A Spectroscopic DRIFT-FTIR Study on the Friction-Reducing Properties and Bonding of Railway Leaf Layers
Abstract
1. Introduction
2. Materials and Methods
2.1. Tribological Twin-Disc Methodology
2.2. DRIFT-FTIR Spectroscopy Methodology
3. Results
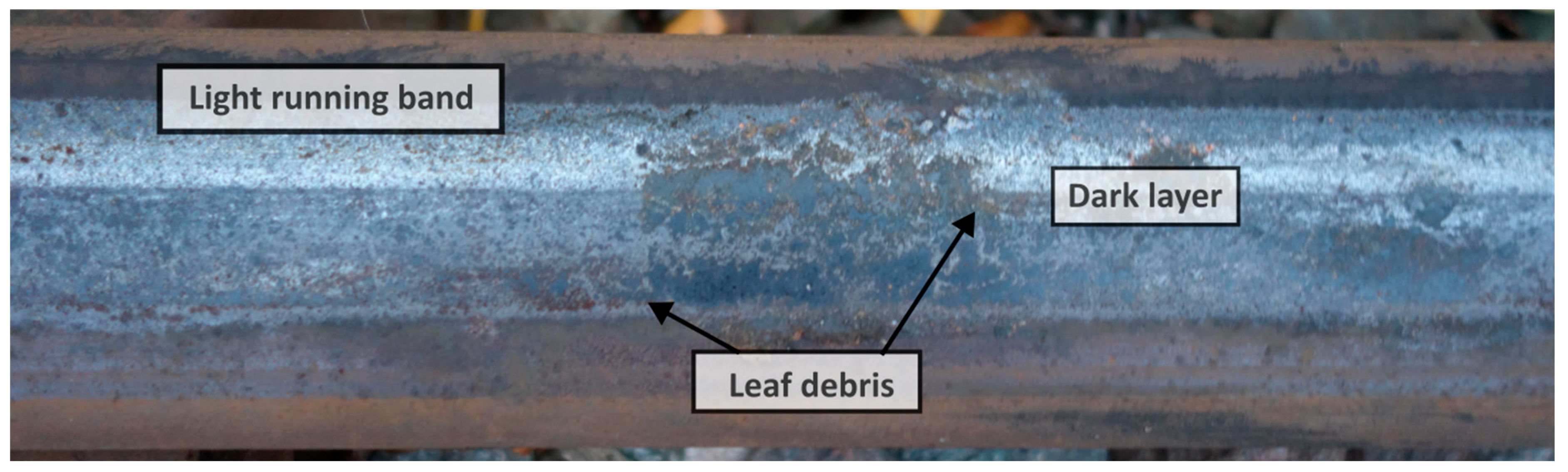
3.1. Tribological Twin-Disc Results
3.2. DRIFT-FTIR Spectroscopy Results
3.2.1. Raw Powdered Material (Sample 1)
3.2.2. Ejected Material Found on Side of Test Specimen (Sample 2)
3.2.3. Dark Material (Sample 3)
3.2.4. Light Material (Sample 4)
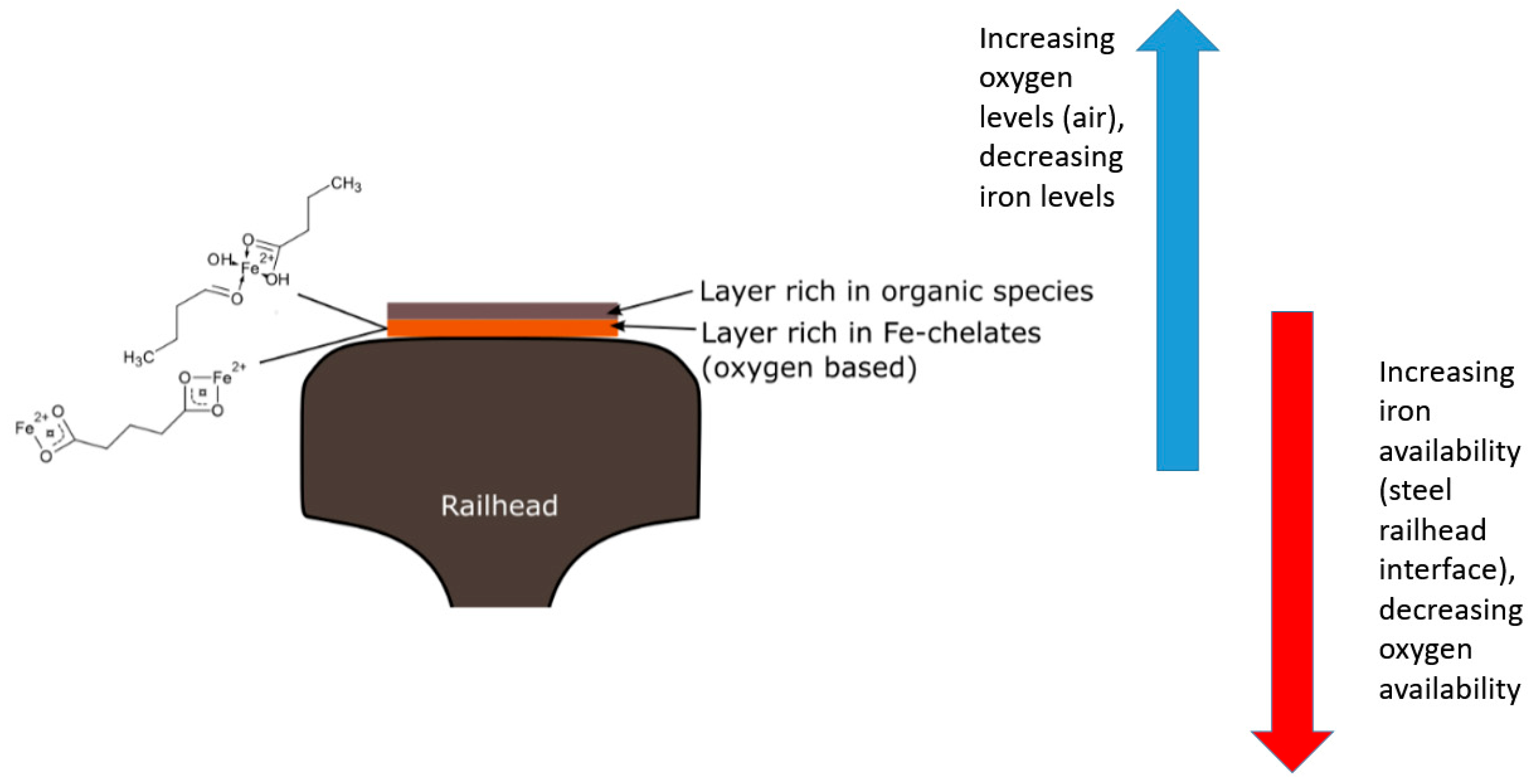
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- RSSB. ADHERE Strategic Case; Rail Safety and Standards Board: London, UK, 2019. [Google Scholar]
- Ishizaka, K.; Lewis, S.R.; Hammond, D. Lewis R. Chemistry of black leaf films synthesised using rail steels and their influence on the low friction. R. Soc. Chem. 2018, 8, 32506–32521. [Google Scholar]
- Watson, M.; White, B.; Lanigan, J.; Slatter, T.; Lewis, R. The composition and friction-reducing properties of leaf layers. Proc. R. Soc. A Math. Phys. Eng. Sci. 2020, 476, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Olofsson, U.; Nilsson, R. A field test study of leaf contamination on railhead surfaces. Proc. Inst. Mech. Eng. Part F J. Rail Rapid Transit 2012, 228, 71–84. [Google Scholar] [CrossRef]
- Gallagher, P.K.; Sinclair, W.R.; Fastnacht, R.A.; Luongo, J.P. Thermal characterization of iron oxide films. Thermochim. Acta 1974, 8, 141–148. [Google Scholar] [CrossRef]
- Xia, S.; Cai, N.; Lu, W.; Zhou, H.; Xiao, H.; Chen, X.; Chen, Y.; Yang, H.; Wang, X.; Wang, S.; et al. Reaction kinetics, mechanism, and product analysis of the iron catalytic graphitization of cellulose. J. Clean. Prod. 2021, 329, 129735. [Google Scholar] [CrossRef]
