Synthesis and Evaluation of Sunflower-Oil-Based Esters as Biolubricant Base Oils Using Ca/TEA Alkoxide Catalyst
Abstract
1. Introduction
2. Material and Methods
| Property | Unit | SUNOME | EN 14214 Limits | Standard Method |
|---|---|---|---|---|
| Density (15 °C) | g/cm3 | 0.880 | 0.860–0.900 | EN ISO 12185 [31] |
| Kinematic viscosity (40 °C) | mm2/s | 4.413 | 3.50–5.00 | EN ISO 3104 [32] |
| Water content | mg/kg | 250.4 | Max. 500 | EN ISO 12937 [33] |
| Acid value (AV) | mg KOH/g | 0.27 | Max. 0.50% | EN 14104 [34] |
| Ester content | m/m | 97.6% | Min. 96.5% | EN 14103 [35] |
| Methanol content | m/m | 0.06% | Max. 0.2% | EN 14110 [36] |
| Linolenic acid methyl ester content | m/m | 0.4% | Max. 12% | EN 14103 [35] |
| Fatty Acid | Molecular Formula | SUNOME | |
|---|---|---|---|
| Caproic | CH3(CH2)4COOCH3 | C6:0 | 0.0% |
| Caprylic | CH3(CH2)6COOCH3 | C8:0 | 0.0% |
| Capric | CH3(CH2)8COOCH3 | C10:0 | 0.0% |
| Lauric | CH3(CH2)10COOCH3 | C12:0 | 0.0% |
| Myristic | CH3(CH2)12COOCH3 | C14:0 | 0.4% |
| Myristoleic | CH3(CH2)3CH=CH(CH2)7CO2CH3 | C14:1 | 0.2% |
| Palmitic | CH3(CH2)14COOCH3 | C16:0 | 7.5% |
| Palmitoleic | CH3(CH2)5CH=CH(CH2)7CO2CH3 | C16:1 | 0.1% |
| Margaric | CH3(CH2)15COOCH3 | C 17:0 | 0.0% |
| Stearic | CH3(CH2)16COOCH3 | C18:0 | 3.9% |
| Oleic | CH3(CH2)7CH=CH(CH2)7CO2CH3 | C18:1 | 32.9% |
| Linoleic | CH3(CH2)4CH=CHCH2CH=CH- (CH2)7CO2 CH3 | C18:2 | 52.9% |
| Linolenic | CH3(CH2CH=CH)3(CH2)7CO2CH3 | C18:3 | 0.4% |
| Arachidic | CH3(CH2)18COO CH3 | C20:0 | 0.0% |
| Eicosenoic | CH3(CH2)7CH=CH(CH2)9CO2CH3 | C20:1 | 0.0% |
| Behenic | C21H43COOCH3 | C22:0 | 0.8% |
| Erucic | CH3(CH2)7CH=CH(CH2)11CO2CH3 | C22:1 | 0.0% |
| Lignoceric | C23H47COOCH3 | C24:0 | 0.9% |
- For isooctane-assisted reactions: 200 °C at atmospheric pressure;
- For vacuum-assisted reactions: 120 °C under 0.05 bar vacuum.
3. Results
4. Discussion
- Higher viscosities and oxidation stability were consistently observed under vacuum conditions.
- The vacuum method avoids the use of organic solvents like isooctane, improving environmental safety and reducing downstream processing.
- Operating at lower reaction temperatures (100–120 °C), the vacuum route reduces energy demand and minimizes thermal stress on sensitive unsaturated chains.
- Methanol removal is efficient via cold-trapping under vacuum, driving the reaction forward without the need for solvent-based azeotropes.
- Waste prevention (Principle 1): The clean reaction pathway minimizes byproducts and post-processing steps.
- Safer chemicals (Principle 4): It replaces hazardous CH3ONa, minimizing fire risk and handling hazards.
- Solvent reduction (Principle 5): Vacuum synthesis avoids VOCs like isooctane.
