The Impact of Curcumin, Gingerol, Piperine, and Proanthocyanidin on the Oxidative Stability of Sunflower and Soybean Oils for Developing Bio-Lubricants
Abstract
1. Introduction
- -
- Carotenoids: This group includes valuable subclasses such as carotenes and xanthophylls, which are known for their strong antioxidant properties that can significantly improve oil stability.
- -
- Phenolics: This category encompasses a range of subclasses, including phenolic acids, stilbenes, flavonoids, lignans, and tannins. These compounds effectively combat oxidative stress and enhance the oil’s overall quality.
- -
- Alkaloids: This group includes indoles, isoquinolines, tropanes, and pyrrolidines. Alkaloids contribute powerful antioxidant effects, playing a crucial role in oil preservation.
- -
- Glucosinolates: This group features subclasses like indole, aliphatic, and aromatic glucosinolates, which are recognized for their strong antioxidant capabilities and are crucial in oil preservation. These bio-antioxidants enhance the quality and stability of vegetable oils.
2. Materials and Methods
2.1. Lubricants
2.2. Analysis of Fatty Acid Composition of Vegetable Oils
2.3. Extraction of Bio-Additive Compounds
2.4. Determination of Oxidative Stability Using RapidOxy 100 Apparatus
2.5. FTIR Investigations
3. Results and Discussions
3.1. Characterization of Vegetable Oils
3.2. The Unsaturation Degree (UD)
3.3. Bio-Additive Compounds
3.4. Oxidative Stability Investigations
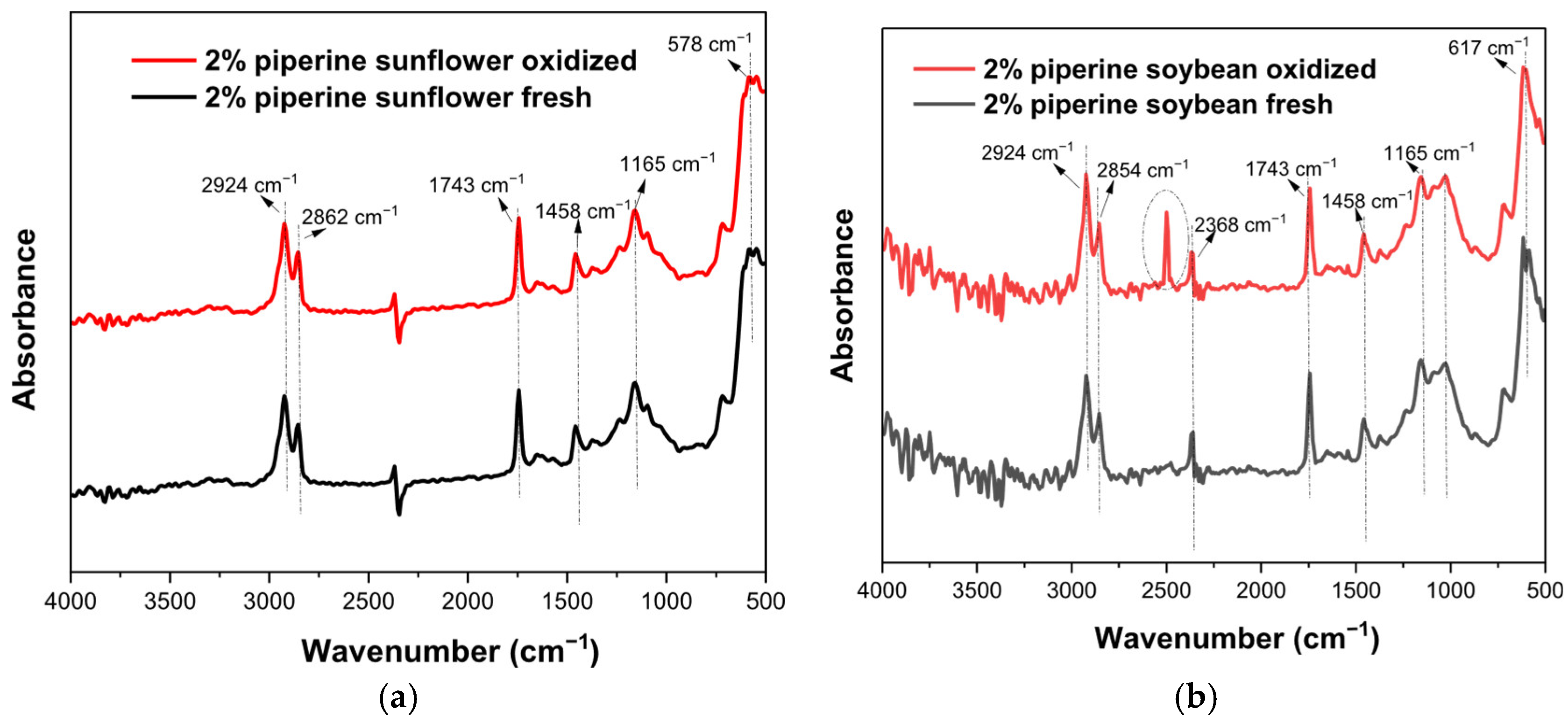
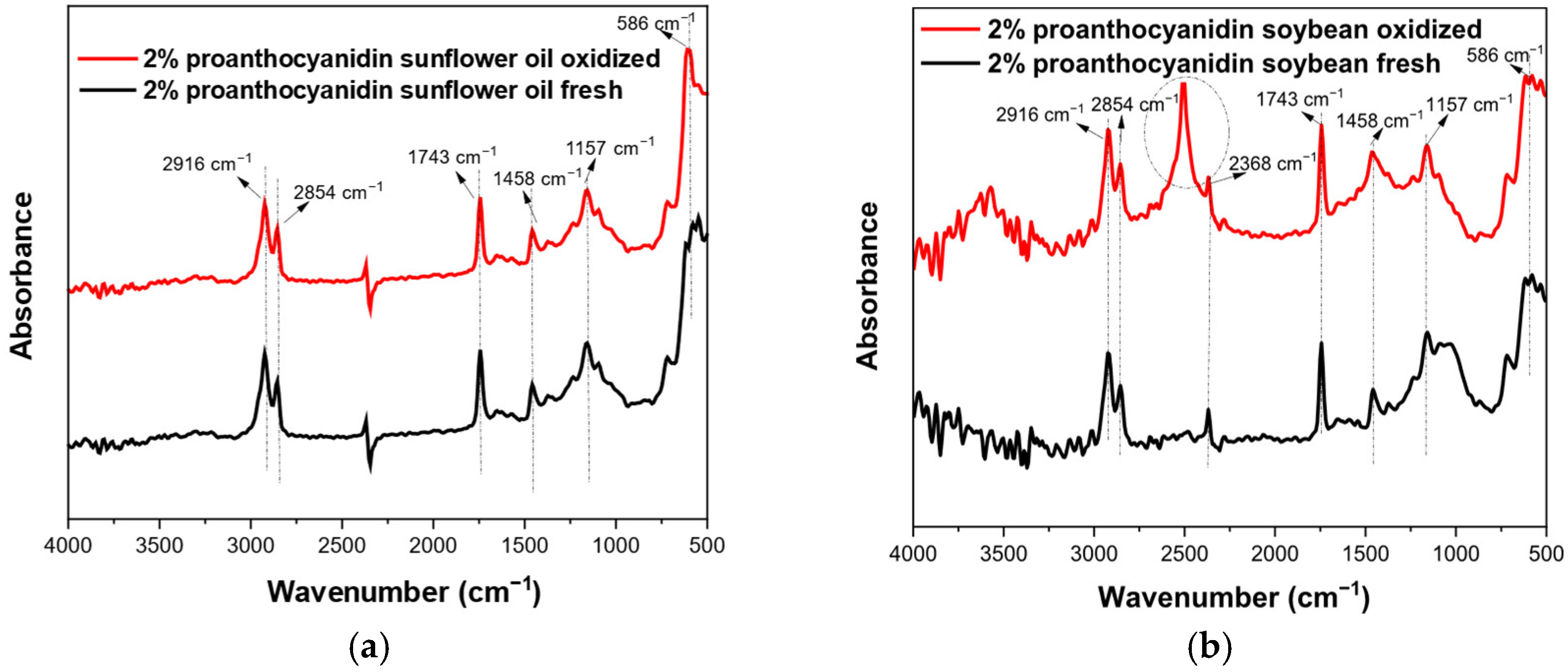
3.5. FTIR Results
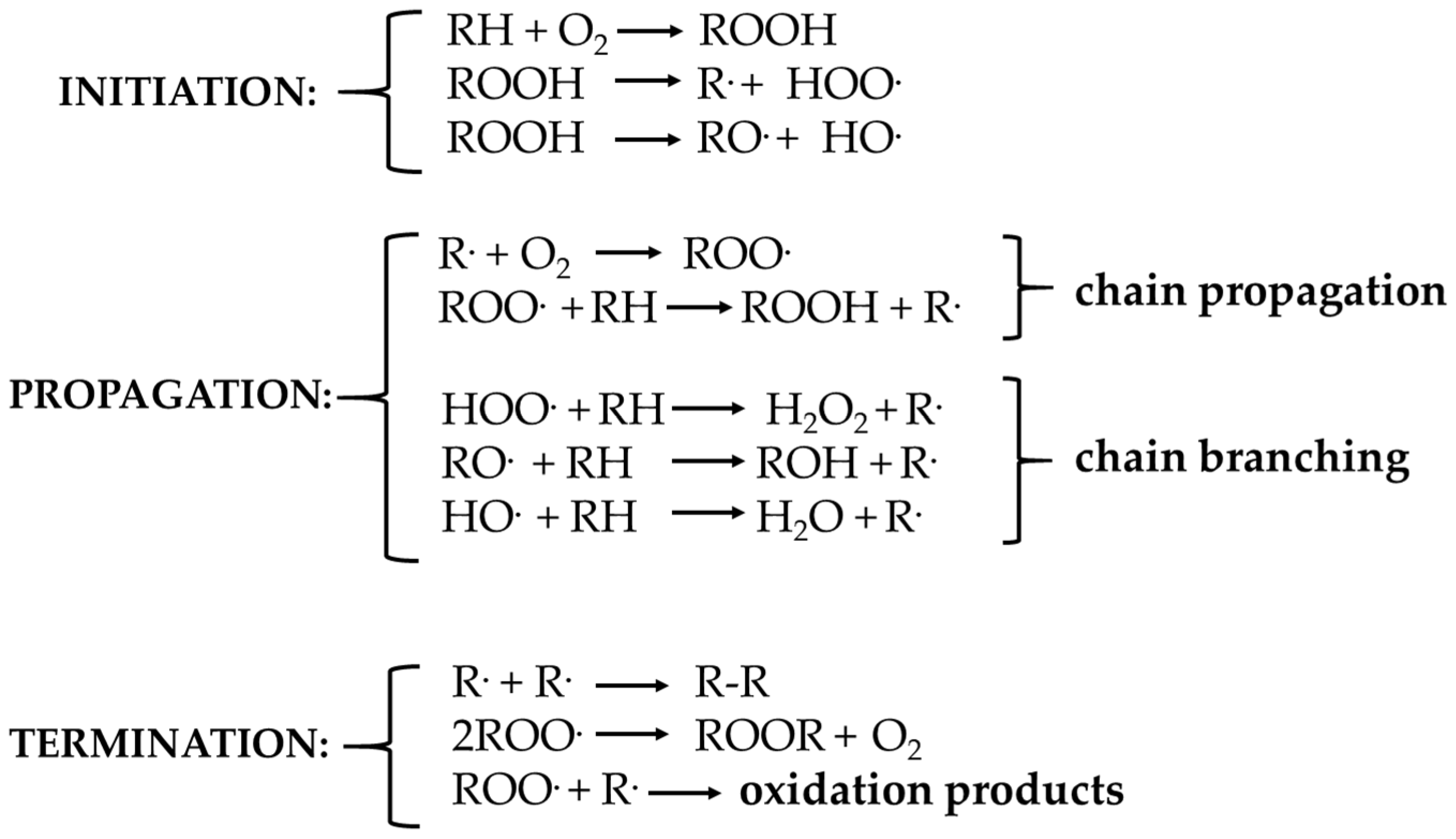
3.6. Oxidation Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sergio Nogales-Delgado, S.; Encinar, J.M.; González, J.F. A Review on Biolubricants Based on Vegetable Oils through Transesterification and the Role of Catalysts: Current Status and Future Trends. Catalysts 2023, 13, 1299. [Google Scholar] [CrossRef]
- Chan, C.H.; Tang, S.W.; Mohd, N.K.; Lim, W.H.; Yeong, S.K.; Idris, Z. Tribological behavior of biolubricant base stocks and additives. Renew. Sustain. Energy Rev. 2018, 93, 145–157. [Google Scholar] [CrossRef]
- Kurre, S.K.; Yadav, J. A review on bio-based feedstock, synthesis, and chemical modification to enhance tribological properties of biolubricants. Ind. Crops Prod. 2023, 193, 116122. [Google Scholar] [CrossRef]
- Cecilia, J.A.; Plata, D.B.; Alves Saboya, R.M.; Tavares de Luna, F.M.; Cavalcante, C.L., Jr.; Rodríguez-Castellón, E. An Overview of the Biolubricant Production Process: Challenges and Future Perspectives. Processes 2020, 8, 257. [Google Scholar] [CrossRef]