- Vrancken, E.; Campagne, J.M. Organic Transformations Promoted by Lewis Acid Iron Catalysts. In Patai’s Chemistry of Functional Groups; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- Emery, J.A.; Schroeder, H.A. Iron-catalyzed oxidation of wood carbohydrates. Wood Sci. Technol. 1974, 8, 123–137. [Google Scholar] [CrossRef]
- Thompson, E.; Danks, A.E.; Bourgeoisa, L.; Schnepp, Z. Iron-catalyzed graphitization of biomass. Green Chem. 2015, 17, 551–556. [Google Scholar] [CrossRef]
- Butcher, T. Understanding and Modelling Low Adhesion Risk in the Wheel-Rail Interface. Ph.D. Thesis, The University of Sheffield, Sheffield, UK, 2023. [Google Scholar]
- Edgley, J. Managing Low Adhesion; Adhesion Working Group: London, UK, 2018. [Google Scholar]
- Lewis, S.R.; Lewis, R.; Cotter, J.; Lu, X.; Eadie, D.T. A new method for the assessment of traction enhancers and the generation of organic layers in a twin-disc machine. Wear 2016, 366–367, 258–267. [Google Scholar] [CrossRef]
- Buckley-Johnstone, L.; Harmon, M.; Lewis, R.; Hardwick, C.; Stock, R. A comparison of friction modifier performance using two laboratory test scales. Proc. Inst. Mech. Eng. Part F J. Rail Rapid Transit 2019, 233, 201–210. [Google Scholar] [CrossRef]
- White, B.; Lewis, R. Developing Validated Benchmark Tests for Assessing Friction Management Products; RSSB Report (COF-G19–01); The University of Sheffield: Sheffield, UK, 2018. [Google Scholar]
- Magel, E.E. A Survey of Wheel/Rail Friction. U.S. Department of Transportation; Federal Railroad Administration Office: Washington, DC, USA, 2017. [Google Scholar]
- Meierhofer, A.; Hardwick, C.; Lewis, R.; Six, K.; Dietmaier, P. Third body layer-experimental results and a model describing its influence on the traction coefficient. Wear 2014, 314, 148–154. [Google Scholar] [CrossRef]
