Effect of Humidity on Friction and Wear—A Critical Review
Abstract
1. Introduction
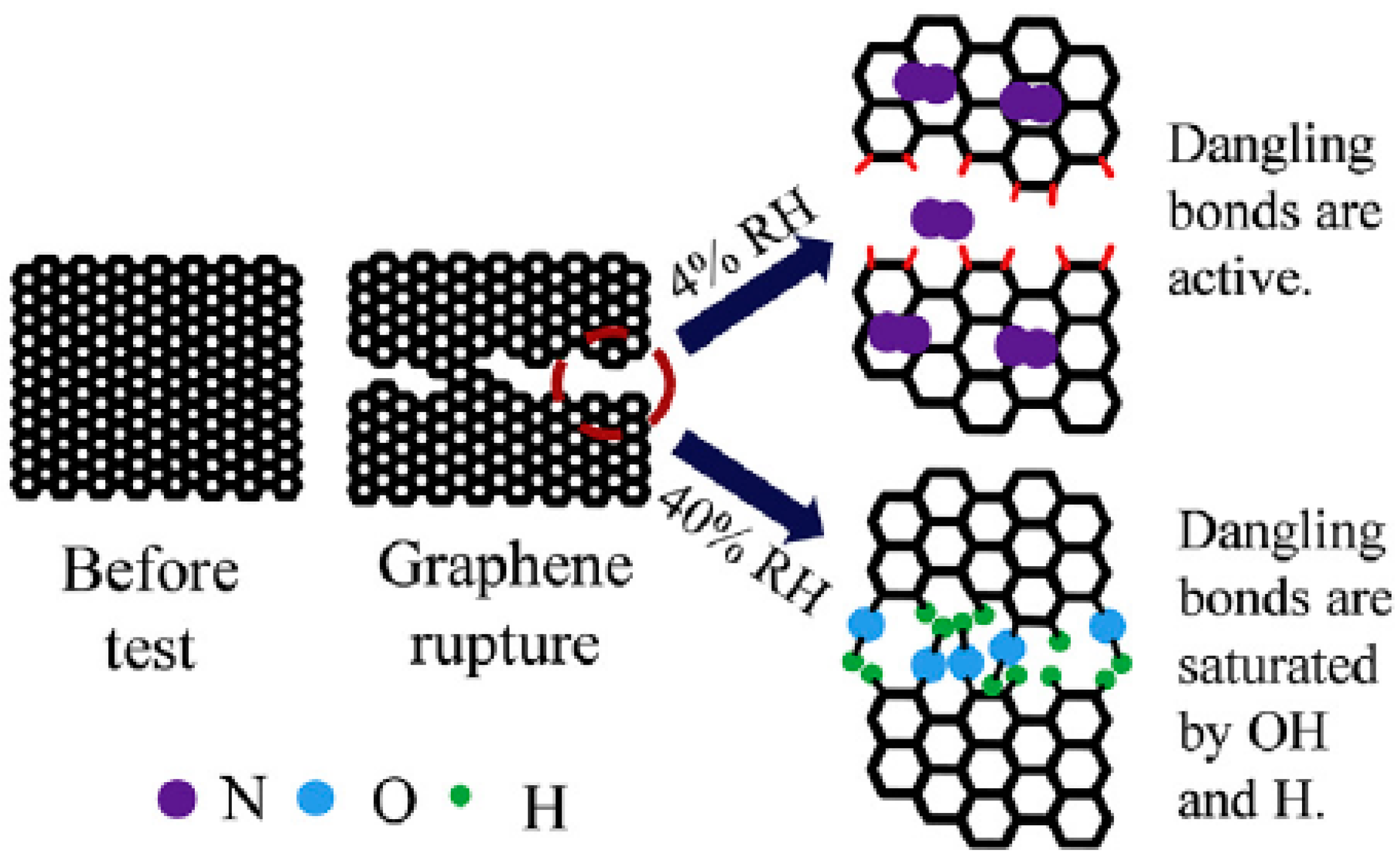
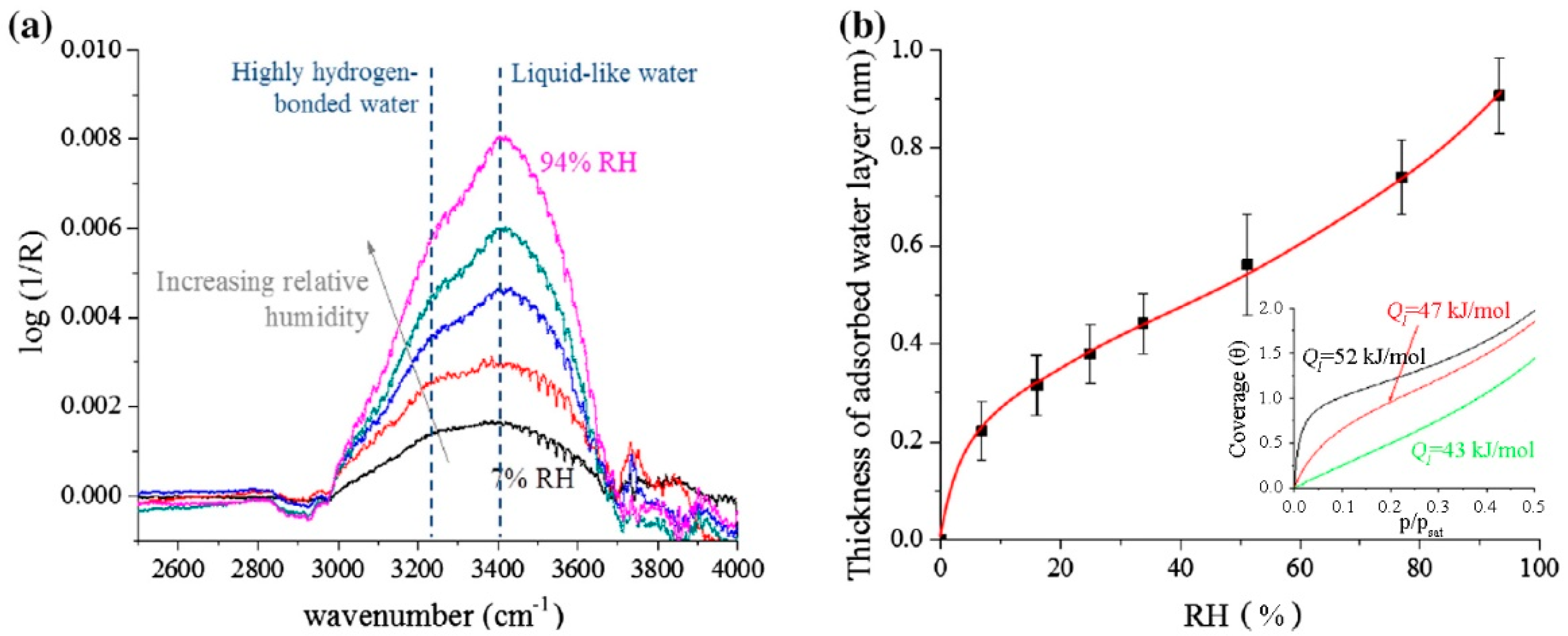
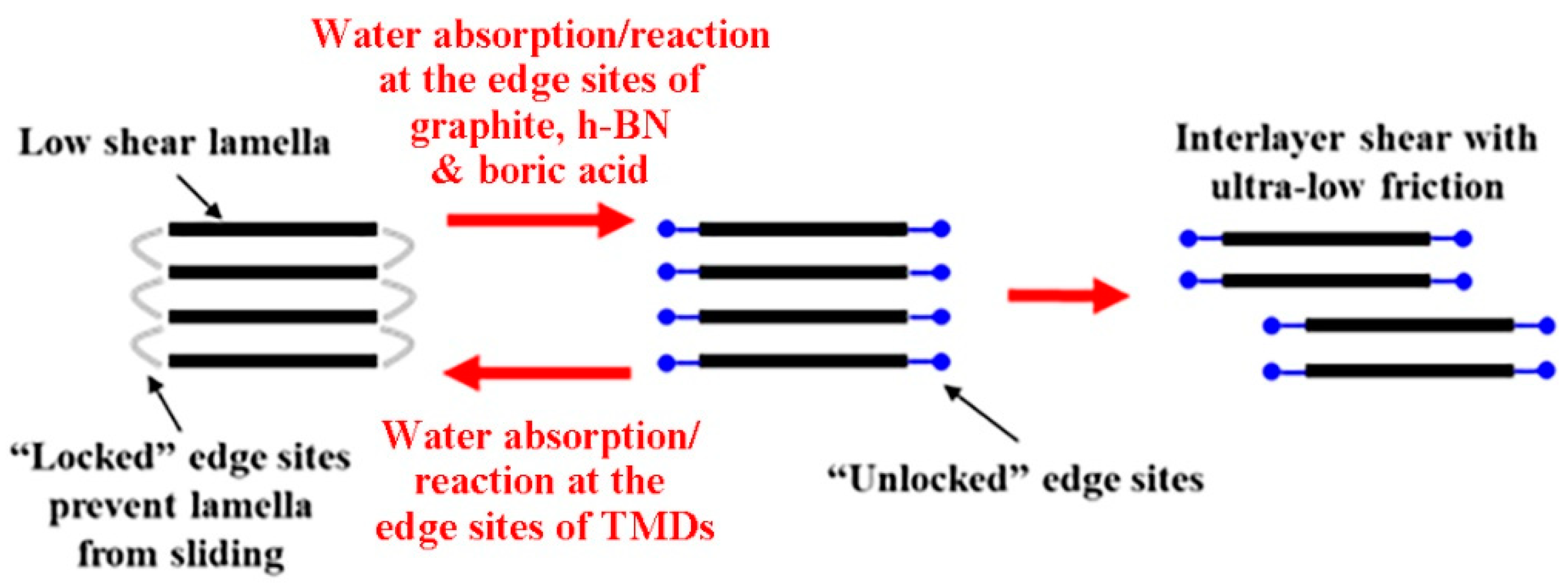
2. Graphite and Graphene
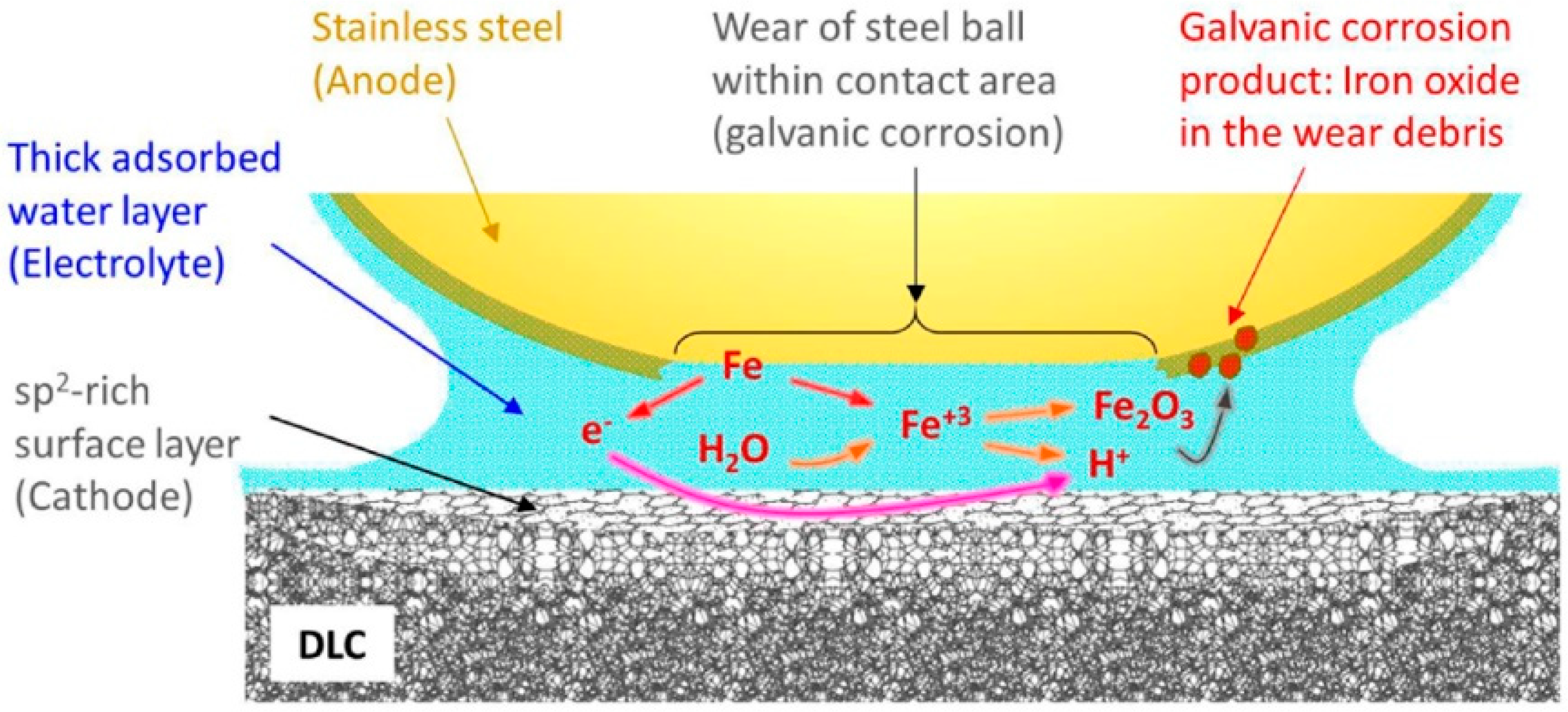
3. Diamond-Like Carbon (DLC)
4. Ultra-Nanocrystalline Diamond (UNCD)
5. Transition Metal Dichalcogenides (TMDs)
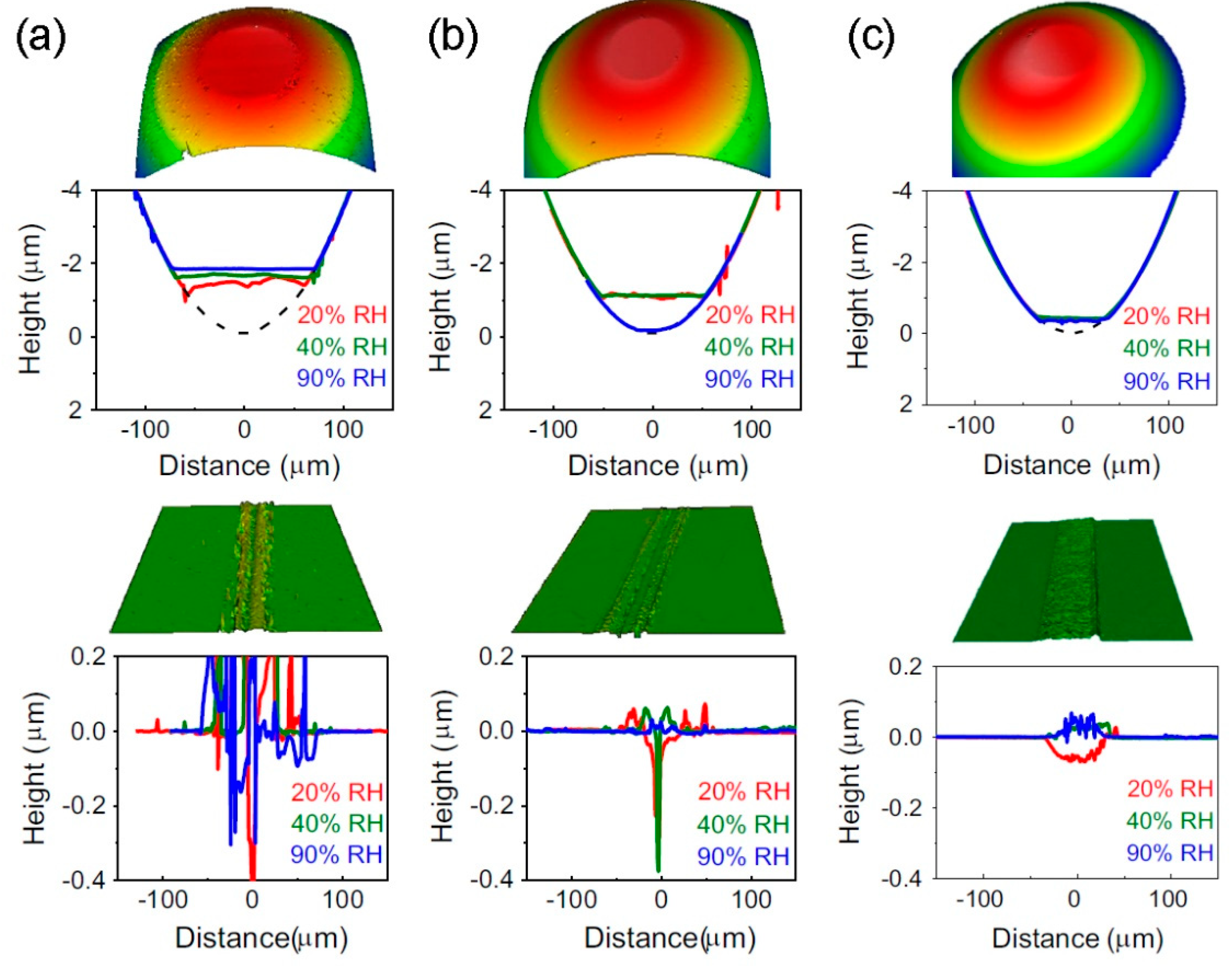
6. Boron-Based Materials
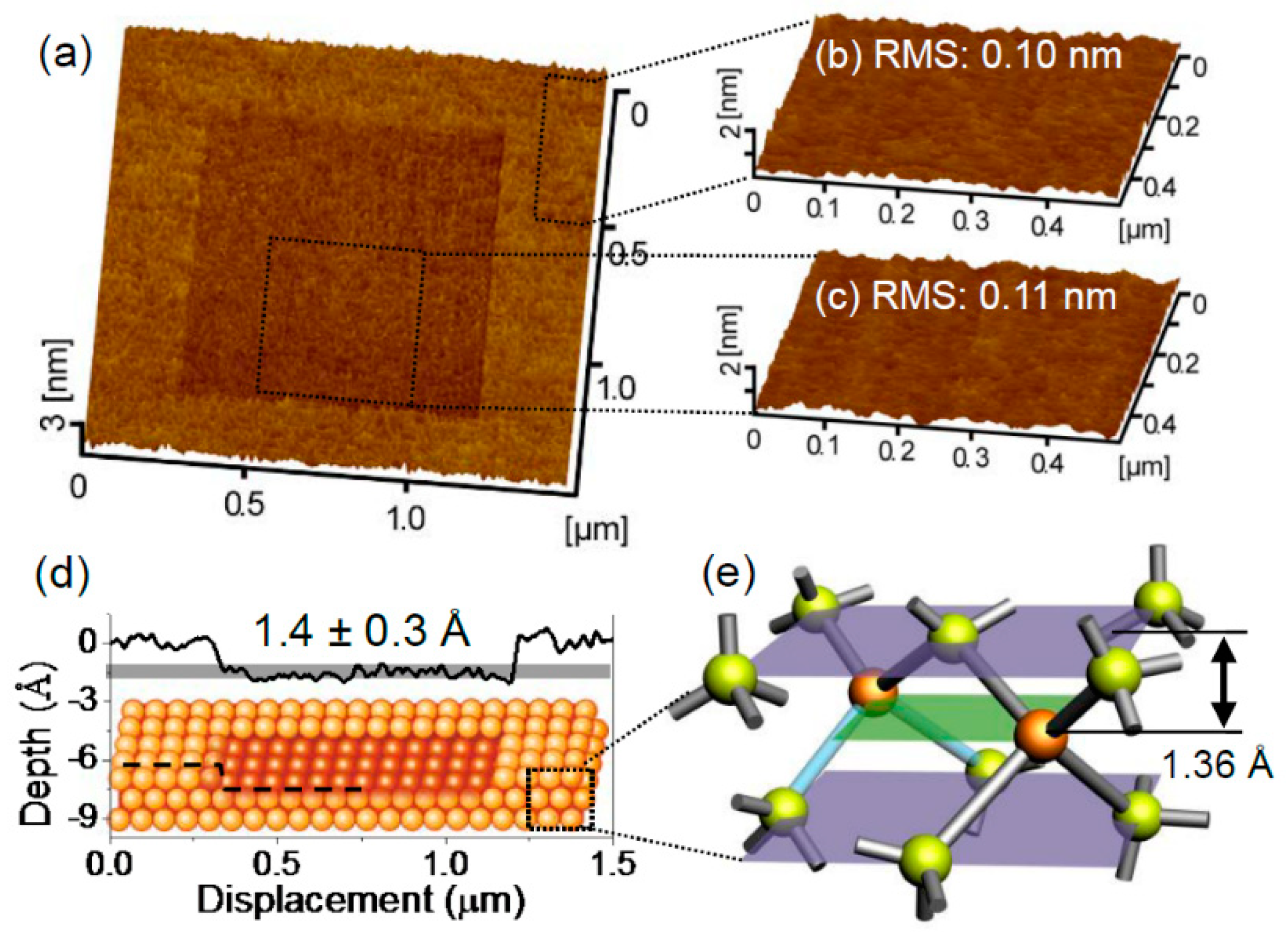
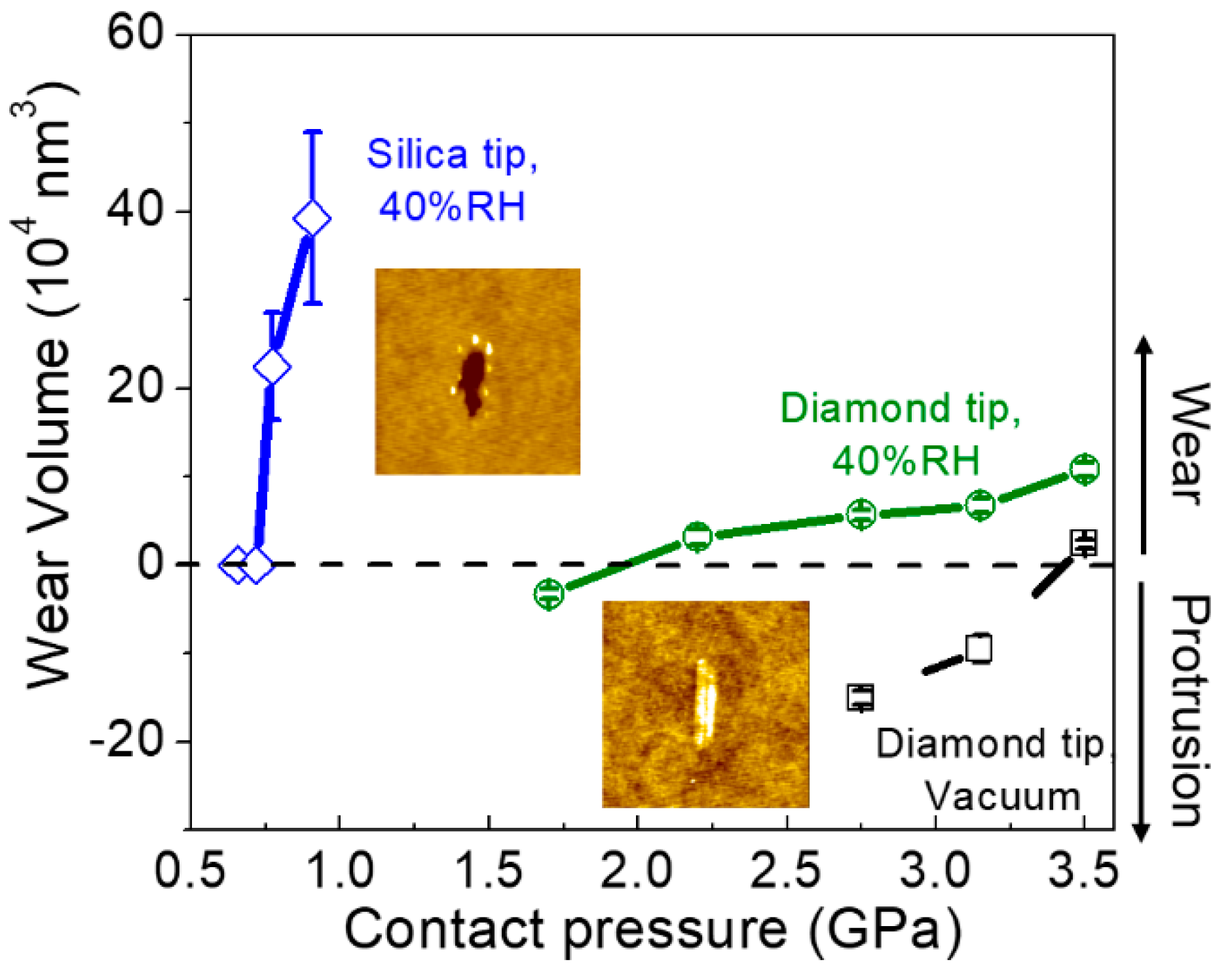
7. Silicon and Silicon Oxides
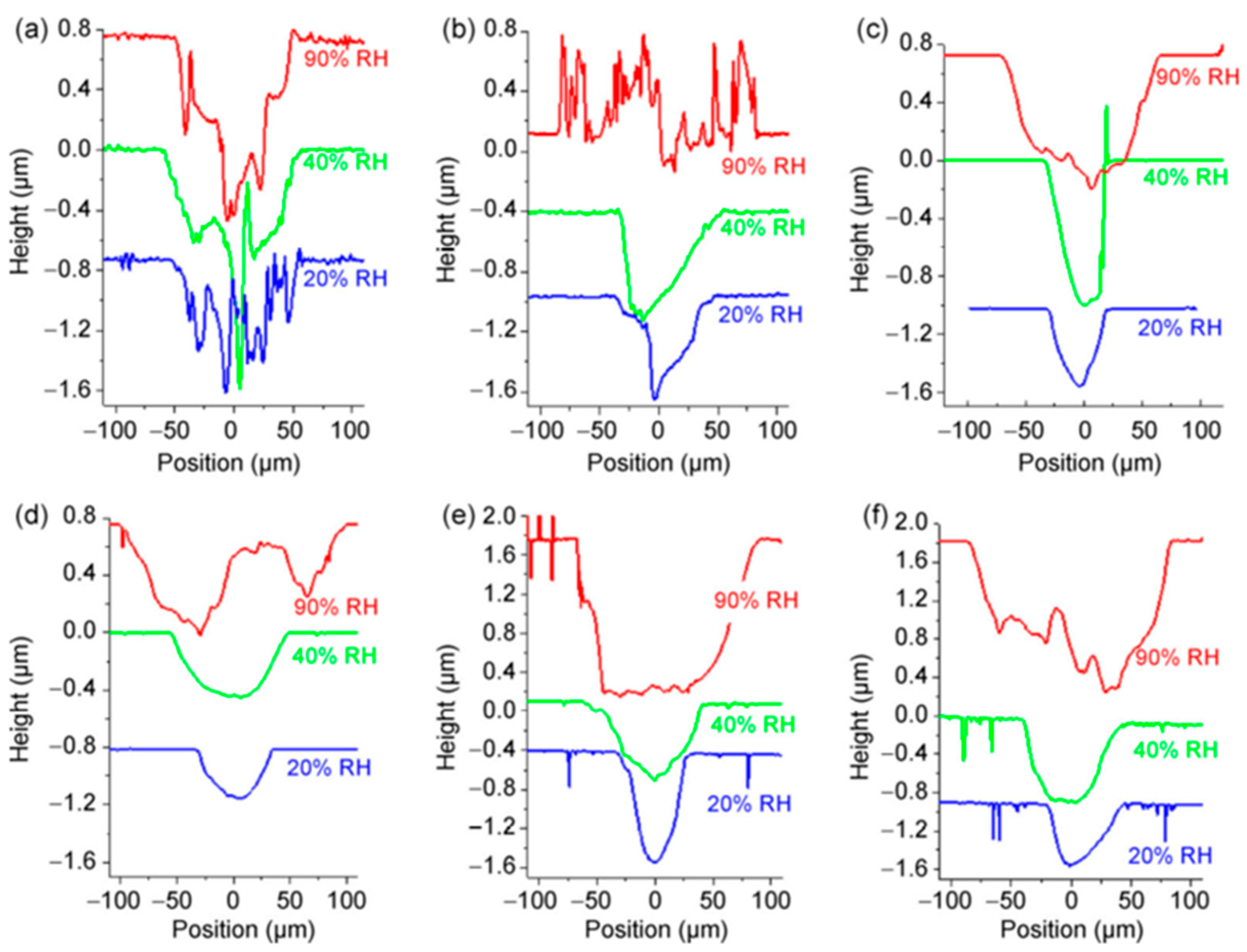
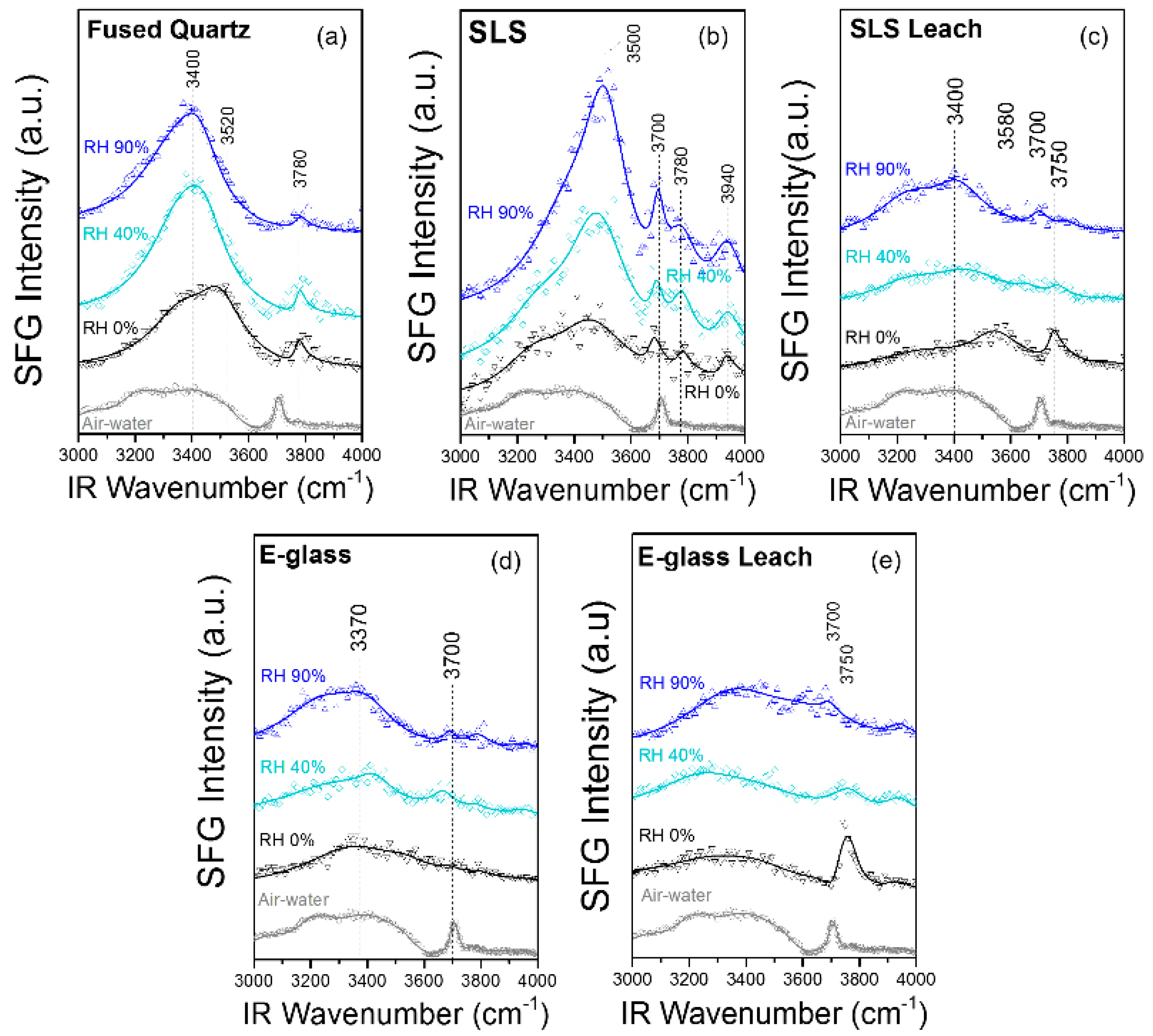
8. Silicates
9. Advanced Ceramics
10. Metals
11. Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Lancaster, J.K. A review of the influence of environmental humidity and water on friction, lubrication and wear. Tribol. Int. 1990, 23, 371–389. [Google Scholar] [CrossRef]
- Bracken, E.F. Humidity control prevents ac brush disintegration. Electr. World 1933, 102, 410. [Google Scholar]
- Dobson, J. The effect of humidity on brush operation. Electr. J. 1935, 32, 134. [Google Scholar]
- Ramadanoff, D.; Glass, S.W. High-altitude brush problem. Electr. Eng. 1944, 63, 825–829. [Google Scholar] [CrossRef]
- Savage, R.H. Graphite lubrication. J. Appl. Phys. 1948, 19, 1–10. [Google Scholar] [CrossRef]
- Bowden, F.P.; Young, J.E. Friction of diamond, graphite, and carbon and the influence of surface films. Proc. R. Soc. Lond. Ser. A 1951, 208, 444–455. [Google Scholar] [CrossRef]
- Savage, R.H.; Schaefer, D.L. Vapor lubrication of graphite sliding contacts. J. Appl. Phys. 1956, 27, 136–138. [Google Scholar] [CrossRef]
- Deacon, R.F.; Goodman, J.F. Lubrication by lamellar solids. Proc. R. Soc. Lond. Ser. A 1958, 243, 464–482. [Google Scholar] [CrossRef]
- Rowe, G. Some observations on the frictional behaviour of boron nitride and of graphite. Wear 1960, 3, 274–285. [Google Scholar] [CrossRef]
- Bryant, P.; Gutshall, P.; Taylor, L. A study of mechanisms of graphite friction and wear. Wear 1964, 7, 118–126. [Google Scholar] [CrossRef]
- Kumar, N.; Dash, S.; Tyagi, A.K.; Raj, B. Super low to high friction of turbostratic graphite under various atmospheric test conditions. Tribol. Int. 2011, 44, 1969–1978. [Google Scholar] [CrossRef]
- Arnell, R.D.; Teer, D.G. Lattice parameters of graphite in relation to friction and wear. Nature 1968, 218, 1155–1156. [Google Scholar] [CrossRef]
- Yen, B.K.; Schwickert, B.E.; Toney, M.F. Origin of low-friction behavior in graphite investigated by surface X-ray diffraction. Appl. Phys. Lett. 2004, 84, 4702–4704. [Google Scholar] [CrossRef]
- Lancaster, J.K. Transitions in the friction and wear of carbons and graphites sliding against themselves. ASLE Trans. 1975, 18, 187–201. [Google Scholar] [CrossRef]
- Lancaster, J. The wear of carbons and graphites. In Treatise on Materials Science & Technology; Scott, D., Ed.; Academic Press: New York, NY, USA, 1979; Volume 13, pp. 141–174. [Google Scholar]
- Lancaster, J.K.; Pritchard, J.R. On the dusting wear regime of graphite sliding against carbon. J. Phys. D Appl. Phys. 1980, 13, 1551–1564. [Google Scholar] [CrossRef]
- Lancaster, J.K.; Pritchard, J.R. The influence of environment and pressure on the transition to dusting wear of graphite. J. Phys. D Appl. Phys. 1981, 14, 747–762. [Google Scholar] [CrossRef]
- Lepage, J.; Zaïdi, H. Influence of water vapour adsorption on the boundary conditions in tribology. In Proceedings of the 14th Leeds-Lyon Symposium on Interface Dynamics; Dowson, D., Taylor, C.M., Godet, M., Berthe, D., Eds.; Elsevier: Amsterdam, The Netherlands, 1988; pp. 259–266. [Google Scholar]
- Allouche, A.; Ferro, Y. Dissociative adsorption of small molecules at vacancies on the graphite (0001) surface. Carbon 2006, 44, 3320–3327. [Google Scholar] [CrossRef]
- Cabrera-Sanfelix, P.; Darling, G.R. Dissociative adsorption of water at vacancy defects in graphite. J. Phys. Chem. C 2007, 111, 18258–18263. [Google Scholar] [CrossRef]
- Gurel, H.H.; Ozcelik, V.O.; Ciraci, S. Dissociative adsorption of molecules on graphene and silicene. J. Phys. Chem. C 2014, 118, 27574–27582. [Google Scholar] [CrossRef]
- Levita, G.; Restuccia, P.; Righi, M.C. Graphene and MoS2 interacting with water: A comparison by ab initio calculations. Carbon 2016, 107, 878–884. [Google Scholar] [CrossRef]
- Rietsch, J.C.; Brender, P.; Dentzer, J.; Gadiou, R.; Vidal, L.; Vix-Guterl, C. Evidence of water chemisorption during graphite friction under moist conditions. Carbon 2013, 55, 90–97. [Google Scholar] [CrossRef]
- Kim, K.S.; Lee, H.J.; Lee, C.; Lee, S.K.; Jang, H.; Ahn, J.H.; Kim, J.H.; Lee, H.J. Chemical vapor deposition-grown graphene: The thinnest solid lubricant. ACS Nano 2011, 5, 5107–5114. [Google Scholar] [CrossRef] [PubMed]
- Berman, D.; Erdemir, A.; Sumant, A.V. Few layer graphene to reduce wear and friction on sliding steel surfaces. Carbon 2013, 54, 454–459. [Google Scholar] [CrossRef]
- Bhowmick, S.; Banerji, A.; Alpas, A.T. Role of humidity in reducing sliding friction of multilayered graphene. Carbon 2015, 87, 374–384. [Google Scholar] [CrossRef]
- Huang, Y.H.; Yao, Q.Z.; Qi, Y.Z.; Cheng, Y.; Wang, H.T.; Li, Q.Y.; Meng, Y.G. Wear evolution of monolayer graphene at the macroscale. Carbon 2017, 115, 600–607. [Google Scholar] [CrossRef]
- Li, Z.Y.; Yang, W.J.; Wu, Y.P.; Wu, S.B.; Cai, Z.B. Role of humidity in reducing the friction of graphene layers on textured surfaces. Appl. Surf. Sci. 2017, 403, 362–370. [Google Scholar] [CrossRef]
- Egberts, P.; Ye, Z.J.; Liu, X.Z.; Dong, Y.L.; Martini, A.; Carpick, R.W. Environmental dependence of atomic-scale friction at graphite surface steps. Phys. Rev. B 2013, 88, 035409. [Google Scholar] [CrossRef]
- Liu, S.W.; Wang, H.P.; Xu, Q.; Ma, T.B.; Yu, G.; Zhang, C.; Geng, D.; Yu, Z.; Zhang, S.; Wang, W.; et al. Robust microscale superlubricity under high contact pressure enabled by graphene-coated microsphere. Nat. Commun. 2017, 8, 14029. [Google Scholar] [CrossRef] [PubMed]
- Jacob, W.; Möller, W. On the structure of thin hydrocarbon films. Appl. Phys. Lett. 1993, 63, 1771–1773. [Google Scholar] [CrossRef]
- Kumar, S.; Sarangi, D.; Dixit, P.N.; Panwar, O.S.; Bhattacharyya, R. Diamond-like carbon films with extremely low stress. Thin Solid Films 1999, 346, 130–137. [Google Scholar] [CrossRef]
