Tribo-Catalytic Degradation of Methyl Orange Dye via Cu/Al2O3 Nanoparticles
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis
2.3. Characterization of Cu/Al2O3 Nanoparticles
2.4. Evaluation of Tribo-Catalytic Activity
3. Results and Discussion
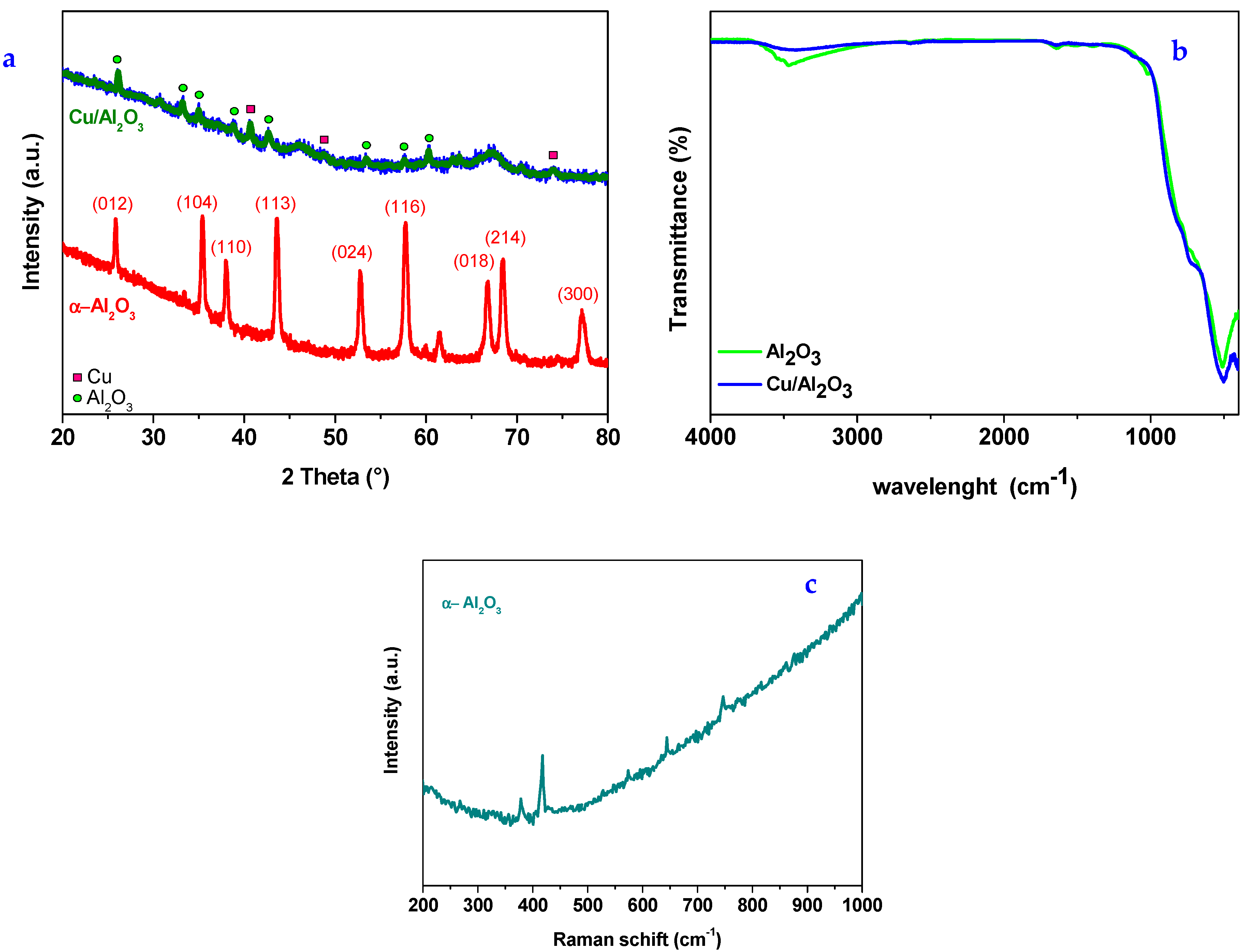
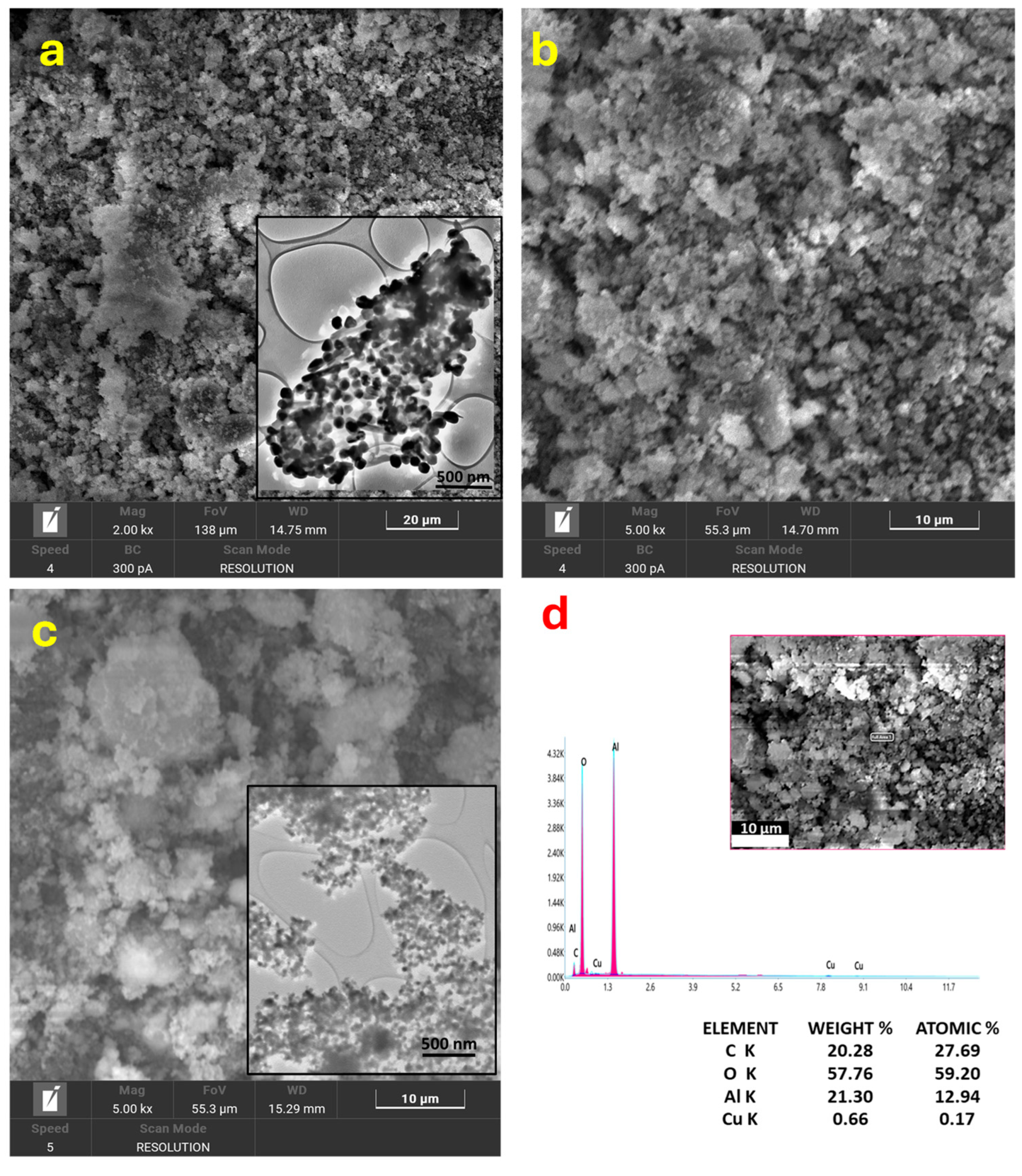
3.1. Characterization of Nanoparticles
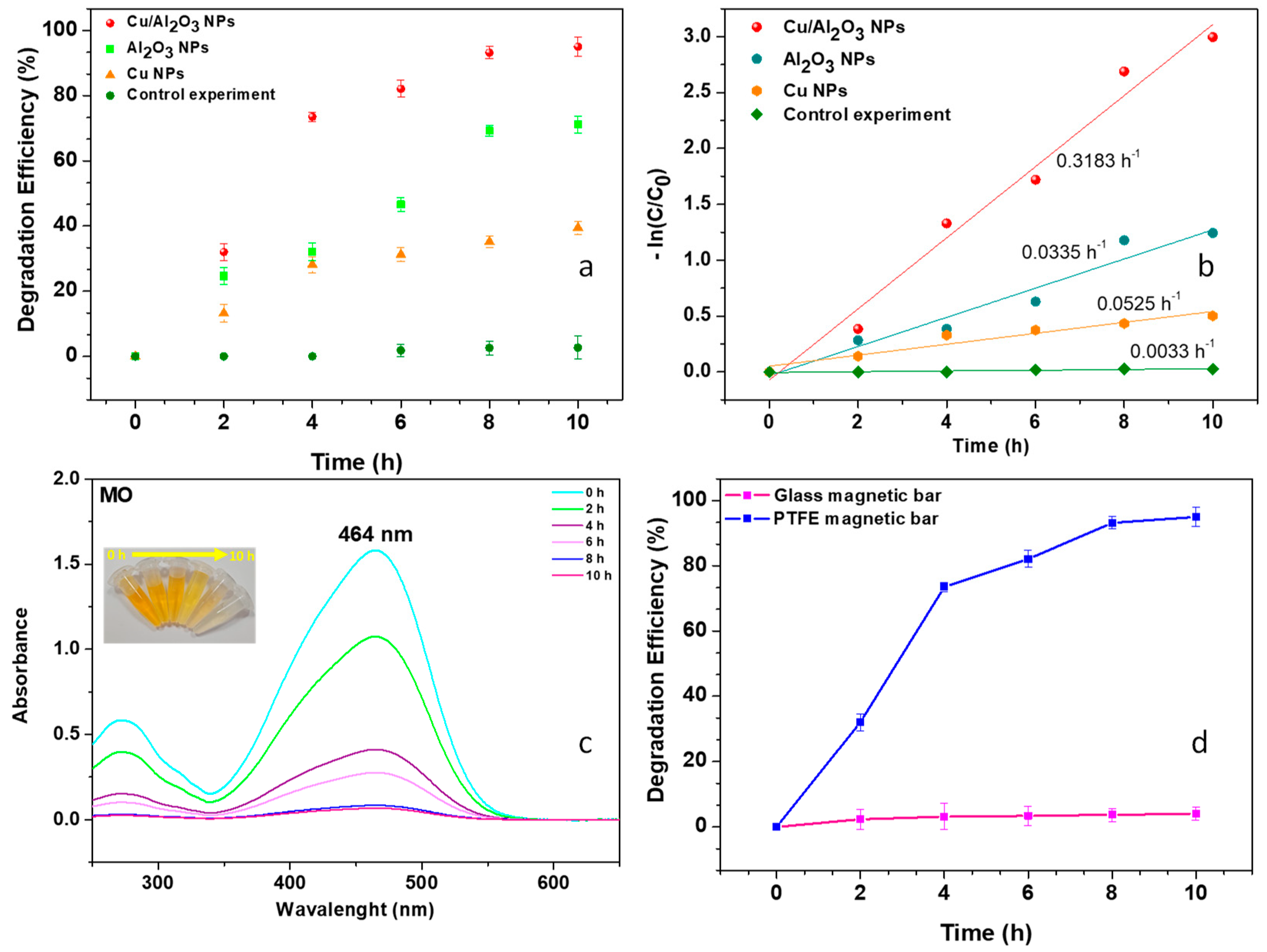
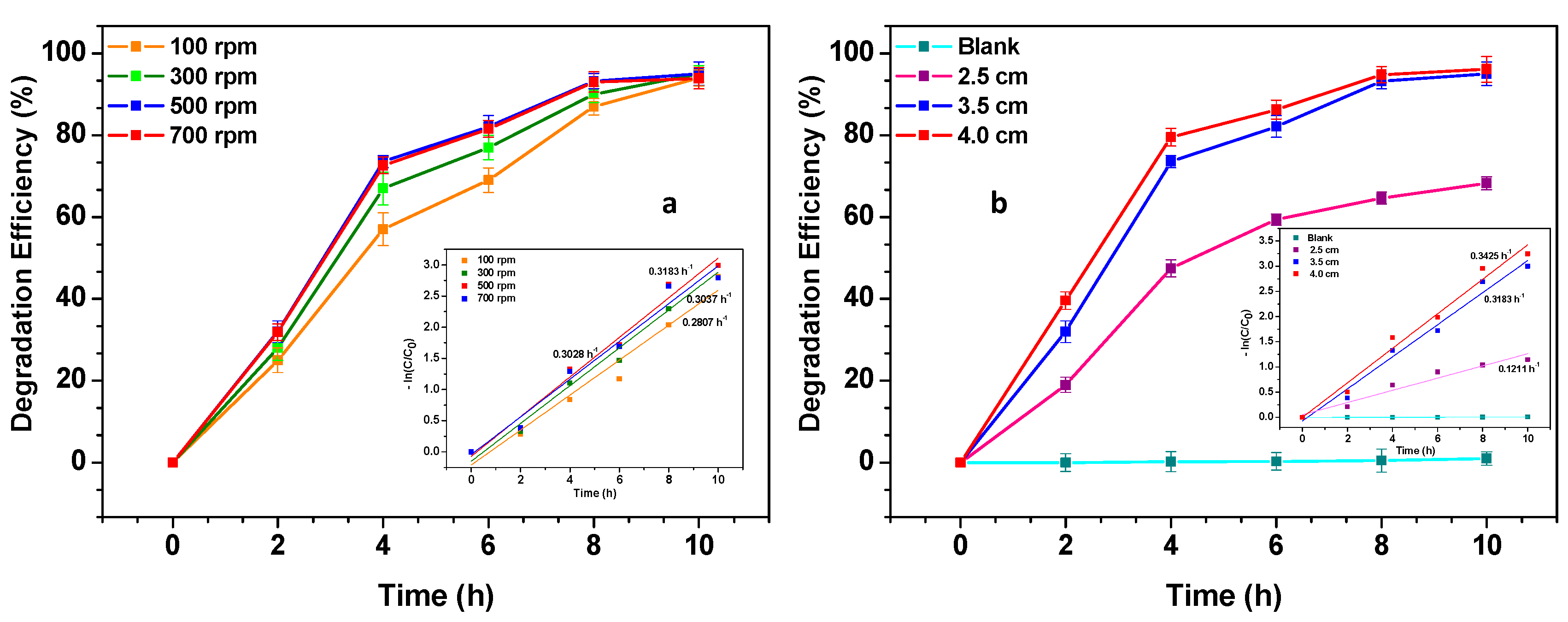
3.2. Tribo-Catalytic Efficiency of Cu/Al2O3 NPs
3.3. Possible Mechanism for Tribo-Catalytic Degradation of MO
- -
- Generation of hydroxyl radicals through hole-induced oxidation of hydroxide ions:OH− + h+ → ·OH
- -
- Formation of superoxide radicals by electron transfer to dissolved oxygen:O2 + e− → ·O2−
- -
- Oxidative attack on MO dye molecules by ROS:
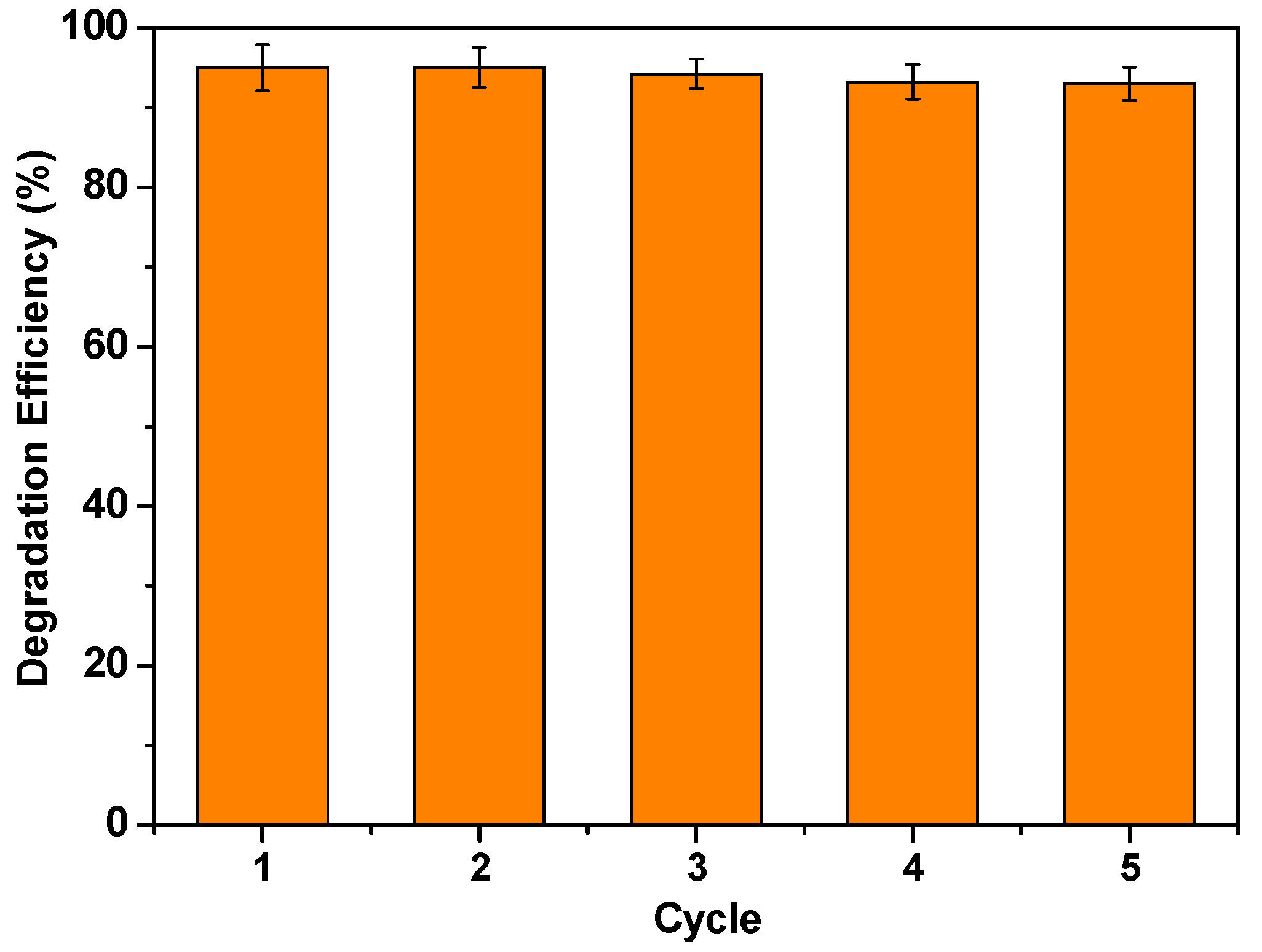
3.4. Reusability of the Cu/Al2O3 Catalyst
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xiao, L.; Xu, X.; Wu, Z.; Sun, T.; He, X.; Xu, X.; Qin, L.; Chen, D. Recent progress and prospect of friction-driven-tribocatalysis: From basic principle to material design. Surf. Interfaces 2024, 56, 105557. [Google Scholar] [CrossRef]
- Li, X.; Tong, W.; Shi, J.; Chen, Y.; Zhang, Y.; An, Q. Tribocatalysis mechanisms: Electron transfer and transition. J. Mater. Chem. A 2023, 11, 4458–4472. [Google Scholar] [CrossRef]
- Sun, C.; Guo, X.; Hu, C.; Liu, L.; Fang, L.; Cheng, Z.; Luo, N. Tribocatalytic degradation of dyes by tungsten bronze ferroelectric Ba2.5Sr2.5Nb8Ta2O30 submicron particles. RSC Adv. 2021, 11, 13386–13395. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, Y.; Yi, Y.; Zhou, B.; Sun, P.; Dong, X. Regulation of friction pair to promote conversion of mechanical energy to chemical energy on Bi2WO6 and realization of enhanced tribocatalytic activity to degrade different pollutants. J. Hazard. Mater. 2023, 459, 132147. [Google Scholar] [CrossRef]
- Yuan, A.; Lei, H.; Xi, F.; Liu, J.; Qin, L.; Chen, Z.; Dong, X. Graphene quantum dots decorated graphitic carbon nitride nanorods for photocatalytic removal of antibiotics. J. Colloid Interface Sci. 2019, 548, 56–65. [Google Scholar] [CrossRef]
- Yao, C.; Yuan, A.; Zhang, H.; Li, B.; Liu, J.; Xi, F.; Dong, X. Facile surface modification of textiles with photocatalytic carbon nitride nanosheets and the excellent performance for self-cleaning and degradation of gaseous formaldehyde. J. Colloid Interface Sci. 2018, 533, 144–153. [Google Scholar] [CrossRef]