- Malik, M.A.I.; Kalam, M.A.; Mujtaba, M.A.; Almomani, F. A review of recent advances in the synthesis of environmentally friendly, sustainable, and nontoxic bio-lubricants: Recommendations for the future implementations. Environ. Technol. Innov. 2023, 32, 103366. [Google Scholar] [CrossRef]
- McNutt, J.; He, Q.S. Development of biolubricants from vegetable oils via chemical modification. J. Ind. Eng. Chem. 2016, 36, 1–12. [Google Scholar] [CrossRef]
- Gemsprim, M.S.; Babu, N.; Udhayakumar, S. Tribological evaluation of vegetable oil-based lubricant blends. Mater. Today: Proc. 2021, 37, 2660–2665. [Google Scholar] [CrossRef]
- Singh, Y.; Farooq, A.; Raza, A.; Mahmood, M.A.; Jain, S. Sustainability of a non-edible vegetable oil-based bio-lubricant for automotive applications: A review. Process Saf. Environ. Prot. 2017, 111, 701–713. [Google Scholar] [CrossRef]
- Heikal, E.K.; Elmelawy, M.S.; Khalil, S.A.; Elbasuny, N.M. Manufacturing of environment friendly biolubricants from vegetable oils. Egypt. J. Pet. 2017, 26, 53–59. [Google Scholar] [CrossRef]
- Syahir, A.Z.; Zulkifli, N.W.M.; Masjuki, H.H.; Kalam, M.A.; Alabdulkarem, A.; Gulzar, M.; Khuong, L.S.; Harith, M.H. A review on bio-based lubricants and their applications. J. Clean. Prod. 2017, 168, 997–1016. [Google Scholar] [CrossRef]
- Almasi, S.; Ghobadian, B.; Najafi, G.; Soufi, M.D. A review on bio-lubricant production from non-edible oil-bearing biomass resources in Iran: Recent progress and perspectives. J. Clean. Prod. 2021, 290, 125830. [Google Scholar] [CrossRef]
- Quinchia, L.A.; Delgado, M.A.; Reddyhoff, T.; Gallegos, C.; Spikes, H.A. Tribological studies of potential vegetable oil-based lubricants containing environmentally friendly viscosity modifiers. Tribol. Int. 2014, 69, 110–117. [Google Scholar] [CrossRef]
- Owuna, F.J.; Dabai, M.U.; Sokoto, M.A.; Dangoggo, S.M.; Bagudo, B.U.; Birnin-Yauri, U.A.; Hassan, L.G.; Sada, I.; Abubakar, A.L.; Jibrin, M.S. Chemical modification of vegetable oils for the production of biolubricants using trimethylolpropane: A review. Egypt. J. Pet. 2020, 29, 75–82. [Google Scholar] [CrossRef]
- Attia, N.K.; El-Mekkawi, S.A.; Elardy, O.A.; Abdelkader, E.A. Chemical and rheological assessment of produced biolubricants from different vegetable oils. Fuel 2020, 271, 117578. [Google Scholar] [CrossRef]
- Zainal, N.A.; Zulkifli, N.W.M.; Gulzar, M.; Masjuki, H.H. A review on the chemistry, production, and technological potential of bio-based lubricants. Renew. Sustain. Energy Rev. 2018, 82, 80–102. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, G.; Deng, Z.; Zhang, B.; Li, H. Effects of Endogenous Anti-Oxidative Components from Different Vegetable Oils on Their Oxidative Stability. Foods 2023, 12, 2273. [Google Scholar] [CrossRef]
