Study of Surface Treatment by Ionic Plasma and Self-Protective Pastes of AISI 304 and 316L Stainless Steels: Chemical, Microstructural, and Nanohardness Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Surface Treatment with Self-Protecting Paste (SNP)
2.3. The Ionic Plasma Nitriding (IPN) Process
2.4. Surface Characterization
3. Results
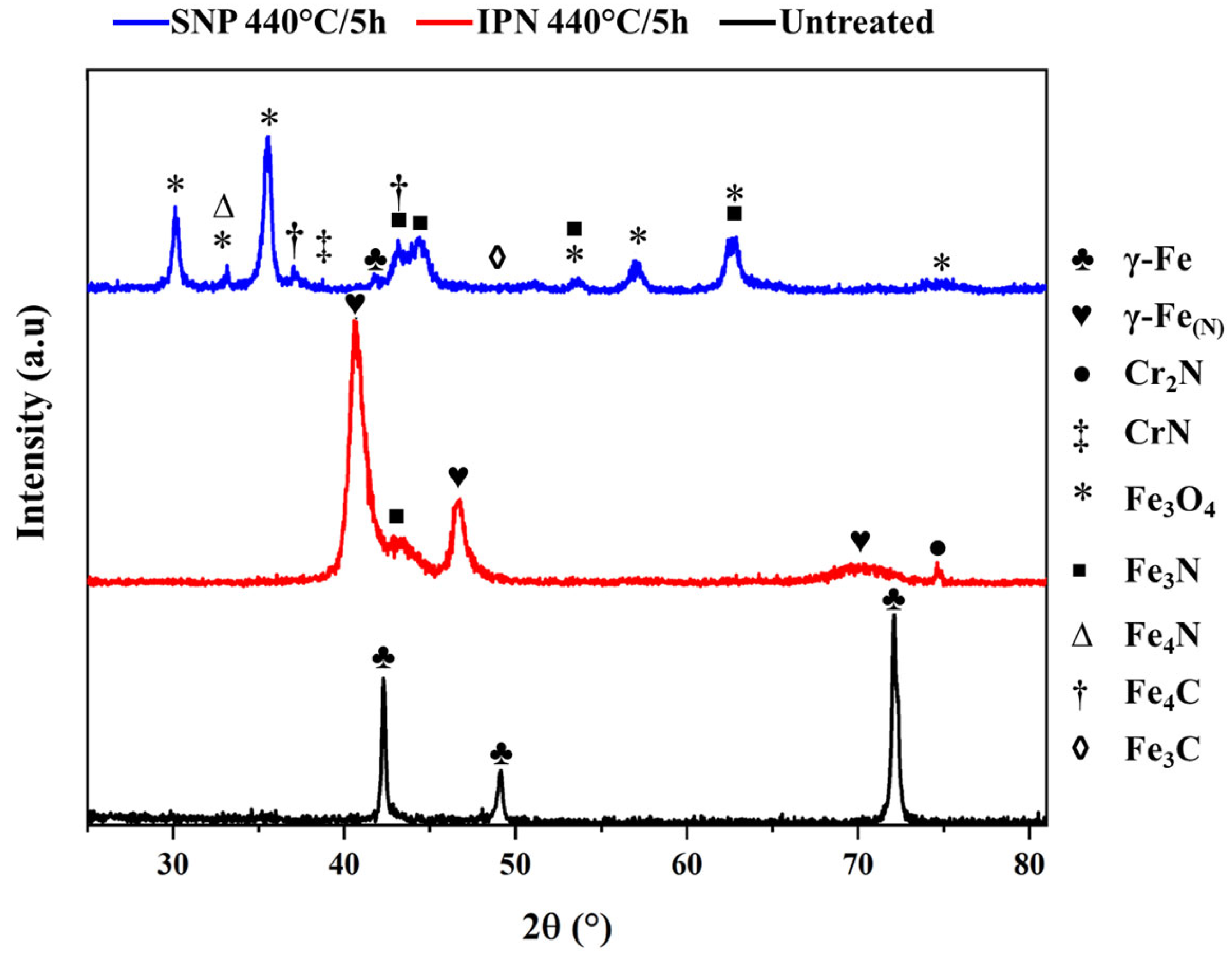
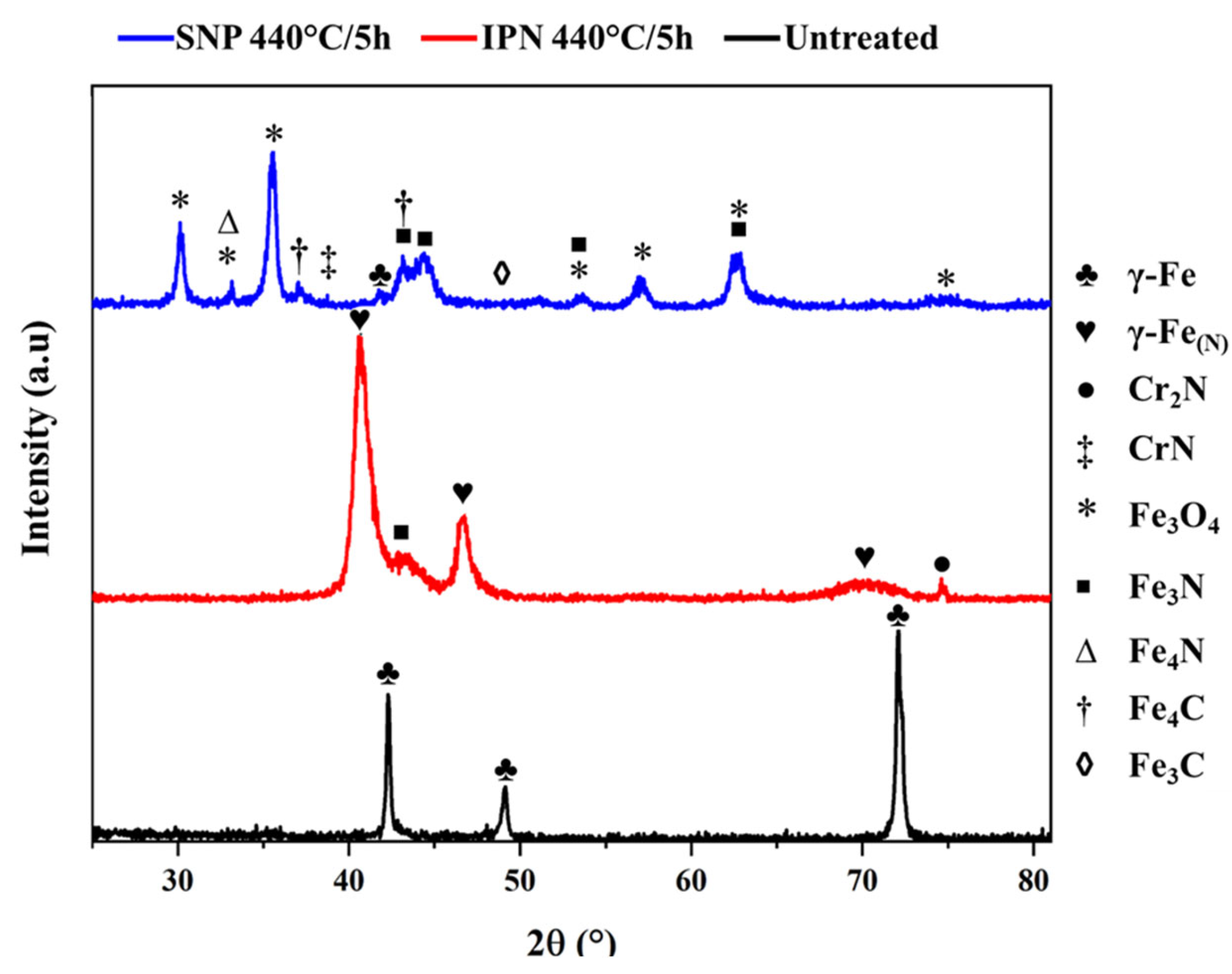
3.1. X-Ray Diffraction Analysis
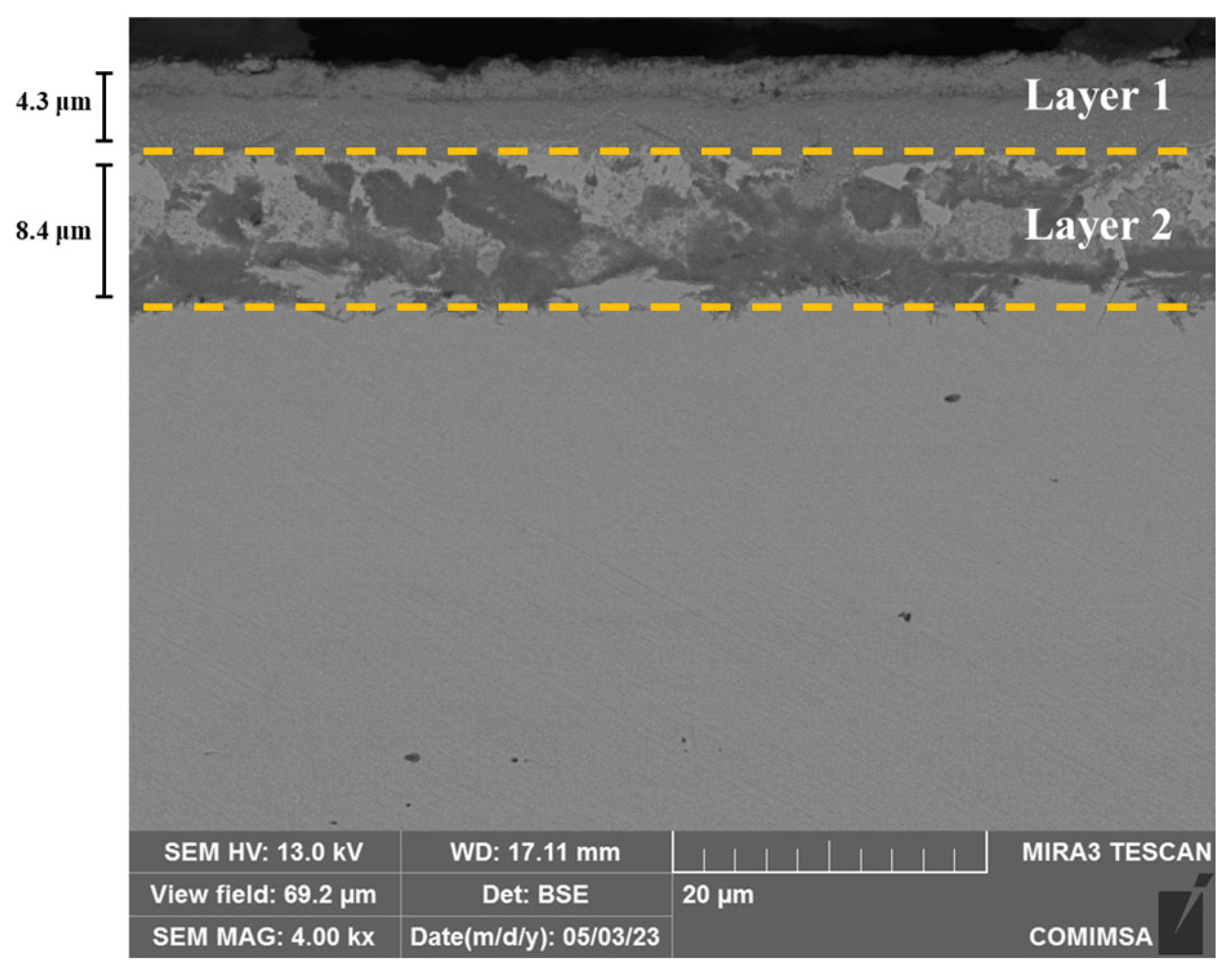
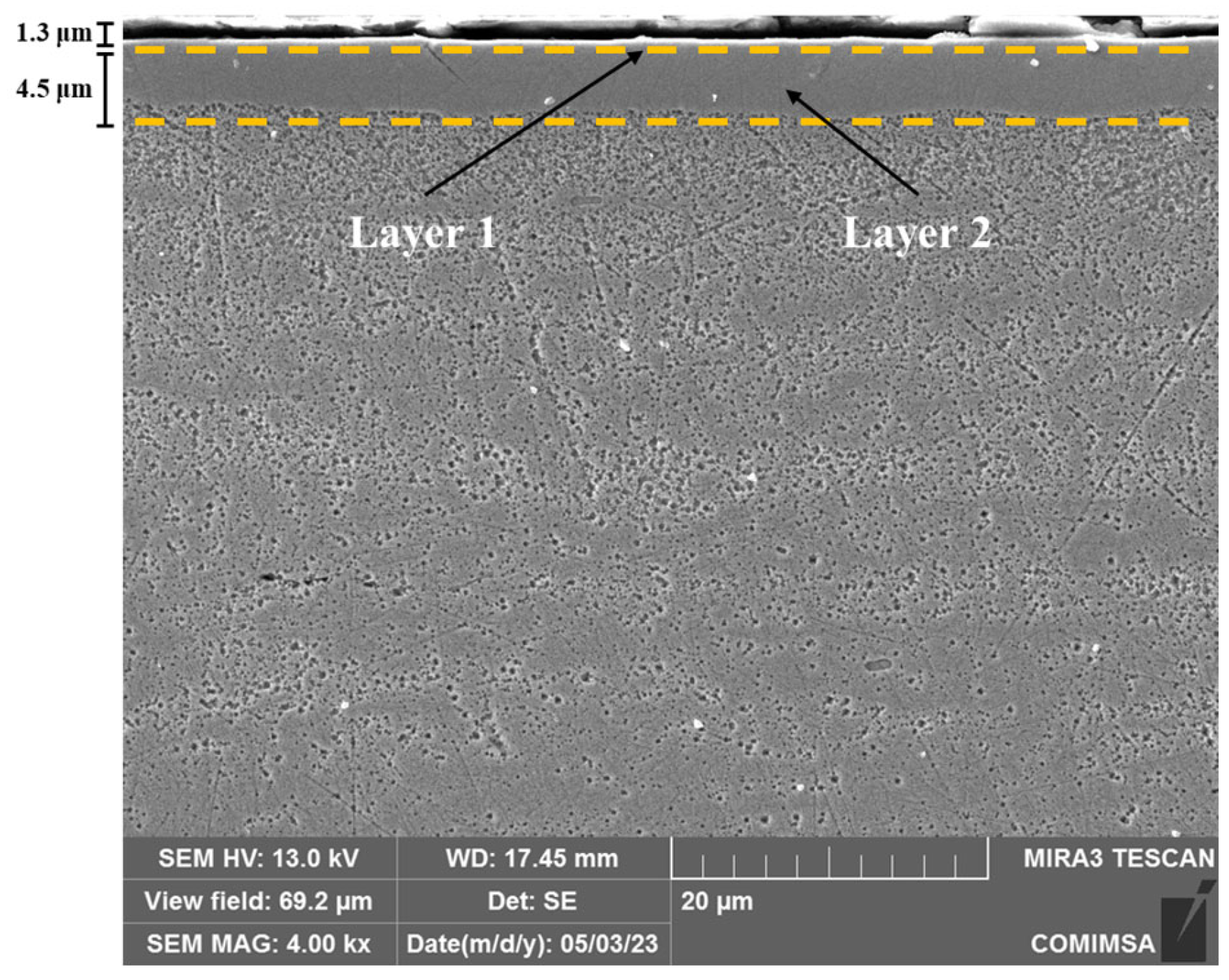
3.2. Microstructure Analysis
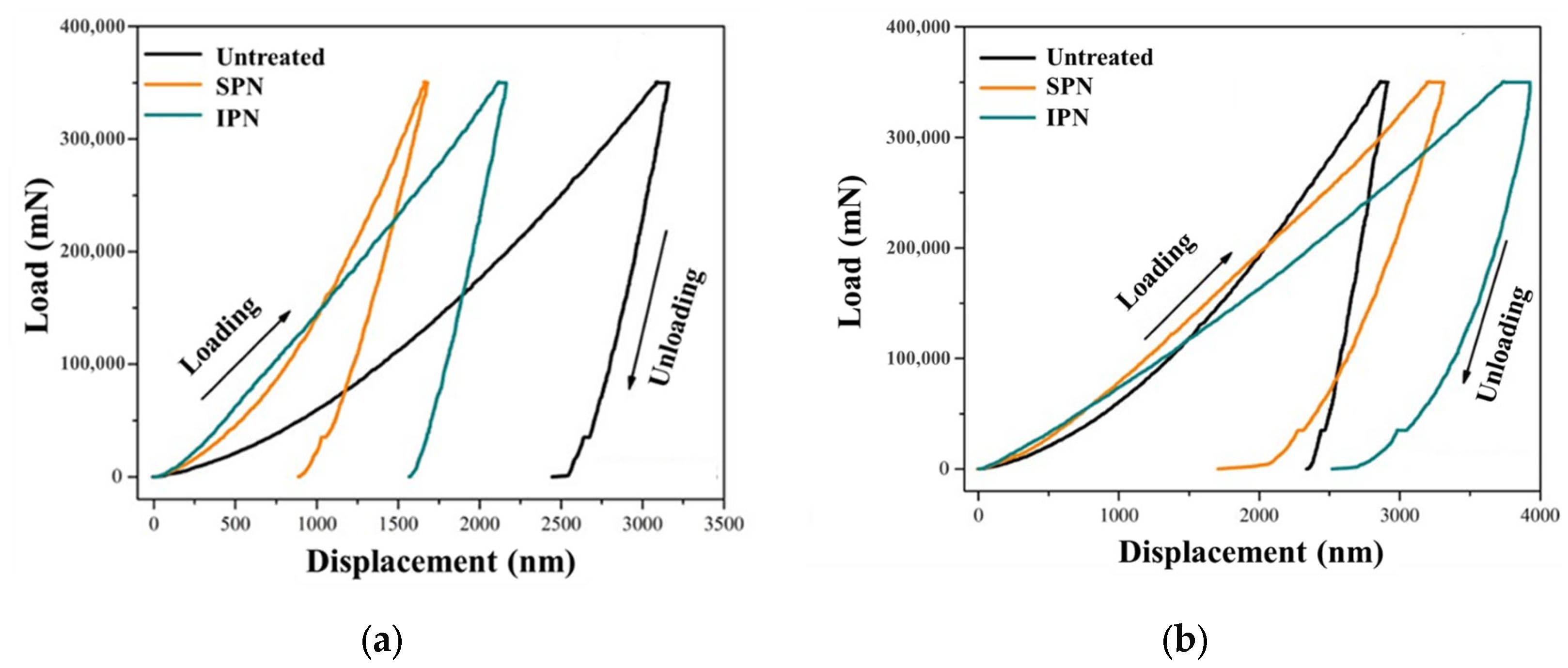
3.3. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SS | Stainless steel |
| SNP | Self-Protective Paste Nitriding |
| IPN | Ion Plasma Nitriding |
| H/E | Hardness-to-Elastic Modulus Ratio |
References
- Dhaiveegan, P.; Elangovan, N.; Nishimura, T.; Rajendran, N. Corrosion behavior of 316L and 304 stainless steels exposed to industrial-marine-urban environment: Field study. RSC Adv. 2016, 6, 47314–47324. [Google Scholar] [CrossRef]
- Rajesh, K.D.; Buddi, T.; Kanth, P.R.; Satyanarayana, K. Microstructural and corrosion resistance study on plasma arc welded joints of AISI 304 and AISI 316. Adv. Mater. Process. Technol. 2020, 6, 189–205. [Google Scholar] [CrossRef]