- Henríquez, R.; Nogales, P.S.; Moreno, P.G.; Cartagena, E.M.; Bongiorno, P.L.; Navarrete-Astorga, E.; Dalchiele, E.A. One-Step hydrothermal synthesis of Cu2ZnSnS4 nanoparticles as an efficient visible light photocatalyst for the degradation of Congo Red Azo dye. Nanomaterials 2023, 13, 1731. [Google Scholar] [CrossRef] [PubMed]
- Henríquez, R.; Nogales, P.S.; Moreno, P.G.; Cartagena, E.M.; Bongiorno, P.L.; Garate, P.Z.; Navarrete-Astorga, E.; Dalchiele, E.A. Solvothermal synthesis of Cu2ZnSnSe4 nanoparticles and their Visible-Light-Driven photocatalytic activity. Nanomaterials 2024, 14, 1079. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Qin, N.; Bao, D. Effective enhancement of piezocatalytic activity of BaTiO3 nanowires under ultrasonic vibration. Nano Energy 2017, 45, 44–51. [Google Scholar] [CrossRef]
- Wu, J.M.; Sun, Y.; Chang, W.; Lee, J. Piezoelectricity induced water splitting and formation of hydroxyl radical from active edge sites of MoS2 nanoflowers. Nano Energy 2018, 46, 372–382. [Google Scholar] [CrossRef]
- Shao, Y.; Shen, M.; Zhou, Y.; Cui, X.; Li, L.; Zhang, Y. Nanogenerator-based self-powered sensors for data collection. Beilstein J. Nanotechnol. 2021, 12, 680–693. [Google Scholar] [CrossRef]
- You, H.; Jia, Y.; Wu, Z.; Xu, X.; Qian, W.; Xia, Y.; Ismail, M. Strong piezo-electrochemical effect of multiferroic BiFeO3 square micro-sheets for mechanocatalysis. Electrochem. Commun. 2017, 79, 55–58. [Google Scholar] [CrossRef]
- Hong, K.; Xu, H.; Konishi, H.; Li, X. Piezoelectrochemical Effect: A New Mechanism for Azo Dye Decolorization in Aqueous Solution through Vibrating Piezoelectric Microfibers. J. Phys. Chem. C 2012, 116, 13045–13051. [Google Scholar] [CrossRef]
- Tian, J.; He, Y.; Li, F.; Peng, W.; He, Y. On the mechanism of contact electrification: A comprehensive review. J. Mater. Chem. A 2024, 15, 2505–2536. [Google Scholar] [CrossRef]
- Che, J.; Gao, Y.; Wu, Z.; Ma, J.; Wang, Z.; Liu, C.; Jia, Y.; Wang, X. Review on tribocatalysis through harvesting friction energy for mechanically-driven dye decomposition. J. Alloys Compd. 2024, 1002, 175413. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, N.; Xue, Y.; Shi, H.; Xu, H.; Li, M.; Sun, C.; Xing, Y.; Gao, B.; Ma, B. Challenges and perspectives of tribocatalysis in the treatment for dye wastewater. J. Water Process Eng. 2024, 63, 105455. [Google Scholar] [CrossRef]