- Kamalak, A.; Hassan, K.G.; Ameen, S.M.; Zebari, H.M.; Hasan, A.H.; Aslan, F. Determination of Chemical Composition, Potential Nutritive Value and Methane Emission of Oak Tree (Quercus coccifera) Leaves and Nuts. Korean J. Anesth. 2015, 70, 22–26. [Google Scholar]
- Somashekara, N.; Saiyed, Z.M.; Ramchand, C.N. Biochemistry of Railhead Leaf Film Contamination; Railways Safety and Standards Board: London, UK, 2006; pp. 1–91. [Google Scholar]
- D’Antonio, M.C.; Wladimirsky, A.; Palacios, D.; Coggiolaa, L.; González-Baró, A.C.; Baran, E.J.; Mercader, R.C. Spectroscopic Investigations of Iron(II) and Iron(III) Oxalates. J. Braz. Chem. Soc. 2009, 20, 445–450. [Google Scholar] [CrossRef]
- Jaffe, J.; White, B.; Lanigan, J.; Lewis, R. A novel methodology for developing ultra-low adhesion leaf layers on a full-scale wheel/rail rig. Proc. Inst. Mech. Eng. Part F J. Rail Rapid Transit 2024, 238, 736–741. [Google Scholar] [CrossRef]
- Lewis, R.; Trummer, G.; Six, K.; Stow, J.; Alturbeh, H.; Bryce, B.; Shackleton, P.; Johnstone, L.B. Leaves on the Line: Characterising Leaf based Low Adhesion on Railway Rails. Tribol. Int. 2023, 185, 108529. [Google Scholar] [CrossRef]
- Lanigan, J.L.; Faas, L.; Butcher, T.; Skipper, W.A.; Silva, M.P.; Lewis, R.; Gomez, L.D. Pressure induced transformation of biomass to a highly durable, low friction film on steel. Proc. R. Soc. A Math. Phys. Eng. Sci. 2024, 480, 20230450. [Google Scholar] [CrossRef]
- Pritchard, C.; Tanvir, M. British Rail Technical Note- Further Observations of Leaves; British Rail Archives: Derby, UK, 1973. [Google Scholar]
- Goldblatt, I.L. Model for lubrication behavior of polynuclear aromatics. Ind. Eng. Chem. Prod. Res. Dev. 1971, 10, 270–278. [Google Scholar] [CrossRef]