- Zheng, Z.; Zheng, Y.G. Effects of surface treatments on the corrosion and erosion-corrosion of 304 stainless steel in 3.5% NaCl solution. Corros. Sci. 2016, 112, 657–668. [Google Scholar] [CrossRef]
- Kim, I.-J. Wear Behaviours and Mechanisms. In Engineering Metrology for Pedestrian Falls Prevention and Protection; Springer International Publishing: Berlin/Heidelberg, Germany, 2022; pp. 153–191. [Google Scholar] [CrossRef]
- López-Ojeda, L.; Vargas-Gutiérrez, G. High wear resistance and better pitting corrosion resistance of AISI 316L stainless steel by a self-protective oxy-nitrocarburizing paste. J. Mater. Res. Technol. 2022, 16, 1803–1813. [Google Scholar] [CrossRef]
- Sah, J.; Joseph, A.; Jhala, G.; Mukherjee, S. On the Effects of H2 and Ar on Dual Layer Formed by Plasma Nitrocarburizing on Austenitic Stainless Steels. J. Mater. Eng. Perform. 2022, 31, 2664–2677. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Z.; Shao, M.; Dou, H.; He, Y.; Li, Y.; Luo, J. Structural and tribological properties of sulfonitrocarburizing layers prepared by plasma nitrocarburizing and low temperature ion sulfurizing. Surf. Coat. Technol. 2024, 478, 130470. [Google Scholar] [CrossRef]
