Abstract
This overview covers the most recent developments in the field of ionic nanofluid lubricants, defined as dispersions of nanoparticles with ionic liquids through the activation of nanophases. The nanophases range from metal nanoparticles and ceramic inorganic nanoparticles, to different carbon nanophases. The combinations with room-temperature ionic liquids can be in the form of mixtures, dispersions, surface-modified nanophases, or chemically-functionalized nanophases. The new ionic nanofluids can be used as base lubricants, as lubricant additives, or as anti-friction and wear-reducing additives in new nanocomposite materials.
Keywords:
nanoprticles; carbon nanotubes; grapheme; ionic liquids; friction; lubrication; wear; surfaces; interphases 1. Introduction
Nanomaterials have become one of the main additive groups added to base oils to enhance their tribological performance with effective friction-reducing and anti-wear behavior. In fact, according to a recent review [1] the main anti-friction additives for liquid lubricant oils can be broadly classified into three main types: organic compounds; metal-organic derivatives, mainly transition metal complexes; and nanomaterials.
The lubricant nanoadditives could, in turn, be classified into the following categories: inorganic nanophases, comprising metal, metal oxide and other metal-based nanoparticles, such as fullerene-like nanomaterials; and carbon-based nanophases, such as fullerenes, nanodiamonds, carbon nanotubes, and graphene nanostructures.
2. Ionic Liquids
Ionic liquids (ILs) are molten salts [2] which are in the liquid state at room temperature, and are molecules generally formed by bulk organic cations and organic or inorganic anions. ILs show a unique combination of properties. From the tribological point of view, some of the most relevant properties are their low volatility and inflammability, and their high thermal stability.
ILs have shown an excellent performance as lubricants and lubricant additives in lubrication of metallic and ceramic materials under severe sliding conditions. ILs have also been used for the reduction of friction coefficients and wear rates of thermoplastic polymers and epoxy resins.
The first molten salt which was in the liquid state at room temperature, ethylammonium nitrate ([C2H5NH3]NO3), was described by Walden in 1914 ([2], and cited by [3]). However, it was only in 1992 that Wilkes and Zaworotko [4] reported the first ionic liquids which were stable in air and, the alkylimidazolium derivatives with the tetrafluoroborate or hexafluorophosphate anions. These ILs were the first ones studied as lubricants by Liu et al. in 2001 [5]. From then to the present moment, the number of groups dedicated to the research of the tribological performance of ILs has been growing steadily [6,7,8,9,10,11,12,13,14,15]. As the properties and applications of ILs in tribology have been the object of a number of previous reviews [6,8,9,10,11,12,13], this aspect will not be the purpose of the present review.
The discovery of the lubricating ability of ILs in 2001 [5] was closely followed by the description of the interactions between ILs and single-walled carbon nanotubes (SWCNTs) by Fukushima et al., in 2003 [16]. The ability of ILs to modify, disperse, and functionalize [17,18,19] carbon nanotubes gave rise to a new family of nanofluids [20].
The present review will focus on the tribological applications of the new IL-nanophase hybrid nanomaterials. In the first place, it is necessary to distinguish the different kinds of nanomaterials [21], the results of their interactions with ILs and, finally, the different mechanisms that could explain their tribological performance.
3. Nanophases
3.1. Nanometals
Transition metal nanoparticles [21,22,23], particularly those composed of Group 11 noble metals copper, silver, and gold, but also of other heavy metals, such as cerium or zinc, have been used as lubricant additives. The excellent tribological performance described has been explained by the low shear stress of the nanometals and by their ability to form a thin deposition layer on the worn surfaces. However, to achieve these results it is necessary to modify the nanometal particles’ surface in order to disperse them either in base lubricants or in fully-formulated oils.
IL-based friction modifiers have shown outstanding performance as compared with traditional friction modifiers [5,6,7,8,9,10,11,12,13,14,15]. The surface-modification ability of ILs has opened a new line of development of nanoadditives by enabling the dispersion of inorganic nanoparticles which, otherwise, would aggregate when added to the base oils, giving rise to large agglomerates and, thus, losing their good tribological behavior.
Chinas-Castillo and Spikes [21] investigated the effect of particle size using gold particles of five and 20 nm. The results showed that gold particles of 20 nm were more effective in reducing friction and wear than gold particles of 5 nm. This difference might be because the tiny 5 nm particles allow more asperity interactions than do the 20 nm particles.
Among the wider group of nanofluids, nanoparticle liquids (NPLs) are defined as monolithic hybrid materials composed of an inorganic nanometal core modified by an organic low viscosity corona.
Patton et al. [22] synthesized a (NPL) lubricant and deposited it on microelectromechanical systems (MEMS) switch contacts. In this case, the NPLs used were based on Au or Pt nanoparticles as core and a mercaptoethanesulfonate ionic liquid as the corona (Figure 1). The mercaptoethanesulfonate anion forms a covalent bond with the gold nanoparticles by S-H bond breaking due to the strong affinity between the thiol group and gold. The quaternary ammonium cation prevents agglomeration of the nanoparticles due to its long hydrocarbon chains. The NPL lubricants showed the ability to fill the contact area.
