Lubricants in Pharmaceutical Solid Dosage Forms
Abstract
:1. Introduction
2. Fundamentals of Lubrication
2.1. Friction
2.2. Friction and Adhesion
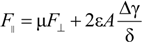
2.3. Lubrication
2.3.1. Lubrication in Pharmaceutical Processes
2.3.1.1. Wall Friction
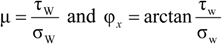
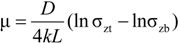
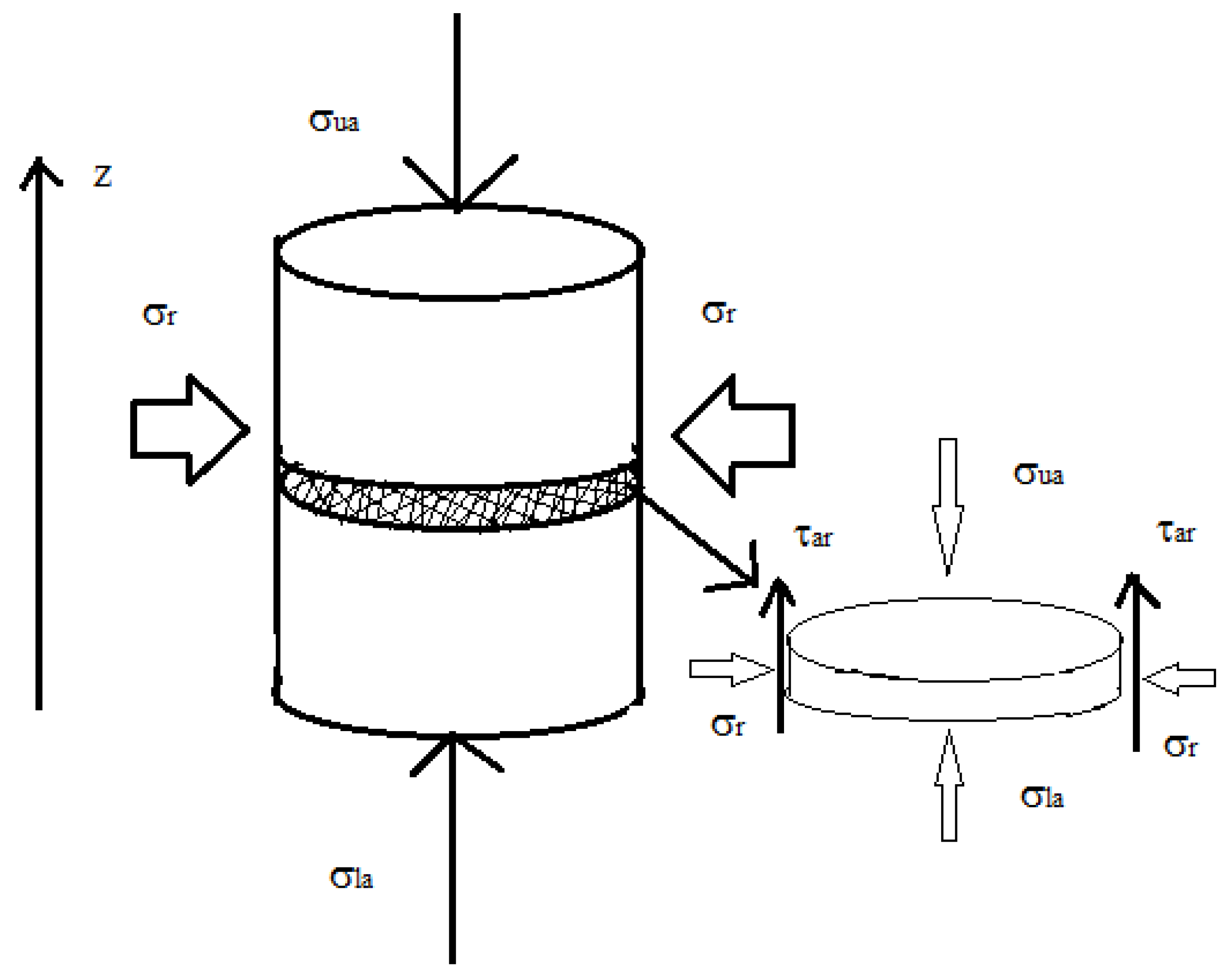
2.3.1.2. Powder Flow
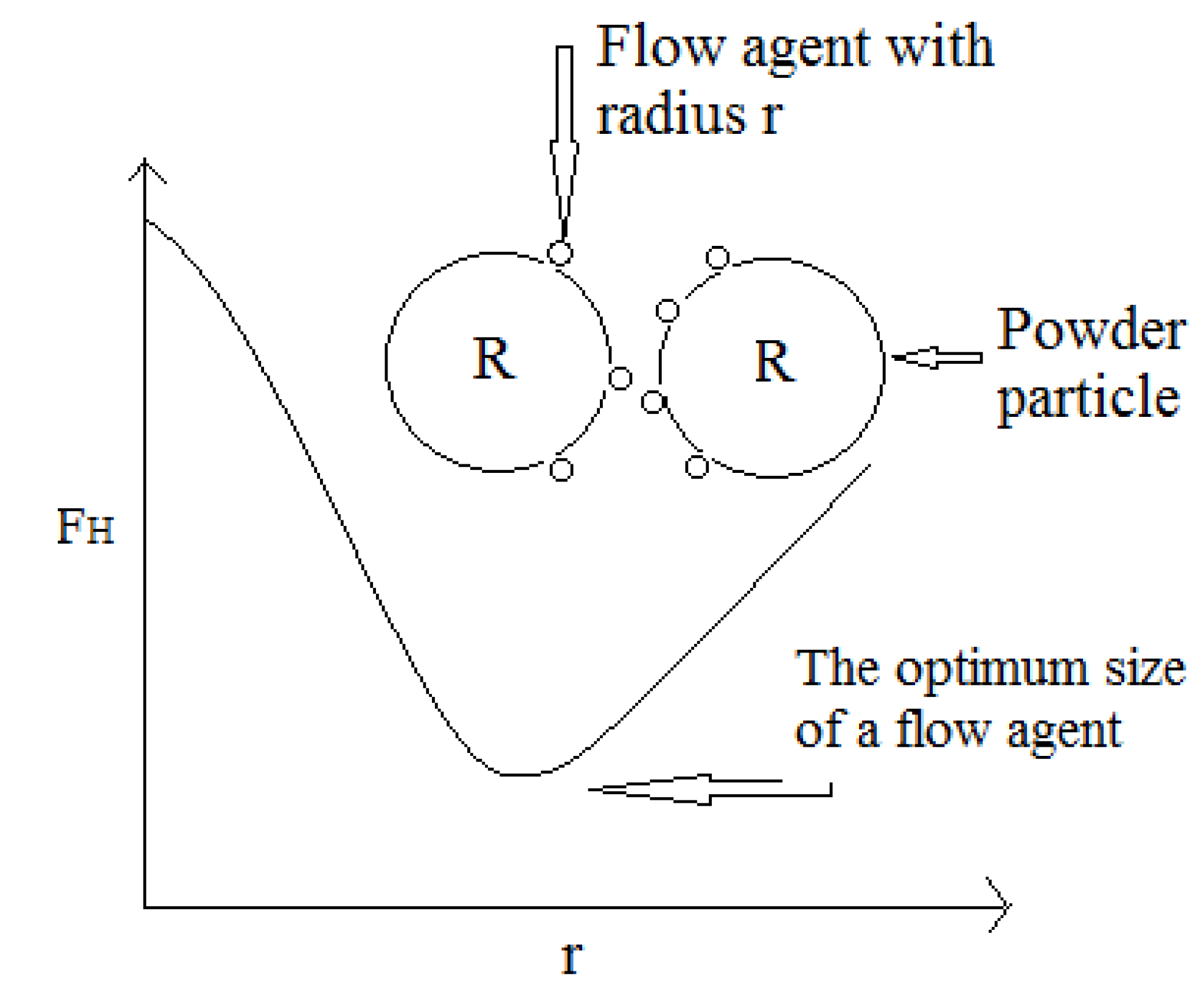
3. Common Lubricants Used in Drug Development
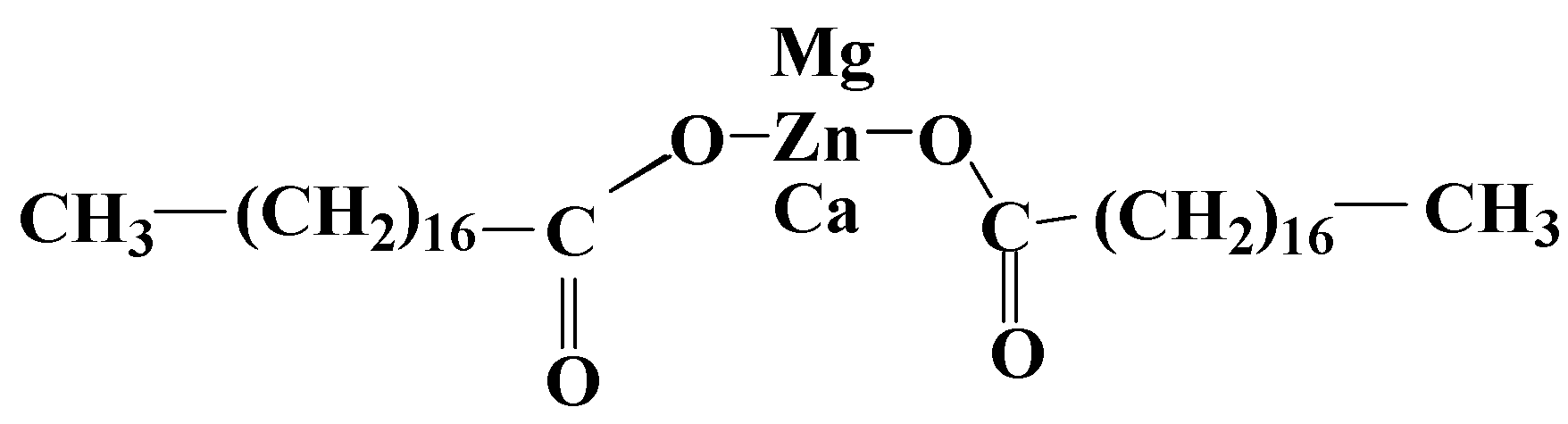
3.1. Metallic Salts of Fatty Acids
3.2. Fatty Acids
| Fatty acid | Formula | Molecular weight | Melting point (°C) | Boiling point at 16 mm (°C) |
|---|---|---|---|---|
| Stearic | CH3(CH2)16COOH | 284 | 69.6 | 240 |
| Palmitic | CH3(CH2)14COOH | 256 | 62.9 | 222 |
| Myristic | CH3(CH2)12COOH | 228 | 54.4 | 202 |
| Surfaces | Lubricant | Coefficient of friction at 20 °C | Breakdown temperature (°C) |
|---|---|---|---|
| Copper | 1% stearic acid Smear copper stearate | 0.08, smooth 0.08 | 90 94 |
| Platinum & cadmium | 1% stearic acid Cadmium stearate | 0.05 0.04 | 130 140 |
| Platinum & steel | Smear sodium stearate | 0.1 | 280 |
3.3. Fatty Acid Esters
3.4. Inorganic Materials and Polymers
4. Magnesium Stearate
4.1. Effect of Pseudo-Polymorph [20,21]
| Formula | Magnesium stearate ratio | Pre-Compression force | Main compression force | Ejection force | Porosity | Overall ranking |
|---|---|---|---|---|---|---|
| 1 | A50:M50 | F | F | P | P | 12th |
| 2 | A25:M75 | P | G | P | P | 11th |
| 3 | A75:M25 | P | G | F | F | 5th |
| 4 | D50:A50 | P | G | G | G | 5th |
| 5 | D75:A25 | F | G | G | G | 3rd |
| 6 | D25:A75 | F | P | G | G | 4th |
| 7 | D50:M50 | F | P | P | F | 8th |
| 8 | D75:M25 | F | F | F | P | 8th |
| 9 | D25:M75 | F | F | F | P | 8th |
| 10 | Anhydrous | G | F | G | F | 2nd |
| 11 | Monohydrate | F | P | P | P | 7th |
| 12 | Dihydrate | G | F | G | F | 1st |
4.2. Effect of Powder Properties on Lubrication
4.3. Effect on the Mechanical Properties of Compressed Products [33,34,35]
4.4. Online Monitoring of Magnesium Stearate in Blending