- Iuliano, M.; Cirillo, C.; Astorga, E.N.; Sarno, M. A new nanocomposite as adsorbent and catalyst for enhanced removal of methylene blue. Surf. Interfaces 2024, 51, 104582. [Google Scholar] [CrossRef]
- Iuliano, M.; Ponticorvo, E.; Cirillo, C.; Sarno, M. A New Nanocomposite from Vesuvian Slope Pinecones for Azo-Dyes Removal. Ind. Eng. Chem. Res. 2022, 61, 1965–1976. [Google Scholar] [CrossRef]
- Zafar, S.; Bukhari, D.A.; Rehman, A. Azo dyes degradation by microorganisms—An efficient and sustainable approach. Saudi J. Biol. Sci. 2022, 29, 103437. [Google Scholar] [CrossRef] [PubMed]
- Iuliano, M.; Ponticorvo, E.; Cirillo, C.; Adami, R.; Sarno, M. Catalytic hydrogenation of organic dyes by Ag and Au magnetic nanoparticles supported on nanocellulose from waste pistachio shells. Mol. Catal. 2023, 544, 113179. [Google Scholar] [CrossRef]
- Forgacs, E.; Cserháti, T.; Oros, G. Removal of synthetic dyes from wastewaters: A review. Environ. Int. 2004, 30, 953–971. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, W.; Li, Y.; Wang, K.; Ge, L.; Zhang, X.; Zhao, J.; Shen, Z.; Chen, W.; Hu, Y. Tribocatalytic degradation of concentrated methyl orange solutions by BiFeO3 nanoparticles prepared through a sol–gel method. RSC Adv. 2025, 15, 23089–23096. [Google Scholar] [CrossRef]
- Kishor, R.; Purchase, D.; Saratale, G.D.; Ferreira, L.F.R.; Hussain, C.M.; Mulla, S.I.; Bharagava, R.N. Degradation mechanism and toxicity reduction of methyl orange dye by a newly isolated bacterium Pseudomonas aeruginosa MZ520730. J. Water Process Eng. 2021, 43, 102300. [Google Scholar] [CrossRef]
- Cui, X.; Li, P.; Lei, H.; Tu, C.; Wang, D.; Wang, Z.; Chen, W. Greatly enhanced tribocatalytic degradation of organic pollutants by TiO2 nanoparticles through efficiently harvesting mechanical energy. Sep. Purif. Technol. 2022, 289, 120814. [Google Scholar] [CrossRef]
- Lei, H.; Cui, X.; Jia, X.; Qi, J.; Wang, Z.; Chen, W. Enhanced tribocatalytic degradation of organic pollutants by ZNO nanoparticles of high crystallinity. Nanomaterials 2022, 13, 46. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, L.; Luo, W.; Li, H.; Wu, Z.; Xu, Z.; Zhang, Y.; Zhang, H.; Yuan, G.; Gao, J.; et al. Strong tribo-catalysis of zinc oxide nanorods via triboelectrically-harvesting friction energy. Ceram. Int. 2020, 46, 25293–25298. [Google Scholar] [CrossRef]