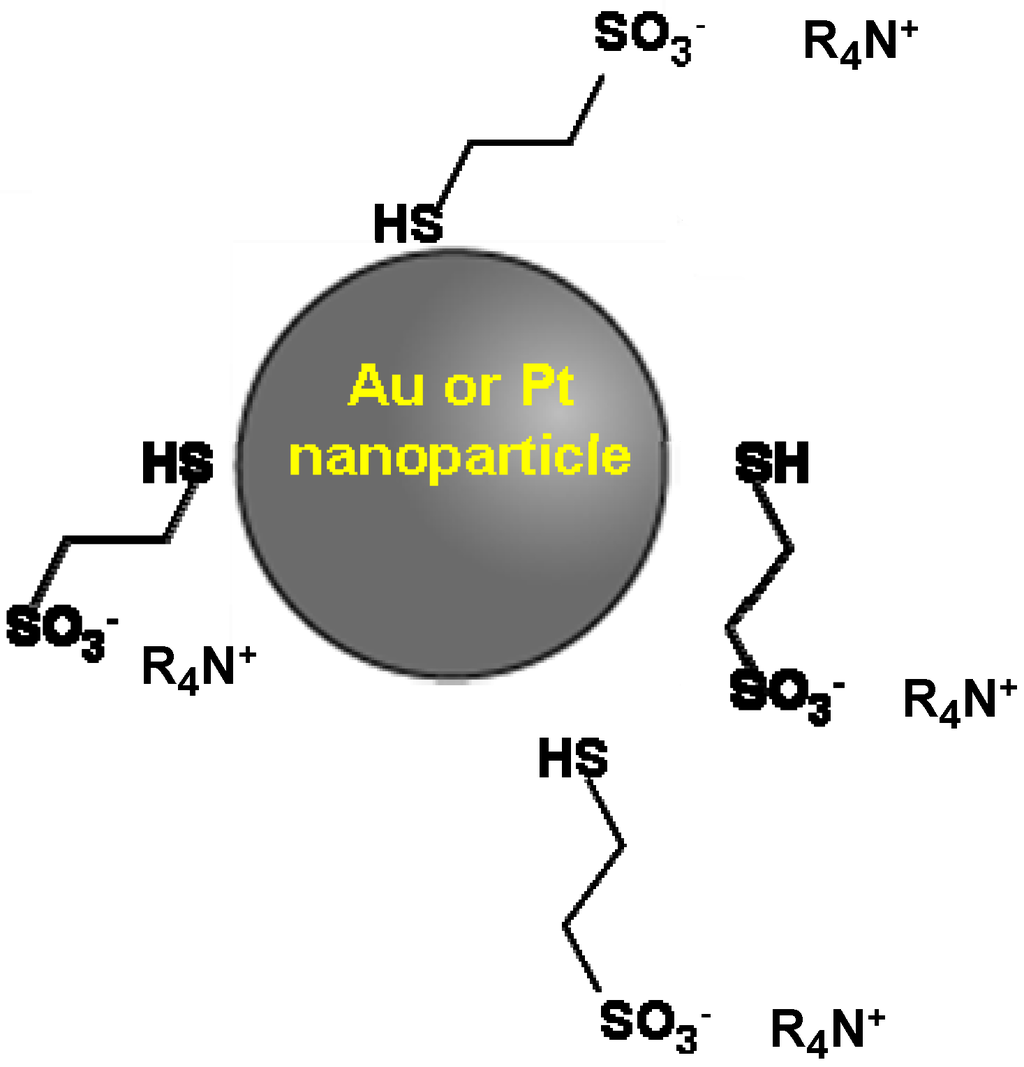
Figure 1.
Nanoparticle liquid (NPL) lubricant with a metal core and an ionic liquid corona [22].
3.2. Metal Oxide Nanoparticles
The heavy metals used as nanoadditives are also used as lubricant additives in the form of metal oxides.
The use of spherical metal oxide nanoparticles such as CuO, ZnO [23], Al2O3, and TiO2 as additives in base oil lubricants, such as polyalphaolefines (PAO) has been described. The friction and wear reductions have been attributed to the formation of a third body and to a tribo-sinterization mechanism. Other authors used nanoparticles as additives in lubricating oils, also reporting good tribological performances [24,25].
Smith et al., [26] found that pure ethylammonium nitrate IL prevents the formation of aggregates from suspensions of 1 μm diameter SiO2 spherical nanoparticles. However, these dispersions are not stable in the presence of small amounts of water. The stability of the suspensions in the IL can be explained by repulsions between solvation layers, which are weakened in the presence of water. Other protic ILs such as ethylammonium formate and diethylmethyl-ammonium formate also form stable suspensions, whose behavior depends on the ILs interfacial order.
CuO nanorods stabilized by ILs containing imidazolium and ammonium cations exhibited excellent friction and wear reduction (up to a 43%) compared to engine oil [27]. The good lubricating performance of the CuO nanorods in ILs was attributed to their good dispersion stability and rolling effect mechanism (Figure 2a). Interaction with IL molecules produced morphological changes in copper oxide nanorods and modified their aspect ratio. It was found that the anti-friction and wear ability of the nanorods increased with increasing aspect ratio and dispersion stability.
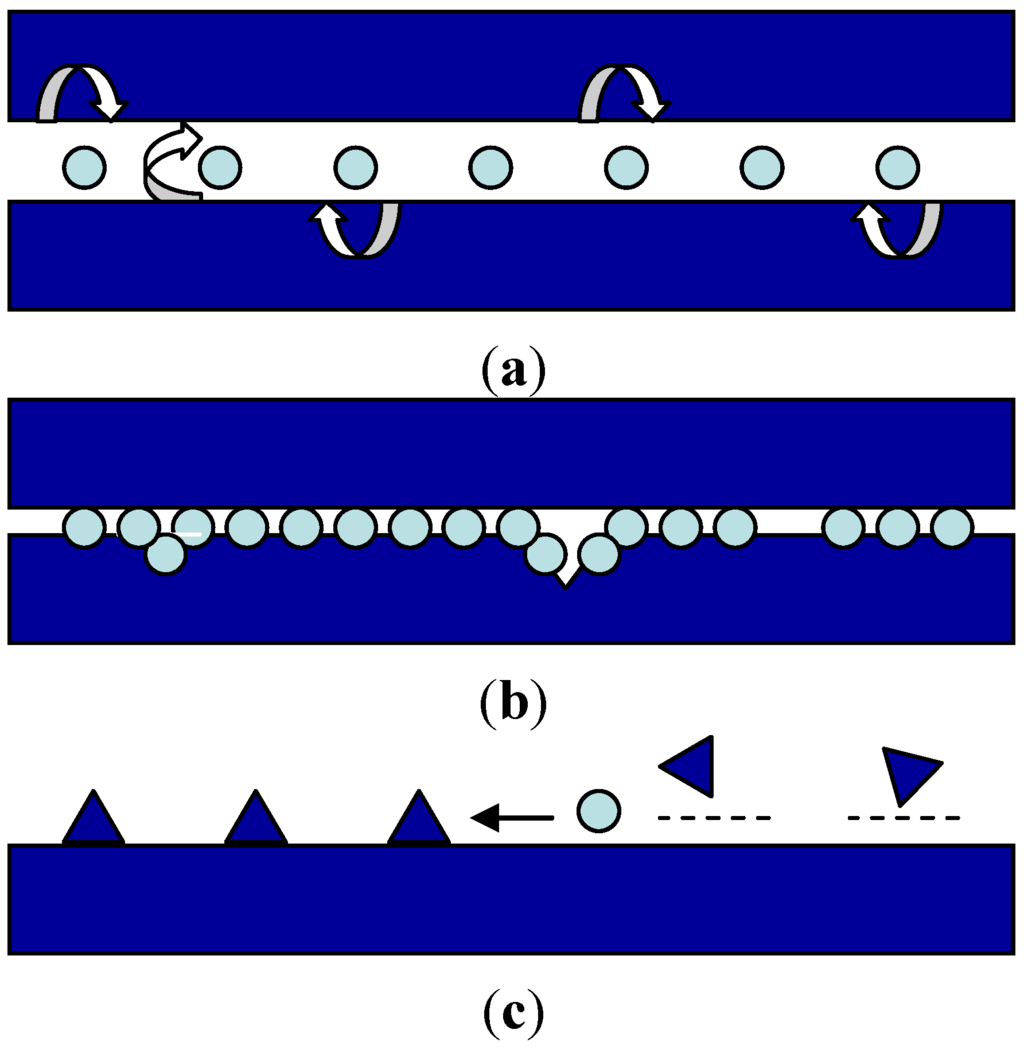
Figure 2.
