1. Introduction
Friction is common in both daily life and industry. Appropriate friction facilitates life, and too much friction leads to unnecessary waste. In machining, friction between parts accelerates wear and tear. For example, the prolonged friction of the piston of an automobile engine results in a decrease in accuracy and longevity, requiring frequent maintenance and replacement, thus increasing costs [
1,
2]. Friction inevitably leads to energy dissipation through heat generation, which substantially diminishes the overall efficiency of mechanical systems. This energy loss mechanism highlights the critical need for advanced lubrication solutions to mitigate frictional losses [
3,
4]. Lubrication can reduce frictional wear and prolong the service life of machinery, such as reducing the wear rate of gears and bearings. It can also reduce energy consumption, reduce energy waste caused by frictional heat and improve equipment efficiency. It also dissipates heat and prevents components from failing due to high temperatures. In addition, it can improve the smoothness of operation, reduce noise, improve the reliability and user experience of the device, and is a key means of mechanical maintenance and performance optimization. Lubricants are divided into two main categories according to their basic components: water-based lubricants and oil-based lubricants [
5,
6]. Lubricating oil is based on mineral oil or synthetic oil. It has good lubrication performance and strong adhesion. It is suitable for high-temperature and high-pressure environments. Water-based lubricant takes water as dispersion medium and adds a surfactant and an abrasive. They are eco-friendly, easy to clean and have strong compatibility with a variety of materials, leaving no oil stains. Widely used in food processing, health care and other areas requiring high levels of cleanliness. In addition, their fast heat dissipation feature can effectively reduce the operating temperature of equipment. Water-based nano-lubricant developed on this basis has more impressive advantages [
7,
8,
9]. The high specific surface area and unique physico-chemical properties of nanomaterials enhance lubricant stability and bearing capacity [
10]. At the same time, they maintain the environmental protection of water-based lubricants, reduce environmental pollution, and show great application potential in the field of precision machinery, aerospace, and other high-end manufacturing areas [
11]. Specifically, in aerospace applications, these nano-lubricants can significantly reduce wear in turbine bearings and other high-load components due to their excellent thermal stability and extreme pressure properties. In precision machinery, their uniform interlayer sliding characteristics ensure the smooth operation of high-precision components such as optical lens positioning systems. Among the many nanolubrication additives, silicate-based materials exhibit special advantages due to their unique layered structure, environmental friendliness, and adjustable surface chemistry.
Silicate minerals, fundamentally structured around the silicon–oxygen tetrahedron, exhibit notable differences in hardness, varied crystal formations, and consistent chemical characteristics [
12]. These crystals typically appear in multi-layered, chain-like, or structural shapes, characterized by vibrant colors and sheen, with some exhibiting superior electrical insulation and absorption capabilities. Current scholarly articles indicate that common multi-layered silicate additives like asbestos, mica, talc, kaolinite, montmorillonite, and zeolite are effective in enhancing the tribological characteristics of lubricants [
13,
14,
15]. Within the realm of friction, each of these minerals exhibits distinct advantages. Talc, known for its minimal friction coefficient, plays a crucial role in the production of conventional solid lubricants; mica boosts composite materials’ resistance to wear and is incorporated into brake pads for better friction steadiness; asbestos, previously a part of friction material production, is slowly being substituted due to health hazards; kaolinite and montmorillonite are known to refine the rheological attributes of lubricating greases and amplify their wear-resistant properties; and zeolite is also effective as a modifier for friction, enhancing the friction properties of lubricating oils. Silicate minerals are vital in diminishing mechanical friction and creating materials resistant to wear, achieved by modifying the friction interface’s physical and chemical characteristics, thereby effectively curtailing wear and managing friction coefficients [
16].
Montmorillonite (MMT) is a 2:1-type layered silicate clay mineral with unique crystal structure and excellent properties. It is characterized by large surface area, strong adsorption capacity, high cation exchange capacity, water absorption and expansion between layers after contact with water, with good dispersion and suspension. In application, montmorillonite is widely used in chemical engineering, building materials, environmental protection, and other fields. The modification methods of montmorillonite include ion exchange modification, organic modification, and intercalation modification. Ion exchange modification alters surface properties by replacing the interlayer cations; organic modification uses organic surfactants to enhance compatibility with organic substances; and interlayer modification inserts polymers or other functional materials between layers to expand their functions. In the field of friction, montmorillonite can significantly improve material performance. After modification, lubricating oil can form lubricating film on friction surface and reduce friction coefficient and wear speed [
17]. However, unmodified montmorillonite still faces problems such as insufficient dispersion stability and weak bonding to metal surfaces in lubrication applications. These challenges can be addressed simultaneously by modifying zwitterionic surfactants such as betaine.
Betaine-based surfactants are hermaphroditic and contain cationic (e.g., quaternary ammonium salts) and anion (e.g., carboxylate and sulfonate) groups in their molecules. Features include the following: their pH is adaptable and stable in acidic, neutral, and alkaline environments; they have low toxicity and high biocompatibility, making them suitable for medical and food industries; they have strong salt tolerance and good surface activity in high-salt solutions; and their system stability is synergistically enhanced when combined with anionic or cationic surfactants. In the field of tribology, they can be used as lubricant additives to reduce friction and wear by forming an adsorption film on metal surfaces through their amphoteric groups, such as in water-based cutting fluids. They can be modified into nanoscale lubricants to improve the dispersion of nanoparticles. They can also be used to develop biobased lubrication materials suitable for high environmental protection requirements, providing an efficient and less toxic solution for tribology [
18,
19].