- Shabashov, V.A.; Gavrilov, N.V.; Kozlov, K.A.; Makarov, A.V.; Titova, S.G.; Voronin, V.I. Structure of the Surface Layers of Metastable Austenitic Stainless Steel Nitrided in Electron Beam Plasma. Phys. Met. Metallogr. 2018, 119, 755–763. [Google Scholar] [CrossRef]
- Galdikas, A.; Moskalioviene, T. The anisotropic stress-induced diffusion and trapping of nitrogen in austenitic stainless steel during nitriding. Metals 2020, 10, 1319. [Google Scholar] [CrossRef]
- Shen, L.; Wang, L.; Xu, J.J. Plasma nitriding of AISI 304 austenitic stainless steel assisted with hollow cathode effect. Surf. Coat. Technol. 2013, 228, S456–S459. [Google Scholar] [CrossRef]
- Jafarpour, S.M.; Dalke, A.; Biermann, H. Different approaches for plasma nitrocarburizing of austenitic stainless steel using a plasma-activated solid carbon precursor in a hot-wall reactor. Mater. Res. Technol. 2025, 34, 1791–1802. [Google Scholar] [CrossRef]
- Kurelo, B.C.E.S.; Lepienski, C.M.; de Oliveira, W.R.; de Souza, G.B.; Serbena, F.C.; Cardoso, R.P.; das Neves, J.C.K.; Borges, P.C. Identification of Expanded Austenite in Nitrogen-Implanted Ferritic Steel through In Situ Synchrotron X-ray Diffraction Analyses. Metals 2023, 13, 1744. [Google Scholar] [CrossRef]
- Jimenez, L.B.V.; Umemura, M.T.; Calderón-Hernández, J.W.; Magnabosco, R.; Pinedo, C.E.; Tschiptschin, A.P. Plasma nitriding of 410S ferritic/martensitic stainless steel: Microstructure, wear and corrosion properties. Tecnol. Metal. Mater. Min. 2023, 20, e2830. [Google Scholar] [CrossRef]