- Fadda, A.; Sanna, D.; Sakar, E.H.; Gharby, S.; Mulas, M.; Medda, S.; Yesilcubuk, N.S.; Karaca, A.C.; Gozukirmizi, C.K.; Lucarini, M.; et al. Innovative and Sustainable Technologies to Enhance the Oxidative Stability of Vegetable Oils. Sustainability 2022, 14, 849. [Google Scholar] [CrossRef]
- Deng, W.; Chen, Y.; Sun, X.; Wang, L. AODB: A comprehensive database for antioxidants including small molecules, peptides and proteins. Food Chem. 2023, 418, 135992. [Google Scholar] [CrossRef]
- Nassef, B.G.; Pape, F.; Poll, G. Enhancing the Performance of Rapeseed Oil Lubricant for Machinery Component Applications through Hybrid Blends of Nanoadditives. Lubricants 2023, 11, 479. [Google Scholar] [CrossRef]
- Aroor, G.; Khan, M.A.; Shetty, A.R.; Rai, R.; Ganesha, H.; Navada, M.K. From chemistry to performance: How nano additives are transforming bio-lubricants for enhanced tribological applications. J. Mol. Liq. 2025, 425, 127242. [Google Scholar] [CrossRef]
- Martín-Torres, S.; González-Casado, A.; Medina-García, M.; Medina-Vázquez, M.S.; Cuadros-Rodríguez, L. A Comparison of the Stability of Refined Edible Vegetable Oils under Frying Conditions: Multivariate Fingerprinting Approach. Foods 2023, 12, 604. [Google Scholar] [CrossRef]
- Symoniuk, E.; Wroniak, M.; Napiórkowska, K.; Brzezinska, R.; Ratusz, K. Oxidative Stability and Antioxidant Activity of Selected Cold-Pressed Oils and Oils Mixtures. Foods 2022, 11, 1597. [Google Scholar] [CrossRef]
- Sanna, D.; Fadda, A. Oxidative Stability of Sunflower Oil: Effect of Blending with an Oil Extracted from Myrtle Liqueur By-Product. Antioxidants 2025, 14, 300. [Google Scholar] [CrossRef]
- Panchal, T.M.; Patel, A.; Chauhan, D.D.; Thomas, M.; Patel, J.V. A methodological review on bio-lubricants from vegetable oil based resources. Renew. Sustain. Energy Rev. 2017, 70, 65–70. [Google Scholar] [CrossRef]
- Hamnas, A.; Unnikrishnan, G. Bio-lubricants from vegetable oils: Characterization, modifications, applications and challenges—Review. Renew. Sustain. Energy Rev. 2023, 182, 113413. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Haq, I.U.; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Suleria, H.A.R. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Moreno, K.J.; Hernández-Sierra, M.T.; Báez, J.E.; Rodríguez-deLeón, E.; Aguilera-Camacho, L.D.; García-Miranda, J.S. On the Tribological and Oxidation Study of Xanthophylls as Natural Additives in Castor Oil for Green Lubrication. Materials 2021, 14, 5431. [Google Scholar] [CrossRef]