5. Chemical Stability and Compatibility
5.1. Potential Interactions with Impurities (MgO)
5.2. Hydrolytic Degradation at Basic pH
5.3. Oxidation
5.4. Metal Ion-Mediated Degradation
5.5. Reaction with Amines
5.6. Other Interactions between Magnesium Stearate and Drugs
5.7. Stearic Acid
5.8. Sodium Stearyl Fumarate
6. Considerations for Selecting a Lubricant
| Water soluble lubricant | Amount in formulation (%) | Water insoluble lubricant | Amount in formulation (%) |
|---|---|---|---|
| Boric acid | 1 | Metal (Mg, Ca, Na) stearate | 0.25–2 |
| Carbowax (PEG) 4000/6000 | 1–5 | Stearic acid | 0.25–2 |
| Sodium oleate | 5 | Sterotex | 0.25–1 |
| Sodium benzoate | 5 | Talc | 1–5 |
| Sodium acetate | 5 | Waxes | 1–5 |
| Sodium lauryl sulfate | 1–5 | Stear-O-Wet | 1–5 |
| Mg-Lauryl sulfate | 1–2 | Glyceryl behenate (Compritol 888) | 0.5–3 |
7. Conclusions
Acknowledgments
Conflicts of Interest
References
- Bowden, F.P.; Tabor, D. The Friction and Lubrication of Solids; Clarendon Press: Oxford, UK, 2001. [Google Scholar]
- Wang, J.; Wen, H.; Desai, D. Lubrication in tablet formulations. Eur. J. Pharm. Biopharm. 2010, 75, 1–15. [Google Scholar] [CrossRef]
- Bolhuis, G.K.; Hölzer, A.W. Lubricant Sensitivity. In Pharmaceutical Powder Compaction Technology, 1st ed.; Alderborn, G., Nyström, C., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 1996; pp. 517–560. [Google Scholar]
- Goldberg, R.; Klein, J. Liposomes as lubricants: Beyond drug delivery. Chem. Phys. Lipids 2012, 165, 374–381. [Google Scholar] [CrossRef]
- Faghihnejad, A.; Zeng, H. Fundamentals of Surface Adhesion, Friction, and Lubrication. In Polymer Adhesion, Friction, and Lubrication; Zheng, H., Ed.; Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 1–57. [Google Scholar]
- Israelachvili, J.N. Intermolecular and Surface Forces, 3rd ed.; Elsevier: Burlington, MA, USA, 2011; pp. 469–499. [Google Scholar]
- Pietsch, W. Size Enlargement by Agglomeration. In Handbook of Powder Science & Technology, 2nd ed.; Fayed, M.E., Otten, L., Eds.; Chapman & Hall: New York, NY, USA, 1997; pp. 202–295. [Google Scholar]
