1. Introduction
The threats of climate change and depletion of fossil fuels have urged the adoption of policies that can reduce greenhouse gas emissions and dependency on gasoline and diesel. One approach used by some countries—in particular Brazil [
1], USA [
2], Belgium [
3], Sweden [
4], and Canada [
5]—are national programs to stimulate the production and use of renewable liquid fuels [
6]. In spark ignition engines, the most widely used such renewable fuel is ethanol. Despite concerns regarding the large amount of arable land required for crops and ethanol production’s impact on grain supply, CO
2 emissions for ethanol fuel are lower than gasoline even when the total ethanol production cycle is considered [
7]. In addition, the presence of particulates in the exhaust system is reduced when the fuel contains ethanol [
7,
8].
However, analysis of lubricants after engine tests shows that unburnt ethanol fuel can contaminate the lubricant [
9,
10,
11,
12], thus resulting in tribological challenges to the engines. It is common knowledge among users that engine parts running on fuels containing large proportions of ethanol tend to suffer more severe wear when compared with gasoline-fueled engines. For this reason, recent work measured the thickness of boundary films formed by the antiwear additive zinc dialkyldithiophosphate (ZDDP) in lubricants contaminated with ethanol. When compared with neat lubricants, the contaminated lubricants presented much lower rates of ZDDP tribofilm formation, resulting in very thin tribofilms. In other tests where a thick and stable tribofilm was allowed to form from the neat lubricant and ethanol was later added, rubbing in the presence of ethanol led to significant removal of the pre-formed tribofilm [
13]. Advanced chemical analysis of the tribofilms formed in the presence of ethanol has suggested oxidation of sulfur species into sulfites and reduction in the length of the phosphate chains [
14]. Water contamination has also been shown to reduce the thickness and change the structure of ZDDP tribofilms, apparently by reducing the length of the polyphosphate chains [
15].
On the other hand, the effects of ethanol contamination on friction are not always detrimental. De Silva and coworkers have shown that in combination with water, ethanol can produce a considerable friction reduction [
12,
16]. Our previous work has also shown friction reduction for lubricants contaminated with anhydrous ethanol and hydrated ethanol under certain conditions because ethanol fuel can be either anhydrous (when supplied dissolved in gasoline) or hydrated when used on its own. Film thickness was measured using ultrathin interferometry and Stribeck curves were obtained using a mini-traction-machine (MTM) test rig. Ethanol reduced film thickness and friction in the full-film and mixed elastohydrodynamic lubrication (EHD) regimes because the lubricant viscosity was lowered. In the boundary regime, film thickness increased and friction was reduced for ethanol/water in base oil, but the opposite (thinner boundary film and higher boundary friction) was observed in a formulated oil without friction modifiers. For the base oil, apparent oxidation reduced boundary friction, whereas for the formulated oil ethanol and water hindered the formation of detergent and ZDDP tribofilms [
17]. However, none of these studies addressed how ethanol interacts with friction modifiers present in the lubricant.
Friction modifiers (FMs) are added to engine lubricants to reduce friction in the boundary lubrication regime. The two main groups of FMs are organic friction modifiers (OFMs) and organomolybdenum-based additives [
18]. OFMs are long chain surfactants with a polar group that adsorbs onto metal surfaces preventing metal-to-metal contact and a lipophilic group (long alkyl chain) that enables additive solution in the lubricant, as well as providing a “cushioning effect” in the adsorbed layer that further prevents metallic contact [
19]. The adsorbed film can consist of either monolayers or thick viscous layers. For example, copper carboxylate, which is widely used as organic friction modifier, tends to form thick boundary films. Also, it has been shown that for other OFMs such as carboxylic acids, the presence of water induces the formation of thick boundary films, apparently by the formation of an iron carboxylate film [
18]. Therefore, a strong interaction between OFMs, ethanol, and water might be expected, particularly considering the strong interaction between alcohols and additives reported in the literature [
20].
Mo-based friction modifiers were originally developed as anti-wear additives, but can also provide very low boundary friction under certain contact conditions and thus have been widely used as friction modifiers in engine oils since the 1980s [
18]. Oil-soluble organo-molybdenum additives differ from colloidal molybdenum disulphide (MoS
2) used in oils and greases since the 1940s [
21] because they dissolve in the lubricant and only form low-friction MoS
2 when rubbing between the surfaces occurs. Molybdenum dialkyldithiocarbamate (MoDTC) is the most widely used oil-soluble organo-molybdenum friction modifier additive. The additive functions by forming nanosheets of MoS
2 in the tribofilm. Although these are very small and only cover a small proportion of the surface they form preferentially on the tips of the asperities and therefore are very efficient in reducing friction [
22]. Initial friction for lubricants containing MoDTC is high but it then drops to much lower values when the formation of MoS
2 is activated. Higher temperatures and rougher surfaces tend to favor the decomposition of MoDTC and the formation of low-friction MoS
2. However, whereas fully-formulated oils containing MoDTC showed friction reduction in sliding reciprocating tests, when these oils were contaminated with ethanol no friction drop was observed [
18]. These results suggest that ethanol can have a negative impact on MoS
2 formation. However, since the oils were fully formulated, it was difficult to assess the interaction of ethanol solely with MoDTC.
The present work aims to investigate how ethanol affects friction and film formation of both organic and soluble organo Mo-based friction modifier solutions in mineral base oil.
2. Materials and Methods
2.1. Materials
In order to investigate the interaction between friction modifiers and ethanol, the friction modifiers were added to an otherwise additive-free Group I base oil, thereby precluding possible interactions with other engine oil additives. Small amounts of ethanol (5 wt %), typical of those reached in practice, were employed. Both anhydrous and hydrated ethanol were studied, since for engines running on neat ethanol this is generally hydrated with up to 5.5 wt % water, the azeotrope, to reduce production costs, whereas when ethanol is used in a gasoline/ethanol blend it must be anhydrous because water is not soluble in gasoline. In fact, it is plausible that in a real engine the ethanol contaminating the lubricant is more likely to be hydrated even if the fuel is anhydrous ethanol due to the water generated from the combustion and the large affinity between water and ethanol. However, even if that is the case, the investigation of both anhydrous and hydrated ethanol is highly desirable because it enables one to observe effects that are exclusively due to the presence of water.