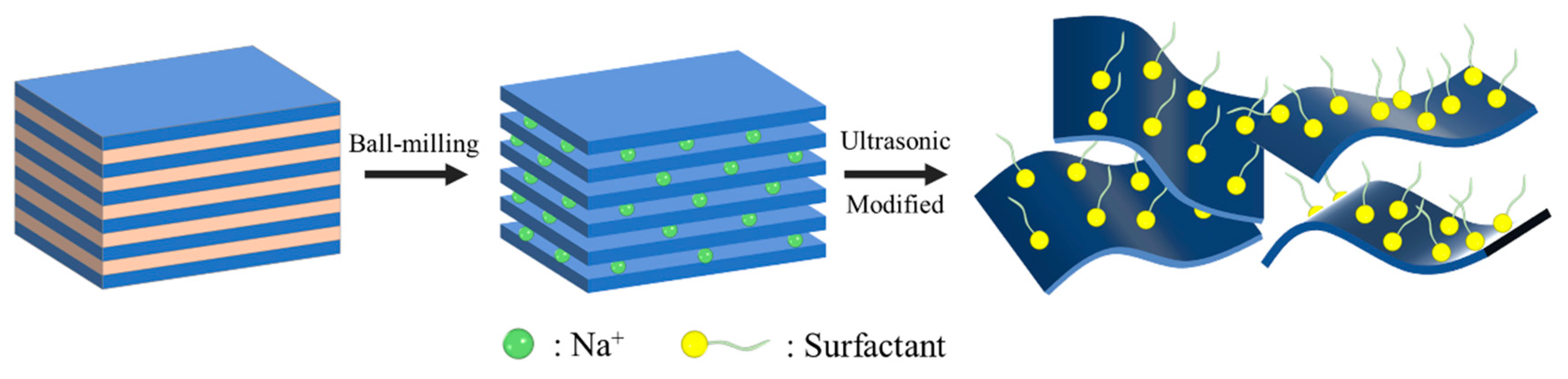
In this study, two betaine-based surfactants were successfully prepared by modifying metal-based surfactants by one-step mechanical chemistry. The modified MMT exhibited good dispersion stability in aqueous solutions and optimized the tribological properties of aqueous lubricant. The results show that under high pressure and high frequency conditions, the interlayer sliding is uniform and the microstructure is thin and loose. The addition of betaine-based surfactants significantly improved the thermal stability and extreme pressure properties of the material. It was found that the special size of nanoparticles, combined with the adsorption film and reaction film formed on the friction surface, can improve the friction reduction resistance of MMT. The preparation process is simple, safe, and environmentally friendly, not only providing a new option for MMT but also laying a foundation for its broad application in other water-based lubrication systems.
3. Results and Discussion
The XRD spectrum in
Figure 2a shows that Na-MMT exhibits a distinct (001) crystal plane diffraction peak at 2θ = 5.8°. By using the Bragg equation (2d sin θ = nλ, λ = 1.5418 Å, n = 1), the layer spacing of Na-MMT is calculated to be 1.523 nm, which is consistent with the typical structural characteristics of Na
+-type montmorillonite. After being modified with betaine, the (001) diffraction peaks of BS-MMT and CAB-MMT shift towards lower angles to less than 5°, and the corresponding layer spacings increase. This significant increase in layer spacing indicates that the 3-sulfotetradecyldimethyl betaine and coconut fatty acid amine propyl betaine molecules have been successfully inserted into the interlayer of montmorillonite. It is worth noting that the intensity of the modified diffraction peaks has decreased, which may be due to the insertion of the organic molecules that disrupted the original regular layered structure of montmorillonite [
20].
The infrared spectrum results (
Figure 2b) show that the absorption peaks at 3624 cm
−1 and 3435 cm
−1 of Na-MMT are attributed to the O-H stretching vibration of the hydroxyl groups on the surface of montmorillonite and the interlayer water, respectively, and the peak at 1633 cm
−1 is the bending vibration of water molecules [
21]. In the modified samples, in addition to retaining these characteristic peaks, new absorption peaks were observed at 2920 cm
−1 and 2850 cm
−1, corresponding to the antisymmetric and symmetric stretching vibrations of -CH
2 in the betaine molecule. In particular, the tensile vibration of C-N
+ in the betaine molecule resulted in characteristic peaks near 1471 cm
−1, providing direct evidence of the successful insertion of organic molecules. In BS-MMT, the weak peak near 1180 cm
−1 may result from S=O tensile vibration of the sulfonate group. The thermogravimetric curve indicates that Na-MMT exhibits two distinct weight loss stages at 25–200 °C and 600–700 °C, corresponding to the removal of adsorbed water and the loss of structural hydroxyl groups, respectively, with an overall weight loss rate of 15.5%. Modified samples show a new phase of weight loss between 200 °C and 500 °C due to the pyrolysis of the layered betaine molecules. The thermal stability of Na-MMT above 300 °C (
Figure 2c) contrasts with bulk water degradation at 100 °C due to two factors: structural -OH groups in the octahedral layer remain intact until 600 °C, and the nanoconfinement of interlayer water by electrostatic and geometric effects delays its evaporation. This behavior is characteristic of phyllosilicates and has been leveraged in high-temperature lubrication applications. The total weight loss of BS-MMT and CAB-MMT at 800 °C was 53.2% and 52.2%, respectively, significantly higher than Na-MMT, further confirming the successful insertion of organic molecules. The insertion and stratification of Na-MMT is shown in
Figure 3. Notably, the weight loss behavior of the modified samples at high temperature may reflect the differences in the thermal stability of different betaine molecules in the montmorillonite layers.