- Robertson, J. Diamond-like amorphous carbon. Mater. Sci. Eng. R 2002, 37, 129–281. [Google Scholar] [CrossRef]
- Ban, M.; Hasegawa, T.; Fujii, S.; Fujioka, J. Stress and structural properties of diamond-like carbon films deposited by electron beam excited plasma CVD. Diamond Relat. Mater. 2003, 12, 47–56. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Raman spectroscopy of amorphous, nanostructured, diamond-like carbon, and nanodiamond. Philos. Trans. R. Soc. A 2004, 362, 2477–2512. [Google Scholar] [CrossRef] [PubMed]
- Oka, Y.; Kirinuki, M.; Nishimura, Y.; Azuma, K.; Fujiwara, E.; Yatsuzuka, M. Measurement of residual stress in DLC films prepared by plasma-based ion implantation and deposition. Surf. Coat. Technol. 2004, 186, 141–145. [Google Scholar] [CrossRef]
- Ferrari, A.C. Non-destructive characterisation of carbon films. In Tribology of Diamond-Like Carbon Films; Donnet, C., Erdemir, A., Eds.; Springer: Boston, MA, USA, 2008; pp. 25–82. [Google Scholar]
- Yang, M.; Marino, M.J.; Bojan, V.J.; Eryilmaz, O.L.; Erdemir, A.; Kim, S.H. Quantification of oxygenated species on a diamond-like carbon (DLC) surface. Appl. Surf. Sci. 2011, 257, 7633–7638. [Google Scholar] [CrossRef]
- Ala’A, A.-A.; Eryilmaz, O.; Erdemir, A.; Kim, S.H. Nano-texture for a wear-resistant and near-frictionless diamond-like carbon. Carbon 2014, 73, 403–412. [Google Scholar]
- Erdemir, A.; Switala, M.; Wei, R.; Wilbur, P. A tribological investigation of the graphite-to-diamond-like behavior of amorphous-carbon films ion-beam deposited on ceramic substrates. Surf. Coat. Technol. 1991, 50, 17–23. [Google Scholar] [CrossRef]
- Donnet, C.; Belin, M.; Auge, J.C.; Martin, J.M.; Grill, A.; Patel, V. Tribochemistry of diamond-like carbon coatings in various environments. Surf. Coat. Technol. 1994, 68, 626–631. [Google Scholar] [CrossRef]
- Ronkainen, H.; Koskinen, J.; Likonen, J.; Varjus, S.; Vihersalo, J. Characterization of wear surfaces in dry sliding of steel and alumina on hydrogenated and hydrogen-free carbon-films. Diamond Relat. Mater. 1994, 3, 1329–1336. [Google Scholar] [CrossRef]
- Harris, S.J.; Weiner, A.M.; Meng, W.J. Tribology of metal-containing diamond-like carbon coatings. Wear 1997, 211, 208–217. [Google Scholar] [CrossRef]
- Voevodin, A.A.; Donley, M.S.; Zabinski, J.S. Pulsed laser deposition of diamond-like carbon wear protective coatings: A review. Surf. Coat. Technol. 1997, 92, 42–49. [Google Scholar] [CrossRef]
- Donnet, C.; Le Mogne, T.; Ponsonnet, L.; Belin, M.; Grill, A.; Patel, V.; Jahnes, C. The respective role of oxygen and water vapor on the tribology of hydrogenated diamond-like carbon coatings. Tribol. Lett. 1998, 4, 259–265. [Google Scholar] [CrossRef]
- Venkatraman, C.; Brodbeck, C.; Lei, R. Tribological properties of diamond-like nanocomposite coatings at high temperatures. Surf. Coat. Technol. 1999, 115, 215–221. [Google Scholar] [CrossRef]
- Kim, H.I.; Lince, J.R.; Eryilmaz, O.L.; Erdemir, A. Environmental effects on the friction of hydrogenated DLC films. Tribol. Lett. 2006, 21, 51–56. [Google Scholar] [CrossRef]
- Eryilmaz, O.L.; Erdemir, A. Surface analytical investigation of nearly-frictionless carbon films after tests in dry and humid nitrogen. Surf. Coat. Technol. 2007, 201, 7401–7407. [Google Scholar] [CrossRef]
- Erdemir, A. The role of hydrogen in tribological properties of diamond-like carbon films. Surf. Coat. Technol. 2001, 146, 292–297. [Google Scholar] [CrossRef]
- Erdemir, A. Genesis of superlow friction and wear in diamondlike carbon films. Tribol. Int. 2004, 37, 1005–1012. [Google Scholar] [CrossRef]
- Andersson, J.; Erck, R.A.; Erdemir, A. Friction of diamond-like carbon films in different atmospheres. Wear 2003, 254, 1070–1075. [Google Scholar] [CrossRef]
- Andersson, J.; Erck, R.A.; Erdemir, A. Frictional behavior of diamondlike carbon films in vacuum and under varying water vapor pressure. Surf. Coat. Technol. 2003, 163, 535–540. [Google Scholar] [CrossRef]
- Enke, K.; Dimigen, H.; Hubsch, H. Frictional properties of diamondlike carbon layers. Appl. Phys. Lett. 1980, 36, 291–292. [Google Scholar] [CrossRef]
- Le Huu, T.; Zaidi, H.; Paulmier, D.; Voumard, P. Transformation of sp3 to sp2 sites of diamond like carbon coatings during friction in vacuum and under water vapour environment. Thin Solid Films 1996, 290, 126–130. [Google Scholar] [CrossRef]
- Ronkainen, H.; Varjus, S.; Holmberg, K. Friction and wear properties in dry, water- and oil-lubricated DLC against alumina and DLC against steel contacts. Wear 1998, 222, 120–128. [Google Scholar] [CrossRef]
- Zhang, W.; Tanaka, A.; Wazumi, K.; Koga, Y. Tribological properties of diamond-like carbon films in low and high moist air. Tribol. Lett. 2003, 14, 123–130. [Google Scholar] [CrossRef]
- Li, H.; Xu, T.; Wang, C.; Chen, J.; Zhou, H.; Liu, H. Tribochemical effects on the friction and wear behaviors of diamond-like carbon film under high relative humidity condition. Tribol. Lett. 2005, 19, 231–238. [Google Scholar] [CrossRef]
- Li, H.; Xu, T.; Wang, C.; Chen, J.; Zhou, H.; Liu, H. Friction-induced physical and chemical interactions among diamond-like carbon film, steel ball and water and/or oxygen molecules. Diamond Relat. Mater. 2006, 15, 1228–1234. [Google Scholar] [CrossRef]
- Uchidate, M.; Liu, H.; Iwabuchi, A.; Yamamoto, K. Effects of water environment on tribological properties of DLC rubbed against stainless steel. Wear 2007, 263, 1335–1340. [Google Scholar] [CrossRef]
- Wu, X.; Ohana, T.; Tanaka, A.; Kubo, T.; Nanao, H.; Minami, I.; Mori, S. Tribochemical investigation of DLC coating in water using stable isotopic tracers. Appl. Surf. Sci. 2008, 254, 3397–3402. [Google Scholar] [CrossRef]
- Scharf, T.W.; Singer, I.L. Role of the transfer film on the friction and wear of metal carbide reinforced amorphous carbon coatings during run-in. Tribol. Lett. 2009, 36, 43–53. [Google Scholar] [CrossRef]
- Cui, L.C.; Lu, Z.B.; Wang, L.P. Environmental effect on the load-dependent friction behavior of a diamond-like carbon film. Tribol. Int. 2015, 82, 195–199. [Google Scholar] [CrossRef]