- Lei, H.; Wu, M.; Mo, F.; Ji, S.; Dong, X.; Wu, Z.; Gao, J.; Yang, Y.; Jia, Y. Tribo-catalytic degradation of organic pollutants through bismuth oxyiodate triboelectrically harvesting mechanical energy. Nano Energy 2020, 78, 105290. [Google Scholar] [CrossRef]
- Gaur, A.; Moharana, A.K.; Porwal, C.; Chauhan, V.S.; Vaish, R. Degradation of organic dyes by utilizing CaCu3Ti4O12 (CCTO) nanoparticles via tribocatalysis process. J. Ind. Eng. Chem. 2023, 129, 341–351. [Google Scholar] [CrossRef]
- Zhu, M.; Song, J.; Ke, S.; Gu, Y.; Bing, L.; Shen, Z.; Chen, W. TI Coating-Enhanced tribocatalytic degradation of organic dyes by CDS nanoparticles. Inorganics 2025, 13, 46. [Google Scholar] [CrossRef]
- Trueba, M.; Trasatti, S.P. Γ-Alumina as a support for Catalysts: A Review of Fundamental Aspects. Eur. J. Inorg. Chem. 2005, 2005, 3393–3403. [Google Scholar] [CrossRef]
- Sun, Y.; Li, S.; Zhao, Q.; Cong, Z.; Xia, Y.; Jiao, X.; Chen, D. Recent advancements in Alumina-Based High-Temperature insulating Materials: Properties, applications, and future Perspectives. High-Temp. Mater. 2025, 2, 10001. [Google Scholar] [CrossRef]
- Jähnichen, T.; Carstens, S.; Franz, M.; Laufer, O.; Wenzel, M.; Matysik, J.; Enke, D. Towards High Surface Area α-Al2O3–Mn-Assisted Low Temperature Transformation. Materials 2023, 16, 3047. [Google Scholar] [CrossRef] [PubMed]
- Ke, S.; Mao, C.; Luo, R.; Zhou, Z.; Hu, Y.; Zhao, W.; Chen, W. Surprising effects of Al2O3 coating on tribocatalytic degradation of organic dyes by CDS nanoparticles. Coatings 2024, 14, 1057. [Google Scholar] [CrossRef]
- Das, A.; Ren, Y.; Hessin, C.; Murr, M.D. Copper catalysis with redox-active ligands. Beilstein J. Org. Chem. 2020, 16, 858–870. [Google Scholar] [CrossRef]
- Cui, X.; Wang, H.; Lei, H.; Jia, X.; Jiang, Y.; Fei, L.; Jia, Y.; Chen, W. Surprising Tribo-catalytic Conversion of H2O and CO2 into Flammable Gases utilizing Frictions of Copper in Water. ChemistrySelect 2023, 8, e202204146. [Google Scholar] [CrossRef]
- Song, Y.; Chen, W.; Zhao, C.; Li, S.; Wei, W.; Sun, Y. Metal-Free Nitrogen-Doped mesoporous carbon for electroreduction of CO2 to ethanol. Angew. Chem. Int. Ed. 2017, 56, 10840–10844. [Google Scholar] [CrossRef]
- Chen, M.; Jia, Y.; Li, H.; Wu, Z.; Huang, T.; Zhang, H. Enhanced pyrocatalysis of the pyroelectric BiFeO3/g-C3N4 heterostructure for dye decomposition driven by cold-hot temperature alternation. J. Adv. Ceram. 2021, 10, 338–346. [Google Scholar] [CrossRef]
