Lube Oil Wear Reduction via Organic Tribofilms
Abstract
:1. Introduction
2. Experimental Methods
2.1. Materials
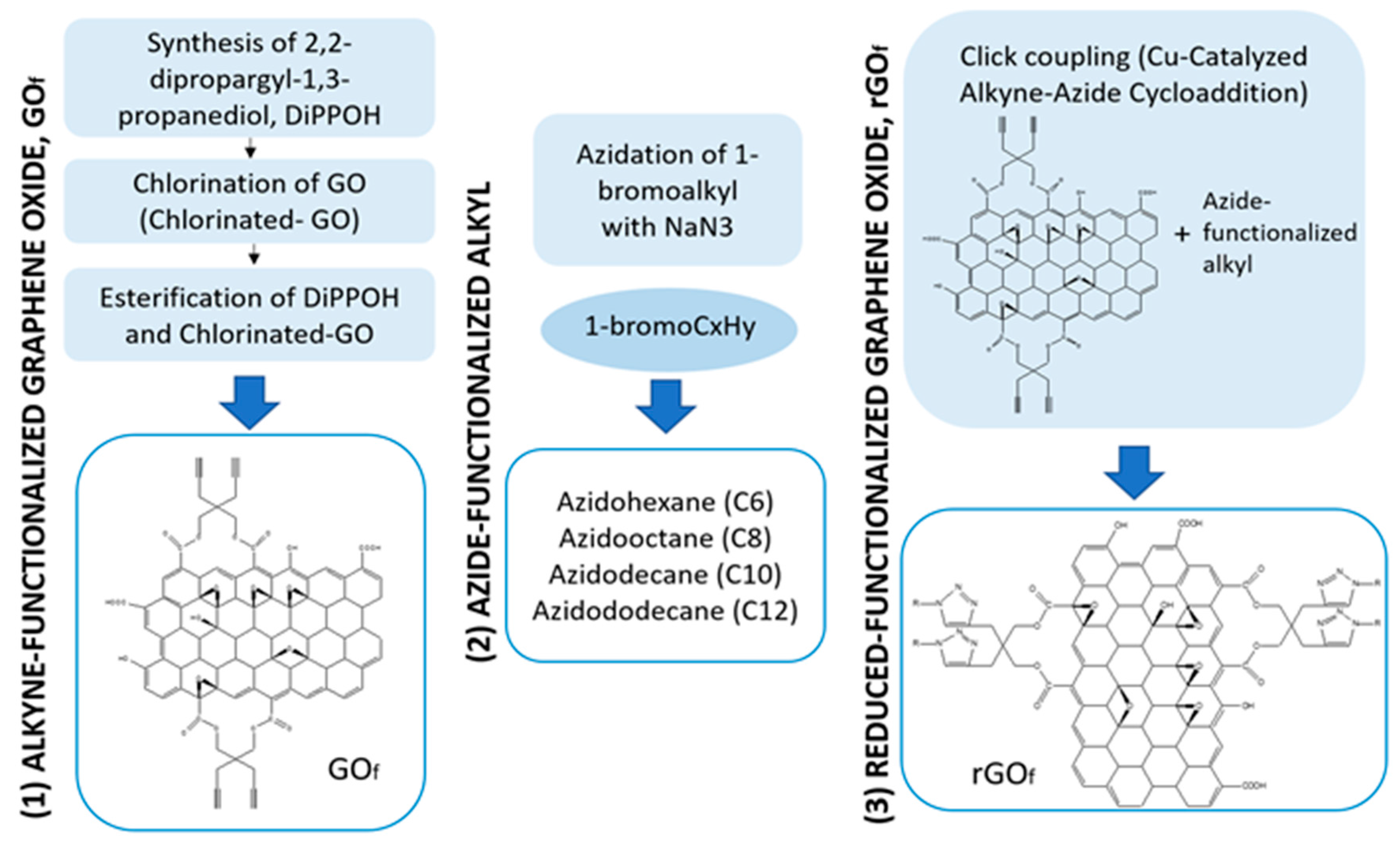
2.2. Materials Preparation
2.3. Materials Characterization
2.4. Tribological Characterization
3. Results and Discussion
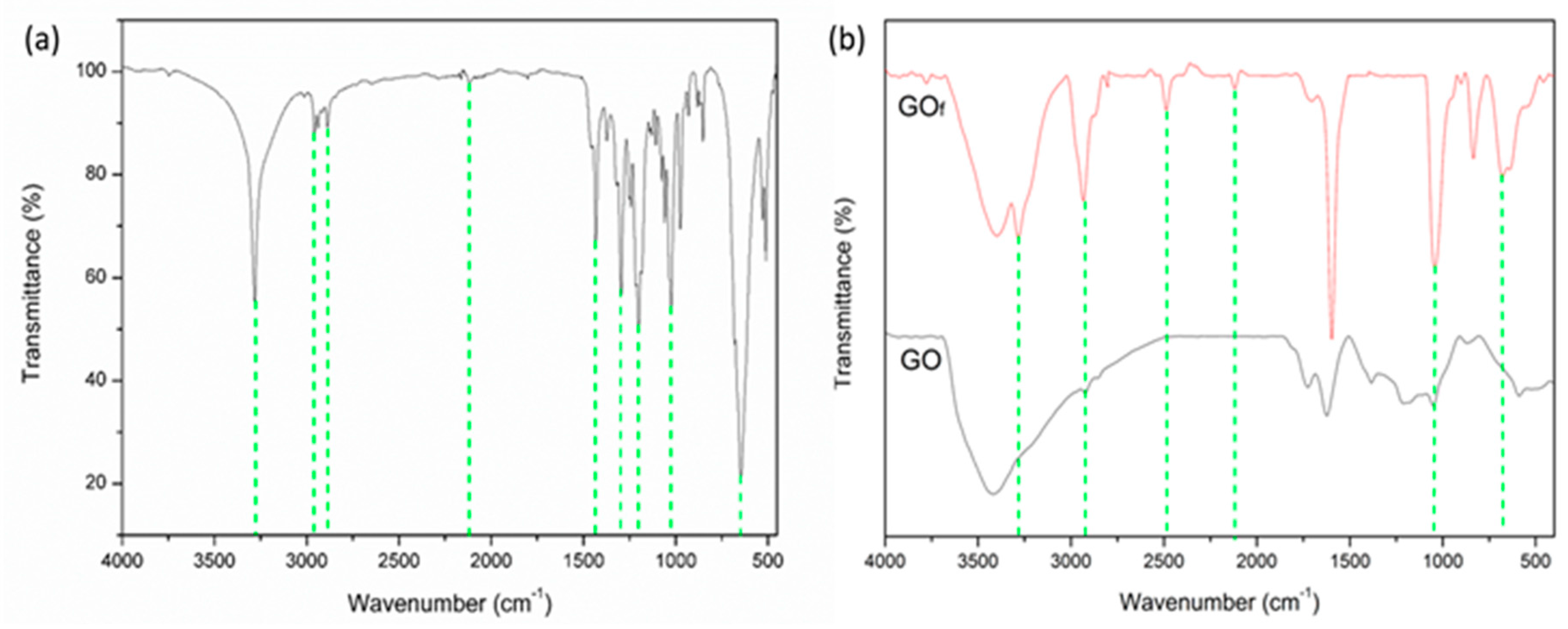
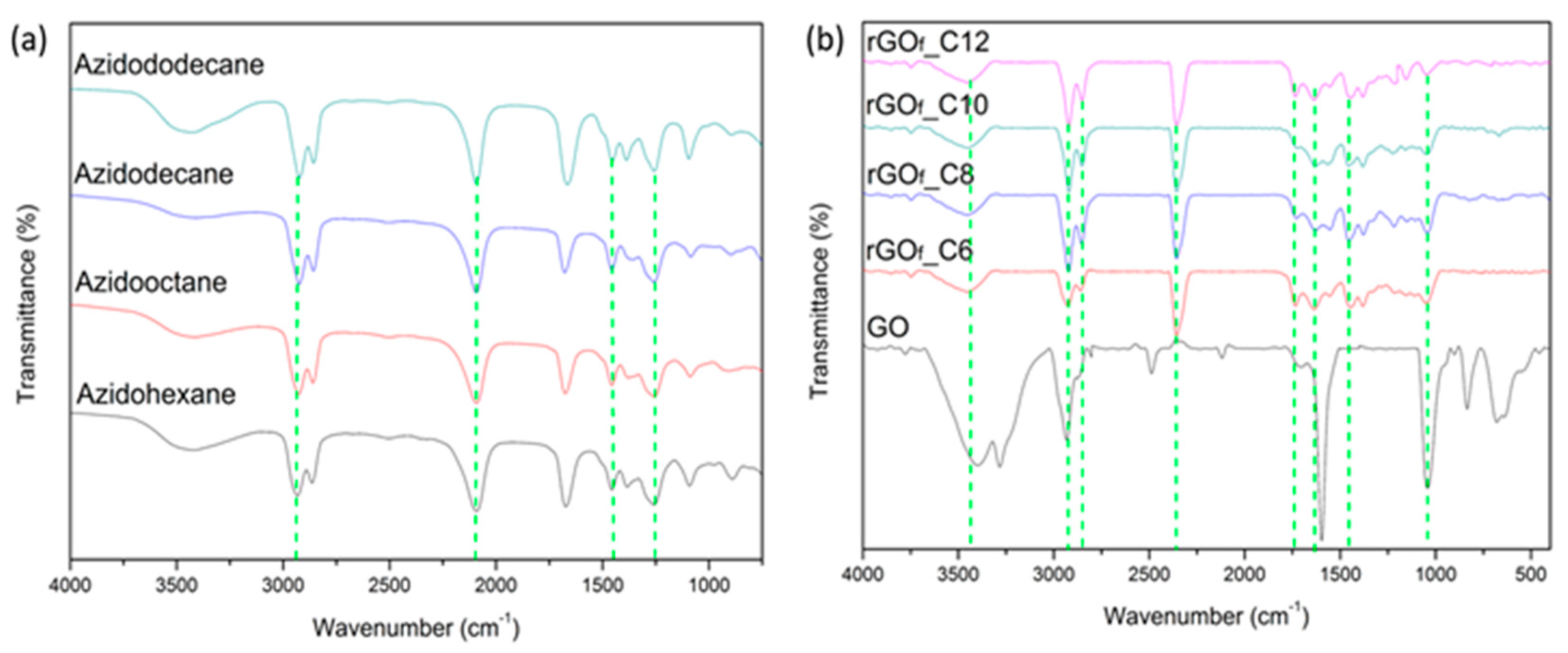
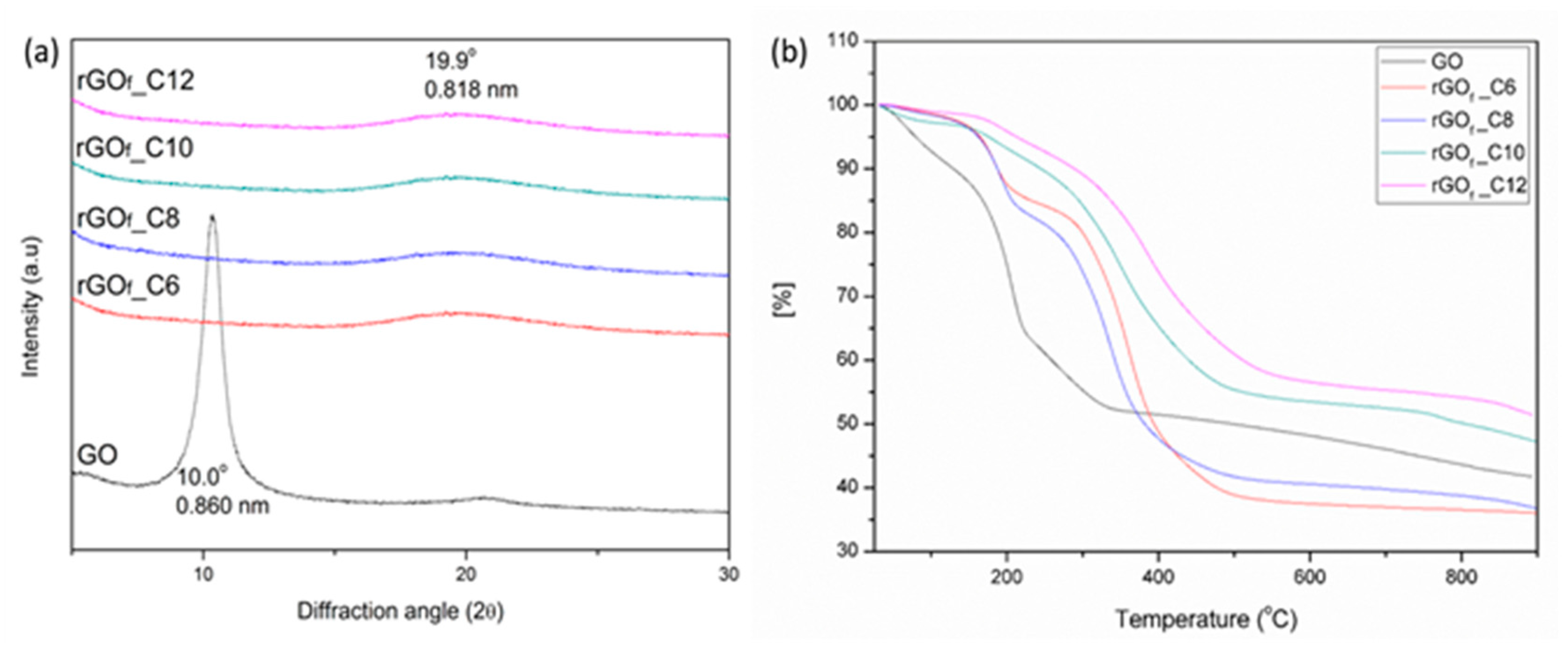
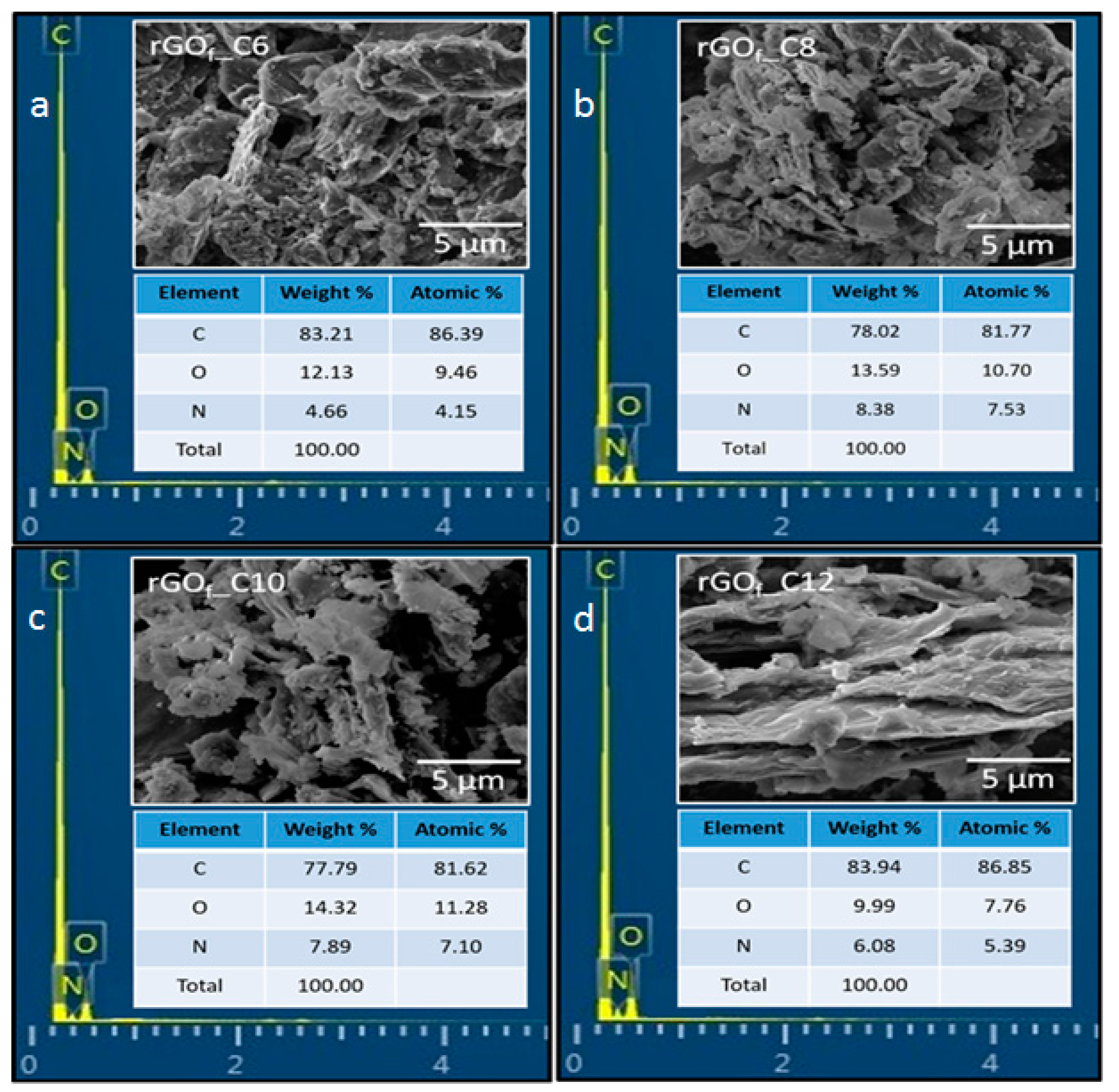
3.1. Materials Characterization
3.2. Dispersibility Test
3.3. Tribological Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Holmberg, K.; Andersson, P.; Erdemir, A. Global energy consumption due to friction in passenger cars. Tribol. Int. 2012, 47, 221–234. [Google Scholar] [CrossRef]
- Holmberg, K.; Erdemir, A. Global impact of friction on energy consumption, economy and environment. FME Trans. 2015, 43, 181–185. [Google Scholar]
- Berman, D.; Erdemir, A.; Sumant, A.V. Graphene: A new emerging lubricant. Mater. Today 2014, 17, 31–42. [Google Scholar] [CrossRef]
- Eswaraiah, V.; Sankaranarayanan, V.; Ramaprabhu, S. Graphene-based engine oil nanofluids for tribological applications. ACS Appl. Mater. Interfaces 2011, 3, 4221–4227. [Google Scholar] [CrossRef] [PubMed]