The zeta potential showed that Na-MMT had a zeta potential of −41.0 mV in aqueous solution, indicating a strong negative charge on its surface. The zeta potential of BS-MMT and CAB-MMT is −54.6 mV and −98.2 mV, respectively, after betaine modification. This trend indicates that although the betaine molecules have positive charge groups, the overall surface charge of montmorillonite does not change significantly due to their amphiphilic ion characteristics [
22]. The absolute zeta potential of all samples was greater than 30 mV, indicating good dispersion stability in aqueous solution. The dispersion of montmorillonite in water was shown in
Figure 4 (the dispersion stability was evaluated by the static observation method, the sample concentration was 1%, and the sedimentation condition was observed every seven days), with Na-MMT showing significant aggregation and deposition after 7 days of dispersion, while BS-MMT shows slight aggregation and stratification after 14 days of dispersion. However, throughout the process, CAB-MMT has maintained a stable dispersion, laying the foundation for its application as a lubricant additive. Through the above characterization, it is confirmed that the betaine molecules enter the montmorillonite interlayer successfully by ion exchange and intercalation and significantly increase the interlayer spacing. The modified montmorillonite retains the original hydrophilicity, introduces organic functional groups, and is an additive of water-based lubricant with excellent properties. Especially at high temperatures, the modified montmorillonite still has good thermal stability, which provides a possibility for its application in harsh conditions.
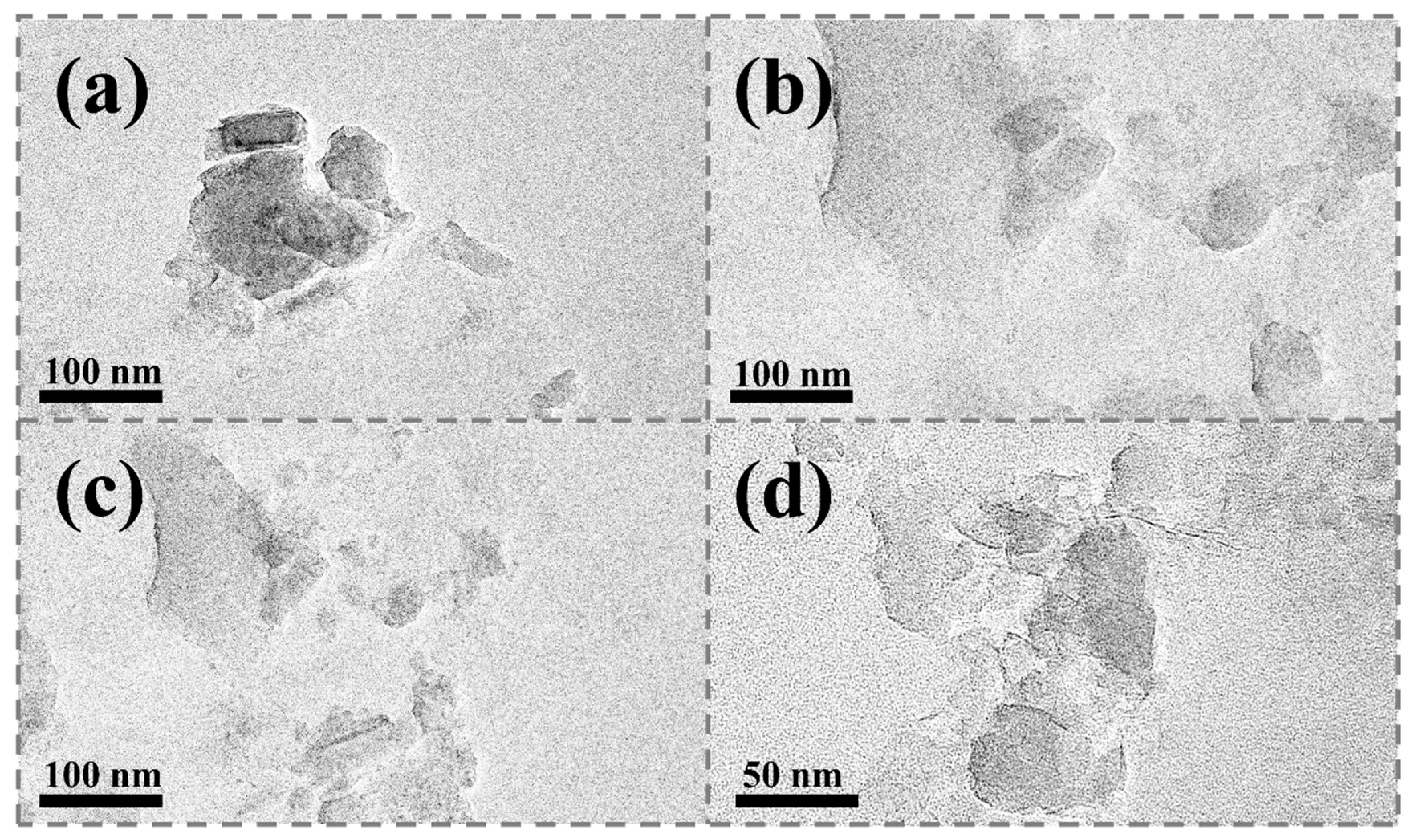
Transmission electron microscopy (TEM) analysis revealed that Na-MMT exhibited a typical tightly layered stacking structure, with clear edges but dense arrangement, and a small amount of agglomeration (
Figure 5a). After being modified with 3-sulfotetradecyldimethyl betaine, the lamellae of BS-MMT became significantly loose, presenting more separated single-layer structures, with slight surface curling and improved dispersibility (
Figure 5b), while CAB-MMT modified with coconut fatty amide propyl betaine demonstrated the optimal peeling effect, forming highly peeled nanosheet structures, with uniform size distribution, intact edges, and almost no agglomeration (
Figure 5c). High-resolution images (
Figure 5d) further revealed the existence of ultra-thin single-layer structures, with smooth and flat surfaces and flexible wavy edges. These morphological differences confirmed that there were significant differences in the intercalation capabilities of different betaine molecules, among which the coconut fatty amide propyl betaine with amide groups exhibited stronger peeling ability, and this highly peeled ultra-thin nanosheet structure provided an ideal physical basis for it to be used as a lubricant additive.