- Buckley, D.H. Friction Differences Between Aliphatic and Aromatic Structures in Lubrication of Titanium; NASA Technical Note; NASA: Washington, DC, USA, 1975. [Google Scholar]
- Kim, H.J.; Kim, D.E. Water lubrication of stainless steel using reduced graphene oxide coating. Sci. Rep. 2015, 5, 17034. [Google Scholar] [CrossRef]
- Bowden, F.P.; Young, J.E. Friction of diamond, graphite, and carbon and the influence of surface films. Proceedings of the Royal Society of London. Ser. A Math. Phys. Sci. 1951, 208, 444–455. [Google Scholar]
- De Barros Bouchet, M.I.; Matta, C.; Martin, J.M.; Joly-Pottuz, L. Superlubricity of steel surfaces in presence of polyhydric alcohols. In Proceedings of the STLE/ASME International Joint Tribology Conference, Miami, FL, USA, 20–22 October 2008; pp. 181–183. [Google Scholar]
- Tomala, A.; Karpinska, A.; Werner, W.S.M.; Olver, A.; Störi, H. Tribological properties of additives for water-based lubricants. Wear 2010, 269, 804–810. [Google Scholar] [CrossRef]
- Iglesias, J.; García de Saldaña, E.; Jaén, J.A. On the tannic acid interaction with metallic iron. Hyperfine Interact. 2001, 134, 109–114. [Google Scholar] [CrossRef]
- Suzumura, J.; Sone, Y.; Ishizaki, A.; Yamashita, D.; Nakajima, Y.; Ishida, M. In situ X-ray analytical study on the alteration process of iron oxide layers at the railhead surface while under railway traffic. Wear 2011, 271, 47–53. [Google Scholar] [CrossRef]
- Gust, J.; Suwalski, J. Use of Mossbauer spectroscopy to study reaction products of polyphenols and iron compounds. Corrosion 1994, 50, 355–365. [Google Scholar] [CrossRef]
- Delplancke-Ogletree, M.P.; Monteiro, O.R. Wear behavior of diamond-like carbon/metal carbide multilayers. Surf. Coat. Technol. 1998, 108–109, 484–488. [Google Scholar] [CrossRef]