- Hernández-Sierra, M.T.; Báez, J.E.; Aguilera-Camacho, L.D.; García-Miranda, J.S.; Moreno, K.J. Friction and wear improvement by using Curcumin as a natural additive in green lubricants. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2022, 237, 578–588. [Google Scholar] [CrossRef]
- Jiang, T.; Ghosh, R.; Charcosset, C. Extraction, purification and applications of curcumin from plant materials—A comprehensive review. Trends Food Sci. Technol. 2021, 112, 419–430. [Google Scholar] [CrossRef]
- Kanda, H.; Zhu, L.; Zhu, W.; Wang, T. Ethanol-free extraction of curcumin and antioxidant activity of components from wet Curcuma longa L. by liquefied dimethyl ether. Arab. J. Chem. 2023, 16, 104585. [Google Scholar] [CrossRef]
- Zielińska, A.; Alves, H.; Marques, V.; Durazzo, A.; Lucarini, M.; Alves, T.F.; Morsink, M.; Willemen, N.; Eder, P.; Chaud, M.V.; et al. Properties, Extraction Methods, and Delivery Systems for Curcumin as a Natural Source of Beneficial Health Effects. Medicina 2020, 56, 336. [Google Scholar] [CrossRef]
- Bagal, M.V.; Deshmukh, A.; Thakur, N.; Valiyare, A. Curcumin Extraction using Ultra sonication: A Review. J. Emerg. Technol. Innov. Res. 2020, 7, 1110–1114. [Google Scholar]
- ASTM D1298-12b; Standard Test Method for Density, Relative Density, or API Gravity of Crude Petroleum and Liquid Petroleum Products by Hydrometer Method. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D445-23; Standard Test Method for Standard Test Method for Kinematic Viscosity of Transparent and Opaque Liquids (and Calculation of Dynamic Viscosity). ASTM International: West Conshohocken, PA, USA, 2023.
- ASTM D2270-24; Standard Practice for Calculating Viscosity Index from Kinematic Viscosity at 40°C and 100°C. ASTM International: West Conshohocken, PA, USA, 2024.
- ASTM D92; Standard Test Method for Flash and Fire Points by Cleveland Open Cup Tester. ASTM International: West Conshohocken, PA, USA, 2018.
- ASTM D97-17; Standard Test Method Standard Test Method for Pour Point of Petroleum Products. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D130; Standard Test Method for Corrosiveness to Copper from Petroleum Products by Copper Strip Test. ASTM International: West Conshohocken, PA, USA, 1922.
- ASTM D974-22; Standard Test Method for for Acid and Base Number by Color-Indicator Titration. ASTM International: West Conshohocken, PA, USA, 2022.
- ASTM D2272-22; Standard Test Method for Oxidation Stability of Steam Turbine Oils by Rotating Pressure Vessel. ASTM International: West Conshohocken, PA, USA, 2022.
- ASTM D4172-94; Standard Test Method for Wear Preventive Characteristics of Lubricating Fluid (Four-Ball Method). ASTM International: West Conshohocken, PA, USA, 2014.