- Alazizi, A.; Draskovics, A.; Ramirez, G.; Erdemir, A.; Kim, S.H. Tribochemistry of carbon films in oxygen and humid environments: Oxidative wear and galvanic corrosion. Langmuir 2016, 32, 1996–2004. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Fischer, T.; Gallois, B. The effects of oxygen and humidity on friction and wear of diamond-like carbon films. In Metallurgical Coatings and Thin Films; McGuire, G.E., McIntyre, D.C., Hofmann, S., Eds.; Elsevier: Amsterdam, The Netherlands, 1991; pp. 537–542. [Google Scholar]
- Liu, E.; Ding, Y.F.; Li, L.; Blanpain, B.; Celis, J.P. Influence of humidity on the friction of diamond and diamond-like carbon materials. Tribol. Int. 2007, 40, 216–219. [Google Scholar] [CrossRef]
- Wang, X.; Wang, P.; Zhang, B.; Yang, S.R.; Zhang, J.Y. The tribological properties of fullerene-like hydrogenated carbon (FL-C:H) film under different humidity conditions. Tribol. Trans. 2009, 52, 354–359. [Google Scholar] [CrossRef]
- Yi, J.; Kim, J.K.; Moon, M.W.; Lee, K.R.; Kim, S.S. Tribological performance of alternating-layered Si-DLC/DLC films under humid conditions. Tribol. Lett. 2009, 34, 223–228. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, K.R.; Ko, D.H. Tribochemical reaction of hydrogenated diamond-like carbon films: A clue to understand the environmental dependence. Tribol. Int. 2004, 37, 913–921. [Google Scholar] [CrossRef]
- Erdemir, A.; Eryilmaz, O.L.; Nilufer, I.B.; Fenske, G.R. Synthesis of superlow-friction carbon films from highly hydrogenated methane plasmas. Surf. Coat. Technol. 2000, 133, 448–454. [Google Scholar] [CrossRef]
- Gilmore, R.; Hauert, R. Control of the tribological moisture sensitivity of diamond-like carbon films by alloying with F, Ti or Si. Thin Solid Films 2001, 398, 199–204. [Google Scholar] [CrossRef]
- Yang, S.H.; Kong, H.; Lee, K.R.; Park, S.; Kim, D.E. Effect of environment on the tribological behavior of Si-incorporated diamond-like carbon films. Wear 2002, 252, 70–79. [Google Scholar] [CrossRef]
- Voevodin, A.A.; Phelps, A.W.; Zabinski, J.S.; Donley, M.S. Friction induced phase transformation of pulsed laser deposited diamond-like carbon. Diamond Relat. Mater. 1996, 5, 1264–1269. [Google Scholar] [CrossRef]
- Koskinen, J.; Ronkainen, H.; Varjus, S.; Muukkonen, T.; Holmberg, K.; Sajavaara, T. Low friction ta-C films with hydrogen reservoirs. Diamond Relat. Mater. 2001, 10, 1030–1035. [Google Scholar] [CrossRef]
- Gharam, A.A.; Lukitsch, M.; Qi, Y.; Alpas, A. Role of oxygen and humidity on the tribo-chemical behaviour of non-hydrogenated diamond-like carbon coatings. Wear 2011, 271, 2157–2163. [Google Scholar] [CrossRef]
- Gardos, M.N.; Gabelich, S.A. Atmospheric effects of friction, friction noise and wear with silicon and diamond. Part III. SEM tribometry of polycrystalline diamond in vacuum and hydrogen. Tribol. Lett. 1999, 6, 103–112. [Google Scholar] [CrossRef]
- Sumant, A.V.; Krauss, A.R.; Gruen, D.M.; Auciello, O.; Erdemir, A.; Williams, M.; Artiles, A.F.; Adams, W. Ultrananocrystalline diamond film as a wear-resistant and protective coating for mechanical seal applications. Tribol. Trans. 2005, 48, 24–31. [Google Scholar] [CrossRef]
- Kumar, N.; Panda, K.; Dash, S.; Popov, C.; Reithmaier, J.P.; Panigrahi, B.K.; Tyagi, A.K.; Raj, B. Tribological properties of nanocrystalline diamond films deposited by hot filament chemical vapor deposition. AIP Adv. 2012, 2, 032164. [Google Scholar] [CrossRef]
- Konicek, A.R.; Grierson, D.S.; Sumant, A.V.; Friedmann, T.A.; Sullivan, J.P.; Gilbert, P.U.P.A.; Sawyer, W.G.; Carpick, R.W. Influence of surface passivation on the friction and wear behavior of ultrananocrystalline diamond and tetrahedral amorphous carbon thin films. Phys. Rev. B 2012, 85, 155448. [Google Scholar] [CrossRef]
- Radhika, R.; Kumar, N.; Sankaran, K.J.; Dumpala, R.; Dash, S.; Rao, M.S.R.; Arivuoli, D.; Tyagi, A.K.; Tai, N.H.; Lin, I.N. Extremely high wear resistance and ultra-low friction behaviour of oxygen-plasma-treated nanocrystalline diamond films. J. Phys. D Appl. Phys. 2013, 46, 425304. [Google Scholar] [CrossRef]
- Rani, R.; Kumar, N.; Kozakov, A.T.; Googlev, K.A.; Sankaran, K.J.; Das, P.K.; Dash, S.; Tyagi, A.K.; Lin, I.N. Superlubrication properties of ultra-nanocrystalline diamond film sliding against a zirconia ball. RSC Adv. 2015, 5, 100663–100673. [Google Scholar] [CrossRef]
- Gardos, M.N.; Soriano, B.L. The effect of environment on the tribological properties of polycrystalline diamond films. J. Mater. Res. 1990, 5, 2599–2609. [Google Scholar] [CrossRef]
- Erdemir, A.; Fenske, G.R.; Krauss, A.R.; Gruen, D.M.; McCauley, T.; Csencsits, R.T. Tribological properties of nanocrystalline diamond films. Surf. Coat. Technol. 1999, 120, 565–572. [Google Scholar] [CrossRef]
- Grillo, S.E.; Field, J.E. The friction of cvd diamond at high hertzian stresses: The effect of load, environment and sliding velocity. J. Phys. D Appl. Phys. 2000, 33, 595–602. [Google Scholar] [CrossRef]
- Chromik, R.R.; Winfrey, A.L.; Luning, J.; Nemanich, R.J.; Wahl, K.J. Run-in behavior of nanocrystalline diamond coatings studied by in situ tribometry. Wear 2008, 265, 477–489. [Google Scholar] [CrossRef]
- Konicek, A.R.; Grierson, D.S.; Gilbert, P.U.; Sawyer, W.G.; Sumant, A.V.; Carpick, R.W. Origin of ultralow friction and wear in ultrananocrystalline diamond. Phys. Rev. Lett. 2008, 100, 235502. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Konca, E.; Alpas, A.T. Atmospheric effects on the adhesion and friction between non-hydrogenated diamond-like carbon (DLC) coating and aluminum—A first principles investigation. Surf. Sci. 2006, 600, 2955–2965. [Google Scholar] [CrossRef]
- De Barros Bouchet, M.-I.; Zilibotti, G.; Matta, C.; Righi, M.C.; Vandenbulcke, L.; Vacher, B.; Martin, J.-M. Friction of diamond in the presence of water vapor and hydrogen gas. Coupling gas-phase lubrication and first-principles studies. J. Phys. Chem. C 2012, 116, 6966–6972. [Google Scholar] [CrossRef]
- Salomon, G.; De Gee, A.W.J.; Zaat, J.H. Mechano-chemical factors in MoS2 film lubrication. Wear 1964, 7, 87–101. [Google Scholar] [CrossRef]
- Stewart, T.B.; Fleischauer, P.D. Chemistry of sputtered molybdenum-disulfide films. Inorg. Chem. 1982, 21, 2426–2431. [Google Scholar] [CrossRef]
- Fleischauer, P.D. Effects of crystallite orientation on environmental stability and lubrication properties of sputtered MoS2 thin-films. ASLE Trans. 1984, 27, 82–88. [Google Scholar] [CrossRef]