- Wu, Z.; Luo, W.; Zhang, H.; Jia, Y. Strong pyro-catalysis of shape-controllable bismuth oxychloride nanomaterial for wastewater remediation. Appl. Surf. Sci. 2020, 513, 145630. [Google Scholar] [CrossRef]
- Putri, S.E.; Herawati, N.; Fudhail, A.; Pratiwi, D.E.; Side, S.; Rahman, A.; Desa, S.S.; Ahmad, N.; Junaedi, S.; Surleva, A. Biosynthesis of Copper Nanoparticles Using Hylocereus costaricensis Peel Extract and their Photocatalytic Properties. Karbala Int. J. Mod. Sci. 2023, 9, 13. [Google Scholar] [CrossRef]
- Liu, C.; Shih, K.; Gao, Y.; Li, F.; Wei, L. Dechlorinating transformation of propachlor through nucleophilic substitution by dithionite on the surface of alumina. J. Soils Sediments 2012, 12, 724–733. [Google Scholar] [CrossRef]
- Černík, M.; Padil, V.V.T. Green synthesis of copper oxide nanoparticles using gum karaya as a biotemplate and their antibacterial application. Int. J. Nanomed. 2013, 8, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Thanigachalam, S.; Pathak, M.; Sathiyanarayanan, K.I. Photodegradation of rhodamine-B and methyl orange employing nano-alumina developed from new aluminium(III) complex(es) associated with phenanthridine-salicylaldehyde derived ligands. J. Coord. Chem. 2022, 75, 2189–2213. [Google Scholar] [CrossRef]
- Li, P.; Lei, M.; Tang, W. Raman and photoluminescence properties of α-Al2O3 microcones with hierarchical and repetitive superstructure. Mater. Lett. 2009, 64, 161–163. [Google Scholar] [CrossRef]
- Aldbea, F.W.; Vazquez, C.V.; Abobaker, M.; Alsteeni, A.; Saad, A.; Sharma, A.; Singh, P.K.; Diantoro, M.; May, M.; Abdullah, T.; et al. Structural and Optical Properties of α Aluminum Oxide prepared by sol-gel method. Curr. Appl. Phys. 2024, 71, 85–90. [Google Scholar] [CrossRef]
- Yan, J.; Li, J.; Peng, J.; Zhang, H.; Zhang, Y.; Lai, B. Efficient degradation of sulfamethoxazole by the CuO@Al2O3 (EPC) coupled PMS system: Optimization, degradation pathways and toxicity evaluation. Chem. Eng. J. 2018, 359, 1097–1110. [Google Scholar] [CrossRef]
- Mondal, P.; Sinha, A.; Salam, N.; Roy, A.S.; Jana, N.R.; Islam, S.M. Enhanced catalytic performance by copper nanoparticle–graphene based composite. RSC Adv. 2013, 3, 5615. [Google Scholar] [CrossRef]