One MoDTC and three organic friction modifiers (OFM01, OFM02, and OFM03) were tested. The elements other than H, C, O, and N present in the additives are listed in
Table 1 and except for Mo and S in the MoDTC are at very low concentration. The amounts of friction modifier added followed recommendation from the suppliers: 0.9 wt % for MoDTC, 1.2 wt % for OFM01, 0.5 wt % for OFM02 and OFM03. The oil samples containing the additives were heated to 50 °C and magnetically stirred for 3 hours until total solution occurred. All the fluid samples investigated are summarized in
Table 2, where the symbols that will be used to represent the different test conditions in the graphs presented in the Results section are listed. The test temperatures were 40, 70 and 100 °C. The test temperatures of 40 and 100 °C are commonly used for tests of engine oils. The temperature of 40 °C tries to simulate lubricant temperatures when the engine runs at lower speeds and short distances, such as some urban drive conditions, whereas higher temperatures (100 °C) relate to higher speed driving conditions. The intermediate temperature of 70 °C was chosen because it is close to the evaporation point of ethanol, allowing the simulation of a higher temperature condition without substantial ethanol evaporation from the lubricant.
An SVM3000 Stabinger viscometer (Anton-Paar, Graz, Austria) was used to measure the rheological properties (
Table 3) of the fluids at the test temperatures, 40, 70, and 100 °C, but viscosity and density of the fluids containing ethanol could not be measured at 100 °C due to rapid evaporation of ethanol, which formed numerous bubbles in the measurement tubes. Anhydrous ethanol (AE) reduced the viscosity more than hydrated ethanol (HE), as noted in previous studies [
13,
17].
2.2. Friction Tests
Friction tests were carried out using a mini traction machine (MTM) in which a ball is loaded and rotated against the flat surface of a rotating disc. Ball and disc are driven by two independent DC motors so that any combination of rolling and sliding can be obtained. In this study, friction was measured over a range of entrainment speeds (mean rolling speed) at a fixed slide roll ratio (SRR) to obtain Stribeck curves. SRR, which was held constant at a value of 50%, is defined as (Uball − Udisk)/U, where |Uball − Udisk| is the sliding speed and U is the entrainment or mean rolling speed, given by U = (Uball + Udisk)/2. When SRR = 0% (i.e. Uball = Udisk), pure rolling contact occurs and when SRR = 200% (i.e. either Uball or Udisk = 0), pure sliding contact occurs, while values between 0 and 200% represent mixed sliding/rolling contact. The tests started at a mean rolling speed of 1000 mm/s, which was continuously reduced (in 31 logarithmically-spaced stages) down to 1 mm/s. The disc was fully-immersed in lubricant and the temperature of the lubricant and contact was controlled to a set value within ±0.5 °C. Set temperatures of 40, 70, and 100 °C were studied. The balls were AISI 52100 bearing steel with diameter 19 mm, Young′s modulus 210 GPa and nominal root mean square roughness (Rq) between 10 and 13 nm while the discs were AISI 52100 steel with nominal Rq between 25 and 30 nm. The load applied was 20 N, corresponding to a maximum Hertz pressure of 0.82 GPa. Fresh balls and disks were used in each test, and were ultrasonically cleaned in Analar toluene followed by Analar acetone prior to a test. The rig was cleaned using toluene followed by Analar isopropanol before each test. At least two repeats were carried out for each test condition.
Molybdenum dialkyldithiocarbamate (MoDTC) additives are able to reduce friction coefficient in thin film, boundary lubrication conditions. It is accepted that the tribochemical reactions that lead to the formation of a low friction MoS
2 tribolayer on the surfaces are most likely to occur in low entrainment speed and thus thin film conditions where there is direct sliding asperity–asperity interaction [
18]. Therefore, before obtaining Stribeck curves, the samples were subject to rubbing at a normal load of 20 N and entrainment speed of 20 mm/s for 10 min at a slide-to-roll ratio (
SRR) of 50%. In contrast, organic friction modifiers are expected to operate via adsorption of surfactants, therefore no prior rubbing was carried out for the MTM tests with the oils containing organic friction modifiers. Since the adsorption of surfactants is diffusion controlled, similar tests were carried out leaving the samples immersed in oil after stabilization of the test temperatures for both 10 min and 2 h before the Stribeck curves were obtained, but no significant difference was observed.
2.3. EHL Film Thickness Measurements
Ultrathin interferometry was used to measure EHL and mixed lubrication film thickness. In this, white light shines onto the contact between a flat semi-reflective glass disc and a reflective steel ball. The incident light and the light that travels through the lubricant recombine and optically interfere. The lubricant film thickness is then calculated from the wavelength of maximum constructive interference and from the refractive index of the lubricants measured at all the test temperatures (
Table 4).
These tests were only carried out on the blends with organic friction modifiers since, with MoDTC, the very thin film rubbing conditions necessary for tribofilm formation would damage the semi-reflective coating on the glass disk.
In the ultrathin interferometry tests, the disc was rotated and drove the ball (diameter 19 mm, AISI 52100 steel) in nominally pure rolling. The applied load was 20 N, giving a maximum contact pressure of 0.52 GPa. A new steel ball was used for each test. Disc, ball, and test chamber were thoroughly rinsed using analar toluene followed by analar isopropanol prior to each test. First, a test was carried out at 40 °C, followed by a test at 70 °C and then a final test at 100 °C within the same test sequence, i.e., the balls and disk were not cleaned between the tests, nor was the lubricant sample changed. Mean rolling speed increased from 1 to 1000 mm/s in 31 stages with at least three film thickness values measured and averaged at each speed stage.