- Peterson, M.B.; Johnson, R.L. Friction and Wear Investigation of Molybdenum Disulfide II: Effects of Contaminants and Method of Application; National Advisory Committee for Aeronautics: Washington, DC, USA, 1954. [Google Scholar]
- Fusaro, R.L. Effect of substrate surface finish on the lubrication and failure mechanisms of molybdenum-disulfide films. ASLE Trans. 1982, 25, 141–156. [Google Scholar] [CrossRef]
- Pardee, R.P. The effect of humidity on low-load frictional properties of a bonded solid film lubricant. ASLE Trans. 1972, 15, 130–142. [Google Scholar] [CrossRef]
- Prasad, S.V.; McDevitt, N.T.; Zabinski, J.S. Tribology of tungsten disulfide films in humid environments: The role of a tailored metal-matrix composite substrate. Wear 1999, 230, 24–34. [Google Scholar] [CrossRef]
- Haltner, A.J.; Oliver, C.S. Effect of water vapor on friction of molybdenum disulfide. Ind. Eng. Chem. Fundam. 1966, 5, 348–355. [Google Scholar] [CrossRef]
- Roberts, E.W. Towards an optimised sputtered MoS2 lubricant film. In Proceedings of the 20th Aerospace Mechanics Symposium; NASA, Lewis Research Center: Cleveland, OH, USA, 1986; pp. 103–119. [Google Scholar]
- Uemura, M.; Saito, K.; Nakao, K. A mechanism of vapor effect on friction coefficient of molybdenum-disulfide. Tribol. Trans. 1990, 33, 551–556. [Google Scholar] [CrossRef]
- Khare, H.S.; Burris, D.L. The effects of environmental water and oxygen on the temperature-dependent friction of sputtered molybdenum disulfide. Tribol. Lett. 2013, 52, 485–493. [Google Scholar] [CrossRef]
- Khare, H.S.; Burris, D.L. Surface and subsurface contributions of oxidation and moisture to room temperature friction of molybdenum disulfide. Tribol. Lett. 2014, 53, 329–336. [Google Scholar] [CrossRef]
- Luan, B.Q.; Zhou, R.H. Wettability and friction of water on a MoS2 nanosheet. Appl. Phys. Lett. 2016, 108, 131601. [Google Scholar] [CrossRef]
- Ataca, C.; Ciraci, S. Dissociation of H2O at the vacancies of single-layer MoS2. Phys. Rev. B 2012, 85, 195410. [Google Scholar] [CrossRef]
- Ghuman, K.K.; Yadav, S.; Singh, C.V. Adsorption and dissociation of H2O on monolayered MoS2 edges: Energetics and mechanism from ab initio simulations. J. Phys. Chem. C 2015, 119, 6518–6529. [Google Scholar] [CrossRef]
- Schumacher, A.; Kruse, N.; Prins, R.; Meyer, E.; Luthi, R.; Howald, L.; Guntherodt, H.J.; Scandella, L. Influence of humidity on friction measurements of supported MoS2 single layers. J. Vac. Sci. Technol. B 1996, 14, 1264–1267. [Google Scholar] [CrossRef]
- Zhao, X.; Perry, S.S. The role of water in modifying friction within MoS2 sliding interfaces. ACS Appl. Mater. Interfaces 2010, 2, 1444–1448. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.D.; Peng, J.F.; Liu, S.S.; Zheng, X.J.; Yan, X.Y.; He, W.Y. Influences of thickness, scanning velocity and relative humidity on the frictional properties of WS2 nanosheets. Mater. Res. Express 2018, 5, 10. [Google Scholar] [CrossRef]
- Fox, V.; Renevier, N.; Teer, D.; Hampshire, J.; Rigato, V. The structure of tribologically improved MoS2–metal composite coatings and their industrial applications. Surf. Coat. Technol. 1999, 116, 492–497. [Google Scholar] [CrossRef]
- Renevier, N.M.; Fox, V.C.; Teer, D.G.; Hampshire, J. Coating characteristics and tribological properties of sputter-deposited MoS2/metal composite coatings deposited by closed field unbalanced magnetron sputter ion plating. Surf. Coat. Technol. 2000, 127, 24–37. [Google Scholar] [CrossRef]
- Renevier, N.M.; Fox, V.C.; Teer, D.G.; Hampshire, J. Performance of low friction MoS2/titanium composite coatings used in forming applications. Mater. Des. 2000, 21, 337–343. [Google Scholar] [CrossRef]
- Simmonds, M.C.; Savan, A.; Pfluger, E.; Van Swygenhoven, H. Mechanical and tribological performance of MoS2 co-sputtered composites. Surf. Coat. Technol. 2000, 126, 15–24. [Google Scholar] [CrossRef]
- Renevier, N.M.; Hamphire, J.; Fox, V.C.; Witts, J.; Allen, T.; Teer, D.G. Advantages of using self-lubricating, hard, wear-resistant MoS2-based coatings. Surf. Coat. Technol. 2001, 142, 67–77. [Google Scholar] [CrossRef]
- Teer, D.G. New solid lubricant coatings. Wear 2001, 251, 1068–1074. [Google Scholar] [CrossRef]
- Xu, S.S.; Gao, X.M.; Sun, J.Y.; Hu, M.; Wang, D.S.; Jiang, D.; Zhou, F.; Weng, L.J.; Liu, W.M. Comparative study of moisture corrosion to WS2 and WS2/Cu multilayer films. Surf. Coat. Technol. 2014, 247, 30–38. [Google Scholar] [CrossRef]
- Singh, H.; Mutyala, K.C.; Mohseni, H.; Scharf, T.W.; Evans, R.D.; Doll, G.L. Tribological performance and coating characteristics of sputter-deposited Ti-doped MoS2 in rolling and sliding contact. Tribol. Trans. 2015, 58, 767–777. [Google Scholar] [CrossRef]
- Li, H.; Zhang, G.A.; Wang, L.P. Low humidity-sensitivity of MoS2/Pb nanocomposite coatings. Wear 2016, 350–351, 1–9. [Google Scholar] [CrossRef]
- Ren, S.M.; Li, H.; Cui, M.J.; Wang, L.P.; Pu, J.B. Functional regulation of Pb-Ti/MoS2 composite coatings for environmentally adaptive solid lubrication. Appl. Surf. Sci. 2017, 401, 362–372. [Google Scholar] [CrossRef]
- Ding, X.Z.; Zeng, X.T.; He, X.Y.; Chen, Z. Tribological properties of Cr- and Ti-doped MoS2 composite coatings under different humidity atmosphere. Surf. Coat. Technol. 2010, 205, 224–231. [Google Scholar] [CrossRef]
- Ye, M.; Zhang, G.J.; Ba, Y.W.; Wang, T.; Wang, X.; Liu, Z.N. Microstructure and tribological properties of MoS2+Zr composite coatings in high humidity environment. Appl. Surf. Sci. 2016, 367, 140–146. [Google Scholar] [CrossRef]
- Niederhauser, P.; Hintermann, H.E.; Maillat, M. Moisture-resistant MoS2-based composite lubricant films. Thin Solid Films 1983, 108, 209–218. [Google Scholar] [CrossRef]
- Oñate, J.; Brizuela, M.; García-Luis, A.; Braceras, I.; Viviente, J.; Gomez-Elvira, J. Improved tribological behaviour of MoS2 thin solid films alloyed with WC. In Proceedings of the 9th European Space Mechanisms and Tribology Symposium; ESA Publications Division: Noordwijk, The Netherlands, 2001; pp. 257–262. [Google Scholar]
- Vierneusel, B.; Schneider, T.; Tremmel, S.; Wartzack, S.; Gradt, T. Humidity resistant MoS2 coatings deposited by unbalanced magnetron sputtering. Surf. Coat. Technol. 2013, 235, 97–107. [Google Scholar] [CrossRef]
- Domínguez-Meister, S.; Conte, M.; Igartua, A.; Rojas, T.; Sánchez-López, J. Self-lubricity of WSex nanocomposite coatings. ACS Appl. Mater. Interfaces 2015, 7, 7979–7986. [Google Scholar] [CrossRef] [PubMed]