- Pye, D. Practical Nitriding and Ferritic Nitrocarburizing; ASM International: Materials Park, OH, USA, 2003. [Google Scholar]
- Yuan, X.; Zhao, Y.; Li, X.; Chen, L. Effects of Gas Nitriding Temperature on the Surface Properties of a High Manganese TWIP Steel. Metals 2017, 7, 102. [Google Scholar] [CrossRef]
- Kurelo, B.C.E.S.; Monteiro, J.F.H.L.; de Souza, G.B.; Serbena, F.C.; Lepienski, C.M.; Cardoso, R.P.; Brunatto, S.F. Thermal Evolution of Expanded Phases Formed by PIII Nitriding in Super Duplex Steel Investigated by In Situ Synchrotron Radiation. Metals 2024, 14, 1396. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Lin, Y.; Yan, J.; Zeng, D.-Z.; Huang, R.; Hu, Z. Modification of AISI 304 Stainless Steel Surface by the Low Temperature Complex Salt Bath Nitriding at 430 °C. ISIJ Int. 2012, 52, 1118–1123. [Google Scholar] [CrossRef]
- Gontijo, L.C.; Machado, R.; Casteletti, L.C.; Kuri, S.E.; Nascente, P.A.P. X-ray diffraction characterisation of expanded austenite and ferrite in plasma nitrided stainless steels. Surf. Eng. 2010, 26, 265–270. [Google Scholar] [CrossRef]
- Christiansen, T.; Somers, M.A.J. Decomposition kinetics of expanded austenite with high nitrogen contents. Int. J. Mater. Res. 2006, 97, 79–88. [Google Scholar] [CrossRef]