- Design for energy efficiency (Principle 6): The lower processing temperature in vacuum reactions cuts energy usage.
- Catalysis (Principle 9): The heterogeneous nature of Ca/TEA enhances activity with potential for reuse, reducing waste [20].
5. Conclusions
- They exhibit noteworthy performance characteristics, such as high kinematic viscosities and low pour points—ranging from 33–48 cSt at 40 °C, 7.68–10.03 cSt at 100 °C, to −14 to −7 °C, respectively—which are comparable to or improved over those of mineral oils. These values ensure good flowability, lubricity, and pumpability of the biolubricant at both high and low temperatures.
- They have significantly higher viscosity indices than mineral oils, indicating excellent thermal stability and the ability to function across a wide temperature range.
- Among the two catalytic systems, the superiority of Ca/TEA alkoxide over CH3ONa is evident, as demonstrated by the kinematic viscosity, oxidation stability, and viscosity index measurements.
- It is observed that using Ca/TEA alkoxide instead of CH3ONa enables the synthesis of biolubricants with significantly improved performance—especially when the synthesis is conducted under vacuum conditions, which is particularly advantageous as it allows for both the elimination of isooctane and lower reaction temperatures.
- The oxidation resistance of any biolubricant was significantly lower than that of each mineral oil tested, which limits their use to specific applications such as non-recoverable or high-risk lubrication scenarios that involve low thermal and oxidative loads. However, they can still be used in blends with traditional mineral oils or after the addition of suitable performance-enhancing additives.
- Ca/TEA offers a safer, greener, and more sustainable pathway for biolubricant synthesis, reducing reliance on fossil-derived or hazardous catalytic agents.
- Overall, the vacuum method paired with Ca/TEA represents the most promising configuration in terms of product performance and environmental profile for industrial biolubricant production.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| FAMEs | Fatty acid methyl esters |
| TMP | Trimethylolpropane |
| Ca/TEA | Calcium/triethanolamine alkoxide |
| TEA | Triethanolamine |
| SUNOMEs | Sunflower oil methyl esters |
| FATMPEs | Fatty acid trimethylolpropane esters |
| SUNOTMPEs | Sunflower oil trimethylolpropane esters |
References
- Salih, N.; Salimon, J. A review on eco-friendly green biolubricants from renewable and sustainable plant oil sources. Biointerface Res. Appl. Chem. 2021, 11, 13303–13327. [Google Scholar] [CrossRef]
- Kurre, S.K.; Yadav, J. A review on bio-based feedstock, synthesis, and chemical modification to enhance tribological properties of biolubricants. Ind. Crops Prod. 2023, 193, 116122. [Google Scholar] [CrossRef]
- Cecilia, J.A.; Plata, D.B.; Saboya, R.M.A.; de Luna, F.M.T.; Cavalcante, C.L.; Rodríguez-Castellón, E. An overview of the biolubricant production process: Challenges and future perspectives. Processes 2020, 8, 257. [Google Scholar] [CrossRef]
- Shah, R.; Woydt, M.; Zhang, S. The economic and environmental significance of sustainable lubricants. Lubricants 2021, 9, 21. [Google Scholar] [CrossRef]
- Sharma, B.K.; Adhvaryu, A.; Liu, Z.; Erhan, S.Z. Chemical modification of vegetable oils for lubricant applications. J. Am. Oil Chem. Soc. 2006, 83, 129–136. [Google Scholar] [CrossRef]
- FAO. FAOSTAT Statistical Database: Crops and Livestock Products; Food and Agriculture Organization of the United Nations: Rome, Italy, 2023; Available online: https://www.fao.org/faostat/en/ (accessed on 18 July 2025).
- Böcker, T.; Finger, R.; Dalhaus, T. Opportunities and limitations of revenue insurance for high-oleic sunflower production. Agric. Econ. 2020, 51, 247–259. [Google Scholar] [CrossRef]
- Knothe, G.; Steidley, K.R. Lubricity of components of biodiesel and petrodiesel. Energy Fuels 2005, 19, 1192–1200. [Google Scholar] [CrossRef]
- European Commission. EU Agricultural Markets Data—Sunflower Seeds; European Commission Agriculture and Rural Development: Brussels, Belgium, 2023; Available online: https://agriculture.ec.europa.eu (accessed on 18 July 2025).