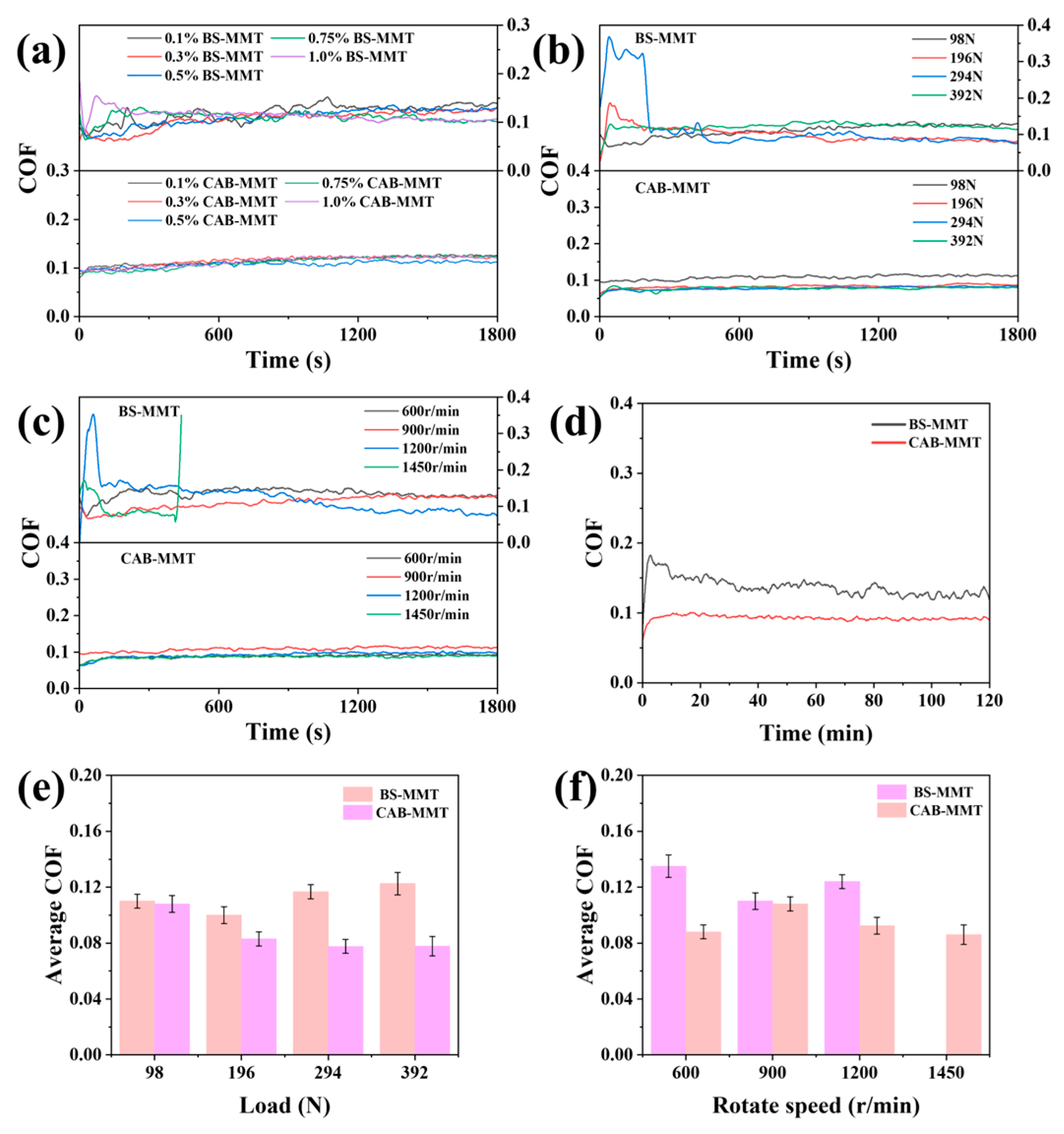
The tribological performance tests results show that the modified MMT nanomaterials, as additives to water-based lubricants, have remarkable friction reduction and wear resistance. As shown in
Figure 6a,b, when lubricated with pure water, the friction coefficient up to 0.25, accompanied by severe abrasive wear (the maximum diameter of the spot of wear). Friction performance did not improve when Na-MMT (0.1–1%) is added, while at optimal concentrations (0.5%), the friction coefficient of BS-MMT and CAB-MMT decreased significantly to 0.15 and 0.10 (71.4% reduction), respectively, and the diameter of wear points decreases by 40% and 58.8%, respectively, in terms of friction performance. It is worth noting that concentrations in all samples tended to decrease and then increase. In summary, combined with the figure shown in
Figure 6c, CAB-MMT has the best comprehensive performance and the most stable low friction state (friction coefficient fluctuation is less than 0.02). This improvement is due to three key factors: first, the increased interlayer spacing promotes interlayer sliding; second, the saccharine acid modification improves the dispersion and surface adsorption ability of the nanoparticles; and third, the ultrathin nanoparticles observed by TEM form effective surface repair membranes [
23].