- Fang, R.; Sun, Q.; Zhou, P.; Yang, W.; Wang, P.; Zhang, D.W. High-performance bilayer flexible resistive random access memory based on low-temperature thermal atomic layer deposition. Nanoscale Res. Lett. 2013, 8, 92. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, L.; Jiang, Y.; Lim, W.; Soh, S. Rationalizing the triboelectric series of polymers. Chem. Mater. 2019, 31, 1473–1478. [Google Scholar] [CrossRef]
- Gallo, C.; Lama, W. Some charge exchange phenomena explained by a classical model of the work function. J. Electrost. 1976, 2, 145–150. [Google Scholar] [CrossRef]
- Diaz, A.; Felix-Navarro, R. A semi-quantitative tribo-electric series for polymeric materials: The influence of chemical structure and properties. J. Electrost. 2004, 62, 277–290. [Google Scholar] [CrossRef]
- Wu, J.; Wang, X.; Li, H.; Wang, F.; Yang, W.; Hu, Y. Insights into the mechanism of metal-polymer contact electrification for triboelectric nanogenerator via first-principles investigations. Nano Energy 2018, 48, 607–616. [Google Scholar] [CrossRef]
- Zhao, P.; Bhattacharya, G.; Fishlock, S.J.; Guy, J.G.; Kumar, A.; Tsonos, C.; Yu, Z.; Raj, S.; McLaughlin, J.A.; Luo, J.; et al. Replacing the metal electrodes in triboelectric nanogenerators: High-performance laser-induced graphene electrodes. Nano Energy 2020, 75, 104958. [Google Scholar] [CrossRef]
- Geng, L.; Qian, Y.; Song, W.; Bao, L. Enhanced tribocatalytic pollutant degradation through tuning oxygen vacancy in BaTiO3 nanoparticles. Appl. Surf. Sci. 2023, 637, 157960. [Google Scholar] [CrossRef]
- Schwab, T.; Thomele, D.; Aicher, K.; Dunlop, J.W.C.; McKenna, K.; Diwald, O. Rubbing powders: Direct spectroscopic observation of triboinduced oxygen radical formation in MGO nanocube ensembles. J. Phys. Chem. C 2021, 125, 22239–22248. [Google Scholar] [CrossRef]
- Alabbad, E.A.; Bashir, S.; Liu, J.L. Efficient removal of direct yellow dye using chitosan crosslinked isovanillin derivative biopolymer utilizing triboelectric energy produced from homogeneous catalysis. Catal. Today 2021, 400–401, 132–145. [Google Scholar] [CrossRef]
- Cui, X.; Guo, Z.; Lei, H.; Jia, X.; Mao, C.; Ruan, L.; Zhou, X.; Wang, Z.; Chen, F.; Chen, W. Tribo-Catalytic degradation of methyl orange solutions enhanced by silicon single crystals. Coatings 2023, 13, 1804. [Google Scholar] [CrossRef]
- Li, J.; Xu, X. Catalytic degradation of organic dyes induced by Tribo-Electrification between insulating films. Materials 2025, 18, 2327. [Google Scholar] [CrossRef]
- Park, J.Y.; Salmeron, M. Fundamental aspects of energy dissipation in friction. Chem. Rev. 2014, 114, 677–711. [Google Scholar] [CrossRef]