- OECD-FAO. OECD-FAO Agricultural Outlook 2021–2030; Organisation for Economic Co-operation and Development and Food and Agriculture Organization of the United Nations: Paris, France, 2021. [Google Scholar] [CrossRef]
- de Sá Parente, E.J.; de Oliveira, L.B.; de Luna, F.M.T.; Cavalcante, C.L. Integrated production of biolubricants and biodiesel: Process simulation and technical–economic analysis. Biomass Convers. Biorefinery 2023, 14, 23709–23732. [Google Scholar] [CrossRef]
- Prema, E.; Sundar, V.S.; Lynch, M.; Sivaraman, K.; Venkatesan, P.R. The need for eco-friendly green bio-lubricants to achieve united nations sustainable development goals: An analysis. AIP Conf. Proc. 2024, 2986, 030079. [Google Scholar] [CrossRef]
- Statista. Market Value of Bio-Based Lubricants Worldwide from 2021 to 2023, with a Forecast Until 2031. Available online: https://www.statista.com/statistics/1461186/global-bio-lubricants-market-size-forecast/ (accessed on 15 June 2025).
- Statista. Lubricants Demand Worldwide. Available online: https://www.statista.com/statistics/411616/lubricants-demand-worldwide/ (accessed on 15 June 2025).
- Ahmad, U.; Naqvi, S.R.; Ali, I.; Naqvi, M.; Asif, S.; Bokhari, A.; Juchelková, D.; Klemeš, J.J. A review on properties, challenges and commercial aspects of eco-friendly biolubricants productions. Chemosphere 2022, 309, 136622. [Google Scholar] [CrossRef] [PubMed]
- Pmarketresearch. Environmentally Friendly Lubricant Market. Pmarketresearch.com. 2023. Available online: https://pmarketresearch.com/chemi/environmentally-friendly-lubricant-market/ (accessed on 15 July 2025).
- Pmarketresearch. Readily Biodegradable Lubricant Market. Pmarketresearch.com. 2023. Available online: https://pmarketresearch.com/chemi/readily-biodegradable-lubricant-market/ (accessed on 15 July 2025).
- Karmakar, G.; Ghosh, P.; Sharma, B.K. Chemically modifying vegetable oils to prepare green lubricants. Lubricants 2017, 5, 44. [Google Scholar] [CrossRef]
- Dodos, G.S.; Anastopoulos, G.; Zannikos, F. Production of Biobased Lubricant Basestocks with Improved Performance; SAE Technical Paper 2012-01-1620; SAE: Warrendale, PA, USA, 2012. [Google Scholar] [CrossRef]
- Nogales-Delgado, S.; Encinar, J.M.; González, J.F. A Review on Biolubricants Based on Vegetable Oils through Transesterification and the Role of Catalysts: Current Status and Future Trends. Catalysts 2023, 13, 1299. [Google Scholar] [CrossRef]
- Hájek, M.; Vávra, A.; de Paz Carmona, H.; Kocík, J. The Catalysed Transformation of Vegetable Oils or Animal Fats to Biofuels and Bio-Lubricants: A Review. Catalysts 2021, 11, 1118. [Google Scholar] [CrossRef]
- Dodos, G.S.; Karonis, D.; Zannikos, F.; Lois, E. Renewable fuels and lubricants from Lunaria annua L. Ind. Crops Prod. 2015, 75, 43–50. [Google Scholar] [CrossRef]
- Narayana Sarma, R.; Vinu, R. Current Status and Future Prospects of Biolubricants: Properties and Applications. Lubricants 2022, 10, 70. [Google Scholar] [CrossRef]
- Sarker, M.I.; Ngo, H.; Sharma, B.K.; Wagner, K.M.; Jones, K.C.; Powell, M.J. Green synthesis of trimethylolpropane triisostearate and triisooleate for usage as bio-lubricants. Tribol. Int. 2023, 186, 108649. [Google Scholar] [CrossRef]