Findings from the tribological performance evaluations reveal that BS-MMT and CAB-MMT, when used as additives in water-based lubricants, demonstrate superior properties in reducing friction across different operational scenarios. When subjected to a constant load (
Figure 7a), both altered MMT exhibit standard concentration-responsive patterns: a rise in concentration from 0.1% to 0.5% leads to a reduction in the steady-state friction coefficients for BS-MMT and CAB-MMT, dropping from 0.15 to 0.10 and 0.12 to 0.08, respectively. Within this group, CAB-MMT demonstrates the most effective reduction in friction at a 0.5% concentration, accompanied by a 75% decrease in friction coefficient. Remarkably, a rebound effect is observed in the friction coefficient when the concentration surpasses 0.75%, a result of nanoparticle clustering, suggesting 0.5% as the ideal addition concentration.
In the tests under different loads (
Figure 7b) and different speeds (
Figure 7c), the modified MMT demonstrated excellent adaptability to various working conditions. As the load increased from 98 N to 392 N, the friction coefficients of BS-MMT and CAB-MMT lubrication only rose from 0.12 to 0.18 and from 0.08 to 0.12, respectively, indicating outstanding extreme pressure performance. The speed sensitivity test showed that within the range of 600–1450 rpm, the friction coefficient of CAB-MMT remained stable between 0.07 and 0.09, significantly outperforming BS-MMT (0.10–0.14). At 1450 rpm, BS-MMT even experienced a sharp increase in the friction coefficient, which forced the experiment to stop.
Figure 7d presents the friction coefficient curves of BS-MMT and CAB-MMT under 120 min of prolonged friction coefficient conditions.
Figure 7e,f are the bar charts corresponding to
Figure 7b,c. The differences in performance were mainly due to the following: first, the higher adsorption capacity of the coconut fatty amide groups on the metal surface; second, the formation of a continuous lubricating film with more complete nano-flake structures; and third, the complementary boundary lubrication effect provided by the long carbon chain alkyl group [
24]. Specifically, CAB-MMT’s distinct coconut fatty amide groups, known for their robust adsorption and extended carbon chain supplementary lubrication, revolutionized the mixed lubrication process in the steel–steel friction duo, laying a crucial groundwork for its use in eco-friendly production engineering [
25]. The features of CAB-MMT allow it to sustain an efficient mixed lubrication condition across various operational scenarios, offering considerable value in engineering applications [
26,
27,
28].
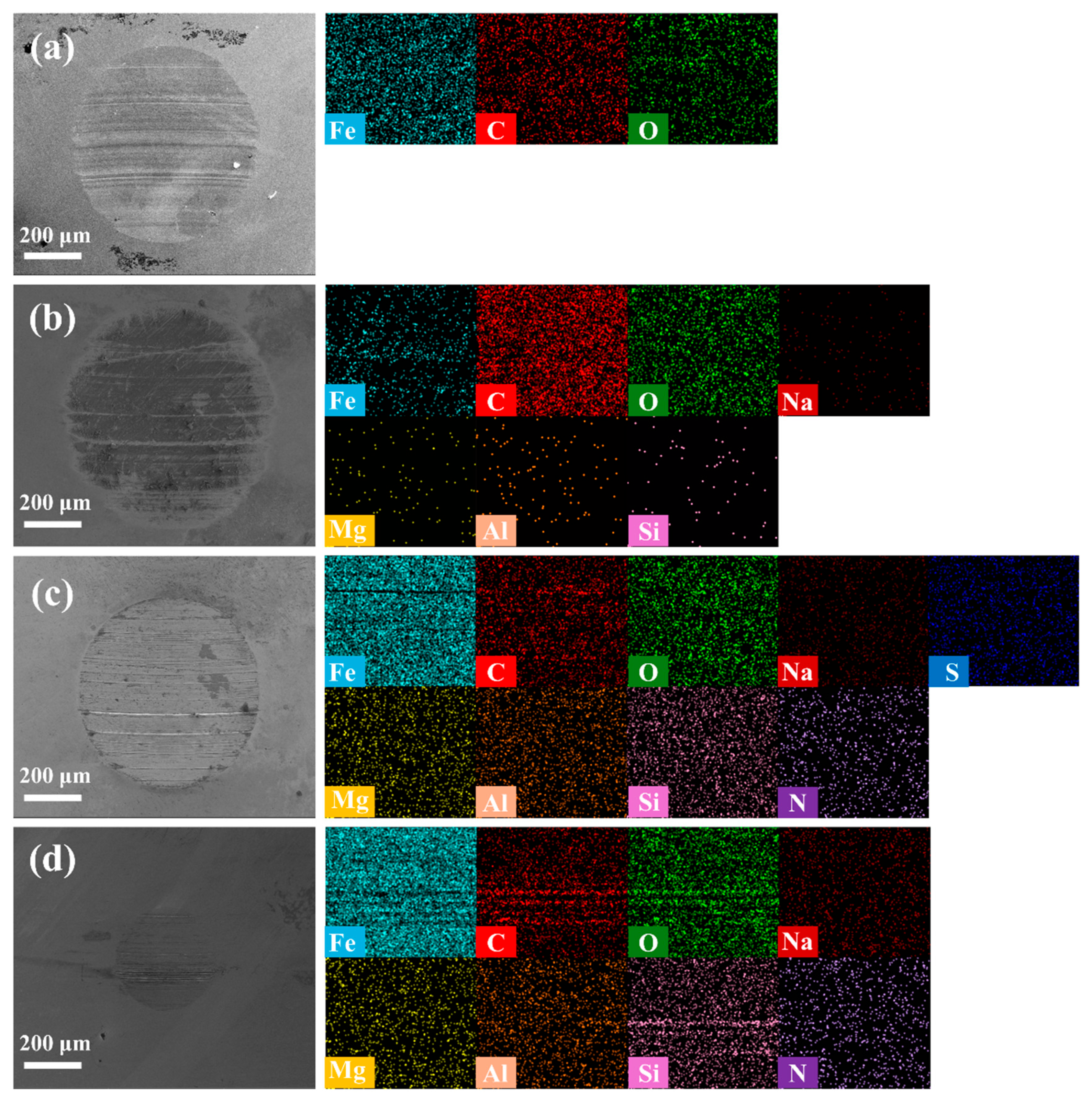