- Gao, X.; Yan, R.; Lv, Y.; Ma, H. In situ pretreatment and self-healing smart anti-corrosion coating prepared through eco-friendly water-base epoxy resin combined with non-toxic chelating agents decorated biomass porous carbon. J. Clean. Prod. 2020, 266, 121920. [Google Scholar] [CrossRef]
- Loehlé, S.; Matta, C.; Minfray, C.; Le Mogne, T.; Iovine, R.; Obara, Y.; Miyamoto, A.; Martin, J.M. Mixed lubrication of steel by C18 fatty acids revisited. Part I: Toward the formation of carboxylate. Tribol. Int. 2015, 82, 218–227. [Google Scholar] [CrossRef]
- White, B.; Lewis, R. Low Adhesion Field Test Protocol, COF-UOS22–04; Rail Safety and Standards Board: London, UK, 2025. [Google Scholar]
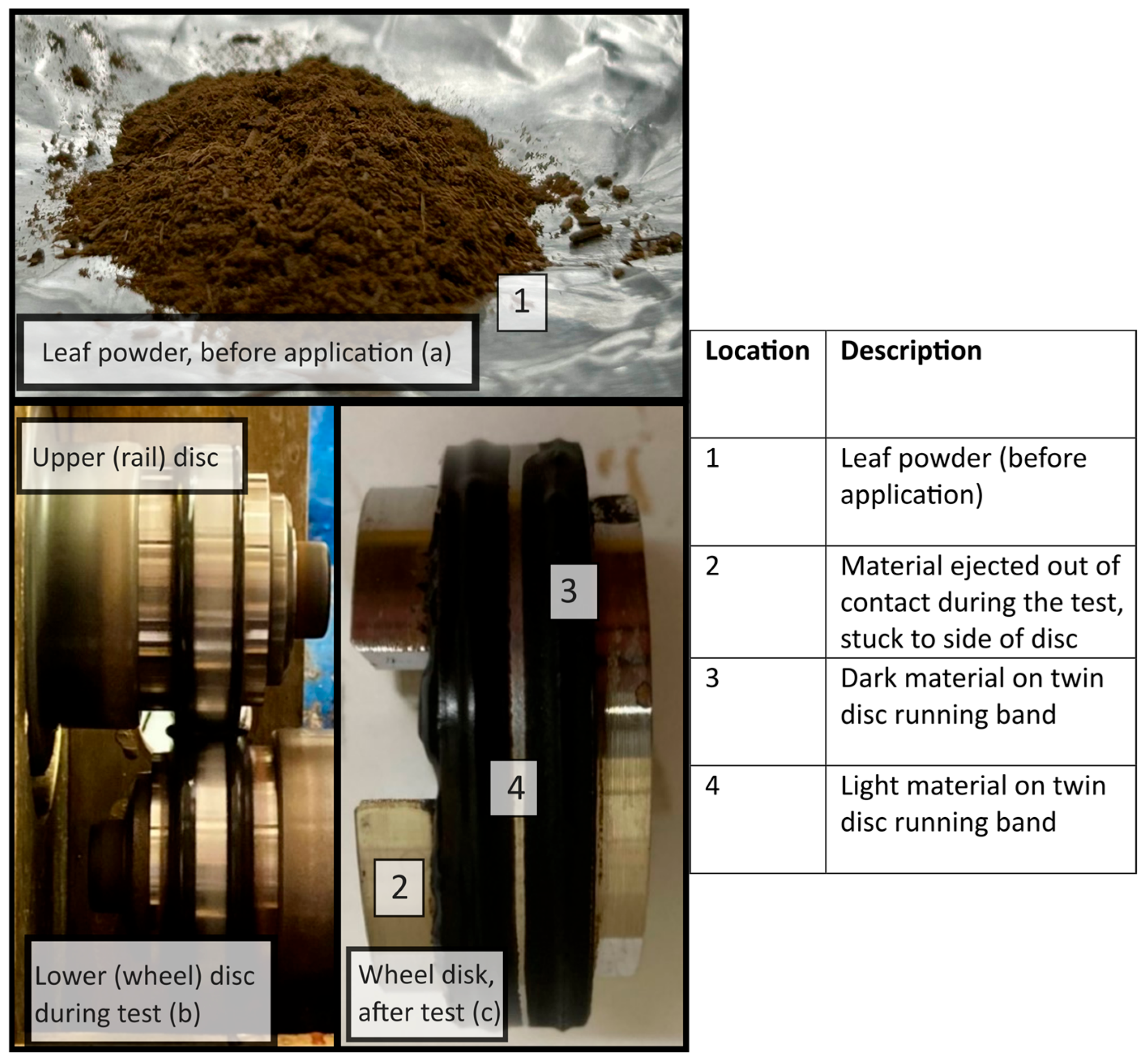

| Technique | Advantages | Disadvantages |
|---|---|---|
| Twin-disc (laboratory) | Easy to remove sample for analysis Realistic contact pressure and real wheel and rail material Standardised leaf layer formation method has been published [12] | Material ejected from contact/curved surface throughout test Typical autumn environmental conditions are difficult to replicate |
| Full-scale wheel/rail test rig (laboratory) | Real rail and wheel geometry. Length of rail used allows for ‘thermal sink’ similar to the mainline Controllable contact conditions “Pocket” rail can be removed for analysis | Typical autumn environmental conditions are difficult to replicate Cannot remove wheel sample for analysis |
| Field, using a locomotive to roll over the layer (friction measured using an OnTrak Tribometer) | Can measure field leaf layers under varying and representative environmental conditions | Difficult to remove sample for analysis Often expensive and time consuming to conduct field trials |
| Oak Leaves | Assignment and Comments | Attributed to… |
|---|---|---|
| Wavenumber/ cm−1 | ||