- Tang, Q.; Zhu, M.D.; Zhang, H.F.; Gao, J.; Kwok, K.W.; Kong, L.B.; Jia, Y.M.; Liu, L.J.; Peng, B.L. Enhanced tribocatalytic degradation of dye pollutants through governing the charge accumulations on the surface of ferroelectric barium zirconium titanate particles. Nano Energy 2022, 100, 107519. [Google Scholar] [CrossRef]
- Liu, S.Y.; Yang, Y.D.; Hu, Y.M.; Rao, W.F. Effect of strontium substitution on the tribocatalytic performance of barium titanate. Materials 2023, 16, 3160. [Google Scholar] [CrossRef]
- Li, P.; Wu, J.; Wu, Z.; Jia, Y.; Ma, J.; Chen, W.; Zhang, L.; Yang, J.; Liu, Y. Strong tribocatalytic dye decomposition through utilizing triboelectric energy of barium strontium titanate nanoparticles. Nano Energy 2019, 63, 103832. [Google Scholar] [CrossRef]
- Yang, B.A.; Chen, H.B.; Guo, X.D.; Wang, L.; Xu, T.; Bian, J.H.; Yang, Y.D.; Liu, Q.D.; Du, Y.P.; Lou, X.J. Enhanced tribocatalytic degradation using piezoelectric CdS nanowires for efficient water remediation. J. Mater. Chem. C 2020, 8, 14845–14854. [Google Scholar] [CrossRef]
- Ruan, L.J.; Jia, Y.M.; Guan, J.F.; Xue, B.; Huang, S.H.; Wang, Z.H.; Fu, Y.H.; Wu, Z. Tribo-electro-catalytic dye degradation driven by mechanical friction using MOF-derived NiCo2O4 double-shelled nanocages. J. Clean. Prod. 2022, 345, 131060. [Google Scholar] [CrossRef]
- Wang, Y.; Arandiyan, H.; Scott, J.; Bagheri, A.; Dai, H.X.; Amal, R. Recent advances in ordered meso/macroporous metal oxides for heterogeneous catalysis: A review. J. Mater. Chem. A 2017, 5, 8825–8846. [Google Scholar] [CrossRef]
- Yousefzadeh, F.; Ghanbari, M.; Salavati-Niasari, M. Sonochemical synthesis and characterization of Sm2CuO4 nanostructures and their application as visible-light photocatalyst for degradation of water-soluble organic pollutants. Chemosphere 2023, 338, 139564. [Google Scholar] [CrossRef]
- Sarfo, D.K.; Kaur, A.; Marshall, D.L.; O’Mullane, A.P. Electrochemical degradation and mineralisation of organic dyes in aqueous nitrate solutions. Chemosphere 2023, 316, 137821. [Google Scholar] [CrossRef]
- Gong, Z.; Wang, J.; Shao, S.; Fan, B.; Shi, Y.; Qian, L.; Lu, K.; Gao, S. H2O2 activation over Cu-Schiff bases nanozyme for the removal of amlodipine: Kinetics, mechanism and toxicity evaluation. Sep. Purif. Technol. 2023, 311, 123329. [Google Scholar] [CrossRef]