- ASTM D6584; Standard Test Method for Determination of Total Monoglycerides, Total Diglycerides, Total Triglycerides, and Free and Total Glycerin in B-100 Biodiesel Methyl Esters by Gas Chromatography. ASTM International: West Conshohocken, PA, USA, 2021.
- Cursaru, D.; Neagu, M.; Bogatu, L. Investigations on the oxidation stability of biodiesel synthesized from different vegetable oils. Rev. De Chim. 2013, 64, 438–441. [Google Scholar]
- Han, J.; Sun, R.; Zeng, X.; Zhang, J.; Xing, R.; Sun, C.; Chen, Y. Rapid Classification and Quantification of Camellia (Camellia oleifera Abel.) Oil Blended with Rapeseed Oil Using FTIR-ATR Spectroscopy. Molecules 2020, 25, 2036. [Google Scholar] [CrossRef]
- Maszewska, M.; Florowska, A.; Dłużewska, E.; Wroniak, M.; Marciniak-Lukasiak, K.; Żbikowska, A. Oxidative Stability of Selected Edible Oils. Molecules 2018, 23, 1746. [Google Scholar] [CrossRef] [PubMed]
- Ansorena, D.; Ramírez, R.; Lopez de Cerain, A.; Azqueta, A.; Astiasaran, I. Oxidative Stability and Genotoxic Activity of Vegetable Oils Subjected to Accelerated Oxidation and Cooking Conditions. Foods 2023, 12, 2186. [Google Scholar] [CrossRef]
- Chowdary, K.; Kotia, A.; Lakshmanan, V.; Elsheikh, A.H.; Ahmed Ali, M.K. A review of the tribological and thermophysical mechanisms of bio-lubricants based nanomaterials in automotive applications. J. Mol. Liq. 2021, 339, 116717. [Google Scholar] [CrossRef]
- Eunok Choe, E.; Min, D.B. Mechanisms and Factors for Edible Oil Oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Gharby, S.; Asbbane, A.; Ahmed, M.N.; Gagour, J.; Hallouch, O.; Oubannin, S.; Bijla, L.; Goh, K.W.; Bouyahya, A.; Ibourki, M. Vegetable oil oxidation: Mechanisms, impacts on quality, and approaches to enhance shelf life. Food Chem. X 2025, 28, 102541. [Google Scholar] [CrossRef] [PubMed]