- Fleischauer, P.; Hilton, M. Applications of space tribology in the USA. Tribol. Int. 1990, 23, 135–139. [Google Scholar] [CrossRef]
- Peng, D.-X.; Chen, C.-H.; Kang, Y.; Chang, Y.-P.; Chang, S.-Y. Size effects of SiO2 nanoparticles as oil additives on tribology of lubricant. Ind. Lubr. Tribol. 2010, 62, 111–120. [Google Scholar] [CrossRef]
- Chen, S.; Liu, W.; Yu, L. Preparation of ddp-coated pbs nanoparticles and investigation of the antiwear ability of the prepared nanoparticles as additive in liquid paraffin. Wear 1998, 218, 153–158. [Google Scholar] [CrossRef]
- Lu, H.F.; Fei, B.; Xin, J.H.; Wang, R.H.; Li, L.; Guan, W.C. Synthesis and lubricating performance of a carbon nanotube seeded miniemulsion. Carbon 2007, 45, 936–942. [Google Scholar] [CrossRef]
- Holmberg, K.; Ronkainen, H.; Matthews, A. Tribology of thin coatings. Ceram. Int. 2000, 26, 787–795. [Google Scholar] [CrossRef]
- Crawford, J.; Psaila, A.; Orszulik, S. Miscellaneous additives and vegetable oils. In Chemistry and Technology of Lubricants; Springer: Dordrecht, The Netherlands, 1997; pp. 181–202. [Google Scholar]