- Lei, X.; You, M.; Pan, F.; Liu, M.; Yang, P.; Xia, D.; Li, Q.; Wang, Y.; Fu, J. CuFe2O4@GO nanocomposite as an effective and recoverable catalyst of peroxymonosulfate activation for degradation of aqueous dye pollutants. Chin. Chem. Lett. 2019, 30, 2216–2220. [Google Scholar] [CrossRef]



| Catalysts | Dosage (mg/L) | Pollutants | Stirring Speed (rpm) | Degradation (%) | Degradation Time (h) | Kinetic Rate (h−1) | Cycle Degradation | Ref. |
|---|---|---|---|---|---|---|---|---|
| Ba(Zr0.05Ti0.95)O3 | 50 | RhB 5 mg/L | 1200 | 94.3 | 6 | 0.4482 | 3 (92%) | [59] |
| Ba0.8Sr0.2TiO3 | 30 | RhB 5 mg/L | 400 | 88.0 | 8 | 0.2613 | 3 (83%) | [60] |
| BaSrTiO3 NPs | 30 | MO 5 mg/L | 300 | 80.0 | 24 | - | - | [61] |
| BiOIO3 | 30 | RhB 5 mg/L | 500 | 98.5 | 12 | 0.3760 | 5 (96%) | [27] |
| CdS nanowires | 30 | RhB 10 mg/L | 400 | 98.0 | 7 | 0.3200 | 5 (95%) | [62] |
| NiCo2O4 | 30 | RhB 5 mg/L | 400 | 98.6 | 56 | 0.0740 | 10 (91%) | [63] |
| PTFE | 30 | RhB 5 mg/L | 500 | 97.0 | 12 | 0.0056 | 5 (90%) | [64] |
| Cu/Al2O3 | 50 | MO 20 mg/L | 500 | 95.0 | 10 | 0.3183 | 5 (91%) | This work |
| Technologies | Catalyst | Pollutants | Efficiency (%) | Ref. |
|---|---|---|---|---|
| Photocatalysis | Sm2CuO4 nanostructures | MO | 91.4 | [65] |
| Electrocatalysis | Cu electrode | MO | 100 | [66] |
| Fenton-like | Cu@SB nanozyme | Amlodipine | 100 | [67] |
| PMS activation | CuFe2O4@GO | Dyes | >90 | [68] |
| Tribo-catalyst | Cu/Al2O3 | MO | 95 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cirillo, C.; Iuliano, M.; Abrar, S.; Navarrete Astorga, E.; Sarno, M. Tribo-Catalytic Degradation of Methyl Orange Dye via Cu/Al2O3 Nanoparticles. Lubricants 2025, 13, 418. https://doi.org/10.3390/lubricants13090418
Cirillo C, Iuliano M, Abrar S, Navarrete Astorga E, Sarno M. Tribo-Catalytic Degradation of Methyl Orange Dye via Cu/Al2O3 Nanoparticles. Lubricants. 2025; 13(9):418. https://doi.org/10.3390/lubricants13090418
Chicago/Turabian StyleCirillo, Claudia, Mariagrazia Iuliano, Sana Abrar, Elena Navarrete Astorga, and Maria Sarno. 2025. "Tribo-Catalytic Degradation of Methyl Orange Dye via Cu/Al2O3 Nanoparticles" Lubricants 13, no. 9: 418. https://doi.org/10.3390/lubricants13090418
APA StyleCirillo, C., Iuliano, M., Abrar, S., Navarrete Astorga, E., & Sarno, M. (2025). Tribo-Catalytic Degradation of Methyl Orange Dye via Cu/Al2O3 Nanoparticles. Lubricants, 13(9), 418. https://doi.org/10.3390/lubricants13090418