- Abdel Hamid, E.M.; Amer, A.M.; Mahmoud, A.K.; Mokbl, E.M.; Hassan, M.A.; Abdel-Monaim, M.O.; Amin, R.H.; Tharwat, K.M. Box-Behnken design (BBD) for optimization and simulation of biolubricant production from biomass using aspen plus with techno-economic analysis. Sci. Rep. 2024, 14, 21769. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.S.; Freire, C.C.C.; Brandão, L.M.S.; Pereira, E.B.; Mendes, A.A.; Pereira, M.M.; Lima, Á.S.; Soares, C.M.F. Biolubricant production under zero-waste Moringa oleifera Lam biorefinery approach for boosting circular economy. Ind. Crops Prod. 2021, 167, 113542. [Google Scholar] [CrossRef]
- Barbera, E.; Hirayama, K.; Maglinao, R.L.; Davis, R.W.; Kumar, S. Recent developments in synthesizing biolubricants—A review. Biomass Convers. Biorefinery 2024, 14, 2867–2887. [Google Scholar] [CrossRef]
- Shahabuddin, M.; Mofijur, M.; Kalam, M.A.; Masjuki, H.H. Study on the friction and wear characteristics of bio-lubricant synthesized from second generation jatropha methyl ester. Tribol. Ind. 2020, 42, 41–49. [Google Scholar] [CrossRef]
- Saha, C.; Pradhan, S. Bio-Lubricants. Lubricants from Renewable Feedstocks; Wiley: Hoboken, NJ, USA, 2024; pp. 445–471. [Google Scholar] [CrossRef]
- DIN EN 14214:2019; Liquid Petroleum Products—Fatty Acid Methyl Esters (FAME) for Use in Biodiesel Engines and Heating Applications–Requirements and Test Methods (Includes Amendment: 2019). Deutsche Institut für Normung e.V. (DIN): Berlin, Germany, 2019.
- EN ISO 12185: 1996; Petroleum Products—Determination of Density—Oscillating U-tube Method. International Organization for Standardization (ISO): Geneva, Switzerland, 1996.
- EN ISO 3104: 2017; Petroleum Products—Transparent and Opaque Liquids—Determination of Kinematic Viscosity and Calculation of Dynamic Viscosity. International Organization for Standardization (ISO): Geneva, Switzerland, 2017.
- EN ISO 12937: 2000; Petroleum Products—Determination of Water—Karl Fischer Method. International Organization for Standardization (ISO): Geneva, Switzerland, 2000.
- EN 14104: 2011; Fat and Oil Derivatives—Fatty Acid Methyl Esters (FAME)—Determination of Acid Value. European Committee for Standardization (CEN): Brussels, Belgium, 2011.
- EN 14103: 2011; Fat and Oil Derivatives—Fatty Acid Methyl Esters (FAME)—Determination of Ester Content and Ester Distribution. European Committee for Standardization (CEN): Brussels, Belgium, 2011.
- EN 14110; Fat and Oil Derivatives—Fatty Acid Methyl Esters (FAME)—Determination of Methanol Content. European Committee for Standardization (CEN): Brussels, Belgium, 2003.
- Encinar, J.M.; Nogales-Delgado, S.; Pinilla, A. Biolubricant production through double transesterification: Reactor design for the implementation of a biorefinery based on rapeseed. Processes 2021, 9, 1224. [Google Scholar] [CrossRef]
- Qiao, S.; Shi, Y.; Wang, X.; Lin, Z.; Jiang, Y. Synthesis of Biolubricant Trimethylolpropane Trioleate and Its Lubricant Base Oil Properties. Energy Fuels 2017, 31, 7185–7190. [Google Scholar] [CrossRef]
- Elmelawy, M.S.; El-Meligy, A.; Mawgoud, H.A.; Morshedy, A.S.; Hanafy, S.A.; El-Sayed, I.E. Synthesis and kinetics study of trimethylolpropane fatty acid triester from oleic acid methyl ester as potential biolubricant. Biomass Convers. Biorefinery 2023, 13, 1645–1657. [Google Scholar] [CrossRef]
- ASTM D97: 2020; Standard Test Method for Pour Point of Petroleum Products. ASTM International: West Conshohocken, PA, USA, 2020.