- Butt, H.J.; Graf, K.; Kappl, M. Physics and Chemistry of Interfaces; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Bowden, F.P.; Tabor, D. Friction: An Introduction to Tribology; Doubleday & Company, Inc.: Garden City, NY, USA, 1973. [Google Scholar]
- Schulze, D. Powder and Bulk Solids: Behavior, Characterization, Storage and Flow; Springer-Verlag: Heidelberg, Germany, 2008. [Google Scholar]
- Gethin, D.T.; Solimanjad, N.; Doremus, P.; Korachkin, D. Friction and Its Measurement in Powder-Compaction Processes. In Modelling of Powder Die Compaction; Brewin, P.R., Coube, O., Doremus, P., Tweed, J.H., Eds.; Springer-Verlag: London, UK, 2008; pp. 105–129. [Google Scholar]
- Zimmermann, I.; Eber, M.; Meyer, K. Nanomaterials as flow regulators in dry powders. Z. Phys. Chem. 2004, 218, 52–102. [Google Scholar]
- Miller, T.A.; York, P. Pharmaceutical tablet lubrication. Int. J. Pharm. 1988, 41, 1–19. [Google Scholar] [CrossRef]
- O’Rourke, S.E.; Morris, R.H. Metallic stearate: A review of their function and use as release agents for rubber compounds. Prog. Rubber Plast. Technol. 1998, 14, 238–247. [Google Scholar]
- Abramovici, B.; Gromenil, J.C.; Molard, F.; Blanc, F. Comparative study on the lubricating properties of a new additive: The glycerol tribehenate (Compritol® 888) compared to magnesium stearate. Bull. Tech. Gattefosse 1985, 78, 75–85. [Google Scholar]
- Aoshima, H.; Miyagisnima, A.; Nozawa, Y.; Sadzuka, Y.; Sonobe, T. Glycerin fatty acid esters as a new lubricant of tablets. Int. J. Pharm. 2005, 293, 25–34. [Google Scholar] [CrossRef]
- Jannin, V.; Bérard, V.; N’Diaye, A.; Andrés, C.; Pourcelot, Y. Comparative study of the lubricant performance of Compritol® 888 ATO either used by blending or by hot melt coating. Int. J. Pharm. 2003, 262, 39–45. [Google Scholar] [CrossRef]
- Dawoodbhai, S.S.; Chueh, H.R.; Rhodes, C.T. Glidants and lubricant properties of several types of talcs. Drug Dev. Ind. Pharm. 1987, 13, 2441–2467. [Google Scholar] [CrossRef]
- Lapeyre, F.; Cuiné, A.; Chulia, D.; Vérain, A. Quantitative evaluation of tablet sticking anti-adherent properties of some tablet lubricants. STP Pharma 1988, 4, 106–110. [Google Scholar]