- Jiang, W.; Nadeau, G.; Zaghib, K.; Kinoshita, K. Thermal analysis of the oxidation of natural graphite—Effect of particle size. Thermochim. Acta 2000, 351, 85–93. [Google Scholar] [CrossRef]
- Ren, P.-G.; Yan, D.-X.; Ji, X.; Chen, T.; Li, Z.-M. Temperature dependence of graphene oxide reduced by hydrazine hydrate. Nanotechnology 2010, 22, 055705. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Li, Q.; Kalb, W.; Liu, X.-Z.; Berger, H.; Carpick, R.W.; Hone, J. Frictional characteristics of atomically thin sheets. Science 2010, 328, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Senatore, A.; D’Agostino, V.; Petrone, V.; Ciambelli, P.; Sarno, M. Graphene oxide nanosheets as effective friction modifier for oil lubricant: Materials, methods, and tribological results. ISRN Tribol. 2013, 2013, 425809. [Google Scholar] [CrossRef]
- Wintterlin, J.; Bocquet, M.-L. Graphene on metal surfaces. Surf. Sci. 2009, 603, 1841–1852. [Google Scholar] [CrossRef]
- Ramanathan, T.; Abdala, A.; Stankovich, S.; Dikin, D.; Herrera-Alonso, M.; Piner, R.; Adamson, D.; Schniepp, H.; Chen, X.; Ruoff, R. Functionalized graphene sheets for polymer nanocomposites. Nat. Nanotechnol. 2008, 3, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wang, L.; Chen, G. Modification of graphene platelets and their tribological properties as a lubricant additive. Tribol. Lett. 2011, 41, 209–215. [Google Scholar] [CrossRef]
- Bhushan, B.; Gupta, B.K. Handbook of Tribology: Materials, Coatings, and Surface Treatments; Krieger Publishing Company: Malabar, FL, USA, 1991. [Google Scholar]
- Huang, H.; Tu, J.; Gan, L.; Li, C. An investigation on tribological properties of graphite nanosheets as oil additive. Wear 2006, 261, 140–144. [Google Scholar] [CrossRef]
- Martorana, P.; Bayer, I.S.; Steele, A.; Loth, E. Effect of graphite and carbon nanofiber additives on the performance efficiency of a gear pump driven hydraulic circuit using ethanol. Ind. Eng. Chem. Res. 2010, 49, 11363–11368. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, M.; Zhu, H.; Tian, Y.; Wang, K.; Wei, J.; Ji, F.; Li, X.; Li, Z.; Zhang, P. Tribological properties of oleic acid-modified graphene as lubricant oil additives. J. Phys. D Appl. Phys. 2011, 44, 205303. [Google Scholar] [CrossRef]