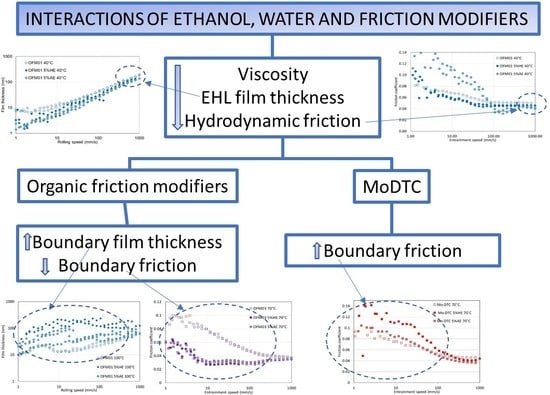
4. Discussion
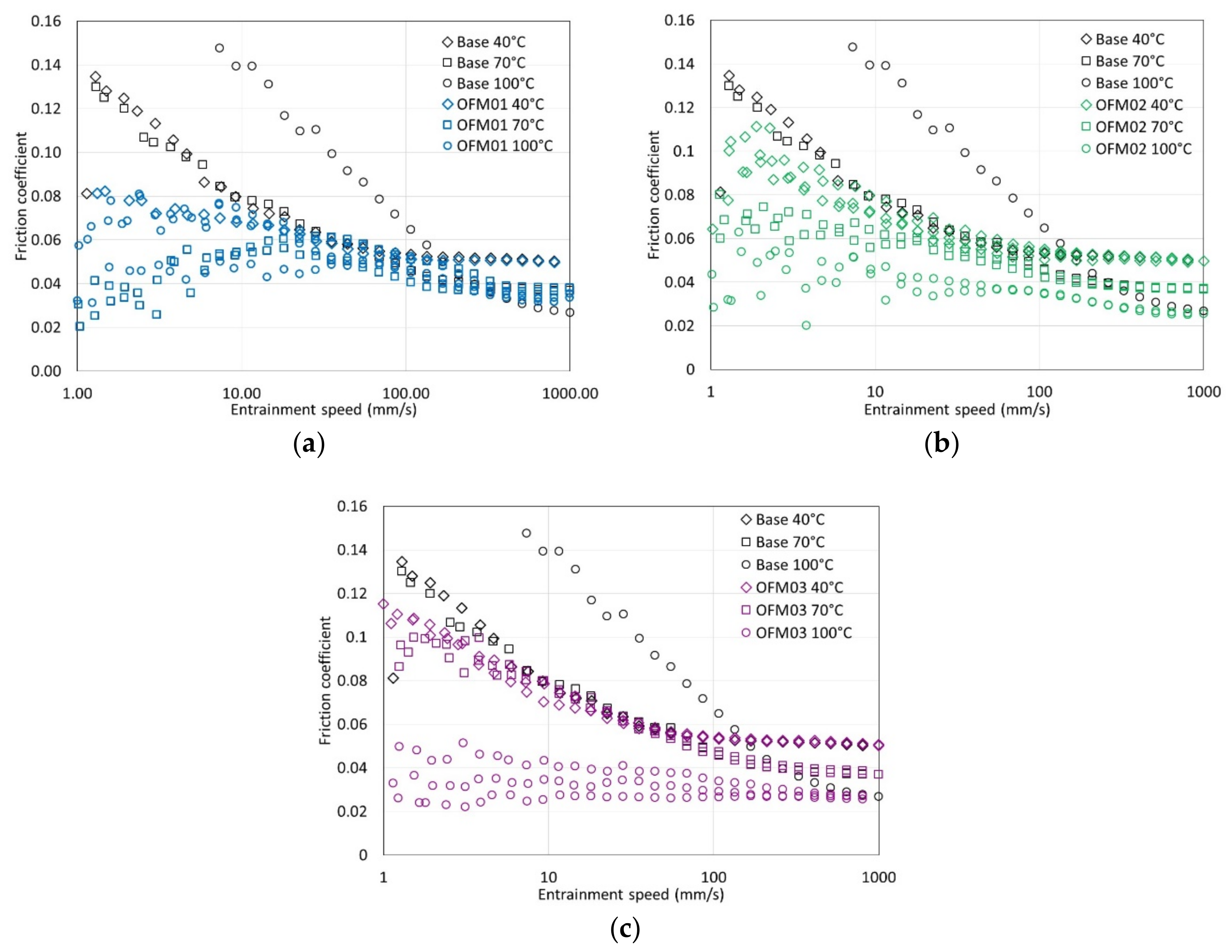
This work shows interactions between ethanol, water, and different types of friction modifiers. Regarding the organic friction modifiers, three different commercial additives were tested. Analyzing the neat lubricants without ethanol, all three organic friction modifiers gave low boundary friction, which could be associated with a measurable boundary film, but there were only small differences between them. OFM01 showed some friction reduction at 40 °C, but this reduction was more significant at 70 and 100 °C. Film thickness measurements showed that the thickness of the boundary film increased with test temperature. At 100 °C the boundary film appeared to be viscous in that it initially developed as speed increased as though being entrained. Many commercial OFMs, such as copper carboxylate, show similar behaviour at higher test temperatures, based on thick boundary-film formation due to a surface deposit which is entrained at low speeds but lost at high speeds [
18]. OFM02 and OFM03 also showed lower boundary friction as temperature increased, but the thickness of the boundary film did not increase with test temperature or speed. This might suggest that the boundary film results from a chemical reaction forming ferrous-based films [
18].