- Okoye, P.; Wu, S.H.; Dave, R.H. To evaluate the effect of various magnesium stearate polymorphs using powder rheology and thermal analysis. Drug Dev. Ind. Pharm. 2012, 38, 1470–1478. [Google Scholar] [CrossRef]
- Bracconi, P.; Andrés, C.; Ndiaye, A. Structural properties of magnesium stearate pseudo-polymorphs: Effect of temperature. Int. J. Pharm. 2003, 262, 109–124. [Google Scholar] [CrossRef]
- Rao, K.P.; Chawla, G.; Kaushal, A.M.; Bansal, A.K. Impact of solid-state properties on lubricant efficacy of magnesium stearate. Pharm. Dev. Technol. 2005, 10, 423–437. [Google Scholar] [CrossRef]
- Ertel, K.D.; Carstensen, J.T. Chemical, physical, and lubricant properties of magnesium stearate. J. Pharm. Sci. 1988, 77, 625–629. [Google Scholar] [CrossRef]
- Barra, J.; Somma, R. Influence of the physicochemical variability of magnesium stearate on its lubricant properties: Possible solutions. Drug Dev. Ind. Pharm. 1996, 22, 1105–1120. [Google Scholar] [CrossRef]
- Dansereau, R.; Peck, G.E. The effect of the variability in the physical and chemical properties of magnesium stearate on the properties of compressed tablets. Drug Dev. Ind. Pharm. 1987, 13, 975–999. [Google Scholar] [CrossRef]
- Soebagyo, S.S. The effect of the particle size of magnesium stearate on the dissolution of dexamethasone from interactive mix tablet. Maj. Farm. Indones. 1994, 5, 52–58. [Google Scholar]
- Kushner, J.I.V.; Langdon, B.A.; Hiller, J.I.; Carlson, G.T. Examining the impact of excipient material property variation on drug product quality attributes: A quality-by-design study for a roller compacted, immediate release tablet. J. Pharm. Sci. 2011, 100, 2222–2239. [Google Scholar] [CrossRef]
- Morin, G.; Briens, L. The effect of lubricants on powder flowability for pharmaceutical application. AAPS PharmSciTech 2013, 14, 1158–1168. [Google Scholar] [CrossRef]
- Faqih, A.M.N.; Mehrotra, A.; Hammond, S.V.; Muzzio, F.J. Effect of moisture and magnesium stearate concentration on flow properties of cohesive granular materials. Int. J. Pharm. 2007, 336, 338–345. [Google Scholar] [CrossRef]