The friction mechanism of the modified MMT nanomaterials can be understood by analyzing the surface morphology and elemental distribution of abrasion point (
Figure 8). The circular wear diameters in
Figure 8a–d are 624 μm, 643 μm, 582 μm and 286 μm. Under pure water lubrication conditions (
Figure 8a), the abrasion point surface has the characteristic of severe abrasive wear, which is characterized by deep and wide grooves and a large amount of material shedding. EDS detection showed only C, O, and Fe signaling, indicating a lack of effective surface protection. After adding Na-MMT (
Figure 8b), the surface of the abrasion point improved slightly, and elements such as Al and Si were detected to be characteristic of MMT but thinly and unevenly distributed, which was associated with a low friction reduction friction coefficient (which decreased by only 14%). Abrasion points lubricated by BS-MMT (
Figure 8c) exhibit markedly different characteristics. First, the surface morphology flattened significantly, and the depth and density of the grooves decreased significantly. Next, EDS detection revealed a uniform distribution of S elements (from the sulfonic acid group), confirming the effective adsorption of the BS-MMT nanosheets on friction surface. Finally, Al and Si signaled increased strength and more uniform distribution, suggesting the formation of a continuous boundary lubrication film [
29]. These observation results are highly consistent with its frictional properties (a 57% reduction in friction coefficient). CAB-MMT surface repair was best (
Figure 8d). First, the surface of the abrasion spot is the smoothest, with few noticeable grooves or shedding observed. Second, the aluminum and silicon elements show a highly uniform surface distribution, suggesting the formation of a dense nanolubrication film. Third, the signature elements have the highest signal strength and determine the most complete surface cover. Of particular note is that under CAB-MMT lubrication, there is a significant concentration of elements at the edge of the abrasion point, which may be a special boundary lubrication structure formed by long carbon chain molecules at the edge of the contact area [
30]. These surface features are consistent with extremely low friction coefficient and minimum abrasive spot diameter, and the effective expansion of the ultra-thin nanosheets structure friction process is confirmed at the microscopic scale, resulting in a high-performance lubrication film observed by TEM [
31]. The strong correlation between element distribution and friction performance provides a direct experimental basis for understanding the lubrication mechanism of beet root acid-modified MMT.