Main friction-reducing and anti-wear mechanisms for spherical nanoparticles: (a) rolling effect; (b) surface protective film and filling of asperities; and (c) polishing effect [1].
Wu et al., [24] described a superior anti-wear ability of CuO nanoparticles, with respect to other metal oxides and to nanodiamond, due to the deposition of CuO nanoparticles on the worn surface and a decrease in the shearing stress.
ZnO nanoparticles are also effective in reducing friction and wear [28], but their tribological performance was enhanced when the morphology and surface composition of the ZnO nanoparticles was modified by an ionic liquid [29,30,31,32].
3.3. Mechanisms of Friction Reduction and Wear Protection by Nanoparticles
The main mechanisms which explain the friction and wear reduction by nanoparticles [20] can be described as sliding, where the nanoparticles separate the sliding materials, thus preventing direct contact, rolling, protective, and coating films and polishing of the surface asperities (Figure 2).
3.4. Effect of Nanoparticle Concentration and Size
The friction-reduction and anti-wear behaviors are dependent on the characteristics of nanoparticles, such as size, shape, and concentration.
A low concentration of nanoparticles (<1%) is sufficient to improve tribological properties. The optimum concentration varies for each nanophase and lubricant oil.
Most studies use nanoparticles with a size within the range nanoparticles of 2–120 nm [1,21]. A smaller size seems to be more adequate for interactions with the surfaces asperities of the sliding materials in order to form anti-wear surface protective films (Figure 2b).
3.5. Fullerene-Like Nanomaterials
Inorganic fullerene-like (IF) metal dichalcogenides MX2 (M = Mo, W; X = S, Se), with shape similar to carbon fullerenes and nanotubes, were first reported as lubricant additives by Rappoport et al. in 1999 [33]. They found that smaller spherical-shaped IF nanoparticles exhibited superior a rolling ability, a lower affinity to metal surfaces, and decreased contact temperatures.
3.6. Friction Mechanisms for IF Nanomaterials
The friction-reducing ability of IF nanoparticles in oil have been attributed by Rappoport et al., to the following main mechanisms [1,33]:
- (a)
- Effective rolling due to the spherical shape of IF nanoparticles.
- (b)
- The prevention of materials contact due to the intercalation of IF nanoparticles.
- (c)
- The formation of a third body by a mixture of the base oil and additives with wear debris particles.
3.7. Carbon Nanophases
Carbon materials have been the focus of interest in tribology since the discovery of carbon nanotubes (CNTs), fullerenes, and graphene because of their high chemical stability and excellent mechanical properties [34].
3.8. Nanodiamonds
Nanodiamonds have been used as oil additives [35], showing a ball-bearing effect between the rubbing surfaces, a surface polishing effect, and an increase in surface hardness as the main reasons for the good tribological performance with friction and wear reduction.
3.9. Carbon Nanotubes
Ionic liquids/multi-walled carbon nanotubes (IL/MWCNTs) composites can improve the friction reduction and the wear resistant ability as friction modifiers in ionic liquids due to a roller bearing effect played by the composites at the sliding contact [36].
Polyelectrolyte-grafted multiwalled carbon nanotubes (MWCNTsg-PILs), with a hard backbone of MWCNTs and a soft brush-like shell of poly(ionic liquids) (PILs) [37], have been described as excellent anti-wear and friction-reducing additives, due to their good dispersibility and to their core-shell structure (Figure 3).
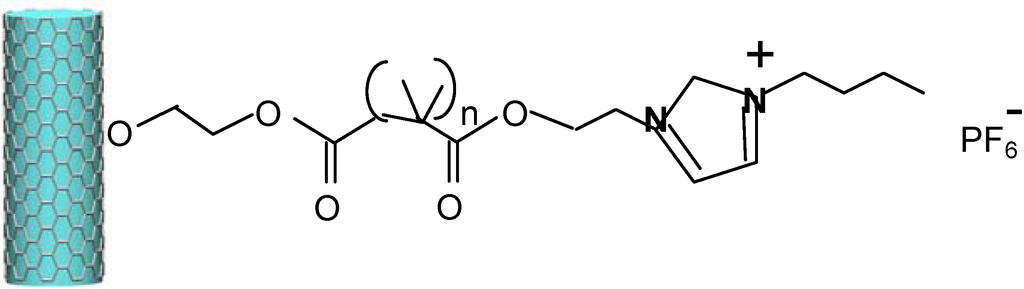
Figure 3.
Surface-functionalized MWCNT from polymerization of 1-butyl-3-methylimidazolium hexafluorophosphate ionic liquid [37].
Wang et al., [38] have described the chemical functionalization of MWCNTs to obtain nanostructures containing an imidazolium hexafluorophosphate unit (Figure 4).