- Liu, L.X.; Marziano, I.; Bentham, A.C.; Lister, J.D.; White, E.T.; Howes, T. Effect of particle propertie on the flowability of ibuprofen powders. Int. J. Pharm. 2008, 362, 109–117. [Google Scholar] [CrossRef]
- Kikuta, J.; Kitamori, N. Effect of mixing time on the lubricating properties of magnesium stearate and the final characteristics of the compressed tablets. Drug Dev. Ind. Pharm. 1994, 20, 343–355. [Google Scholar] [CrossRef]
- Perrault, M.; Bertrand, F.; Chaouki, J. An investigation of magnesium stearate mixing in a V-blender through gamma-ray detection. Powder Technol. 2010, 200, 234–245. [Google Scholar] [CrossRef]
- He, X.R.; Secreast, P.J.; Amidon, G.E. Mechanistic study of the effect of roller compaction and lubrication on tablet mechanical strength. J. Pharm. Sci. 2007, 96, 1342–1355. [Google Scholar] [CrossRef]
- Yu, S.; Adams, M.; Gururajan, B.; Reynolds, G.; Roberts, R.; Wu, C.Y. The effect of lubrication on roll compaction, ribbon milling, and tableting. Chem. Eng. Sci. 2013, 86, 9–18. [Google Scholar] [CrossRef]
- Miguélez-Morán, A.M.; Wu, C.Y.; Seville, J.P.K. The effect of lubrication on density distribution of roller compacted ribbons. Int. J. Pharm. 2008, 362, 52–59. [Google Scholar] [CrossRef]
- Terashita, K. Meeting PAT requirement by evaluating the mixing and distribution of magnesium stearate lubricant and other components in a tablet Blend using on-line analytical method part 2. Pharm. Technol. Jpn. 2012, 28, 1275–1278. [Google Scholar]
- Šašiċ, S.; Ojakovo, P.; Warman, M.; Sanghvi, T. Raman chemical mapping of magnesium stearate delivered by a punch-face lubrication system on the surface of placebo and active tablets. Appl. Spectrosc. 2013, 67, 1073–1079. [Google Scholar] [CrossRef]
- Yoshihashi, Y.; Sato, M.; Kawano, Y.; Yonemochi, E.; Terada, K. Evaluation of physicochemical properties on the blending process of pharmaceutical granules with magnesium stearate by thermal effusivity sensor. J. Therm. Anal. Calorim. 2013, 113, 1281–1285. [Google Scholar] [CrossRef]
- Kararli, T.T.; Needham, T.E.; Seul, C.J.; Finnegan, P.M. Solid state interaction of magnesium oxide and ibuprofen to form a salt. Pharm. Res. 1989, 6, 804–808. [Google Scholar] [CrossRef]