- Samuel, J.; Kaisan, M.U.; Sanusi, Y.S.; Narayan, S.; Menacer, B.; Valenzuela, M.; Salas, A.; Oñate, A.; Mahroogi, F.O.; Tuninetti, V. Assessing Antioxidant and Pour Point Depressant Capacity of Turmeric Rhizome Extract in Biolubricants. Lubricants 2024, 12, 282. [Google Scholar] [CrossRef]
- Wolnicka-Glubisz, A.; Wisniewska-Becker, A. Dual Action of Curcumin as an Anti- and Pro-Oxidant from a Biophysical Perspective. Antioxidants 2023, 12, 1725. [Google Scholar] [CrossRef] [PubMed]
- Alabdali, A.; Kzar, M.; Chinnappan, S.; Mogana, R.; Khalivulla, S.I.; Rahman, H.; Abd Razik, B.M. Antioxidant activity of Curcumin. Res. J. Pharm. Technol. 2021, 14, 6741–6746. [Google Scholar] [CrossRef]
- Wright, J.S. Predicting the antioxidant activity of curcumin and curcuminoids. J. Mol. Struct. Theochem. 2002, 591, 207–217. [Google Scholar] [CrossRef]
- Kusumorini, N.; Nugroho, A.K.; Pramono, S.; Martien, R. Determination of the Potential Antioxidant Activity of Isolated Piperine from White Pepper Using DPPH, ABTS, and FRAP Methods. Maj. Farm. 2022, 18, 454–461. [Google Scholar] [CrossRef]
- Mošovská, S.; Nováková, D.; Kaliňák, M. Antioxidant activity of ginger extract and identification of its active components. Acta Chim. Slovaca 2015, 8, 115–119. [Google Scholar] [CrossRef]
- Lee, H.R.; Bak, M.J.; Jeong, W.S.; Kim, Y.C.; Chung, S.K. Antioxidant Properties of Proanthocyanidin Fraction Isolated from Wild Grape (Vitis amurensis) Seed. J. Korean Soc. Appl. Biol. Chem. 2009, 52, 539–544. [Google Scholar] [CrossRef]