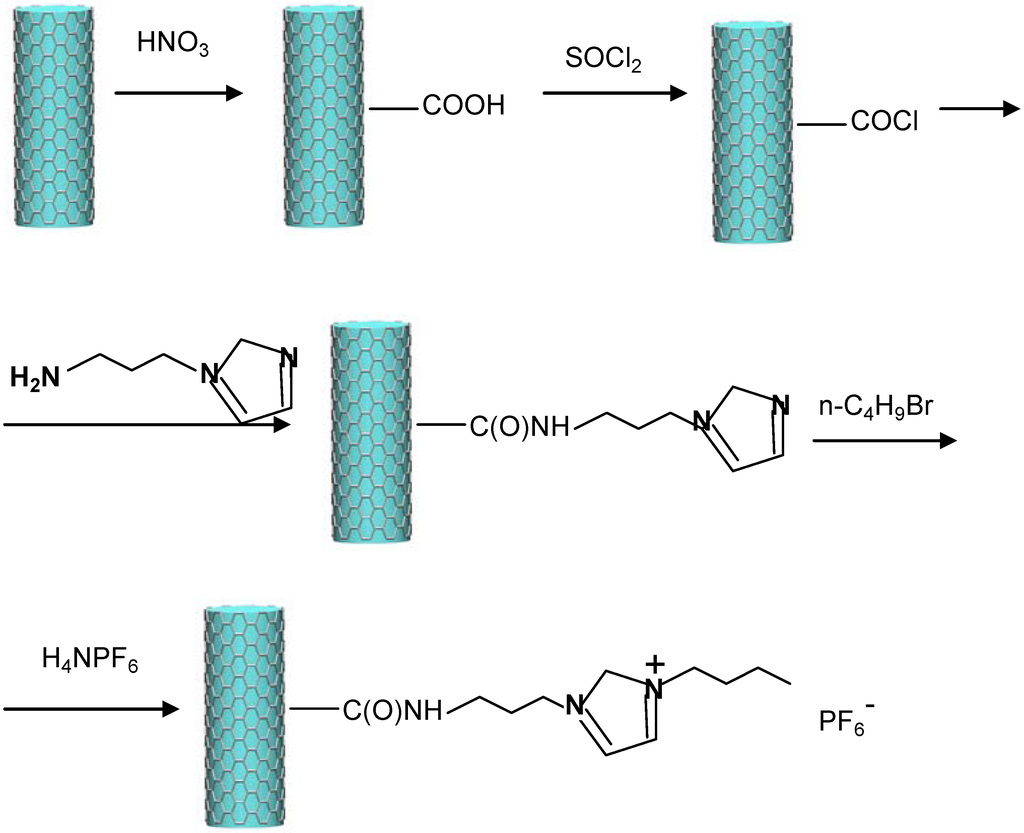
Figure 4.
Synthetic route to obtain chemically-functionalized MWCNT containing the imidazolium hexafluorophosphate ([BMIM] PF6) moiety [39].
The rheological measurements showed that the shear viscosity under high shear rates of the nanofluids was lower than that of the neat IL. This phenomenon was attributed to the self-lubrication of MWCNTs. The new nanofluids also showed friction-reduction and anti-wear ability.
Non-functionalized single-walled carbon nanotubes (SWCNTs) modified by ionic liquids have been used as friction-reducing and anti-wear lubricants [38,40,41].
SWCNTs modified by ILs have also demonstrated a good tribological performance as polymer additives, giving rise to new polymer/CNT/IL nanocomposites, both with SWCNTs [41,42] and MWCNTs [43,44].
3.10. Graphene
Graphene, a monoatomic, two-dimensional sp2 carbon atoms lattice [45], has attracted considerable attention due to its unique structure and remarkable properties, and the tribological applications of graphene have been reported [46,47].
To disperse graphene in non-polar lubricant oils, it is necessary to modify it [48]. Modification with IL molecules is being investigated [49].
The enhanced performance found for the composite graphene/IL nanostructures (Figure 5) can be attributed to the following mechanisms:
- (a)
- A rolling effect between the friction surfaces,
- (b)
- Formation of a tribo-film with a high load-bearing ability, preventing the direct contact between the rubbing surfaces (Figure 6).
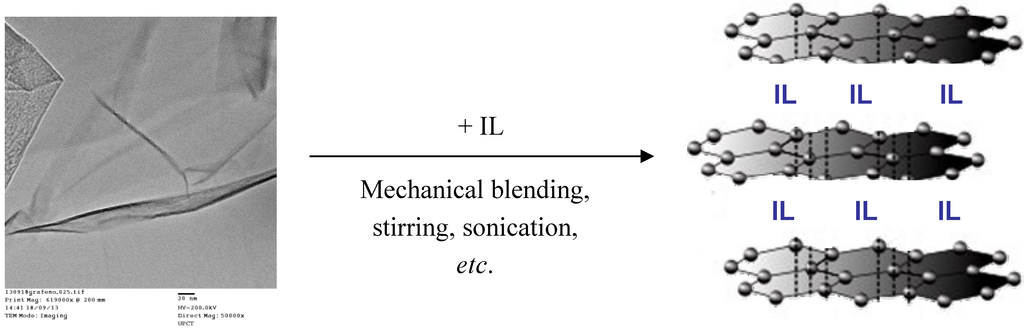
Figure 5.
Modification of graphene with ILs.
Gebbie et al. [50] proposed that ionic liquids form bound and diffuse electric double layers on charged solid surfaces.
The imidazolium cation delocalized positive charge (π+). When the imidazolium cation is in contact with the amorphous carbon surface containing sp2 carbon, a π− π+ interaction could occur at the interface. The formation of a nanometer thick IL layer on a sp2 carbon [42] has been attributed to the existence of a π− π+ stacking between the aromatic imidazolium ring and the surface (Figure 7). This layer was not observed when the imidazolium ring is substituted by an aliphatic cation.

Figure 6.
Formation of graphene additive layers on the sliding surfaces (graphene sheets are represented in black; lubricant film in blue).
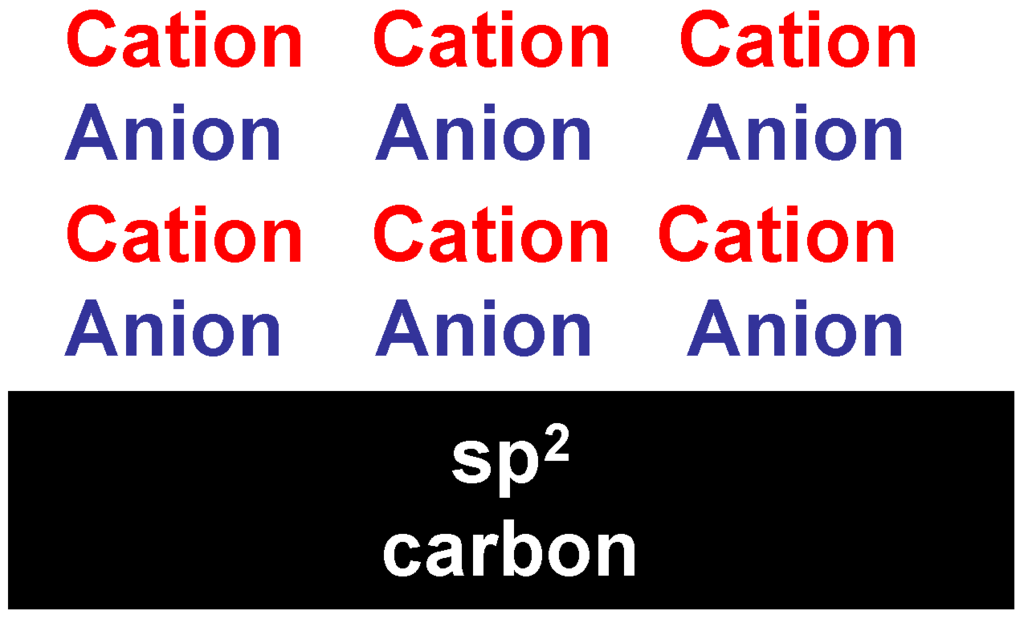
Figure 7.