- Curry, J.F.; Argibay, N.; Babuska, T.; Nation, B.; Martini, A.; Strandwitz, N.C.; Dugger, M.T.; Krick, B.A. Highly oriented MoS2 coatings: Tribology and environmental stability. Tribol. Lett. 2016, 64, 11. [Google Scholar] [CrossRef]
- Kubart, T.; Polcar, T.; Kopecký, L.; Novák, R.; Nováková, D. Temperature dependence of tribological properties of and MoSe2 coatings. Surf. Coat. Technol. 2005, 193, 230–233. [Google Scholar] [CrossRef]
- Dominguez-Meister, S.; Rojas, T.C.; Brizuela, M.; Sanchez-Lopez, J.C. Solid lubricant behavior of MoS2 and WSe2-based nanocomposite coatings. Sci. Technol. Adv. Mater. 2017, 18, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.M.; Lemogne, T.; Chassagnette, C.; Gardos, M.N. Friction of hexagonal boron-nitride in various environments. Tribol. Trans. 1992, 35, 462–472. [Google Scholar] [CrossRef]
- Li, H.; Zeng, X.C. Wetting and interfacial properties of water nanodroplets in contact with graphene and monolayer boron-nitride sheets. ACS Nano 2012, 6, 2401–2409. [Google Scholar] [CrossRef] [PubMed]
- Erdemir, A.; Fenske, G.; Erck, R. A study of the formation and self-lubrication mechanisms of boric acid films on boric oxide coatings. In Metallurgical Coatings and Thin Films, Proceedings of the 17th International Conference on Metallurgical Coatings and 8th International Conference on Thin Films, San Diego, CA, USA; Sartwell, B.D., Zemel, J.N., McGuire, G.E., Bresnock, F.N., Eds.; Elsevier: Amsterdam, The Netherlands, 1990; pp. 588–596. [Google Scholar]
- Bindal, C.; Erdemir, A. Ultralow friction behavior of borided steel surfaces after flash annealing. Appl. Phys. Lett. 1996, 68, 923–925. [Google Scholar] [CrossRef]
- Erdemir, A.; Bindal, C.; Fenske, G.R. Formation of ultralow friction surface films on boron carbide. Appl. Phys. Lett. 1996, 68, 1637–1639. [Google Scholar] [CrossRef]
- Erdemir, A.; Halter, M.; Fenske, G.R. Preparation of ultralow-friction surface films on vanadium diboride. Wear 1997, 205, 236–239. [Google Scholar] [CrossRef]
- Burroughs, B.R.; Kim, J.H.; Blanchet, T.A. Boric acid self-lubrication of B2O3-filled polymer composites. Tribol. Trans. 1999, 42, 592–600. [Google Scholar] [CrossRef]
- Erdemir, A.; Eryilmaz, O.; Fenske, G. Self-replenishing solid lubricant films on boron carbide. Surface Eng. 1999, 15, 291–295. [Google Scholar] [CrossRef]
- Huang, C.B.; Zhang, B.C.; Lan, H.; Du, L.Z.; Zhang, W.G. Friction properties of high temperature boride coating under dry air and water vapor ambiences. Ceram. Int. 2014, 40, 12403–12411. [Google Scholar] [CrossRef]
- Ma, X.; Unertl, W.; Erdemir, A. The boron oxide–boric acid system: Nanoscale mechanical and wear properties. J. Mater. Res. 1999, 14, 3455–3466. [Google Scholar] [CrossRef]
- Barthel, A.J.; Luo, J.W.; Kim, S.H. Origin of ultra-low friction of boric acid: Role of vapor adsorption. Tribol. Lett. 2015, 58. [Google Scholar] [CrossRef]
- Bhushan, B. Nanotribology and nanomechanics of MEMS/NEMS and BioMEMS/BioNEMS materials and devices. Microelectron. Eng. 2007, 84, 387–412. [Google Scholar] [CrossRef]
- Tanaka, M. An industrial and applied review of new MEMS devices features. Microelectron. Eng. 2007, 84, 1341–1344. [Google Scholar] [CrossRef]
- Dong, P.; Chen, Y.-K.; Duan, G.-H.; Neilson, D.T. Silicon photonic devices and integrated circuits. Nanophotonics 2014, 3, 215–228. [Google Scholar] [CrossRef]
- Bhushan, B. Nanotribology and nanomechanics. Wear 2005, 259, 1507–1531. [Google Scholar] [CrossRef]
- Kim, S.H.; Asay, D.B.; Dugger, M.T. Nanotribology and MEMS. Nano Today 2007, 2, 22–29. [Google Scholar] [CrossRef]
- Asay, D.B.; Dugger, M.T.; Kim, S.H. In-situ vapor-phase lubrication of MEMS. Tribol. Lett. 2008, 29, 67–74. [Google Scholar] [CrossRef]
- Achanta, S.; Celis, J.-P. Nanotribology of MEMS/NEMS. In Fundamentals of Friction and Wear on the Nanoscale; Gnecco, E., Meyer, E., Eds.; Springer: New York, NY, USA, 2015; pp. 631–656. [Google Scholar]
- Wang, X.; Yu, J.; Chen, L.; Qian, L.; Zhou, Z. Effects of water and oxygen on the tribochemical wear of monocrystalline Si(100) against SiO2 sphere by simulating the contact conditions in MEMS. Wear 2011, 271, 1681–1688. [Google Scholar] [CrossRef]
- Jin, C.; Yu, B.; Xiao, C.; Chen, L.; Qian, L. Temperature-dependent nanofabrication on silicon by friction-induced selective etching. Nanoscale Res. Lett. 2016, 11, 229. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; He, X.; Liu, H.S.; Qian, L.M.; Kim, S.H. Water adsorption on hydrophilic and hydrophobic surfaces of silicon. J. Phys. Chem. C 2018, 122, 11385–11391. [Google Scholar] [CrossRef]
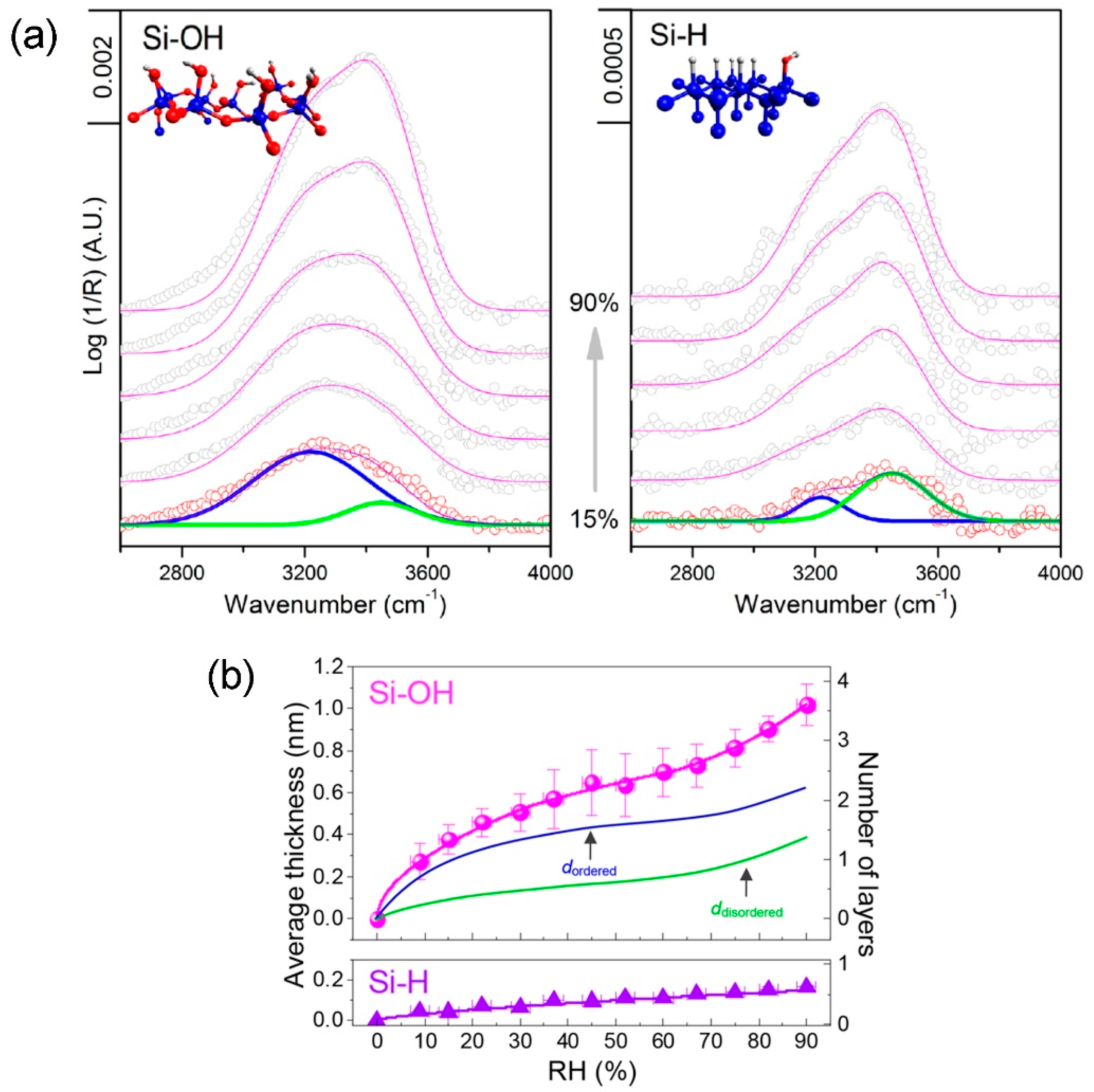
- Asay, D.B.; Kim, S.H. Evolution of the adsorbed water layer structure on silicon oxide at room temperature. J. Phys. Chem. B 2005, 109, 16760–16763. [Google Scholar] [CrossRef] [PubMed]
- Barnette, A.L.; Asay, D.B.; Kim, D.; Guyer, B.D.; Lim, H.; Janik, M.J.; Kim, S.H. Experimental and density functional theory study of the tribochemical wear behavior of SiO2 in humid and alcohol vapor environments. Langmuir 2009, 25, 13052–13061. [Google Scholar] [CrossRef] [PubMed]
- Alazizi, A.; Barthel, A.J.; Surdyka, N.D.; Luo, J.; Kim, S.H. Vapors in the ambient—A complication in tribological studies or an engineering solution of tribological problems? Friction 2015, 3, 85–114. [Google Scholar] [CrossRef]
- Gräf, D.; Grundner, M.; Schulz, R. Reaction of water with hydrofluoric acid treated silicon (111) and (100) surfaces. J. Vac. Sci. Technol. A 1989, 7, 808–813. [Google Scholar] [CrossRef]
- Mizuhara, K.; Hsu, S. Tribochemical reaction of oxygen and water on silicon surfaces. In Wear Particles: From the Cradle to the Grave; Dowson, D., Dalmaz, G., Childs, T.H.C., Taylor, C.M., Godet, M., Eds.; Elsevier: Amsterdam, The Netherlands, 1992; Volume 21, pp. 323–328. [Google Scholar]
- Katsuki, F.; Kamei, K.; Saguchi, A.; Takahashi, W.; Watanabe, J. AFM studies on the difference in wear behavior between Si and SiO2 in KOH solution. J. Electrochem. Soc. 2000, 147, 2328–2331. [Google Scholar] [CrossRef]
- Katsuki, F. Single asperity tribochemical wear of silicon by atomic force microscopy. J. Mater. Res. 2009, 24, 173–178. [Google Scholar] [CrossRef]