- Ou, J.; Wang, J.; Liu, S.; Mu, B.; Ren, J.; Wang, H.; Yang, S. Tribology study of reduced graphene oxide sheets on silicon substrate synthesized via covalent assembly. Langmuir 2010, 26, 15830–15836. [Google Scholar] [CrossRef] [PubMed]
- Gaylord, N.G. Reduction with complex metal hydrides. J. Chem. Educ. 1957, 34, 367. [Google Scholar] [CrossRef]
- Truce, W.E.; Birum, G.; McBee, E. Chlorination of dimethyl sulfide and some of its derivatives with sulfuryl chloride and thionyl chloride1. J. Am. Chem. Soc. 1952, 74, 3594–3599. [Google Scholar] [CrossRef]
- Freeman, J.; Smith, M. The preparation of anhydrous inorganic chlorides by dehydration with thionyl chloride. J. Inorg. Nucl. Chem. 1958, 7, 224–227. [Google Scholar] [CrossRef]
- Liu, D.; Bielawski, C.W. Direct azidation of isotactic polypropylene and synthesis of ‘grafted to’ derivatives thereof using azide–alkyne cycloaddition chemistry. Polym. Int. 2016. [Google Scholar] [CrossRef]
- Namvari, M.; Namazi, H. Sweet graphene i: Toward hydrophilic graphene nanosheets via click grafting alkyne-saccharides onto azide-functionalized graphene oxide. Carbohydr. Res. 2014, 396, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Lai, Z.; Feng, J.; Wu, P. Graphene oxide sheets covalently functionalized with block copolymers via click chemistry as reinforcing fillers. J. Mater. Chem. 2011, 21, 9271–9278. [Google Scholar] [CrossRef]
- Kou, L.; He, H.; Gao, C. Click chemistry approach to functionalize two-dimensional macromolecules of graphene oxide nanosheets. Nano-Micro Lett. 2010, 2, 177–183. [Google Scholar] [CrossRef]
- Wang, H.-X.; Zhou, K.-G.; Xie, Y.-L.; Zeng, J.; Chai, N.-N.; Li, J.; Zhang, H.-L. Photoactive graphene sheets prepared by “click” chemistry. Chem. Commun. 2011, 47, 5747–5749. [Google Scholar] [CrossRef] [PubMed]
- Dondoni, A. Triazole: The keystone in glycosylated molecular architectures constructed by a click reaction. Chem.–Asian J. 2007, 2, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yao, B.; Li, C.; Shi, G. An improved hummers method for eco-friendly synthesis of graphene oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Simic, R.; Kalin, M. Comparison of alcohol and fatty acid adsorption on hydrogenated dlc coatings studied by afm and tribological tests. Stroj. Vestnik-J. Mech. Eng. 2013, 59, 707–718. [Google Scholar] [CrossRef]