IL electric double layer.
The outstanding lubrication performance of graphene nanostructures has been attributed to their laminated structure, which presents a low shear stress and prevents the direct interaction between the sliding surfaces, as graphene platelets could easily enter the contact area.
A graphene-IL hybrid material has been recently described [49] based on 1-butyl-3-methylimidazolium iodide. These authors studied the tribological performance of two nanofluid structures: the supernatant colloidal phase and the sediment after centrifugation of the graphene-IL mixture, in steel-aluminum contacts. Raman spectroscopy results showed that graphene platelets exhibit maximum asymmetry, while the colloidal phase shows minimum asymmetry. This has been attributed to a decrease in the number of layers in the sediment phase and a further decrease in the colloidal supernatant phase, due to interactions with IL molecules.
Mixtures of graphene oxide (GO) sheets and MWCNTs have been recently used [51] as IL lubricants nanoadditives (Figure 8a).
A synergistic effect has been found for the hybrid materials by combining 1D MWCNTs and 2D GO films. In this case, the IL was 1-butyl-3-methylimidazolium tetrafluoroborate.
The optimum graphene concentration found in screen tests was adopted for the additives in the test. The nanofluids were coated on diamond-like carbon (DLC) films by spin coating to obtain a nanofluid layer of approximately 0.5 mm thick. The composite coatings exhibit satisfactory friction performance in simulated space environments [52]. However, friction coefficients and wear rates of DLC/IL coatings under high vacuum conditions remained high, particularly at the running-in stage.
Graphene can also be incorporated into ionic liquids [53] either through direct exfoliation of graphite to create pristine or surface-pure graphene, or through the dispersion of reduced GO sheets by exfoliation of GO [54,55].
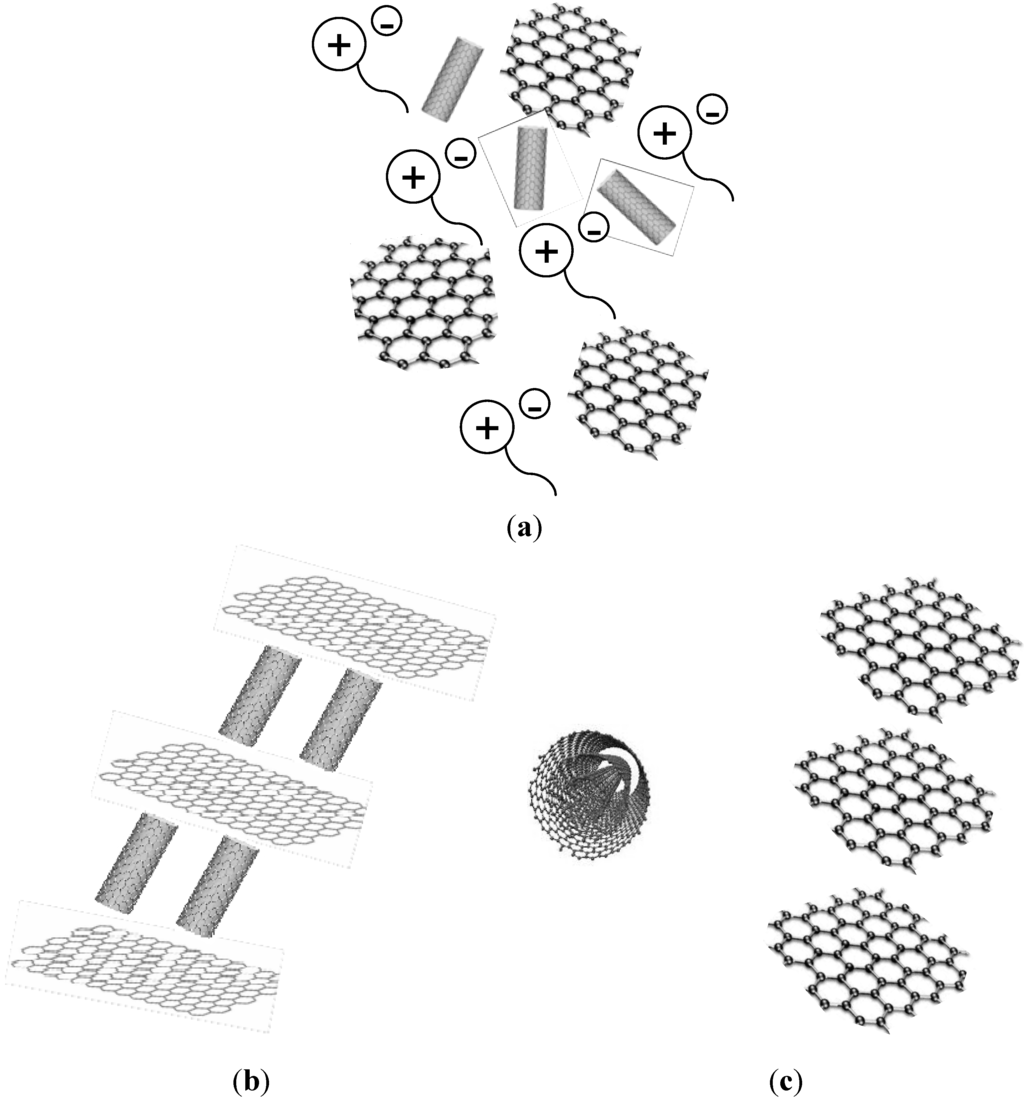
Figure 8.