- Manova, D.; Lotnyk, A.; Mändl, S.; Neumann, H.; Rauschenbach, B. CrN precipitation and elemental segregation during the decay of expanded austenite. Mater. Res. Express 2016, 3, 066502. [Google Scholar] [CrossRef]
- Monteiro, W.A.; Pereira, S.A.L.; Vatavuk, J. Nitriding Process Characterization of Cold Worked AISI 304 and 316 Austenitic Stainless Steels. J. Metall. 2017, 2017, 1052706. [Google Scholar] [CrossRef]
- Nishimoto, A.; Amano, R.; Tamiya, T. Duplex treatment of active screen plasma nitriding and amorphous hydrogenated carbon coating. Appl. Surf. Sci. Adv. 2021, 6, 100129. [Google Scholar] [CrossRef]
- Li, G.Y.; Lei, M. Microstructure and properties of plasma source nitrided AISI 316 austenitic stainless steel. J. Mater. Eng. Perform. 2016, 26, 418–423. [Google Scholar] [CrossRef]
- Bhosale, M.A.; Ummineni, D.; Sasaki, T.; Nishio-Hamane, D.; Bhanage, B.M. Magnetically separable γ-Fe2O3 nanoparticles: An efficient catalyst for acylation of alcohols, phenols, and amines using sonication energy under solvent free condition. J. Mol. Catal. A Chem. 2015, 404–405, 8–17. [Google Scholar] [CrossRef]
- Singh, H.; Kour, S.; Selvaraj, M. Magnetically separable template assisted iron nanoparticle for the enhancement of latent fingerprints. J. Indian Chem. Soc. 2022, 99, 100661. [Google Scholar] [CrossRef]