- ASTM D7042: 2020; Standard Guide for Properties of Diesel Fuels. ASTM International: West Conshohocken, PA, USA, 2020.
- ASTM D2270: 2020; Standard Practice for Calculating Viscosity Index of Petroleum Oils. ASTM International: West Conshohocken, PA, USA, 2020.
- ASTM D7545: 2020; Standard Test Method for Oxidation Stability of Middle Distillate Fuels—Rapid Small Scale Oxidation Test (RSSOT). ASTM International: West Conshohocken, PA, USA, 2020.
- Rizvi, S.Q.A. Lubricant Base Oil and Wax Processing; Marcel Dekker: New York, NY, USA, 2003; pp. 1–35. [Google Scholar]
- Arbain, D.; Salimon, J. Synthesis and characterization of ester trimethylolpropane from renewable feedstock as potential biolubricant base stock. Sci. Res. Essays 2010, 5, 519–525. [Google Scholar]
- Khan, S.; Das, P.; Quadir, M.A.; Thaher, M.; Annamalai, S.N.; Mahata, C.; Hawari, A.H.; al Jabri, H. A comparative physicochemical property assessment and techno-economic analysis of biolubricants produced using chemical modification and additive-based routes. Sci. Total Environ. 2022, 847, 157648. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
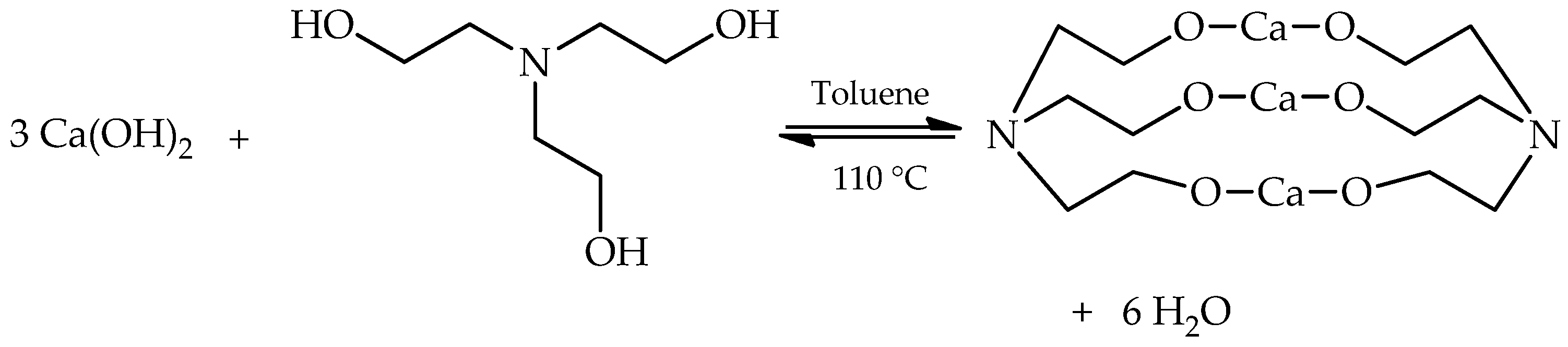



| Property | Unit | 1.0% | 1.5% | 2.0% | 2.5% | 3.0% | Standard Method |
|---|---|---|---|---|---|---|---|
| Acid value | mgKOH/g | 0.09 | 0.07 | 0.07 | 0.10 | 0.11 | EN 14104 [34] |
| Water content | mg/kg | 91 | 97 | 89 | 93 | 78 | EN ISO 12937 [33] |
| Pour point | °C | −12 | −12 | −10 | −10 | −11 | ASTM D 97 [40] |
| Kin. viscosity (40 °C) | mm2/s | 33.67 | 37.12 | 37.75 | 36.78 | 36.00 | ASTM D 7042 [41] |
| Kin. viscosity (100 °C) | mm2/s | 7.981 | 8.521 | 8.517 | 8.271 | 8.212 | |
| Viscosity index | - | 222 | 216 | 213 | 210 | 214 | ASTM D 2270 [42] |
| Density (15 °C) | g/cm3 | 0.9228 | 0.9241 | 0.9260 | 0.9288 | 0.9270 | ASTM D 7042 [41] |
| Oxidation stability (RSSOT 140 °C, 700 kPa) | min | 21.23 | 25.65 | 26.63 | 24.82 | 22.25 | ASTM D7545 [43] |
| Reaction time | h | 11 | 9.0 | 8.0 | 7.5 | 7.5 | - |
| Property | Unit | 2.0% | 2.5% | 3.0% | 3.5% | 4.0% | Standard Method |
|---|---|---|---|---|---|---|---|