The effects of ethanol were different under full film and boundary lubrication conditions. Under full film lubrication, ethanol always reduced film thickness and friction and the reduction in film thickness and friction was larger for AE than for HE. This can be attributed to the effects of ethanol on viscosity shown in
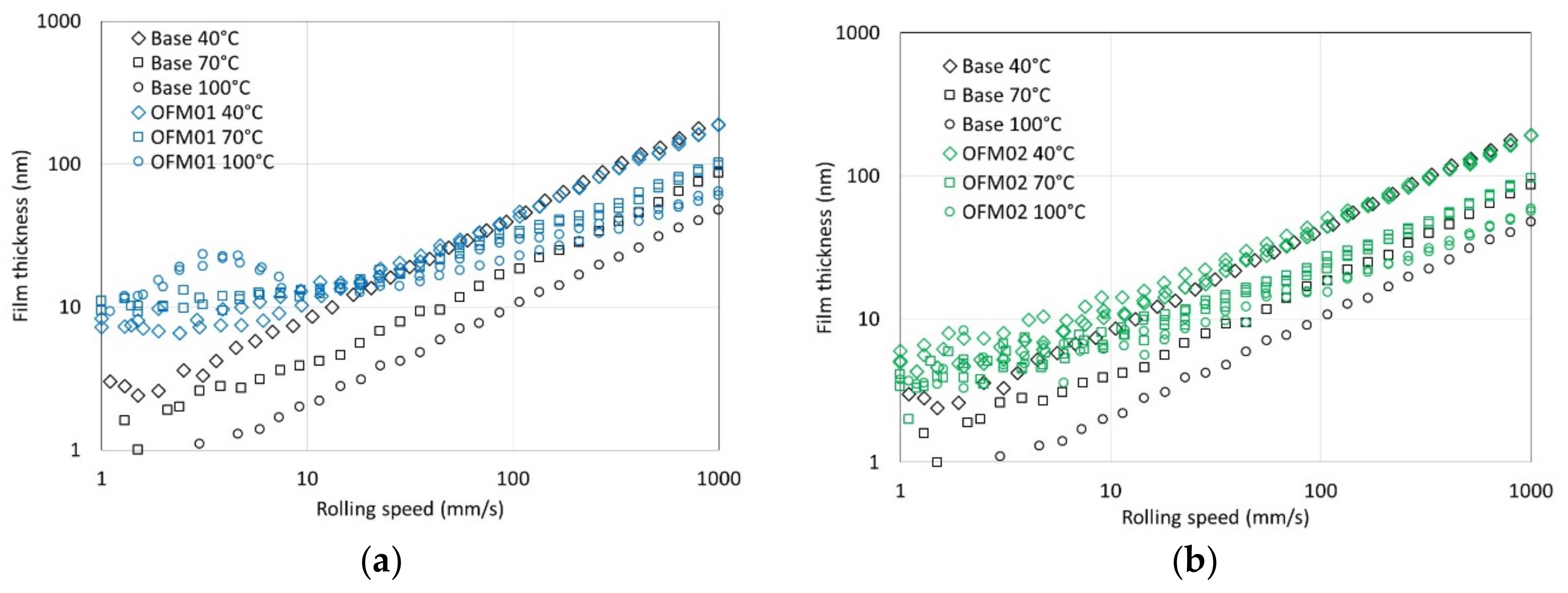
Table 3, probably because water reduces the true solubility of the ethanol in the oil so that some ethanol forms a microemulsion with water, thus having less effect on the lubricant´s viscosity. The reduction in viscosity reduces viscous losses when shearing the lubricant, but it also induces thinner EHL films, increasing the likelihood of asperity contact as speed reduces.
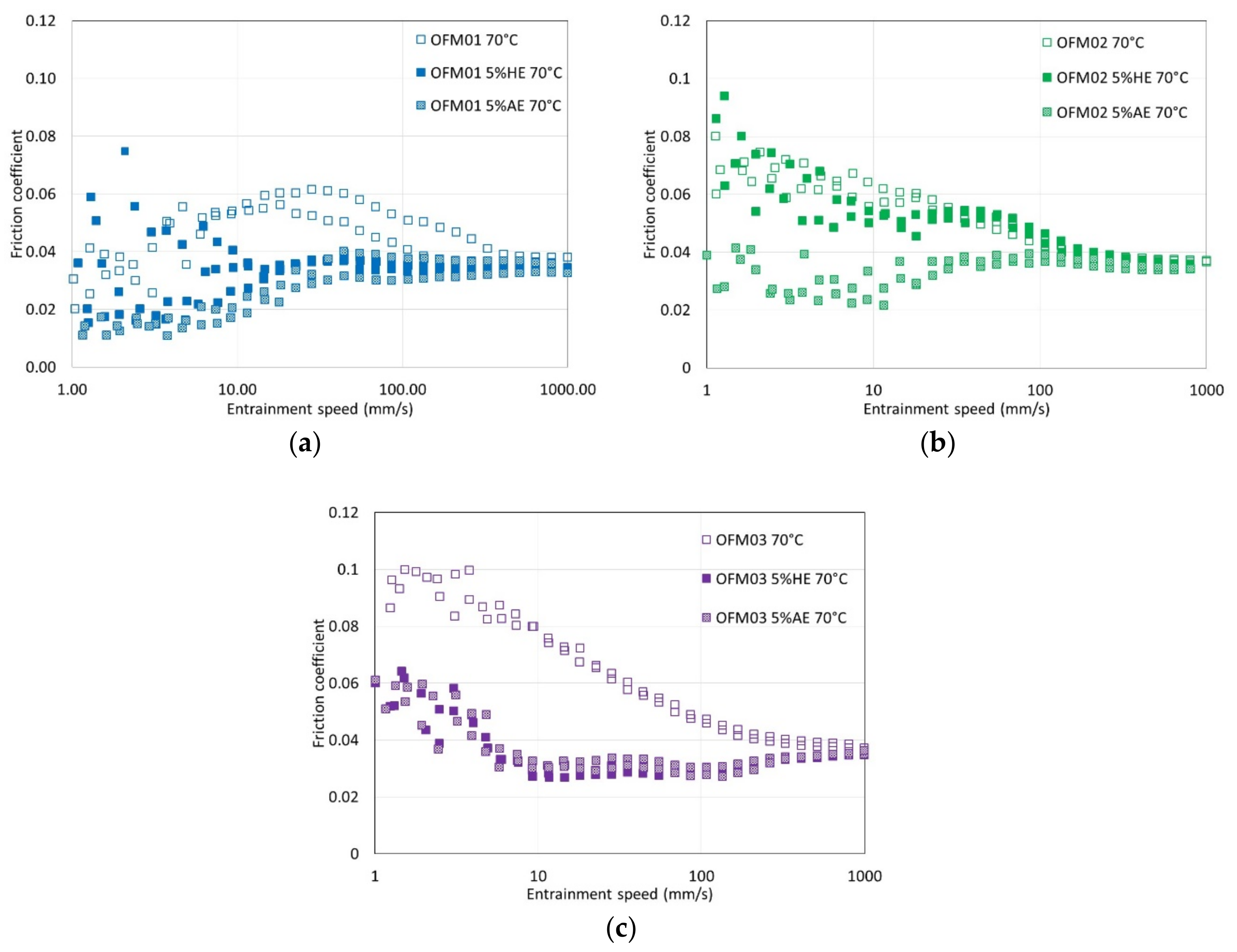