- Naeem, M.; Díaz-Guillén, J.C.; Khalid, A.; Guzmán-Flores, I.; Muñoz-Arroyo, R.; Iqbal, J.; Sousa, R.R.M. Improved wear resistance of AISI-1045 steel by hybrid treatment of plasma nitriding and post-oxidation. Tribol. Int. 2022, 175, 107869. [Google Scholar] [CrossRef]
- Liu, C.; Chen, X.; Chen, T.; Nie, D.; Wang, L. Carbide transformation in carburised zone of 25Cr35NiNb+MA alloy after high-temperature service. Mater. High Temp. 2016, 33, 98–104. [Google Scholar] [CrossRef]
- Ernst, F.; Cao, Y.; Michal, G.M.; Heuer, A.H. Carbide precipitation in austenitic stainless steel carburized at low temperature. Acta Mater. 2007, 55, 1895–1906. [Google Scholar] [CrossRef]
- Maistro, G.; Yao, Y.; Klement, U.; Nyborg, L.; Cao, Y. On surface carbides in low-temperature carburized austenitic stainless steels. Mater. Charact. 2020, 167, 110462. [Google Scholar] [CrossRef]
- McMurdie, H.F.; Morris, M.C.; Evans, E.H.; Paretzkin, B.; Wong-Ng, W.; Hubbard, C.R. Standard X-Ray Diffraction Powder Patterns from The JCPDS Research Associateship. Powder Diffr. 1986, 1, 265–275. [Google Scholar] [CrossRef]