(a) Graphene oxide sheets and carbon nanotubes dispersed in IL; (b) carbon nanotubes-graphene oxide ordered structures formed by stacking of carbon nanotubes and graphene layers at the sliding contact; and (c) unrolling of carbon nanotubes under load to give graphene layers.
Dispersions of reduced GO sheets led to lower viscosities than neat ILs at low temperatures, but its functionalized surface can form hydrogen bonds with the IL ions that can inhibit its viscosity reducing effect.
Zhang et al., have recently described [56] the use of a three-phase nanolubricant fluid formed by the combination of both MWCNTs and graphene with an IL (Figure 8a–c).
Shortening of MWCNTs and breaking of graphene sheets has been found to occur under lower loads at the worn surface, while unrolling of the MWCNTs (Figure 8c) to give rise to graphene-like structures and thickening of graphene multilayer structures have been proposed under higher loads.
The results of the tribological tests have shown that MWCNTs are more effective in improving the friction property of ILs at a low applied load, whereas graphene nanosheets present a better performance under higher loads. The friction reductions found are attributed to the different microstructural variations of MWCNTs and graphene during the friction process. The nanofluids also exhibited improved anti-wear properties due to the transfer of graphene and MWCNTs as a separator.
ILs modification improves the physicochemical properties of GO and graphene in terms of thermal and chemical stability, compatibility, and dispersion in base lubricants [57].
The friction reducing and anti-wear abilities are attributed to the formation of physically adsorbed films, chemisorption, and tribochemical reaction films at the contact areas. Modification by ILs has opened the route to novel graphene hybrid nanomaterials with potential applications in new composite materials and coatings.
However, if an excess graphene is added to an IL, large agglomerates would form, thus losing their lubricating ability. Liu et al. [58] found that an IL for DLC/IL/graphene composite coating found an optimum graphene concentration for the lowest coefficient.
3.11. Graphene/IL Polymer Matrix Composite Materials
As some of the main goals of tribology are to reduce friction coefficients and protect materials from wear, the combinations of ILs and nanophases, which are called nanofluids have been used not only as lubricants or lubricant additives, but also as polymer additives to obtain new nanocomposites.
A new kind of polymer matrix composite materials (Figure 9) based on the addition of graphene nanostructures (G) or graphene modified by ILs have been very recently described [58,59,60].

Figure 9.
New graphene/IL/polymer nanocomposites.
The IL molecules could prevent graphene sheets from agglomeration. The dispersed nanophases would improve the mechanical resistance and thermal stability of the polymer and could also favor the mobility of the polymer chains. The enhancement of the tribological performance [61] of the composites with respect to the polymer matrix depends on the size, concentration, and dispersibility of the nanophases. This is expected to be one of the most active areas in the application of graphene and ILs to improve the tribological performance of light, low resistant materials.
The research results covered in the present overview are fairly recent. Many issues remain to be studied before the true outreach of them can be foreseen. Some key issues such as the feasibility of the practical applications of nanofluids, their long-term stability, or the health risks they may pose should be addressed in parallel to more basic tribological research efforts.
4. Conclusions
The research results covered in the present overview are fairly recent. Many issues remain to be studied before the true outreach of them can be foreseen. The application of graphene and ionic liquids to develop new light, high resistant materials is expected to be one of the most active areas in the near future. Some key issues such as the feasibility of the practical applications of nanofluids, their long-term stability, or the health risks they may pose should be addressed in parallel to more basic tribological research efforts.
Acknowledgments
The authors wish to thank the financial support of the Ministerio de Economía y Competitividad (MINECO, Spain) (MAT2014-55384-P). “Este trabajo es resultado de los proyectos de investigación (19292/PI/14,19544/GERM/14-19877/GERM/14) financiados por la Fundación Séneca-Agencia de Ciencia y Tecnología de la Región de Murcia en el marco del PCTIRM2011-2014”. N. Saurín is grateful to the MINECO for a research grant (BES-2012-056621). T. Espinosa is grateful to the Ministerio de Educación, Cultura y Deporte (MECD, Spain) for a grant research grant (AP2010-3485).
Author Contributions
All authors contributed equally to this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tang, Z.; Li, S. A review of recent developments of friction modifiers for liquid lubricants (2007–present). Curr. Opin. Solid State Mater. Sci. 2014, 18, 119–139. [Google Scholar] [CrossRef]
- Walden, P. Uber die Moleculargrösse und Elektrische Leitfähigkeit einiger geschmolzenen Salze. Bull. Acad. Imp. Sci. St.-Petersb. 1914, 8, 405–422. [Google Scholar]
- Wasserscheid, P.; Welton, T. Ionic Liquids in Synthesis, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar]
- Wilkes, J.S.; Zaworotko, M.J. Air and water stable 1-ethyl-3-methylimidazolium based ionic liquids. Chem. Commun. 1992, 13, 965–967. [Google Scholar] [CrossRef]
- Ye, C.; Liu, W.; Chen, Y.; Yu, L. Room-temperature ionic liquids: A novel versatile lubricant. Chem. Commun. 2001, 2244–2245. [Google Scholar] [CrossRef]