| 1635 | Aromatic skeletal C=O stretch | Lignin |
| 1514 | C=C stretching of aromatic skeletal vibration | Lignin |
| 1448 | C=C and C-H bond. O-H in plane deformation | Lignin and hemicellulose (C-C) |
| 1373 | C-H deformation vibration | Cellulose |
| 1318 | C-H deformation vibration | Cellulose |
| 1235 | Indicates the syringyl ring of synaptyl alcohol (also present in the lignin structure), also C-O stretch) | Lignin and xylan (a hemi-cellulose). |
| 1157 | C-O-C symmetric stretching | Cellulose and Hemicellulose |
| 1049 | C-O stretch | Cellulose and hemicellulose. |
| 836 | Aromatic C-H, out-of-plane deformation | Lignin and tannins. |
| Material from Flat Side 1 | Material from Flat Side 2 | Assignment and Comments | Attributed to… | ||
|---|---|---|---|---|---|
| Peak No. | Wavenumber cm−1 | Peak No. | Wavenumber cm−1 | ||
| 1–20 | 3950–3500 | 1–9 | 3950–3500 | This type of “noise” indicates that free water is present in the sample which was analysed as received. Side 1 appears to be wetter than side 2. | Water |
| 21 | 3359 | 10 | 3406 | Broad group indicating the presence of H-bonded OH groups. The breadth of the peak suggests a multiplicity of chemical environments. | Lignin, cellulose, polysaccharides, and tannins |
| 22 | 2926 | 11 | 2926 | CH3 group (typically terminates an organic molecule or a branch of one). | Lignin |
| 23 | 2853 | Present | CH2 group (typically occurs in the backbone of an organic molecule). | ||
| 24–26 | 2375–2250 | 12–14 | 2375–2250 | Carbon dioxide | |
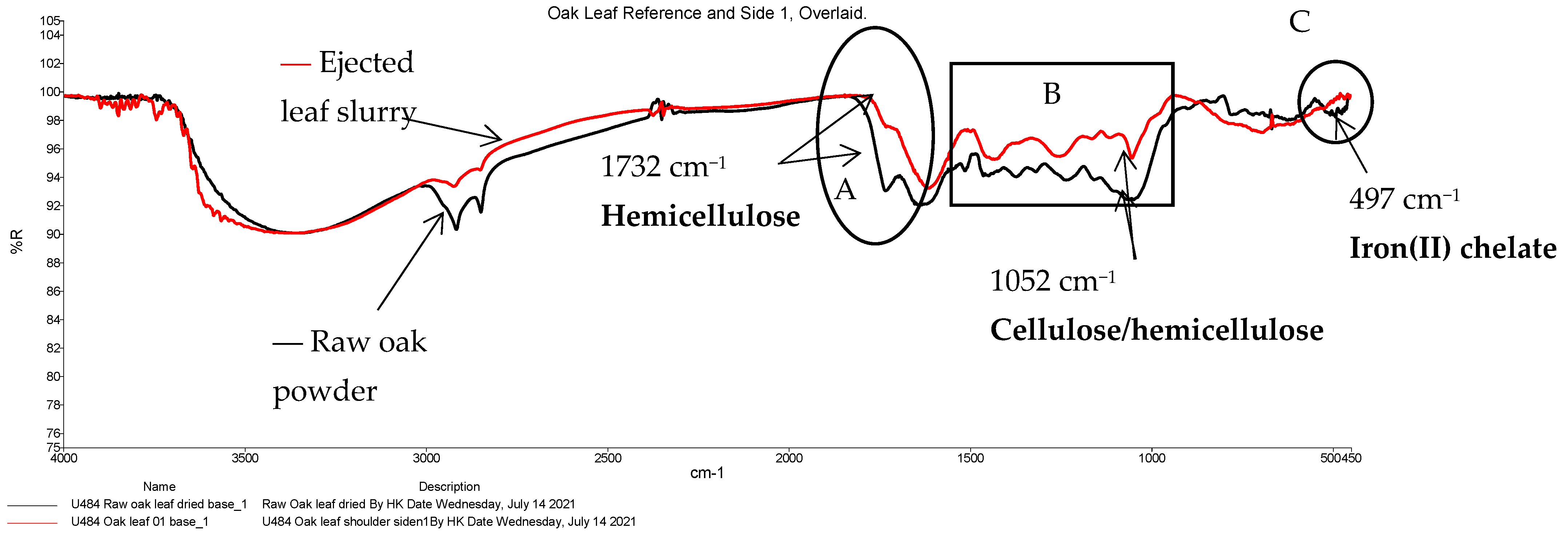
| 28 | 1732 | Present, but indistinct | C=O stretch of acetyl and carbonyl groups | Hemicellulose | |
| 29 | 1618 | 15 | 1621 | Aromatic skeletal and C=O stretch vibration | Lignin |