- Botha, S.A.; Lötter, A.P. Compatibility study between naproxen and tablet excipients using differential scanning calorimetry. Drug Dev. Ind. Pharm. 1990, 16, 673–683. [Google Scholar] [CrossRef]
- Mura, P.; Manderioli, A.; Bramanti, G.; Furlanetto, S.; Pinzauti, S. Utilization of differential scanning calorimetry as a screening technique to determine the compatibility of ketoprofen with excipients. Int. J. Pharm. 1995, 119, 71–79. [Google Scholar] [CrossRef]
- Marcotegui, F.; Sanchez Monge, J.M. Application of differential thermal analysis to study the stability of acetylsalicylic acid in the solid state. I. Hydrolysis of acetylsalicylic acid. Revist. Asoc. Esp. Farm. Hosp. 1981, 5, 5–10. [Google Scholar]
- Ahlneck, C.; Waltersson, J.O.; Lundgren, P. Difference in effect of powdered and granular magnesium stearate on the solid state stability of acetylsalicylic acid. Acta Pharm. Technol. 1987, 33, 21–26. [Google Scholar]
- Fouda, M.A.; Mady, O.Y.; El-Azab, G.A. Stabilization and control of aspirin release via solid dispersion systems. Mansoura J. Pharm. Sci. 1998, 14, 36–70. [Google Scholar]
- Kornblum, S.S.; Zoglio, M.A. Pharmaceutical heterogeneous systems I. Hydrolysis of aspirin in combination with tablet lubricants in an aqueous suspension. J. Pharm. Sci. 1967, 56, 1569–1575. [Google Scholar] [CrossRef]
- Nelson, E.; Eppich, D.; Carstensen, J.T. Topochemical decomposition patterns of aspirin. J. Pharm. Sci. 1974, 63, 755–757. [Google Scholar] [CrossRef]
- Gordon, R.E.; Vankoevering, C.L.; Reits, D.J. Utilization of differential scanning calorimetry in the compatibility screening of ibuprofen with the stearate lubricants and construction of phase diagrams. Int. J. Pharm. 1984, 21, 99–105. [Google Scholar] [CrossRef]
- Pawelczyk, E.; Opielewicz, M. Kinetics of drug decomposition. XLIX. Kinetics of autoxidation of drotaverine hydrochloride in the solid state. Acta Pol. Pharm. 1978, 35, 311–319. [Google Scholar]
- Osawa, Z.; Ishizuka, T. Catalytic action of metal salts in autoxidation and polymerization. X. Effect of various metal stearates on the thermal oxidation of 2,6,10,14-tetramethylpentadecane. J. Appl. Polym. Sci. 1973, 17, 2897–2907. [Google Scholar] [CrossRef]