| Sample | Average Coefficient of Friction, CoF | Difference (%) |
|---|---|---|
| Base oil | 0.077 | Benchmark |
| Base oil with GO | 0.078 | +0.779 |
| Base oil with rGOf_C6 | 0.065 | −14.86 |
| Base oil with rGOf_C8 | 0.065 | −15.39 |
| Base oil with rGOf_C10 | 0.065 | −15.78 |
| Base oil with rGOf_C12 | 0.065 | −15.51 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismail, N.A.; Bagheri, S. Lube Oil Wear Reduction via Organic Tribofilms. Lubricants 2017, 5, 30. https://doi.org/10.3390/lubricants5030030
Ismail NA, Bagheri S. Lube Oil Wear Reduction via Organic Tribofilms. Lubricants. 2017; 5(3):30. https://doi.org/10.3390/lubricants5030030
Chicago/Turabian StyleIsmail, Nurul Athirah, and Samira Bagheri. 2017. "Lube Oil Wear Reduction via Organic Tribofilms" Lubricants 5, no. 3: 30. https://doi.org/10.3390/lubricants5030030
APA StyleIsmail, N. A., & Bagheri, S. (2017). Lube Oil Wear Reduction via Organic Tribofilms. Lubricants, 5(3), 30. https://doi.org/10.3390/lubricants5030030