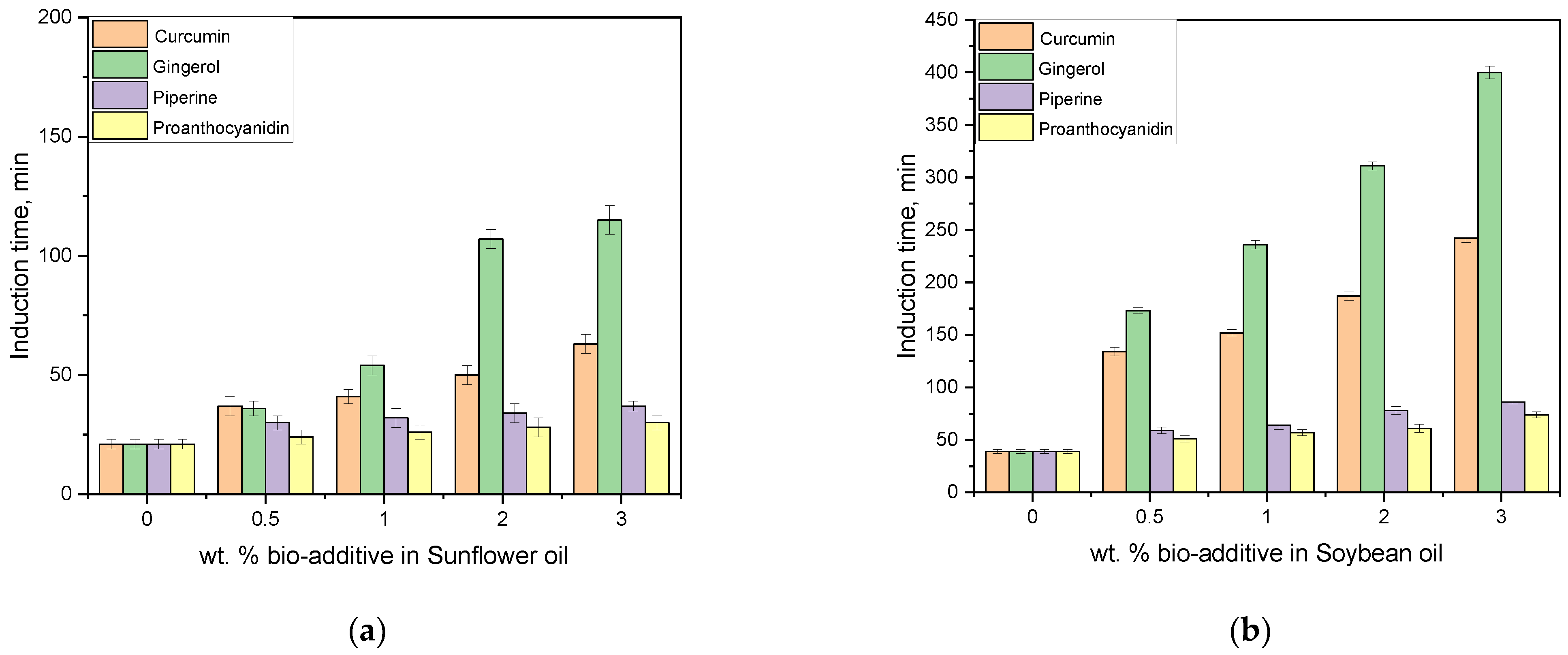
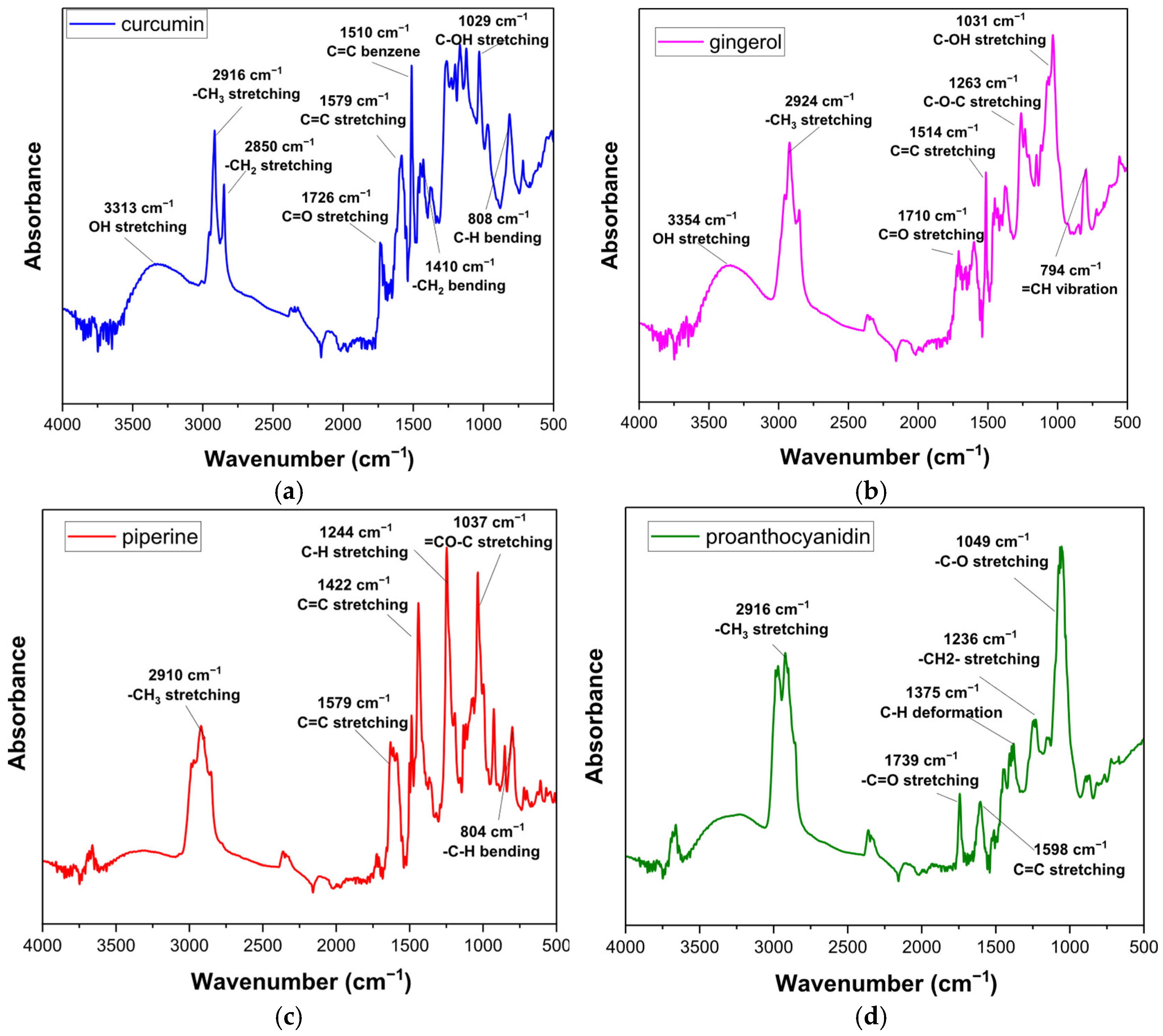
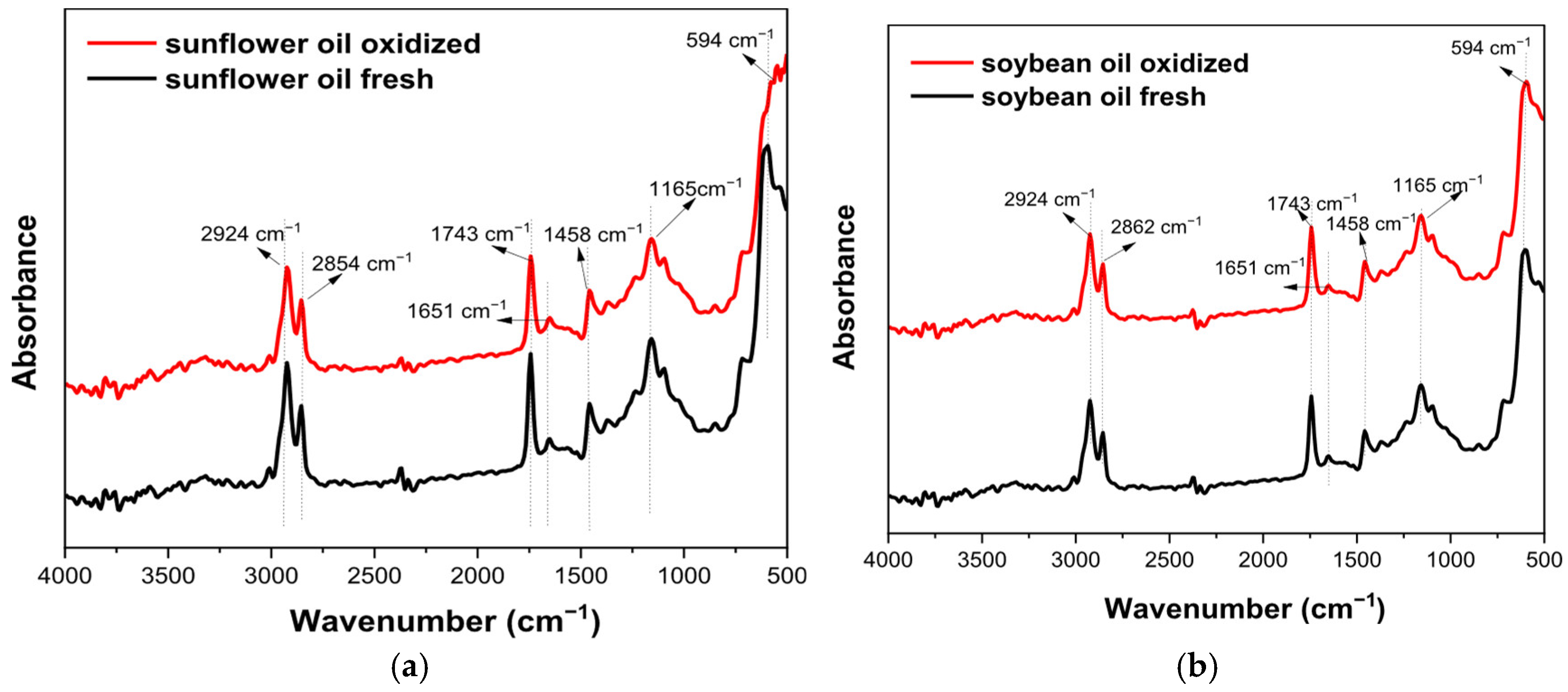
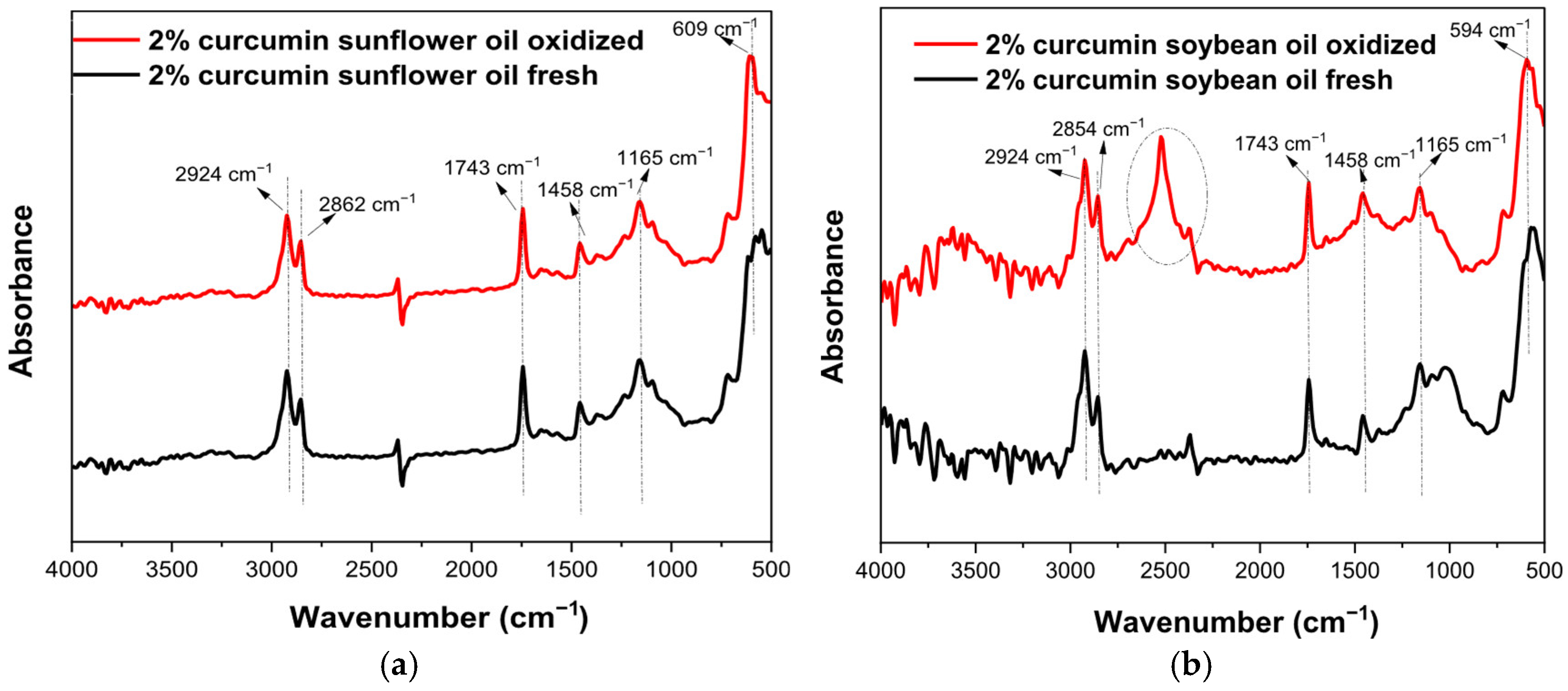


| Properties | SFO | SBO | Methods |
|---|---|---|---|
| Density (20 °C, kg/m3) | 920 | 926 | ASTM D-1298-12b [33] |
| Kinematic viscosity (40 °C, cSt) | 32.33 | 40.99 | ASTM D-445-23 [34] |
| Kinematic viscosity (100 °C, cSt) | 7.65 | 8.92 | ASTM D-445-23 [34] |
| Viscosity index | 219 | 206 | ASTM D-2270-24 [35] |
| Flash point (°C) | 285 | 238 | ASTM D-92 [36] |
| Pour point (°C) | −21 | −17 | ASTM D-97-17 [37] |
| Copper corrosion (at 100 °C) | 1a | 1a | ASTM D-130 [38] |
| Acid value (mg KOH/g) | 0.12 | 1.7 | ASTM D-974-22 [39] |
| Oxidation stability by RBOT, min | 60 | 220 | ASTM D-2272-22 [40] |
| Wear scar diameter according to 4-ball tester, µm (75 °C, 60 min, 1200 RPM, 147N) | 578 | 453 | ASTM D-4172-94 [41] |
| Fatty Acid | Formula | SFO, wt.% | SBO, wt.% |
|---|---|---|---|
| Lauric | C12:0 | 0.0 | 14.5 |
| Myristic | C14:0 | 0.0 | 41.0 |
| Palmitic | C16:0 | 5.8 | 3.5 |
| Stearic | C18:0 | 3.8 | 1.5 |
| Oleic | C18:1 | 28.2 | 14.5 |
| Linoleic | C18:2 | 62.0 | 5.7 |
| Linolenic | C18:3 | 0.1 | 18.9 |
| Others | - | 0.1 | 0.4 |
| Wavenumbers (cm−1) | Functional Group | Mode of Vibration | Possible Structural Units | Absorption Intensity |
|---|---|---|---|---|
| 594 | -C-H- (methoxy group) | Bending | 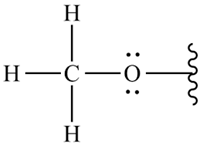 | Strong |
| 723 | -(CH2)n- -HC=CH | Bending (rocking) and out-plane vibration | 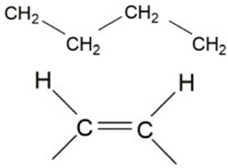 | Medium–weak |
| 1165 | -C-H (CH2) | Bending | 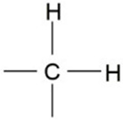 | Strong |