| Acid value | mgKOH/g | 0.17 | 0.13 | 0.09 | 0.10 | 0.11 | EN 14104 [34] |
| Water content | mg/kg | 98 | 77 | 79 | 73 | 78 | EN ISO 12937 [33] |
| Pour point | °C | −11 | −9 | −9 | −10 | −11 | ASTM D 97 [40] |
| Kin. viscosity (40 °C) | mm2/s | 35.66 | 39.71 | 39.49 | 37.59 | 36.23 | ASTM D 7042 [41] |
| Kin. viscosity (100 °C) | mm2/s | 8.201 | 8.728 | 8.601 | 8.388 | 8.224 | |
| Viscosity index | - | 216 | 208 | 204 | 209 | 212 | ASTM D 2270 [42] |
| Density (15 °C) | g/cm3 | 0.9256 | 0.9311 | 0.9309 | 0.9288 | 0.9270 | ASTM D 7042 [41] |
| Oxidation stability (RSSOT 140 °C, 700 kPa) | min | 32.23 | 35.65 | 39.42 | 37.82 | 34.25 | ASTM D7545 [43] |
| Reaction time | h | 9.5 | 8.0 | 6.5 | 7.0 | 7.0 | - |
| Property | Unit | 1.0% | 1.5% | 2.0% | 2.5% | 3.0% | Standard Method |
|---|---|---|---|---|---|---|---|
| Acid value | mgKOH/g | 0.12 | 0.09 | 0.06 | 0.13 | 0.11 | EN 14104 [34] |
| Water content | mg/kg | 103 | 95 | 93 | 97 | 87 | EN ISO 12937 [33] |
| Pour point | °C | −13 | −12 | −10 | −13 | −14 | ASTM D 97 [40] |
| Kin. viscosity (40 °C) | mm2/s | 33.08 | 36.70 | 39.86 | 37.17 | 37.38 | ASTM D 7042 [41] |
| Kin. viscosity (100 °C) | mm2/s | 7.677 | 8.272 | 8.666 | 8.309 | 8.298 | |
| Viscosity index | - | 213 | 211 | 204 | 209 | 207 | ASTM D 2270 [42] |
| Density (15 °C) | g/cm3 | 0.9196 | 0.9201 | 0.9213 | 0.9195 | 0.9179 | ASTM D 7042 [41] |
| Oxidation stability (RSSOT 140 °C, 700 kPa) | min | 21.12 | 23.69 | 26.19 | 21.05 | 20.58 | ASTM D7545 [43] |
| Reaction time | h | 5.5 | 5.0 | 4.0 | 4.5 | 5.0 | - |
| Property | Unit | 2.0% | 2.5% | 3.0% | 3.5% | 4.0% | Standard Method |
|---|---|---|---|---|---|---|---|
| Acid value | mgKOH/g | 0.11 | 0.10 | 0.10 | 0.07 | 0.09 | EN 14104 [34] |
| Water content | mg/kg | 64 | 71 | 58 | 70 | 69 | EN ISO 12937 [33] |
| Pour point | °C | −10 | −9 | −9 | −7 | −8 | ASTM D 97 [40] |
| Kin. viscosity (40 °C) | mm2/s | 37.35 | 40.15 | 47.17 | 48.44 | 42.42 | ASTM D 7042 [41] |
| Kin. viscosity (100 °C) | mm2/s | 8.277 | 8.690 | 9.828 | 10.03 | 9.241 | |
| Viscosity index | - | 206 | 203 | 201 | 200 | 209 | ASTM D 2270 [42] |
| Density (15 °C) | g/cm3 | 0.9288 | 0.9351 | 0.9356 | 0.9381 | 0.9361 | ASTM D 7042 [41] |
