.04 |
| PAO4 + sec-ZDDP + [N888H][DEHP] | 99.16:0.40:0.44 |
| PAO4 + sec-ZDDP + [P8888][DEHP] | 99.08:0.40:0.52 |
| Functional Group | Frequency (cm−1) | Vibrational Mode |
|---|---|---|
| C–CH2–C [22,25,27,28,31,32,33] | 2845–2858 | CH2,s (d+), symmetric methylene stretch |
| 2896–2930 | CH2,as (d−), asymmetric methylene stretch | |
| 2890–2927 | CH2,s,FR (d+FR), symmetric methylene Fermi resonance | |
| C–CH3 [22,25,26,27,28,31,32,33,34] | 2869–2925 | CH3,s (r+), symmetric methyl stretch |
| 2948–2973 | CH3,as (r−), asymmetric methyl stretch | |
| 2884–2942 | CH3,s,FR (r+FR), symmetric methyl Fermi resonance | |
| O–CH2–C, N–CH2–C, P–CH2–C [29,35,36,37] | 2870–2875 | CH2,s (d+), symmetric methylene stretch |
| 2900 | CH2,as (d−), asymmetric methylene stretch | |
| 2938–2954 | CH2,s,FR (d+FR), symmetric methylene Fermi resonance |
| Lubricant | Interfacial Layer | Tribological Property |
|---|---|---|
| PAO4 + IL | IL molecules dominate the interface [11] | Beneficial effects [9,10] |
| PAO4 + OFM | The interface is dominated by OFM molecules and the surface layer structure is very similar to that of silica/OFM interface. | Beneficial effects [9,41] |
| PAO4 + sec-ZDDP | A mixed layer of PAO4 and sec-ZDDP forms at the silica surface and sec-ZDDP dominates this surface layer. | Beneficial effects [50] |
| PAO4 + PIBSI | PIBSI preferential adsorbs to the silica/liquid interface and seems to lie relatively flat at the surface. | Beneficial effects [10,50] |
| PAO4 + OFM + [N888H][DEHP] | A mixed layer of OFM and [N888H][DEHP] is formed at the silica surface and OFM is the major component of this mixed layer. | Synergistic effects [9] |
| PAO4 + OFM + [P8888][DEHP] | The surface layer is dominated by [P8888][DEHP] and its structure is similar to that formed at silica/(PAO4 + [P8888][DEHP]) interface. | Lubrication efficiency was reduced in comparison to that of (PAO4 + OFM) [9] |
| PAO4 + sec-ZDDP + [N888H][DEHP] | [N888H][DEHP] molecules adsorb to the silica surface to form a surface layer that is similar to that of silica/(PAO4 + [N888H][DEHP] interface. | No synergistic effect due to the presence of [N888H]+[6] (In Ref. [6] the base oil was gas-to-liquid (GTL)) |
| PAO4 + sec-ZDDP + [P8888][DEHP] | The surface layer is dominated by [P8888][DEHP] and its structure is very similar to that of silica/(PAO4 + [P8888][DEHP]) interface. | Reduction of friction by ~30% and wear by >70% [6]. The SFG results show that this improved performance must be related to the properties of the tribofilm formed during the tribological test, rather than the initial surface film before tribo-test. |
| PAO4 + PIBSI + [N888H][DEHP] | PIBSI and [N888H][DEHP] form a mixed layer at the silica surface. The presence of PAO4 in the surface layer is insignificant. | Inferior performance, compared to IL only [10] |
| PAO4 + PIBSI + [P8888][DEHP] | PIBSI possibly dominates the silica/mixed liquid interface. | Inferior performance, compared to IL only [10] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngo, D.; He, X.; Luo, H.; Qu, J.; Kim, S.H. Competitive Adsorption of Ionic Liquids Versus Friction Modifier and Anti-Wear Additive at Solid/Lubricant Interface—Speciation with Vibrational Sum Frequency Generation Spectroscopy. Lubricants 2020, 8, 98. https://doi.org/10.3390/lubricants8110098
Ngo D, He X, Luo H, Qu J, Kim SH. Competitive Adsorption of Ionic Liquids Versus Friction Modifier and Anti-Wear Additive at Solid/Lubricant Interface—Speciation with Vibrational Sum Frequency Generation Spectroscopy. Lubricants. 2020; 8(11):98. https://doi.org/10.3390/lubricants8110098
Chicago/Turabian StyleNgo, Dien, Xin He, Huimin Luo, Jun Qu, and Seong H. Kim. 2020. "Competitive Adsorption of Ionic Liquids Versus Friction Modifier and Anti-Wear Additive at Solid/Lubricant Interface—Speciation with Vibrational Sum Frequency Generation Spectroscopy" Lubricants 8, no. 11: 98. https://doi.org/10.3390/lubricants8110098
APA StyleNgo, D., He, X., Luo, H., Qu, J., & Kim, S. H. (2020). Competitive Adsorption of Ionic Liquids Versus Friction Modifier and Anti-Wear Additive at Solid/Lubricant Interface—Speciation with Vibrational Sum Frequency Generation Spectroscopy. Lubricants, 8(11), 98. https://doi.org/10.3390/lubricants8110098