| 30 | 1432 | 16 | 1428 | C=C and C-H bond. O-H in plane deformation | Lignin and Hemicellulose |
| 31 | 1252 | 17 | 1263 | G-ring plus C=O stretch | G-Lignin |
| 32 | 1164 | 18 | 1160 | C-O-C symmetric stretching | Cellulose and Hemicellulose |
| 33 | 1115 | 19 | 1110 | Ring asymmetric valence vibration | Polysaccharides |
| 34 | 1053 | 20 | 1050 | C-O stretch | Cellulose and Hemicellulose |
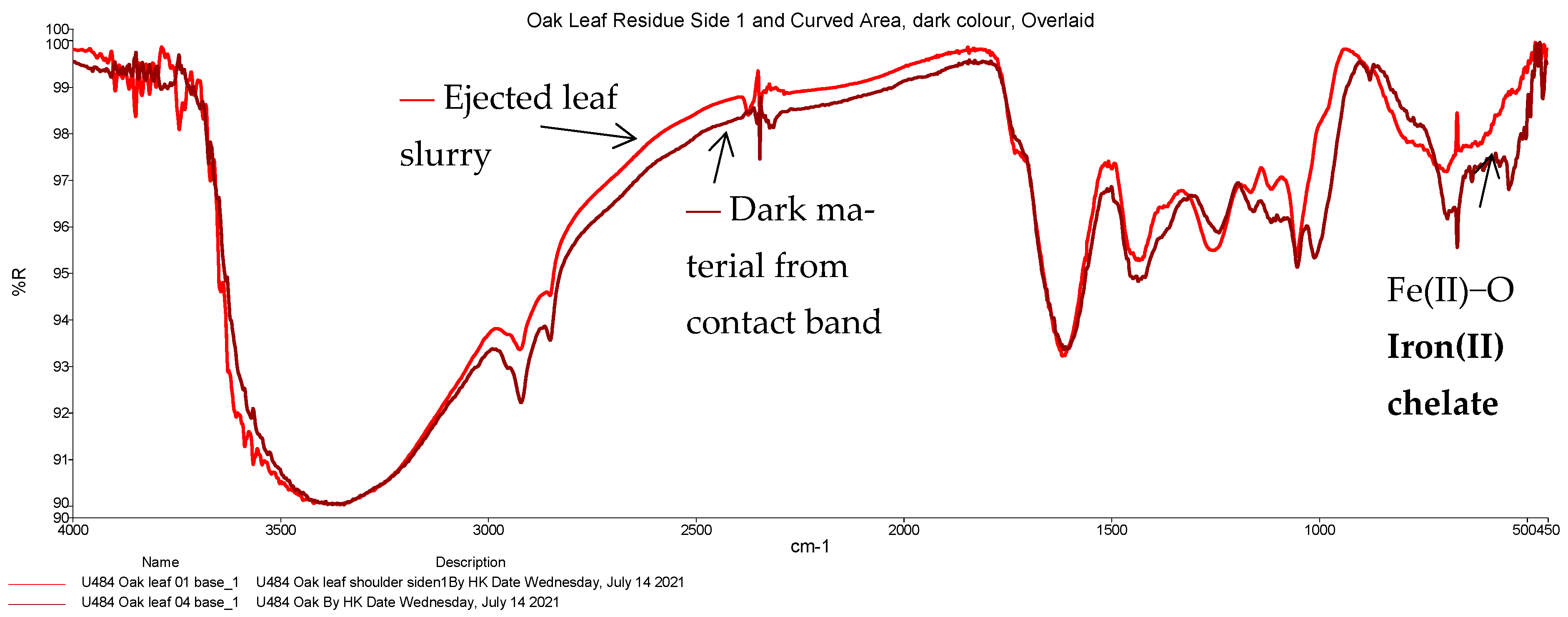
| 39 | 497 | 27 | 502 | Likely Fe(II)-O bond in a chelate | Iron(II) chelate |
| Peak Number | Wavenumber/ cm−1 | Assignment and Comments | Attributed to… |
|---|---|---|---|
| 1–19 | 3960–3550 | Presence of free water | Water |
| 20 | 3350 | Broad group indicating the presence of H-bonded OH groups. | Lignin, cellulose and poly saccharides, tannins |
| 21 | 2923 | CH3 group (typically terminates an organic molecule or a branch of one). | Many organic compounds, Lignin |
| 22 | 2852 | CH2 group (typically occurs in the backbone of an organic molecule). | |
| 23–25 | 2350–2320 | Carbon dioxide | |
| 26 | 1614 | Aromatic skeletal C=O stretch | Lignin |
| 27 | 1435 | C=C, C-H bonds/OH deformation. (C-C), C=C (aryl) in lignin and tannins | Lignin, hemicellulose and tannins |
| 28 | 1240 | Indicates the syringyl ring of synaptyl alcohol (present in the lignin structure), also C-O stretch) | Lignin and xylan (a hemi-cellulose). |
| 29 | 1157 | C-O-C symmetric stretching | Cellulose and Hemicellulose |
| 30 | 1114 | Not assigned | |
| 31 | 1091 | Ring asymmetric valence vibration | Polysaccharides |
| 33 | 1010 | C-O stretch | Cellulose and Hemicellulose |
| 34 | 876 | Aromatic C-H out-of-plane deformation | Cellulose, hemicellulose, and pectin |
| 43 | 508 | Ring deformation | |
| 44 | 500 | Likely Fe(II)-O bond in a chelate | Iron(II) chelate |
| Peak Number | Wavenumber/cm−1 | Assignment and Comments | Attributed to… |