- Torimoto, T.; Tsuda, K.; Okazaki, S.; Kuwabata, S. New frontiers in materials science opened by ionic liquids. Adv. Mater. 2010, 22, 1196–1221. [Google Scholar] [CrossRef] [PubMed]
- Lovelock, K.R.; Villar-García, I.J.; Maier, F.; Steinrück, H.P.; Licence, P. Photoelectron spectroscopy of ionic liquid-based interfaces. Chem. Rev. 2010, 110, 5158–5190. [Google Scholar] [CrossRef] [PubMed]
- Minami, I. Ionic liquids in tribology. Molecules 2009, 14, 2262–2269. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez, M.D.; Jiménez, A.E.; Sanes, J.; Carrión, F.J. Ionic liquids as advanced lubricantes. Molecules 2009, 14, 2888–2908. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Liang, Y.; Liu, W. Ionic liquid lubricants: Designed chemistry for engineering applications. Chem. Soc. Rev. 2009, 38, 2590–2599. [Google Scholar] [CrossRef] [PubMed]
- Van Rensselar, J. Unleashing the potential of ionic liquids. Tribol. Lubr. Technol. 2010, 4, 24–31. [Google Scholar]
- Palacio, M.; Bushan, B. A review of ionic liquids for green molecular lubrication in nanotechnology. Tribol. Lett. 2010, 40, 247–268. [Google Scholar] [CrossRef]
- Somers, A.E.; Howlett, P.C.; MacFarlane, D.R.; Forsyth, M. A Review of Ionic Liquid Lubricants. Lubricants 2013, 1, 3–21. [Google Scholar] [CrossRef]
- Bermúdez, M.D. Introduction to the ionic liquids special issue. Tribol. Lett. 2010, 40, 213. [Google Scholar] [CrossRef]
- Dörr, N. Special issue on ionic liquids as lubricants. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2012, 226, 889–890. [Google Scholar] [CrossRef]
- Fukushima, T.; Kosaka, A.; Ishimura, Y.; Yamamoto, T.; Takigawa, T.; Ishii, N.; Aida, T. Molecular ordering of organic molten salts triggered by single-walled carbon nanotubes. Science 2003, 300, 2072–2074. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, T.; Aida, T. Ionic liquids form soft functional materials with carbon nanotubes. Chemistry 2007, 13, 5048–5058. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chu, H.; Li, Y. Why single-walled carbon nanotubes can be dispersed in imidazoliumbased ionic liquids. ACS Nano 2008, 2, 2540–2546. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.S.; Wu, T.B.; Ding, K.L.; Hu, B.J.; Hou, M.Q.; Han, B.X. The dispersion of carbon nanotubes in water with the aid of very small amounts of ionic liquid. J. Chem. Soc. Chem. Commun. 2009, 14, 1897–1899. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.U.S. Nanofluids: From vision to reality through research. J. Heat Transf. ASME 2009, 131, 033106. [Google Scholar] [CrossRef]
- Chinas-Castillo, F.; Spikes, H.A. Mechanism of action of colloidal solid dispersions. Trans. ASME 2003, 125, 552–557. [Google Scholar] [CrossRef]
- Patton, S.T.; Andrey, A.; Voevodin, A.A.; Vaia, R.A.; Pender, M.; Diamanti, S.J.; Phillips, B. Nanoparticle Liquids for Surface Modification and Lubrication of MEMS Switch Contacts. J. Microelectromech. Syst. 2008, 17, 741–746. [Google Scholar] [CrossRef]
- Hernández-Battez, A.; González, R.; Viesca, J.L.; Fernández, J.E.; Díaz-Fernández, J.M.; Machado, A.; Chou, R.; Riba, J. CuO, ZrO and ZnO nanoparticles as antiwear additive in oil lubricants. Wear 2008, 265, 422–428. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Tsui, W.C.; Liu, T.C. Experimental analysis of tribological properties of lubricating oils with nanoparticle additives. Wear 2007, 262, 819–825. [Google Scholar] [CrossRef]
- Ingole, S.; Charanpahari, A.; Kakade, A.; Umare, S.S.; Bhatt, D.V.; Menghani, J. Tribological behavior of nano TiO as an additive in base oil. Wear 2013, 301, 776–785. [Google Scholar] [CrossRef]
- Smith, J.A.; Werzer, O.; Webber, G.B.; Warr, G.G.; Atkin, R. Surprising Particle Stability and Rapid Sedimentation Rates in an Ionic Liquid. J. Phys. Chem. Lett. 2010, 1, 64–68. [Google Scholar] [CrossRef]
- Gusain, R.; Khatri, O.P. Ultrasound assisted shape regulation of CuO nanorods in ionic liquids and their use as energy efficient lubricant additives. J. Mater. Chem. A 2013, 1, 5612–5619. [Google Scholar] [CrossRef]
- Hernandez-Battez, A.; Fernandez-Rico, J.E.; Navas-Arias, A.; Viesca-Rodriguez, J.L.; Chou Rodriguez, R.; Diaz Fernandez, J.M. The tribological behaviour of ZnO nanoparticles as an additive to PAO 6. Wear 2006, 261, 256–263. [Google Scholar] [CrossRef]
- Sanes, J.; Carrión, F.J.; Bermúdez, M.D. ZnO-ionic liquid nanostructures. Appl. Surf. Sci. 2009, 255, 4859–4862. [Google Scholar] [CrossRef]
- Carrion, F.J.; Sanes, J.; Bermudez, M.D. Effect of ionic liquid on the structure and tribological properties of polycarbonate-zinc oxide nanodispersion. Mater. Lett. 2007, 61, 4531–4535. [Google Scholar] [CrossRef]
- Bermúdez, M.D.; Brostow, W.; Carrión, F.J.; Sanes, J. Scratch resistance of polycarbonate containing ZnO nanoparticles: Effects of sliding direction. J. Nanosci. Nanotechnol. 2010, 10, 6683–6689. [Google Scholar] [CrossRef] [PubMed]
- Sanes, J.; Carrión, F.J.; Bermúdez, M.D. Effect of the addition of room temperature ionic liquid and ZnO nanoparticles on the wear and scratch resistance of epoxy resin. Wear 2010, 268, 1295–1302. [Google Scholar] [CrossRef]
- Rapoport, L.; Feldman, Y.; Homyonfer, M.; Cohen, H.; Sloan, J.; Hutchison, J.L. Inorganic fullerene-like material as additives to lubricants: Structure-function relationship. Wear 1999, 225–229, 975–982. [Google Scholar] [CrossRef]
- Thostenson, E.T.; Ren, Z.; Chou, T.W. Advances in the science and technology of carbon nanotubes and their composites: A review. Compos. Sci. Technol. 2001, 61, 1899–1912. [Google Scholar] [CrossRef]