- Wen, J.L.; Ma, T.B.; Zhang, W.W.; Psofogiannakis, G.; van Duin, A.C.T.; Chen, L.; Qian, L.M.; Hu, Y.Z.; Lu, X.C. Atomic insight into tribochemical wear mechanism of silicon at the Si/SiO2 interface in aqueous environment: Molecular dynamics simulations using ReaxFF reactive force field. Appl. Surf. Sci. 2016, 390, 216–223. [Google Scholar] [CrossRef]
- Yeon, J.; van Duin, A.C.; Kim, S.H. Effects of water on tribochemical wear of silicon oxide interface: Molecular dynamics (MD) study with reactive force field (ReaxFF). Langmuir 2016, 32, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Duan, F.L. Atomic-level wear behavior of sliding between silica (010) surfaces. Appl. Surf. Sci. 2017, 425, 1168–1175. [Google Scholar] [CrossRef]
- Nevshupa, R.; Scherge, M.; Ahmed, S.-U. Transitional microfriction behavior of silicon induced by spontaneous water adsorption. Surf. Sci. 2002, 517, 17–28. [Google Scholar] [CrossRef]
- Stempflé, P.; Domatti, A.; Dang, H.A.; Takadoum, J. Mechanical and chemical wear components in environmental multi-asperity nanotribology. Tribol. Int. 2015, 82, 358–374. [Google Scholar] [CrossRef]
- Yu, B.J.; Li, X.Y.; Dong, H.S.; Chen, Y.F.; Qian, L.M.; Zhou, Z.R. Towards a deeper understanding of the formation of friction-induced hillocks on monocrystalline silicon. J. Phys. D Appl. Phys. 2012, 45, 145301. [Google Scholar] [CrossRef]
- Yu, B.J.; Li, X.Y.; Dong, H.S.; Qian, L.M. Mechanical performance of friction-induced protrusive nanostructures on monocrystalline silicon and quartz. Micro Nano Lett. 2012, 7, 1270–1273. [Google Scholar] [CrossRef]
- Marchand, D.J.; Chen, L.; Meng, Y.; Qian, L.; Kim, S.H. Effects of vapor environment and counter-surface chemistry on tribochemical wear of silicon wafers. Tribol. Lett. 2014, 53, 365–372. [Google Scholar] [CrossRef]
- Chen, C.; Xiao, C.; Wang, X.D.; Zhang, P.; Chen, L.; Qi, Y.Q.; Qian, L.M. Role of water in the tribochemical removal of bare silicon. Appl. Surf. Sci. 2016, 390, 696–702. [Google Scholar] [CrossRef]
- Xiao, C.; Guo, J.; Zhang, P.; Chen, C.; Chen, L.; Qian, L. Effect of crystal plane orientation on tribochemical removal of monocrystalline silicon. Sci. Rep. 2017, 7, 40750. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Kim, S.H.; Yu, B.; Qian, L.; Zhou, Z. Role of tribochemistry in nanowear of single-crystalline silicon. ACS Appl. Mater. Interfaces 2012, 4, 1585–1593. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Chen, C.; Guo, J.; Zhang, P.; Chen, L.; Qian, L. Threshold contact pressure for the material removal on monocrystalline silicon by SiO2 microsphere. Wear 2017, 376–377, 188–193. [Google Scholar] [CrossRef]
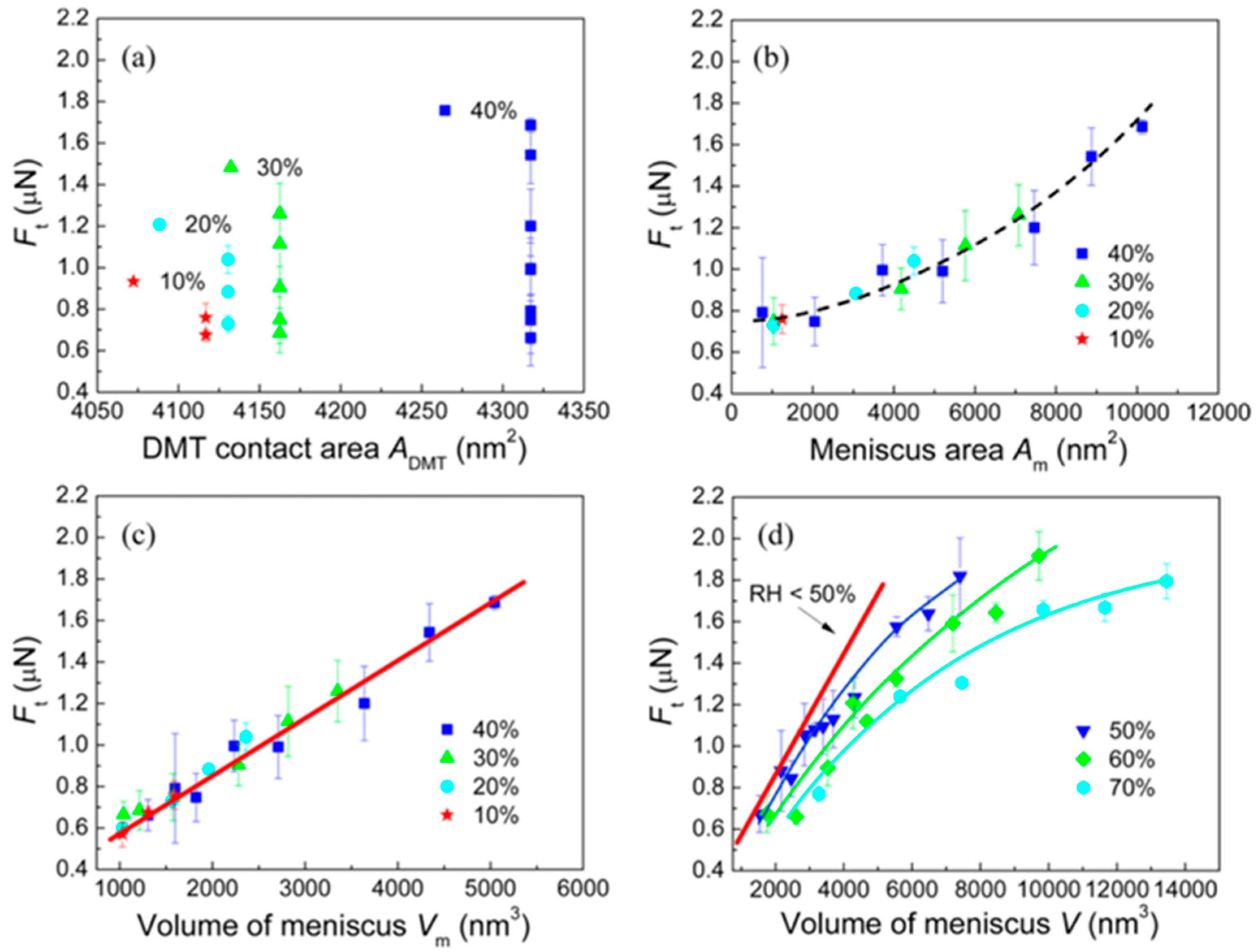
- Chen, L.; Xiao, C.; Yu, B.; Kim, S.H.; Qian, L. What governs friction of silicon oxide in humid environment: Contact area between solids, water meniscus around the contact, or water layer structure? Langmuir 2017, 33, 9673–9679. [Google Scholar] [CrossRef] [PubMed]
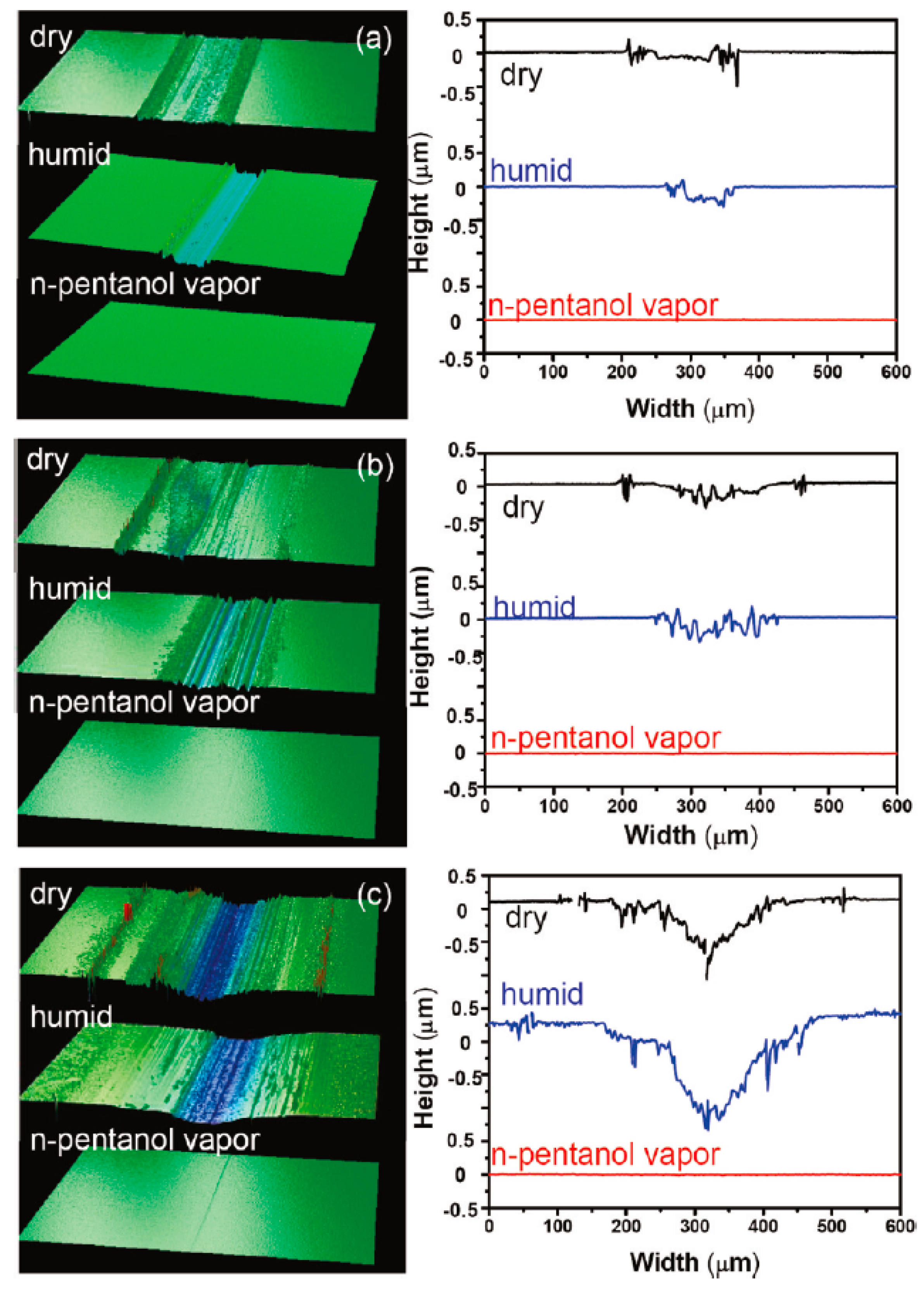
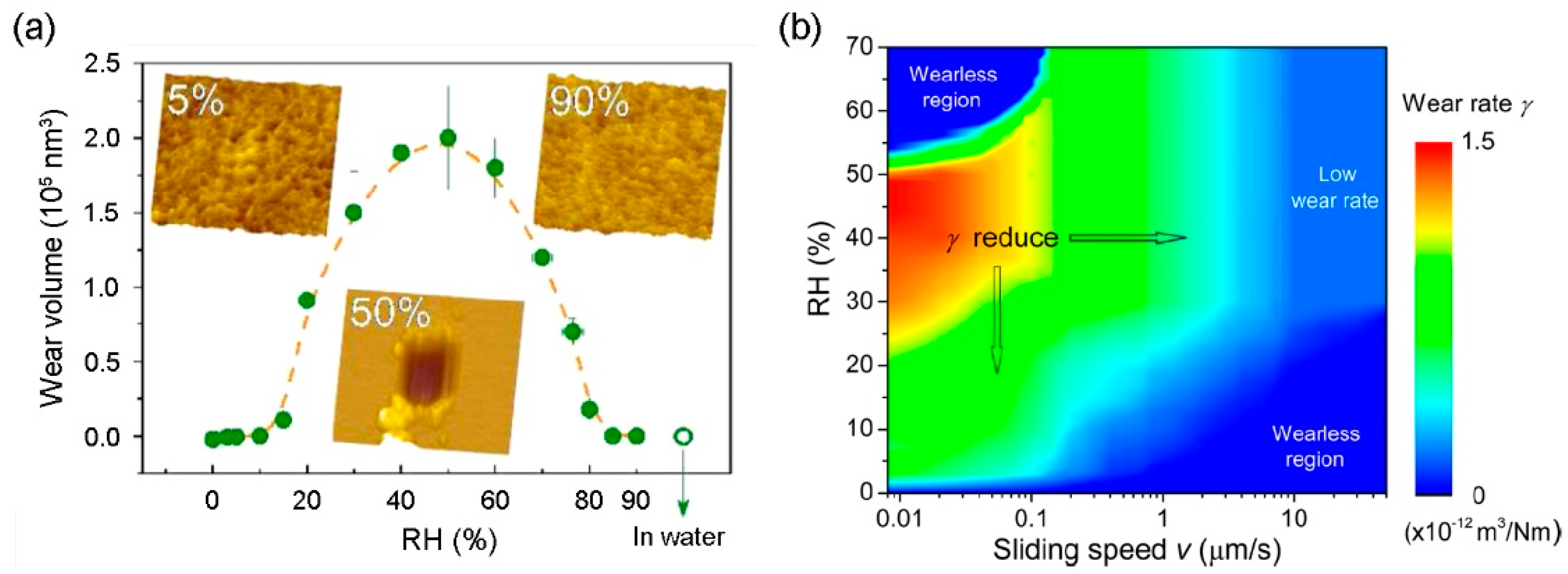
- Wang, X.; Kim, S.H.; Chen, C.; Chen, L.; He, H.; Qian, L. Humidity dependence of tribochemical wear of monocrystalline silicon. ACS Appl. Mater. Interfaces 2015, 7, 14785–14792. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, Y.J.; He, H.T.; Kim, S.H.; Qian, L.M. Effect of coadsorption of water and alcohol vapor on the nanowear of silicon. Wear 2015, 332, 879–884. [Google Scholar] [CrossRef]
- Chen, L.; He, H.; Wang, X.; Kim, S.H.; Qian, L. Tribology of Si/SiO2 in humid air: Transition from severe chemical wear to wearless behavior at nanoscale. Langmuir 2014, 31, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.X.; Chen, L.; Qian, L.M.; Song, D.L.; Cai, Y. Investigation of humidity-dependent nanotribology behaviors of Si(100)/SiO2 pair moving from stick to slip. Appl. Surf. Sci. 2013, 265, 192–200. [Google Scholar] [CrossRef]
- Wang, X.; Song, C.; Yu, B.; Chen, L.; Qian, L. Nanowear behaviour of monocrystalline silicon against SiO2/Si tip in water. Wear 2013, 298, 80–86. [Google Scholar] [CrossRef]
- Wang, X.D.; Guo, J.; Chen, C.; Chen, L.; Qian, L.M. A simple method to control nanotribology behaviors of monocrystalline silicon. J. Appl. Phys. 2016, 119, 044304. [Google Scholar] [CrossRef]
- Baur, W.H. Variation of mean Si–O bond lengths in silicon–oxygen tetrahedra. Acta Crystallogr. Sect. B Struct. Sci. 1978, 34, 1751–1756. [Google Scholar] [CrossRef]
- Asay, D.B.; Kim, S.H. Effects of adsorbed water layer structure on adhesion force of silicon oxide nanoasperity contact in humid ambient. J. Chem. Phys. 2006, 124, 174712. [Google Scholar] [CrossRef] [PubMed]
- Dowson, D. History of Tribology; Addison-Wesley Longman Limited: London, UK, 1979. [Google Scholar]
- Bowden, F.P.; Bowden, F.P.; Tabor, D. The Friction and Lubrication of Solids; Oxford University Press: Oxford, UK, 2001; Volume 1. [Google Scholar]
- Barnette, A.L.; Asay, D.B.; Kim, S.H. Average molecular orientations in the adsorbed water layers on silicon oxide in ambient conditions. Phys. Chem. Chem. Phys. 2008, 10, 4981–4986. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xiao, C.; He, X.; Yu, B.; Kim, S.H.; Qian, L. Friction and tribochemical wear behaviors of native oxide layer on silicon at nanoscale. Tribol. Lett. 2017, 65, 139. [Google Scholar] [CrossRef]
- Chen, L.; Wen, J.; Zhang, P.; Yu, B.; Chen, C.; Ma, T.; Lu, X.; Kim, S.H.; Qian, L. Nanomanufacturing of silicon surface with a single atomic layer precision via mechanochemical reactions. Nat. Commun. 2018, 9, 1542. [Google Scholar] [CrossRef] [PubMed]
- He, H.T.; Qian, L.M.; Pantano, C.G.; Kim, S.H. Mechanochemical wear of soda lime silica glass in humid environments. J. Am. Ceram. Soc. 2014, 97, 2061–2068. [Google Scholar] [CrossRef]
- Shen, L.T.; Zhao, Y.D.; Wang, Y.; Song, R.B.; Yao, Q.; Chen, S.S.; Chai, Y. A long-term corrosion barrier with an insulating boron nitride monolayer. J. Mater. Chem. A 2016, 4, 5044–5050. [Google Scholar] [CrossRef]
- Wiederhorn, S.M.; Bolz, L.H. Stress corrosion and static fatigue of glass. J. Am. Ceram. Soc. 1970, 53, 543–548. [Google Scholar] [CrossRef]
- Michalske, T.A.; Freiman, S.W. A molecular mechanism for stress-corrosion in vitreous silica. J. Am. Ceram. Soc. 1983, 66, 284–288. [Google Scholar] [CrossRef]
- Ciccotti, M. Stress-corrosion mechanisms in silicate glasses. J. Phys. D Appl. Phys. 2009, 42, 214006. [Google Scholar] [CrossRef]