|---|---|---|---|
| 1 | 3338 | Broad group indicating the presence of H-bonded OH groups. Nearly absent in the oak bark sample. | |
| 2 | 2920 | CH3 group (typically terminates an organic molecule or a branch of one). | Many organic compounds, lignin |
| 3 | 2857 | CH2 group (typically occurs in the backbone of an organic molecule). | |
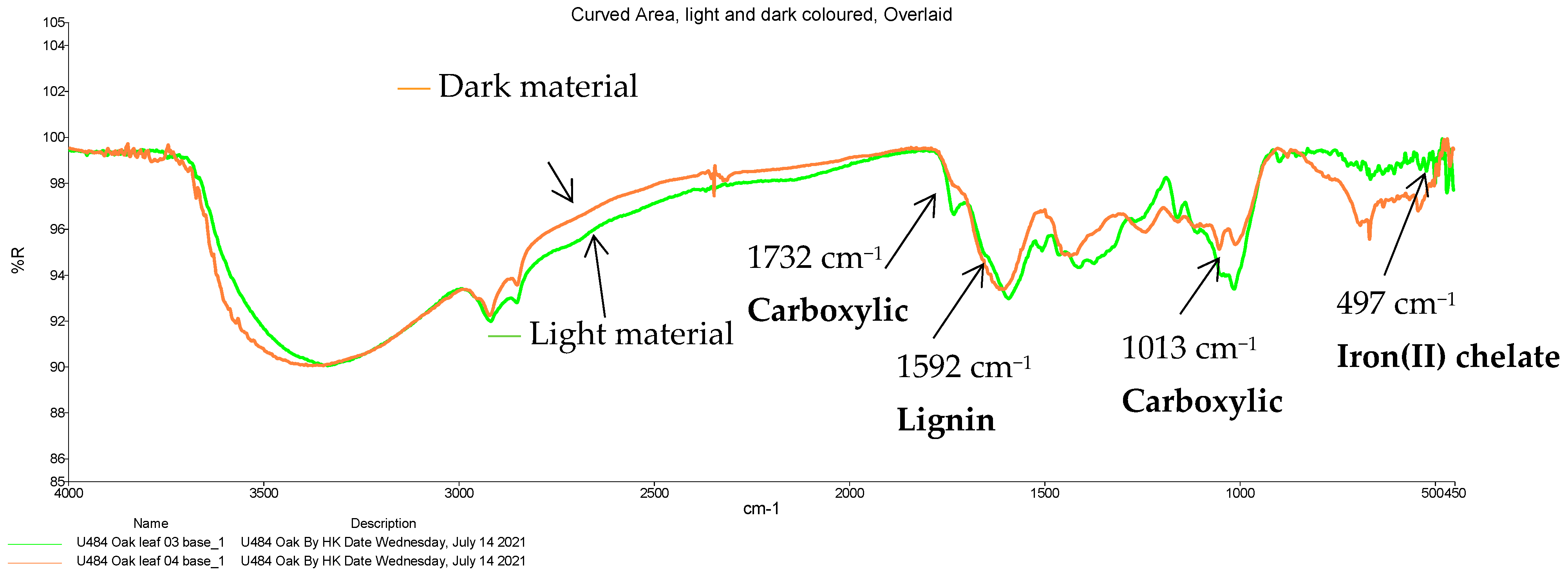
| 4 | 1732 | C=O stretch from acetyl or carbonyl group | Carboxylic acid |
| 5 | 1592 | Aromatic skeletal C=O stretch | Lignin |
| 8 | 1158 | C-O-C symmetric stretching | Cellulose and hemicellulose |
| Not assigned | |||
| 9 | 1013 | C-O stretch | Carboxylic acid |
| 10 | 898 | Aromatic C-H, out-of-plane deformation | Lignin and tannins |
| 520 | Ring deformation | ||
| 497 | Possible Fe(II)-O bond in a chelate | Iron(II) chelate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
White, B.; Lanigan, J.; Lewis, R. A Spectroscopic DRIFT-FTIR Study on the Friction-Reducing Properties and Bonding of Railway Leaf Layers. Lubricants 2025, 13, 329. https://doi.org/10.3390/lubricants13080329
White B, Lanigan J, Lewis R. A Spectroscopic DRIFT-FTIR Study on the Friction-Reducing Properties and Bonding of Railway Leaf Layers. Lubricants. 2025; 13(8):329. https://doi.org/10.3390/lubricants13080329
Chicago/Turabian StyleWhite, Ben, Joseph Lanigan, and Roger Lewis. 2025. "A Spectroscopic DRIFT-FTIR Study on the Friction-Reducing Properties and Bonding of Railway Leaf Layers" Lubricants 13, no. 8: 329. https://doi.org/10.3390/lubricants13080329
APA StyleWhite, B., Lanigan, J., & Lewis, R. (2025). A Spectroscopic DRIFT-FTIR Study on the Friction-Reducing Properties and Bonding of Railway Leaf Layers. Lubricants, 13(8), 329. https://doi.org/10.3390/lubricants13080329