For boundary and mixed conditions, the effects of the addition of ethanol on film thickness depended on the temperature and type of OFM. In none of the conditions tested did the addition of HE or AE stop the formation of low friction boundary films, but their effect varied. In fact, for most of the conditions tested, the addition of ethanol reduced friction coefficient. Also, for all conditions tested, friction reduction due to the addition of AE was generally larger or at least similar to that promoted by HE.
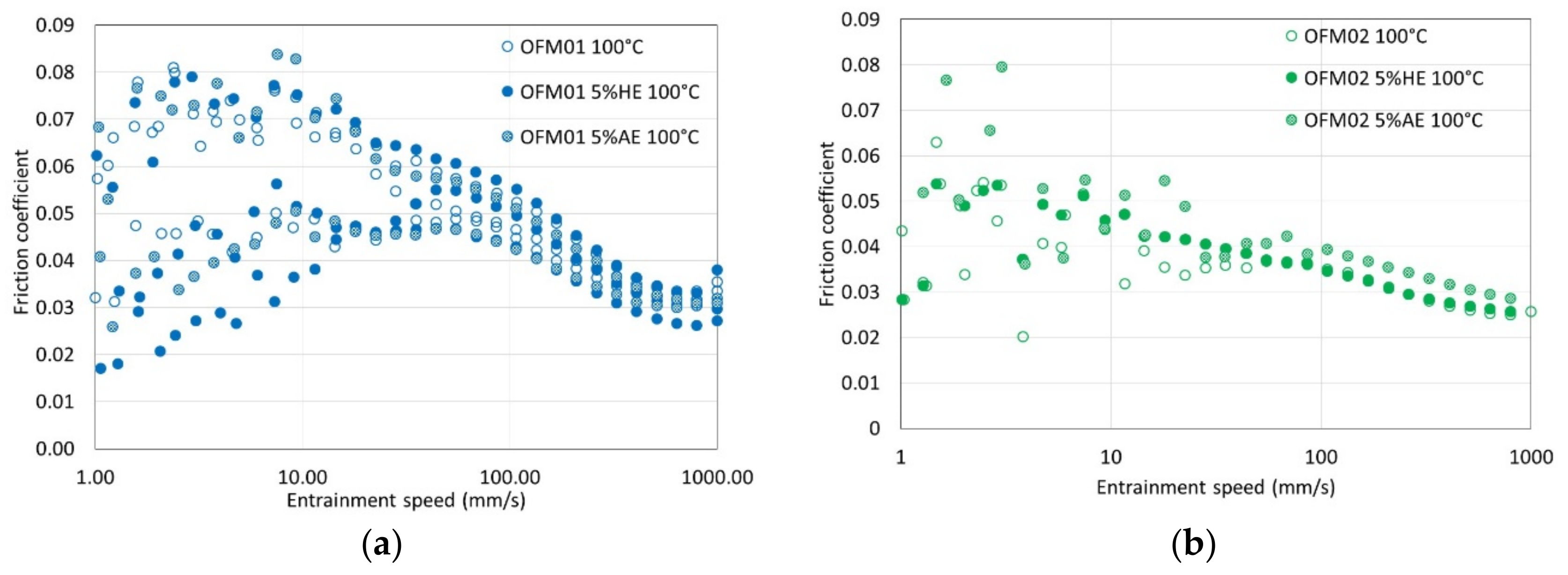
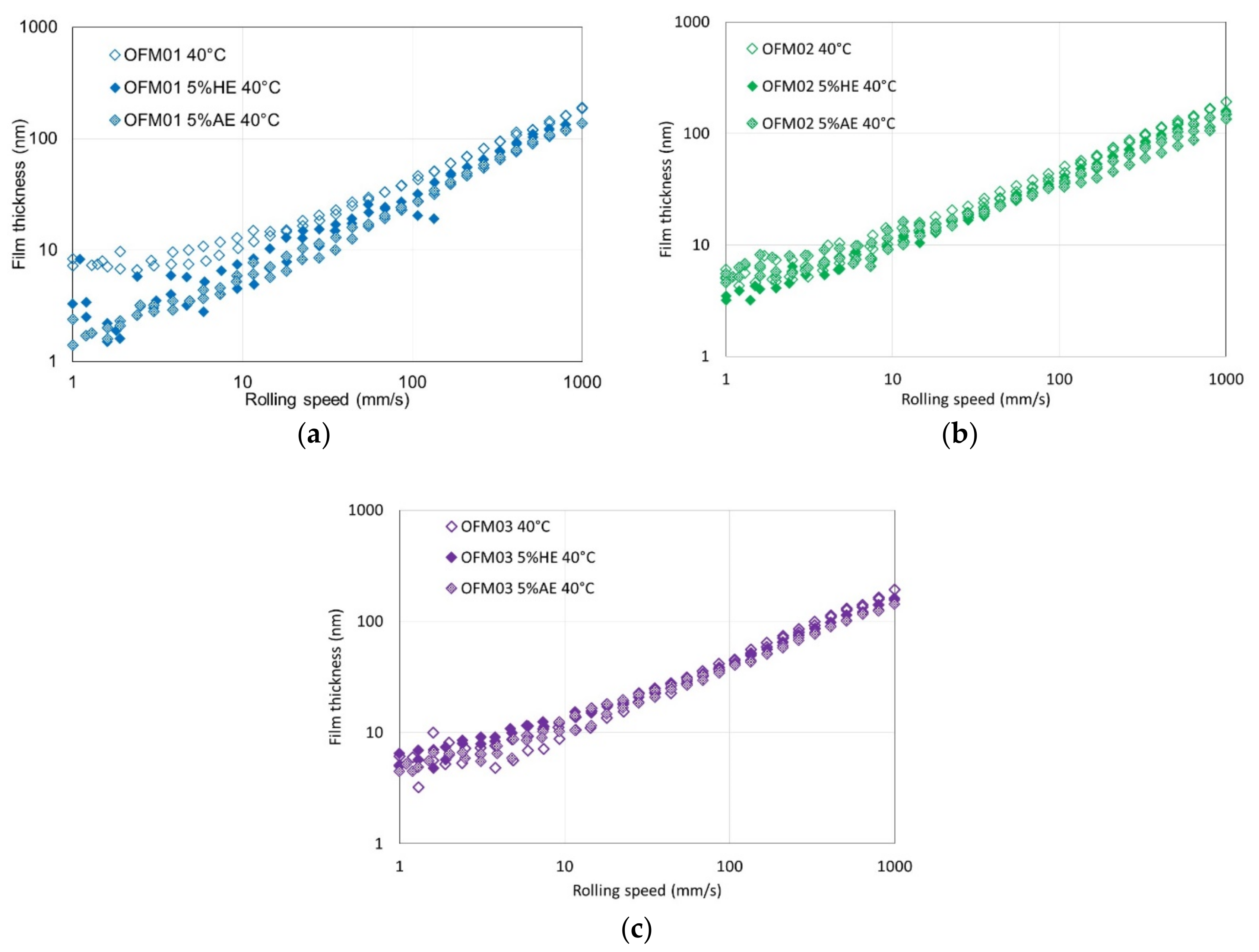
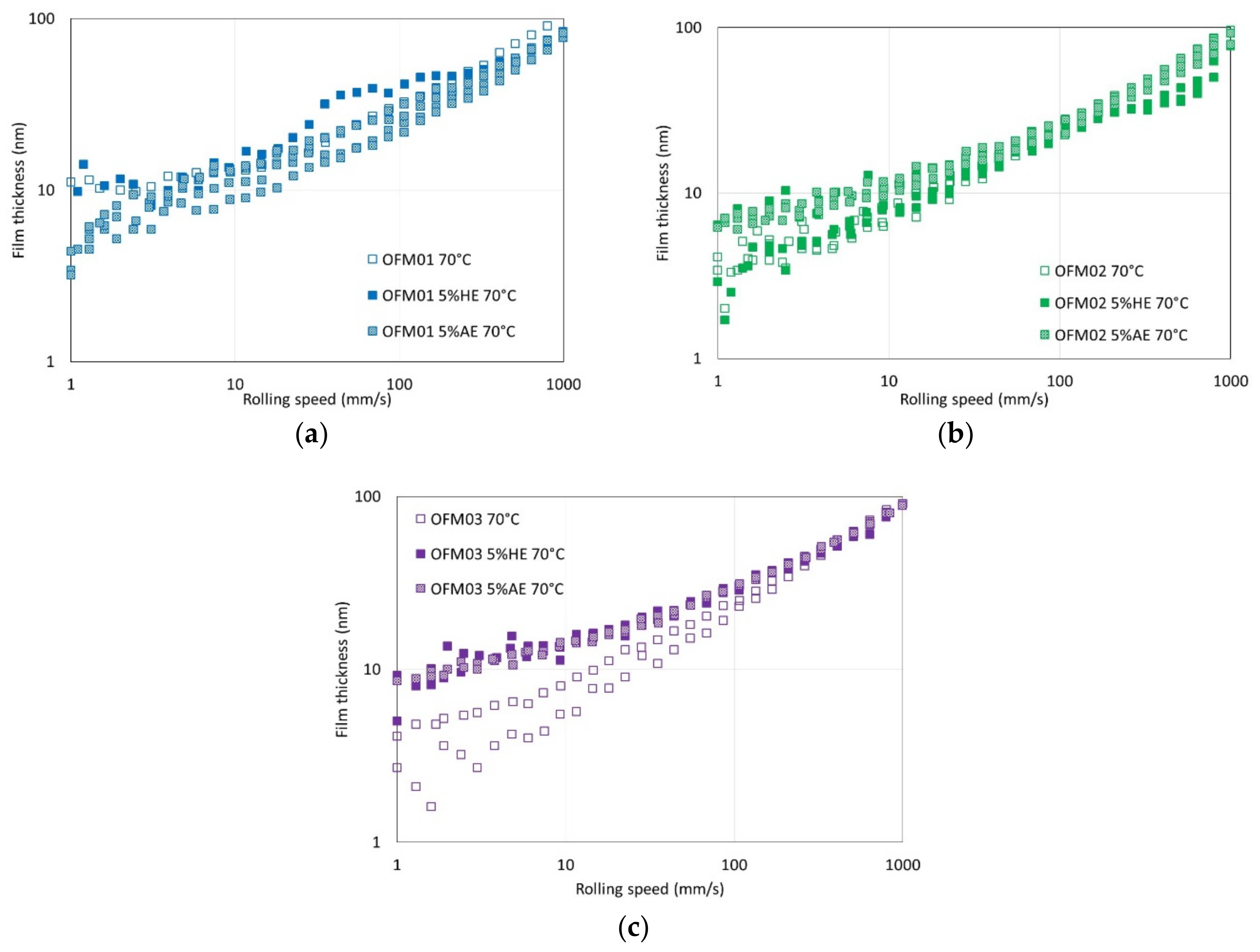
For OFM01 at low temperatures (40 and 70 °C) AE reduced the thickness of the boundary film and the thickness increased with speed, suggesting that pure ethanol might contribute to a viscous boundary film. At 100 °C very thick viscous boundary films were formed, in particular with HE, which resulted in the formation of films with thickness above 100 nm at 100 °C. Boundary friction in the presence of ethanol was also very low at higher temperatures. It can be hypothesized that ethanol at higher temperatures interacts with OFMs to form very thick viscous boundary films, possibly involving chemical reactions that are favored by higher temperature. Since the boundary films were thicker for HE than AE, probably the presence of water interacts strongly with the structure of the boundary film formed. For OFM02 and OFM03, at higher temperatures ethanol also led to thicker boundary films, in particular HE, although much thinner than for OFM01.