| Oxidation stability (RSSOT 140 °C, 700 kPa) | min | 32.39 | 38.38 | 40.07 | 43.56 | 42.98 | ASTM D7545 [43] |
| Reaction time | h | 4.5 | 4.0 | 3.5 | 3.5 | 4.0 | - |
| Property | Unit | CH3ONa (2% wt.)-Isooctane | Ca/TEA (3% wt.)-Isooctane | CH3ONa (2% wt.)-Vacuum | Ca/TEA (3.5% wt.)-Vacuum | SN-150 | SN-500 | Standard Method |
|---|---|---|---|---|---|---|---|---|
| Acid value | mgKOH/g | 0.07 | 0.09 | 0.06 | 0.07 | 0.02 | 0.02 | EN 14104 [34] |
| Water content | mg/kg | 89 | 79 | 93 | 70 | 97 | 98 | EN ISO 12937 [33] |
| Pour point | °C | −10 | −9 | −10 | −7 | −13 | −9 | ASTM D 97 [40] |
| Kin. viscosity (40 °C) | mm2/s | 37.75 | 39.49 | 39.86 | 48.44 | 34.03 | 63.57 | ASTM D 7042 [41] |
| Kin. viscosity (100 °C) | mm2/s | 8.517 | 8.601 | 8.666 | 10.03 | 5.738 | 8.786 | |
| Viscosity index | - | 213 | 204 | 204 | 200 | 107 | 112 | ASTM D 2270 [42] |
| Density (15 °C) | g/cm3 | 0.9260 | 0.9309 | 0.9213 | 0.9381 | 0.8726 | 0.8736 | ASTM D 7042 [41] |
| Oxidation stability (RSSOT 140 °C, 700 kPa) | min | 26.63 | 39.42 | 26.19 | 43.56 | 434.5 | 1461 | ASTM D7545 [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filon, D.; Anastopoulos, G.; Karonis, D. Synthesis and Evaluation of Sunflower-Oil-Based Esters as Biolubricant Base Oils Using Ca/TEA Alkoxide Catalyst. Lubricants 2025, 13, 345. https://doi.org/10.3390/lubricants13080345
Filon D, Anastopoulos G, Karonis D. Synthesis and Evaluation of Sunflower-Oil-Based Esters as Biolubricant Base Oils Using Ca/TEA Alkoxide Catalyst. Lubricants. 2025; 13(8):345. https://doi.org/10.3390/lubricants13080345
Chicago/Turabian StyleFilon, Dimosthenis, George Anastopoulos, and Dimitrios Karonis. 2025. "Synthesis and Evaluation of Sunflower-Oil-Based Esters as Biolubricant Base Oils Using Ca/TEA Alkoxide Catalyst" Lubricants 13, no. 8: 345. https://doi.org/10.3390/lubricants13080345
APA StyleFilon, D., Anastopoulos, G., & Karonis, D. (2025). Synthesis and Evaluation of Sunflower-Oil-Based Esters as Biolubricant Base Oils Using Ca/TEA Alkoxide Catalyst. Lubricants, 13(8), 345. https://doi.org/10.3390/lubricants13080345