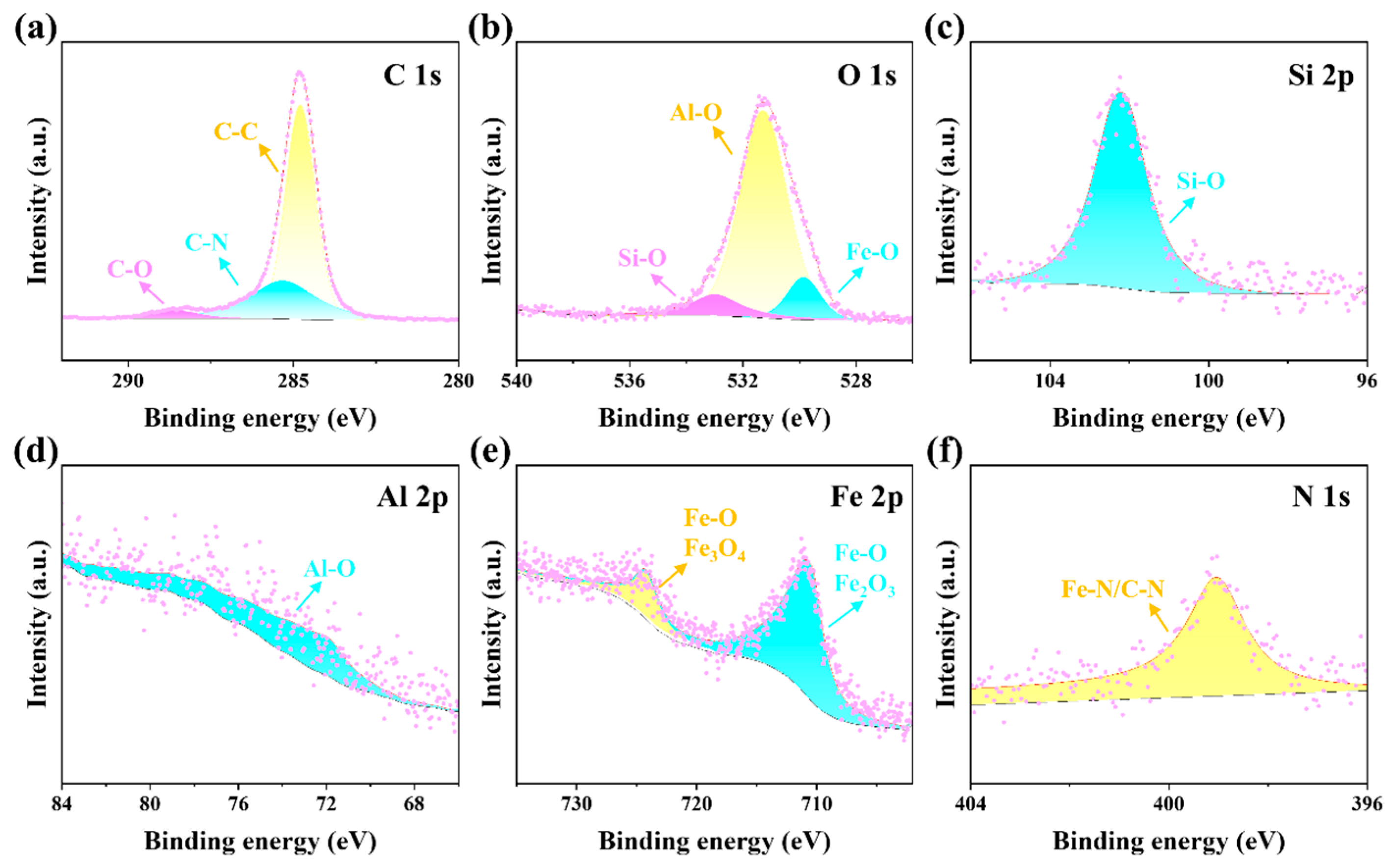
The mechanism of friction chemical reaction of CAB-MMT as lubricant additive was revealed by XPS analysis on the surface of steel ball abrasion spot (
Figure 9a–e). Characteristic peaks of C-C (284.8 eV), C-N (286.2 eV) and C-O (288.5 eV) were detected in the C 1s spectrum (
Figure 9a), confirming the chemical adsorption of betaine molecules based on coconut fatty amine-based betaine molecules on friction surface. Notably, a significant spike of 399.7 eV was observed in the N 1s spectrum compared to the original CAB-MMT (
Figure 9e), suggesting a chemical change in nitrogen-containing functional groups friction process that may form Fe-N coordination bonds. O 1s spectra (
Figure 9b) showed characteristic peaks of 532.6 eV (Si-O), 531.5 eV (Al-O), and 530.1 eV (Fe-O), consistent with the EDS elemental distribution results, confirming the deposition of MMT nanosheets on friction surface. Fe 2p spectra (
Figure 9e) show characteristic peaks of 710.4 eV (Fe
3O
4) and 723.9 eV (Fe
2O
3), suggesting that the addition of CAB-MMT promoted the formation of friction chemical reaction membranes, which are directly related to excellent extreme pressure properties observed in the friction test. It is of particular importance that the characteristic peaks of 102.3 eV (Si-O) and 74.2 eV (Al-O) were detected in the spectra of Si 2p (
Figure 9c) and Al 2p (
Figure 9d), respectively, and it was confirmed at the molecular scale that MMT nanosheets form strong and durable chemical bonds with the steel substrate through the interaction of Si-O-Fe and Al-O-Fe bonds. These changes in surface chemistry are highly consistent with their frictional properties, revealing the microscopic mechanisms by which CAB-MMT achieves super lubrication through synergistic effects of “physical adsorption and chemical bonding”.