| 1458 | -C-H (CH2) -C-H (CH3) | Bending (scissor) and/or deformation |  | Medium |
| 1743 | -C=O (ester group) | Stretching | 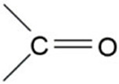 | Very strong |
| 2350–2500 | O-H (carboxylic vibration) | Stretching | 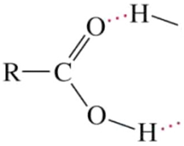 | Very strong |
| 2854 | -C-H (CH2) | Stretching (symmetrical) | 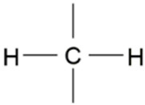 | Very strong |
| 2924 | -C-H (CH2) | Stretching (asymmetrical) | 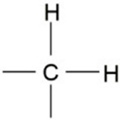 | Very strong |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cursaru, D.-L.; Matei, D. The Impact of Curcumin, Gingerol, Piperine, and Proanthocyanidin on the Oxidative Stability of Sunflower and Soybean Oils for Developing Bio-Lubricants. Lubricants 2025, 13, 302. https://doi.org/10.3390/lubricants13070302
Cursaru D-L, Matei D. The Impact of Curcumin, Gingerol, Piperine, and Proanthocyanidin on the Oxidative Stability of Sunflower and Soybean Oils for Developing Bio-Lubricants. Lubricants. 2025; 13(7):302. https://doi.org/10.3390/lubricants13070302
Chicago/Turabian StyleCursaru, Diana-Luciana, and Dănuța Matei. 2025. "The Impact of Curcumin, Gingerol, Piperine, and Proanthocyanidin on the Oxidative Stability of Sunflower and Soybean Oils for Developing Bio-Lubricants" Lubricants 13, no. 7: 302. https://doi.org/10.3390/lubricants13070302
APA StyleCursaru, D.-L., & Matei, D. (2025). The Impact of Curcumin, Gingerol, Piperine, and Proanthocyanidin on the Oxidative Stability of Sunflower and Soybean Oils for Developing Bio-Lubricants. Lubricants, 13(7), 302. https://doi.org/10.3390/lubricants13070302