- Adams, R.; Mcmillan, P.W. Static fatigue in glass. J. Mater. Sci. 1977, 12, 643–657. [Google Scholar] [CrossRef]
- Orowan, E. The fatigue of glass under stress. Nature 1944, 154, 341–343. [Google Scholar] [CrossRef]
- Bradley, L.C.; Dilworth, Z.R.; Barnette, A.L.; Hsiao, E.; Barthel, A.J.; Pantano, C.G.; Kim, S.H. Hydronium ions in soda-lime silicate glass surfaces. J. Am. Ceram. Soc. 2013, 96, 458–463. [Google Scholar] [CrossRef]
- Surdyka, N.D.; Pantano, C.G.; Kim, S.H. Environmental effects on initiation and propagation of surface defects on silicate glasses: Scratch and fracture toughness study. Appl. Phys. A 2014, 116, 519–528. [Google Scholar] [CrossRef]
- He, H.T.; Qian, L.M.; Pantano, C.G.; Kim, S.H. Effects of humidity and counter-surface on tribochemical wear of soda-lime-silica glass. Wear 2015, 342, 100–106. [Google Scholar] [CrossRef]
- Yu, J. Quantitative investigation on single-asperity friction and wear of phosphate lase glass against aspherical AFM diamond tip. Tribol. Int. 2015, 81, 43–52. [Google Scholar] [CrossRef]
- He, H.; Luo, J.; Qian, L.; Pantano, C.G.; Kim, S.H. Thermal poling of soda-lime silica glass with nonblocking electrodes—Part 2: Effects on mechanical and mechanochemical properties. J. Am. Ceram. Soc. 2016, 99, 1231–1238. [Google Scholar] [CrossRef]
- Yu, J.X.; He, H.T.; Jian, Q.Y.; Zhang, W.L.; Zhang, Y.F.; Yuan, W.F. Tribochemical wear of phosphate laser glass against silica ball in water. Tribol. Int. 2016, 104, 10–18. [Google Scholar] [CrossRef]
- He, H.T.; Yu, J.X.; Qian, L.M.; Pantano, C.G.; Kim, S.H. Enhanced tribological properties of barium boroaluminosilicate glass by thermal poling. Wear 2017, 376, 337–342. [Google Scholar] [CrossRef]
- Yu, J.X.; He, H.T.; Zhang, Y.F.; Hu, H.L. Nanoscale mechanochemical wear of phosphate laser glass against a CeO2 particle in humid air. Appl. Surf. Sci. 2017, 392, 523–530. [Google Scholar] [CrossRef]
- Luo, J.; He, H.; Podraza, N.J.; Qian, L.; Pantano, C.G.; Kim, S.H. Thermal poling of soda-lime silica glass with nonblocking electrodes—Part 1: Effects of sodium ion migration and water ingress on glass surface structure. J. Am. Ceram. Soc. 2016, 99, 1221–1230. [Google Scholar] [CrossRef]
- Sheth, N.; Luo, J.W.; Banerjee, J.; Pantano, C.G.; Kim, S.H. Characterization of surface structures of dealkalized soda lime silica glass using X-ray photoelectron, specular reflection infrared, attenuated total reflection infrared and sum frequency generation spectroscopies. J. Non-Cryst. Solids 2017, 474, 24–31. [Google Scholar] [CrossRef]
- Sheth, N.; Ngo, D.; Banerjee, J.; Zhou, Y.; Pantano, C.G.; Kim, S.H. Probing hydrogen-bonding interactions of water molecules adsorbed on silica, sodium calcium silicate, and calcium aluminosilicate glasses. J. Phys. Chem. C 2018, in press. [Google Scholar] [CrossRef]
- He, H.T.; Kim, S.H.; Qian, L.M. Effects of contact pressure, counter-surface and humidity on wear of soda-lime-silica glass at nanoscale. Tribol. Int. 2016, 94, 675–681. [Google Scholar] [CrossRef]
- Archard, J.F. Contact and rubbing of flat surfaces. J. Appl. Phys. 1953, 24, 981–988. [Google Scholar] [CrossRef]
- Archard, J.F.; Hirst, W. The wear of metals under unlubricated conditions. Proc. R. Soc. Lond. Ser. A 1956, 236, 397–410. [Google Scholar] [CrossRef]
- Kato, K.; Adachi, K. Wear of advanced ceramics. Wear 2002, 253, 1097–1104. [Google Scholar] [CrossRef]
- Fischer, T.E.; Zhu, Z.; Kim, H.; Shin, D.S. Genesis and role of wear debris in sliding wear of ceramics. Wear 2000, 245, 53–60. [Google Scholar] [CrossRef]
- Buckley, D.H.; Miyoshi, K. Friction and wear of ceramics. Wear 1984, 100, 333–353. [Google Scholar] [CrossRef]
- Fischer, T.E. Friction and wear of ceramics. Scr. Metall. Mater. 1990, 24, 833–838. [Google Scholar] [CrossRef]
- Hsu, S.M.; Lim, D.S.; Wang, Y.S.; Munro, R.G. Ceramics wear maps—Concept and method development. Lubr. Eng. 1991, 47, 49–54. [Google Scholar]
- Fischer, T.E.; Mullins, W.M. Chemical aspects of ceramic tribology. J. Phys. Chem. 1992, 96, 5690–5701. [Google Scholar] [CrossRef]
- Lancaster, J.K.; Mashal, Y.A.H.; Atkins, A.G. The role of water in the wear of ceramics. J. Phys. D Appl. Phys. 1992, 25, A205–A211. [Google Scholar] [CrossRef]
- Gogotsi, Y.G.; Yoshimura, M. Water effects on corrosion behavior of structural ceramics. MRS Bull. 1994, 19, 39–45. [Google Scholar] [CrossRef]
- Klaffke, D. On the influence of test parameters on friction and wear of ceramics in oscillating sliding contacts. Tribotest 1995, 1, 311–320. [Google Scholar] [CrossRef]
- Hsu, S.M.; Shen, M.C. Ceramic wear maps. Wear 1996, 200, 154–175. [Google Scholar] [CrossRef]
- Adachi, K.; Kato, K.; Chen, N. Wear map of ceramics. Wear 1997, 203, 291–301. [Google Scholar] [CrossRef]
- Erdemir, A.; Erck, R.; Fenske, G.; Hong, H. Solid/liquid lubrication of ceramics at elevated temperatures. Wear 1997, 203, 588–595. [Google Scholar] [CrossRef]
- Xu, J.G.; Kato, K. Formation of tribochemical layer of ceramics sliding in water and its role for low friction. Wear 2000, 245, 61–75. [Google Scholar] [CrossRef]
- Sugita, T.; Ueda, K.; Kanemura, Y. Material removal mechanism of silicon-nitride during rubbing in water. Wear 1984, 97, 1–8. [Google Scholar] [CrossRef]
- Fischer, T.E.; Tomizawa, H. Interaction of tribochemistry and microfracture in the friction and wear of silicon-nitride. Wear 1985, 105, 29–45. [Google Scholar] [CrossRef]
- Kapsa, P.; Enomoto, Y. Sliding damage on hot-pressed and sintered silicon-nitride caused by a diamond tip under controlled humidity. Wear 1988, 127, 65–83. [Google Scholar] [CrossRef]
- Imada, Y.; Kamamura, K.; Honda, F.; Nakajima, K. The tribological reaction accompanying friction and wear of silicon-nitride containing titanium nitride. J. Tribol. 1992, 114, 230–235. [Google Scholar] [CrossRef]
- Ishigaki, H.; Kawaguchi, I.; Iwasa, M.; Toibana, Y. Friction and wear of hot-pressed silicon-nitride and other ceramics. J. Tribol. 1986, 108, 514–521. [Google Scholar] [CrossRef]
- Sasaki, S. The effects of the surrounding atmosphere on the friction and wear of alumina, zirconia, silicon-carbide and silicon-nitride. Wear 1989, 134, 185–200. [Google Scholar] [CrossRef]
- Perez-Unzueta, A.; Beynon, J.; Gee, M. Effects of surrounding atmosphere on the wear of sintered alumina. Wear 1991, 146, 179–196. [Google Scholar] [CrossRef]