- Tao, X.; Jiazheng, Z.; Kang, X. The ball-bearing effect of diamond nanoparticles as an oil additive. J. Phys. D 1996, 29, 2932–2937. [Google Scholar] [CrossRef]
- Yu, B.; Liu, Z.; Zhou, F.; Liu, W.; Liang, Y. A novel lubricant additive based on carbon nanotubes for ionic liquids. Mater. Lett. 2008, 62, 2967–2969. [Google Scholar] [CrossRef]
- Pei, X.; Xia, Y.; Liu, W.; Bo, Y.; Hao, J. Polyelectrolyte-Grafted Carbon Nanotubes: Synthesis, Reversible Phase-Transition Behavior, and Tribological Properties as Lubricant Additives. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 7225–7237. [Google Scholar] [CrossRef]
- Carrión, F.J.; Espejo, C.; Sanes, J.; Bermúdez, M.D. Single-walled carbon nanotubes modified by ionic liquid as antiwear additives of thermoplastics. Compos. Sci. Technol. 2010, 70, 2160–2167. [Google Scholar] [CrossRef]
- Wang, B.G.; Wang, X.B.; Lou, W.J.; Hao, J.C. Rheological and Tribological Properties of Ionic Liquid-Based Nanofluids Containing Functionalized Multi-Walled Carbon Nanotubes. J. Phys. Chem. C 2010, 114, 8749–8754. [Google Scholar] [CrossRef]
- Carrión, F.J.; Sanes, J.; Bermúdez, M.D.; Arribas, A. New single-walled carbon nanotubes-ionic liquid lubricant. Application to polycarbonate-stainless steel sliding contact. Tribol. Lett. 2011, 41, 199–207. [Google Scholar] [CrossRef]
- Bermúdez, M.D.; Carrión, F.J.; Espejo, C.; Martínez-López, E.; Sanes, J. Abrasive wear under multiscratching of polystyrene plus single-walled carbon nanotube nanocomposites. Effect of sliding direction and modification by ionic liquid. Appl. Surf. Sci. 2011, 257, 9073–9081. [Google Scholar] [CrossRef]
- Gong, X.; Kozbial, A.; Rose, F.; Li, L. Effect of π− π+ Stacking on the Layering of Ionic Liquids Confined to an Amorphous Carbon Surface. ACS Appl. Mater. Interfaces 2015, 7, 7078–7081. [Google Scholar] [CrossRef] [PubMed]
- Espejo, C.; Carrión, F.J.; Bermúdez, M.D. Scratch Resistance of New Polystyrene Nanocomposites with Ionic Liquid-Modified Multi-walled Carbon Nanotubes. Tribol. Lett. 2013, 52, 271–285. [Google Scholar] [CrossRef]
- Espejo, C.; Carrión, F.J.; Martínez, D.; Bermúdez, M.D. Multi-walled Carbon Nanotube-Imidazolium Tosylate Ionic Liquid Lubricant. Tribol. Lett. 2013, 50, 127–136. [Google Scholar] [CrossRef]
- Suárez, I.; Grobert, N.; Ewels, C.P. Nomenclature of sp2 carbon nanoforms. Guest Editorial. Carbon 2012, 50, 741–747. [Google Scholar] [CrossRef]
- Berman, D.; Erdemir, A.; Sumant, A.V. Graphene: A new emerging lubricant. Mater. Today 2014, 17, 31–42. [Google Scholar] [CrossRef]
- Penkov, O.; Kim, H.J.; Kim, H.J.; Kim, D.E. Tribology of Graphene: A Review. Int. J. Precis. Eng. Manuf. 2014, 15, 577–585. [Google Scholar] [CrossRef]
- Lin, J.; Wang, L.; Chen, G. Modification of graphene platelets and their tribological properties as a lubricant additive. Tribol. Lett. 2011, 41, 209–215. [Google Scholar] [CrossRef]
- Khare, V.; Pham, M.Q.; Kumari, N.; Yoon, H.S.; Kim, C.S.; Park, J.I.; Ahn, S.H. Graphene-ionic liquid based hybrid nanomaterials as novel lubricant for low friction and wear. Appl. Mater. Interfaces 2013, 5, 4063–4075. [Google Scholar] [CrossRef] [PubMed]
- Gebbie, M.A.; Valtiner, M.; Banquy, X.; Fox, E.T.; Henderson, W.A.; Israelachvili, J.N. Ionic Liquids Behave as Dilute Electrolyte Solutions. Proc. Natl. Acad. Sci. USA 2013, 110, 9674–9679. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Pu, J.B.; Wang, L.P.; Xue, Q.J. Synergistic Effect of Hybrid Carbon Nanotube-Graphene Oxide as Nanoadditive Enhancing the Frictional Properties of Ionic Liquids in High Vacuum. ACS Appl. Mater. Interfaces 2015, 7, 8592–8600. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, L.; Xue, Q. DLC-based Solid-Liquid Synergetic Lubricating Coatings for Improving Tribological Behavior of Boundary Lubricated Surfaces under High Vacuum Condition. Wear 2011, 271, 889–898. [Google Scholar] [CrossRef]
- Frazier, R.M.; Daly, D.T.; Spear, S.K.; Rogers, R.D. Exfoliation of Graphite Using Ionic Liquids. U.S. Provisional Patent US20110319554 A1, 25 November 2008. [Google Scholar]
- Wang, X.; Fulvio, P.F.; Baker, G.A.; Veith, G.M.; Unocic, R.R.; Mahurin, S.M.; Chi, M.; Dai, S. Direct exfoliation of natural graphite into micrometre size few layers graphene sheets using ionic liquids. Chem. Commun. 2010, 46, 4487–4489. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wu, T.; Ding, K.; Hu, B.; Hou, M.; Han, B. Dispersion of graphene sheets in ionic liquid [bmim][PF6] stabilized by an ionic liquid polymer. Chem. Commun. 2010, 46, 386–388. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Pu, J.B.; Wang, L.P.; Xue, Q.J. Frictional dependence of graphene and carbon nanotube in diamond-like carbon/ionic liquids hybrid films in vacuum. Carbon 2014, 80, 734–745. [Google Scholar] [CrossRef]
- Fan, X.Q.; Wang, L.P. High-performance lubricant additives based on modified graphene oxide by ionic liquids. J. Colloid Interface Sci. 2015, 452, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Pu, J.; Wang, L.; Xue, Q. Novel DLC/ionic liquid/graphene nanocomposite coatings towards high-vacuum related space applications. J. Mater. Chem. A 2013, 1, 3797–3809. [Google Scholar] [CrossRef]
- Kandanur, S.S.; Rafiee, M.A.; Yavari, F.; Schrameyer, M.; Yu, Z.Z.; Blanchet, T.A.; Koratkar, N. Supresion of wear in graphene polymer composites. Carbon 2012, 50, 3178–3183. [Google Scholar] [CrossRef]
- Choudhary, S.; Mungse, H.P.; Khatri, O.P. Dispersion of wear in grapheme polymer composites. J. Mater. Chem. 2012, 22, 21032–21039. [Google Scholar] [CrossRef]
- Saurín, N.; Sanes, J.; Bermúdez, M.D. Effect of Graphene and ionic liquid additives on the tribological performance of epoxy resin. Tribol. Lett. 2014, 56, 133–142. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).