Another relevant remark is that for all the film thickness tests carried out with OFM01 in the presence of HE, the reflective disc appeared severely damaged at the end of the test, although this did not happen when AE was added. On the ball, apparently some solid film was formed both in the presence of AE and HE, and it was not removed by rinsing with toluene. For the tests with OFM02 and OFM03 with addition of HE, visual observation of the ball again showed some apparent film, which was not removed by rinsing with toluene, but the severe damage to the reflective disc observed for OFM01 did not happen. The films formed on the ball were apparently oxidation but they need to be further investigated in future works. Taking into account that the film thickness in the boundary region was larger for HE but friction was lower for AE, it is possible that HE leads to more severe oxidation of the metallic surfaces whereas AE might interfere more directly with the structure of the adsorbed boundary film.
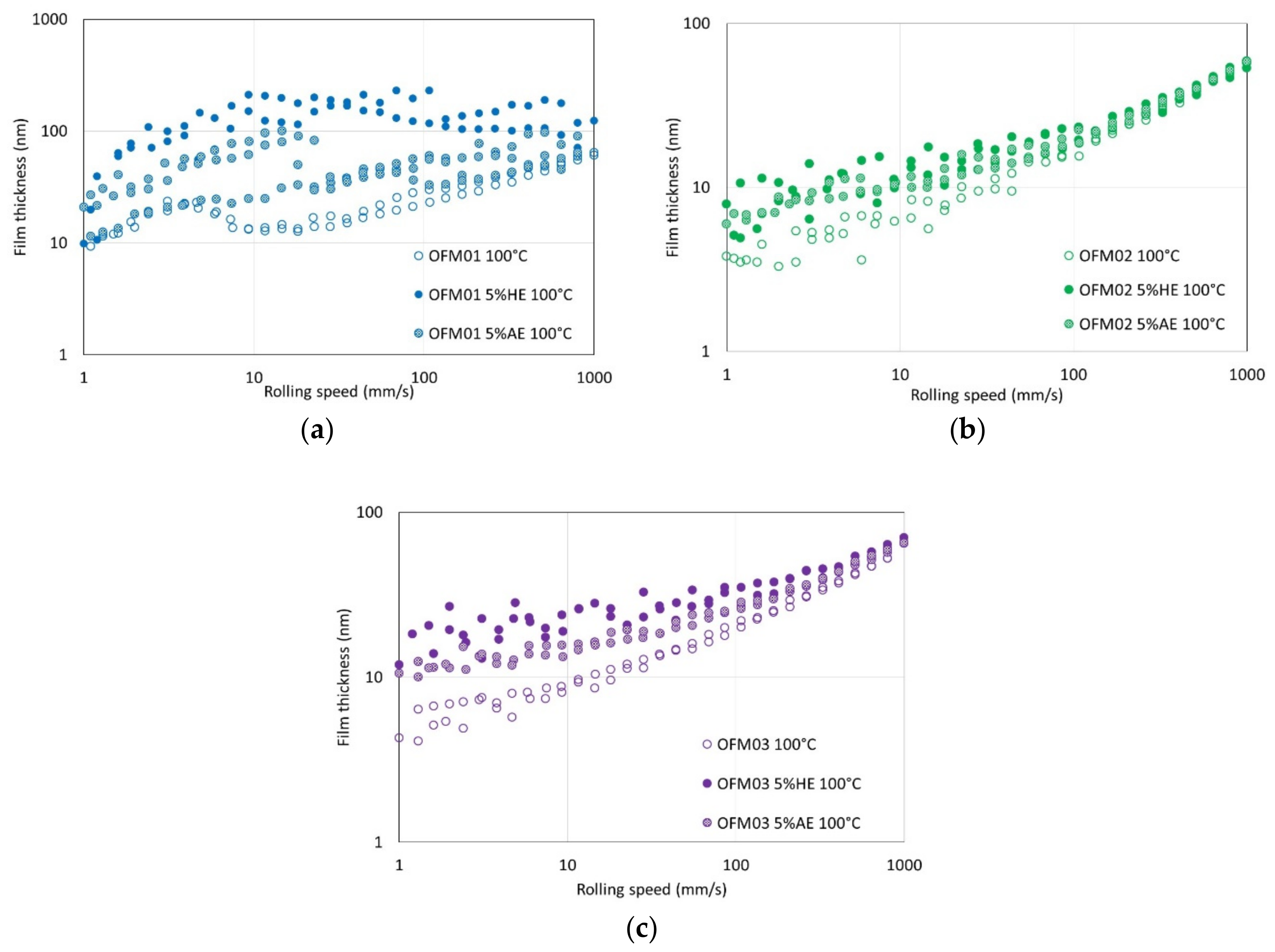
At 100 °C, the effects of ethanol on friction were negligible for the three different OFMs. The most likely reason is ethanol evaporation since this temperature is substantially higher than the boiling point of ethanol. To verify this hypothesis, a simple experimental technique developed in a previous work was employed to estimate the amount of ethanol at the end of the tests [
17]. In this technique, ethanol is extracted from the lubricant by aqueous extraction. After separation from the lubricant, the refractive index of the extraction water is measured. Previous calibration of refractive measurements of water containing different amounts of ethanol allowed to estimate the amount of ethanol in the extraction water. Further details of the technique can be found in [
17]. The amounts of ethanol after the tests using this technique are presented in