- Gee, M.G. The formation of aluminium hydroxide in the sliding wear of alumina. Wear 1992, 153, 201–227. [Google Scholar] [CrossRef]
- Park, D.S.; Danyluk, S.; Mcnallan, M.J. Influence of tribochemical reaction-products on friction and wear of silicon-nitride at elevated-temperatures in reactive environments. J. Am. Ceram. Soc. 1992, 75, 3033–3039. [Google Scholar] [CrossRef]
- Komvopoulos, K.; Li, H. The effect of tribofilm formation and humidity on the friction and wear properties of ceramic materials. J. Tribol. 1992, 114, 131–140. [Google Scholar] [CrossRef]
- Srinivasan, S.R.; Blau, P.J. Effect of relative-humidity on repetitive impact behavior of machined silicon-nitride. J. Am. Ceram. Soc. 1994, 77, 683–688. [Google Scholar] [CrossRef]
- Basu, B.; Vitchev, R.G.; Vleugels, J.; Celis, J.P.; van der Biest, O. Influence of humidity on the fretting wear of self-mated tetragonal zirconia ceramics. Acta Mater. 2000, 48, 2461–2471. [Google Scholar] [CrossRef]
- Murthy, V.S.R.; Kobayashi, H.; Tsurekawa, S.; Tamari, N.; Watanabe, T.; Kato, K. Influence of humidity and doping elements on the friction and wear of SiC in unlubricated sliding. Tribol. Int. 2004, 37, 353–364. [Google Scholar] [CrossRef]
- Wäsche, R.; Klaffke, D.; Troczynski, T. Tribological performance of SiC and TiB2 against SiC and Al2O3 at low sliding speeds. Wear 2004, 256, 695–704. [Google Scholar] [CrossRef]
- Gates, R.S.; Hsu, S.M.; Klaus, E.E. Tribochemical mechanism of alumina with water. Tribol. Trans. 1989, 32, 357–363. [Google Scholar] [CrossRef]
- Jahanmir, S.; Fischer, T.E. Friction and wear of silicon-nitride lubricated by humid air, water, hexadecane and hexadecane + 0.5 percent stearic acid. Tribol. Trans. 1988, 31, 32–43. [Google Scholar] [CrossRef]
- Kimura, Y.; Okada, K.; Enomoto, Y. Sliding damage of silicon-nitride in plane contact. Wear 1989, 133, 147–161. [Google Scholar] [CrossRef]
- Hah, S.R.; Fischer, T.E. Tribochemical polishing of silicon nitride. J. Electrochem. Soc. 1998, 145, 1708–1714. [Google Scholar] [CrossRef]
- Fischer, T.E.; Liang, H.; Mullins, W.M. Tribochemical lubricious oxides on silicon nitride. MRS Online Proc. Libr. 2011, 140, 339–344. [Google Scholar] [CrossRef]
- Wallbridge, N.; Dowson, D.; Roberts, E. The wear characteristics of sliding pairs of high density polycrystalline aluminium oxide under both dry and wet conditions. In Proceedings of the International Conference of Wear of Materials, Reston, VA, USA, 11–14 April 1983. [Google Scholar]
- Fischer, T.E.; Anderson, M.P.; Jahanmir, S.; Salher, R. Friction and wear of tough and brittle zirconia in nitrogen, air, water, hexadecane and hexadecane containing stearic-acid. Wear 1988, 124, 133–148. [Google Scholar] [CrossRef]
- Barnes, A.M.; Bartle, K.D.; Thibon, V.R. A review of zinc dialkyldithiophosphates (ZDDPs): Characterisation and role in the lubricating oil. Tribol. Int. 2001, 34, 389–395. [Google Scholar] [CrossRef]
- Olver, A. Gear lubrication—A review. Proc. Inst. Mech. Eng. Part J. 2002, 216, 255–267. [Google Scholar] [CrossRef]
- Spikes, H. The history and mechanisms of ZDDP. Tribol. Lett. 2004, 17, 469–489. [Google Scholar] [CrossRef]
- Nicholls, M.A.; Do, T.; Norton, P.R.; Kasrai, M.; Bancroft, G.M. Review of the lubrication of metallic surfaces by zinc dialkyl-dithiophosphates. Tribol. Int. 2005, 38, 15–39. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, X.; Liu, Y.; Gunsel, S.; Luo, J. Ultrathin MoS2 nanosheets with superior extreme pressure property as boundary lubricants. Sci. Rep. 2015, 5, 12869. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, Y.; Luo, J. Tribological properties of few-layer graphene oxide sheets as oil-based lubricant additives. Chin. J. Mech. Eng. 2016, 29, 439–444. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Y.; Gunsel, S.; Luo, J. Mechanism of antiwear property under high pressure of synthetic oil-soluble ultrathin MoS2 sheets as lubricant additives. Langmuir 2018, 34, 1635–1644. [Google Scholar] [CrossRef] [PubMed]
- Goto, H.; Buckley, D.H. The influence of water-vapor in air on the friction behavior of pure metals during fretting. Tribol. Int. 1985, 18, 237–245. [Google Scholar] [CrossRef]
- Klaffke, D. On the repeatability of friction and wear results and on the influence of humidity in oscillating sliding tests of steel steel pairings. Wear 1995, 189, 117–121. [Google Scholar] [CrossRef]
- De Baets, P.; Kalacska, G.; Strijckmans, K.; van de Velde, F.; Van Peteghem, A.P. Experimental study by means of thin layer activation of the humidity influence on the fretting wear of steel surfaces. Wear 1998, 216, 131–137. [Google Scholar] [CrossRef]
- Bregliozzi, G.; Di Schino, A.; Kenny, J.M.; Haefke, H. The influence of atmospheric humidity and grain size on the friction and wear of AISI 304 austenitic stainless steel. Mater. Lett. 2003, 57, 4505–4508. [Google Scholar] [CrossRef]
- Oh, H.K.; Yeon, K.H.; Kim, H.Y. The influence of atmospheric humidity on the friction and wear of carbon steels. J. Mater. Process. Technol. 1999, 95, 10–16. [Google Scholar] [CrossRef]
- Wang, Y.; Lei, T.Q. Wear behavior of steel 1080 with different microstructures during dry sliding. Wear 1996, 194, 44–53. [Google Scholar] [CrossRef]
- Wang, Y.; Lei, T.Q.; Liu, J.J. Tribo-metallographic behavior of high carbon steels in dry sliding: I. Wear mechanisms and their transition. Wear 1999, 231, 1–11. [Google Scholar] [CrossRef]
- Goto, H.; Amamoto, Y. Effect of varying load on wear resistance of carbon steel under unlubricated conditions. Wear 2003, 254, 1256–1266. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; He, X.; Xiao, C.; Kim, S.H. Effect of Humidity on Friction and Wear—A Critical Review. Lubricants 2018, 6, 74. https://doi.org/10.3390/lubricants6030074
Chen Z, He X, Xiao C, Kim SH. Effect of Humidity on Friction and Wear—A Critical Review. Lubricants. 2018; 6(3):74. https://doi.org/10.3390/lubricants6030074
Chicago/Turabian StyleChen, Zhe, Xin He, Chen Xiao, and Seong H. Kim. 2018. "Effect of Humidity on Friction and Wear—A Critical Review" Lubricants 6, no. 3: 74. https://doi.org/10.3390/lubricants6030074
APA StyleChen, Z., He, X., Xiao, C., & Kim, S. H. (2018). Effect of Humidity on Friction and Wear—A Critical Review. Lubricants, 6(3), 74. https://doi.org/10.3390/lubricants6030074