- Thakur, A.B.; Morris, K.; Grosso, J.A.; Himes, K.; Thottathil, J.K.; Jerzewski, R.L.; Wadke, D.A.; Carstensen, J.T. Mechanism and kinetics of metal ion-mediated degradation of fosinopril sodium. Pharm. Res. 1993, 10, 800–809. [Google Scholar] [CrossRef]
- Pragatikumar, B.; Sahu, R.; Murphy, K.V.R.; Rao, S.; Ramu, B. A review on mechanism, importance and methods of compatibility testing in the formulation of dosage forms. J. Chem. Pharm. Sci. 2011, 4, 141–151. [Google Scholar]
- Desai, D.S.; Rubitski, B.A.; Varia, S.A.; Newman, A.W. Physical interactions of magnesium stearate with starch-derived disintegrants and their effects on capsule and tablet dissolution. Int. J. Pharm. 1993, 91, 217–226. [Google Scholar] [CrossRef]
- Desai, D.; Kothari, S.; Huang, M. Solid-state interaction of stearic acid with povidone and its effect on dissolution stability of capsules. Int. J. Pharm. 2008, 354, 77–81. [Google Scholar] [CrossRef]
- Wang, J.; Davidovich, M.; Desai, D.; Bu, D.; Hussain, M.; Morris, K. Solid-state interactions of a drug substance and excipients and their impact on tablet dissolution: A thermal-mechanical facilitated process-induced transformation or PIT. J. Pharm. Sci. 2010, 99, 3849–3862. [Google Scholar]
- Hölzer, A.W.; Sjögren, J. Evaluation of sodium stearyl fumarate as a tablet lubricant. Int. J. Pharm. 1979, 2, 145–153. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, J.; Wu, Y. Lubricants in Pharmaceutical Solid Dosage Forms. Lubricants 2014, 2, 21-43. https://doi.org/10.3390/lubricants2010021
Li J, Wu Y. Lubricants in Pharmaceutical Solid Dosage Forms. Lubricants. 2014; 2(1):21-43. https://doi.org/10.3390/lubricants2010021
Chicago/Turabian StyleLi, Jinjiang, and Yongmei Wu. 2014. "Lubricants in Pharmaceutical Solid Dosage Forms" Lubricants 2, no. 1: 21-43. https://doi.org/10.3390/lubricants2010021
APA StyleLi, J., & Wu, Y. (2014). Lubricants in Pharmaceutical Solid Dosage Forms. Lubricants, 2(1), 21-43. https://doi.org/10.3390/lubricants2010021