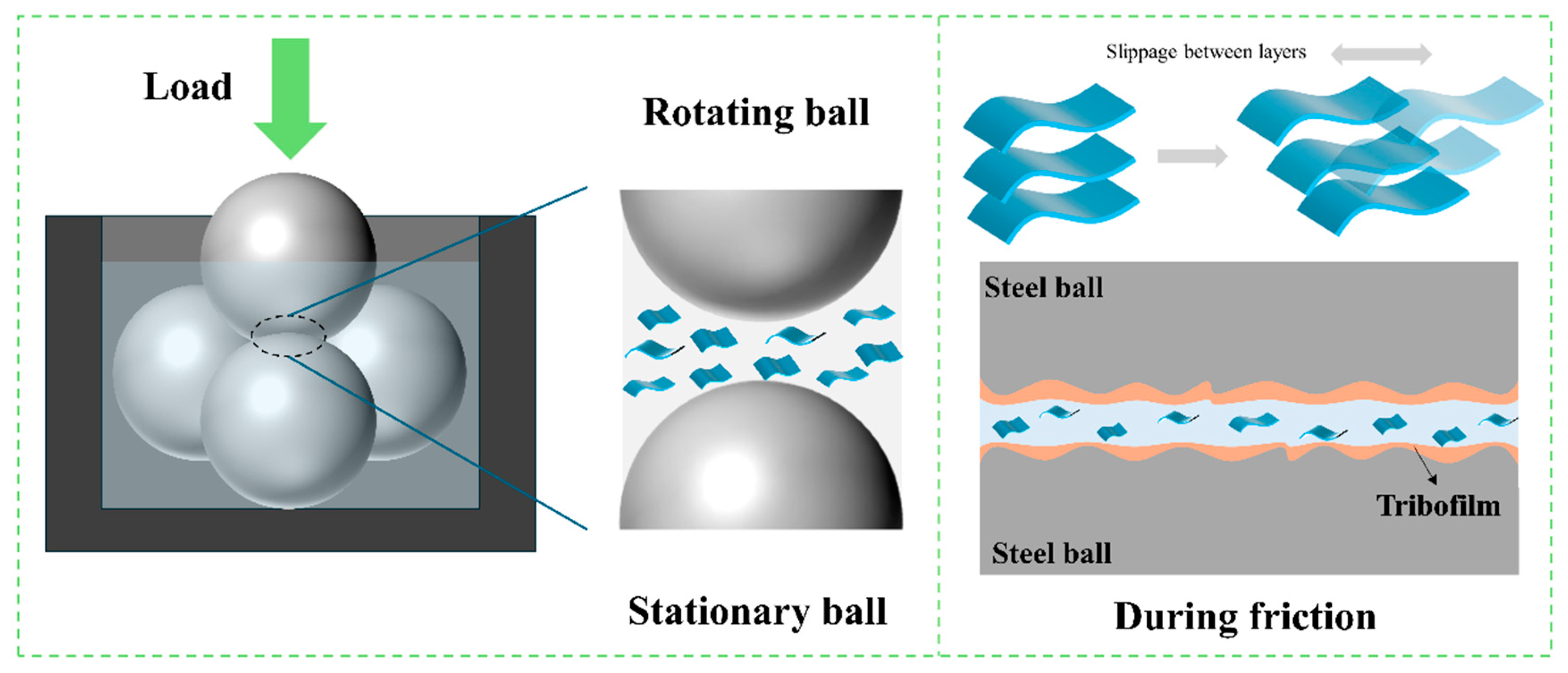
The frictional process of CAB-MMT can be encapsulated as such, following an examination of the friction mechanism diagram and the outcomes of multi-scale characterization. Within the zone of friction contact (
Figure 10), CAB-MMT attains enhanced lubrication via a tripartite cooperative process. At the initial stage lies the physical adsorption layer, characterized by the swift absorption of ultra-thin MMT nanosheets onto the steel ball’s surface, attributed to their extensive specific surface area and negative charge, thereby creating a spatial barrier layer. At the nanosheet’s second tier, the Si-O and Al-O bonds at the nanosheet edges establish covalent links with the base Fe, and the coconut fatty amide chain in the taurine creates a vertical connection via Fe-N coordination (399.7 eV) on the metal, resulting in a composite structure resembling a “nanosheet-molecular brush”. At the third tier, known as the dynamic repair layer, Fe
3O
4 and Fe
2O
3, stimulated by friction, jointly populate the surface micro-defects with MMT layers [
32,
33]. SEM (
Figure 8d) shows the smoothest wear surface under CAB-MMT lubrication, with minimal grooves, confirming the effective coverage by exfoliated MMT nanosheets observed in TEM (
Figure 5c). EDS elemental maps demonstrate the uniform distribution of Al/Si (MMT components) across the wear scar, validating the spatial barrier layer formation via physical adsorption. XPS (
Figure 9) confirms covalent bonding at the interface: Fe–N coordination (399.7 eV in N 1s) correlates with betaine’s amide group adsorption. Si–O–Fe/Al–O–Fe bonds (102.3 eV and 74.2 eV in Si 2p/Al 2p) verify MMT–metal substrate bonding. SEM-EDS (
Figure 8d) shows concentrated Al/Si/N at wear scar edges, matching the nanosheet–molecular brush structure in
Figure 10. The layered lubrication system ensures the contact zone consistently sustains a fluid dynamic layer and retains a minimal friction coefficient even under intense pressure, enhancing its resistance to wear by eightfold (with a 58.8% decrease in the diameter of the wear scar). This process disrupts the conventional “adsorption–desorption” mode of traditional lubricants, paving the way for innovative approaches in creating adaptive nano-lubricant substances [
34,
35].