Table 5. This refractive index-based technique showed that the amount of ethanol remaining in the lubricants at the end of the MTM tests at 100 °C was very small, indicating that ethanol did not affect friction at 100 °C because ethanol evaporates rapidly at this temperature. On the other hand, the amounts of ethanol after the film thickness measurement tests (EHD) were higher. Reasons that could contribute for this are: (i) the EHD test chamber is more hermetically closed than the chamber for the MTM tests; and (ii) the volume of lubricant used in the EHD tests is substantially larger. Also, the EHD tests started at low speeds which then increased, ensuring a large amount of ethanol available when the boundary film formed, whereas the MTM started at higher speeds which then decreased, so that the boundary films would form at the end of the tests, when the amount of ethanol available was substantially smaller. Probably due to this combination of factors, the effects of ethanol on friction were negligible for the oils containing organic friction modifiers at 100 °C, but not on boundary film thickness.
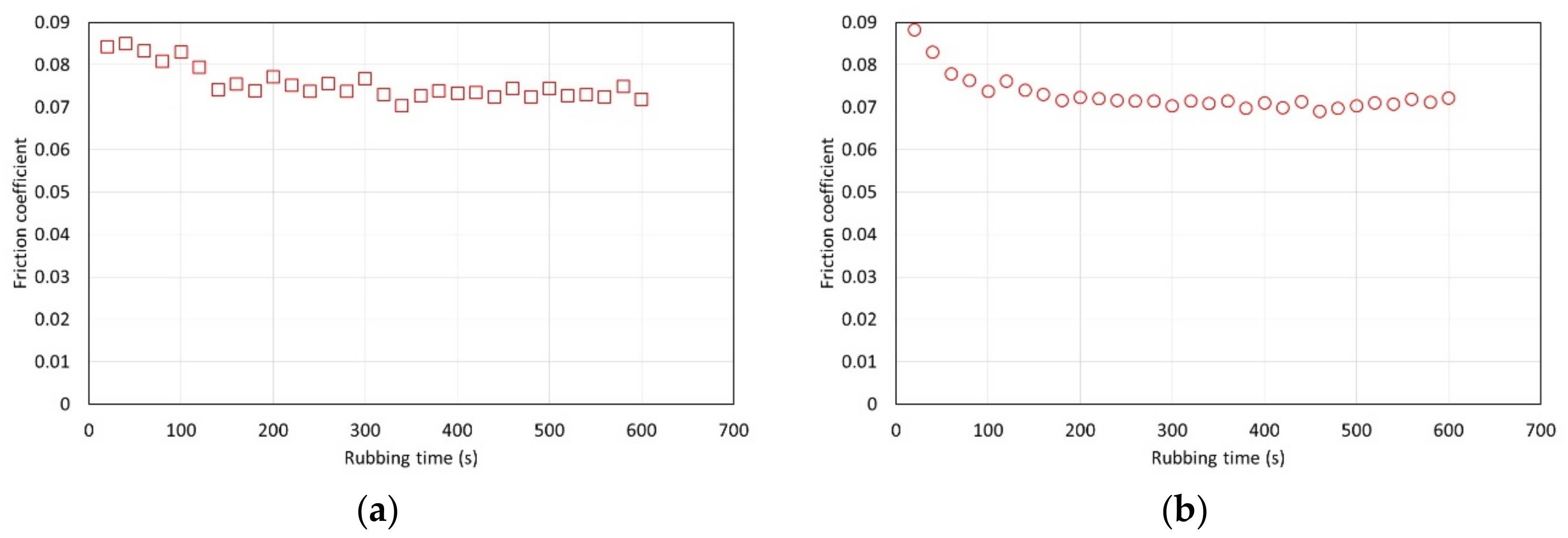
The tests with MoDTC showed that at 40 °C the additive was not properly activated, since the formation of a low boundary film was not observed, but that boundary friction reduced with test temperature. The literature has indeed shown that activation of MoDTC in MTM tests is very challenging and often requires the use of higher test temperatures and rougher disks to induce asperity contact, apparently necessary for the tribochemical reactions involved in the decomposition of the additive and formation of low friction MoS
2 nanosheets [
18].
For the tests with MoDTC at 70 °C the presence of ethanol increased boundary friction compared with the neat lubricant (without ethanol). This suggests that ethanol interferes with the chemical reactions involved in the formation of a low friction tribofilm. Also, this effect is more significant for HE than for AE, indicating that the presence of water is further detrimental to the formation of the low friction film. This could be related for example to oxidation leading to formation of MoO
3 in detriment to MoS
2. At 100 °C, the effects of ethanol on boundary friction disappeared, also probably attributable to the large ethanol evaporation at this temperature (see
Table 5), although the film might also be more stable at this temperature.