- Li, L.; Wang, J.; Yan, J.; Duan, L.; Li, X.; Dong, H. The Effect of Liquid Nitriding on the Corrosion Resistance of AISI 304 Austenitic Stainless Steel in H2S Environments. Metall. Mater. Trans. A 2018, 49, 6521–6532. [Google Scholar] [CrossRef]
- Maurice, V.; Marcus, P. Molybdenum effects on the stability of passive films unraveled at the nanometer and atomic scales. NPJ Mater. Degrad. 2024, 8, 3. [Google Scholar] [CrossRef]
- Wang, Z.; Paschalidou, E.-M.; Seyeux, A.; Zanna, S.; Maurice, V.; Marcus, P. Mechanisms of Cr and Mo Enrichments in the Passive Oxide Film on 316L Austenitic Stainless Steel. Front. Mater. 2019, 6, 232. [Google Scholar] [CrossRef]
- Jauberteau, I.; Bessaudou, A.; Mayet, R.; Cornette, J.; Jauberteau, J.L.; Carles, P.; Merle-Méjean, T.J.C. Molybdenum nitride films: Crystal structures, synthesis, mechanical, electrical and some other properties. Coatings 2015, 5, 656–687. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Dong, H. Response of a molybdenum alloy to plasma nitriding. Int. J. Refract. Met. Hard Mater. 2018, 72, 388–395. [Google Scholar] [CrossRef]
- Li, Y.; He, Y.; Xiu, J.; Wang, W.; Zhu, Y.; Hu, B. Wear and corrosion properties of AISI 420 martensitic stainless steel treated by active screen plasma nitriding. Surf. Coat. Technol. 2017, 329, 184–192. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, J.H.; Yan, M.F.; Zhu, Y.D.; Zhang, Y.X.; Wang, Y.X. Improvement of wear resistance for carburised steel by Ti depositing and plasma nitriding. Surf. Eng. 2018, 34, 132–138. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, X.; Dai, X.Z.; Li, J.; Zhang, S.H.; Zhang, C.S.; Ding, J.C.; Zheng, J. Comparative study of plasma nitriding and plasma oxynitriding for optimal wear and corrosion resistance: Influences of gas composition. J. Mater. Res. Technol. 2021, 15, 448–459. [Google Scholar] [CrossRef]
- Jeyakymar, M.; Munusami, V.; Shanmugam, P.; Boopalan, N.; Boopathi, S.; Sureshkumar, M. An investigation on wear loss and hardness of nitro-carburizing coated stainless-steel grade-316. Mater. Today Proc. 2022, 66, 1398–1404. [Google Scholar] [CrossRef]
- Attabi, S.; Himour, A.; Laouar, L.; Motallebzadeh, A. Mechanical and wear behaviors of 316L stainless steel after ball burnishing treatment. J. Mater. Res. Technol. 2021, 15, 3255–3267. [Google Scholar] [CrossRef]
- Cios, G.; Tokarski, T.; Żywczak, A.; Dziurka, R.; Stępień, M.; Gondek, L.; Marciszko, M.; Pawłowski, B.; Wieczerzak, K.; Bała, P. The Investigation of Strain-Induced Martensite Reverse Transformation in AISI 304 Austenitic Stainless Steel. Met. Mater. Trans. A 2017, 48, 4999–5008. [Google Scholar] [CrossRef]

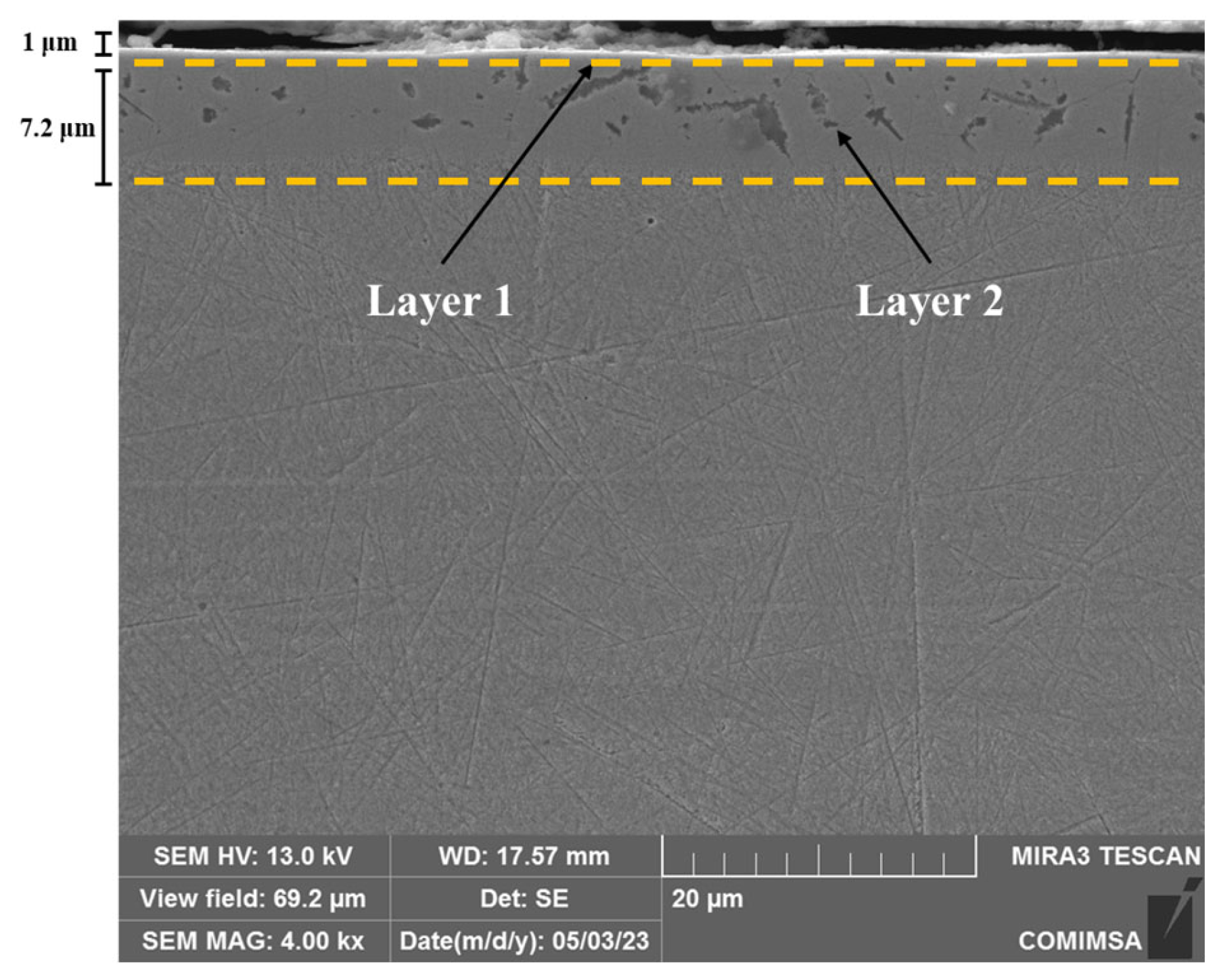
| Element | Elemental Chemical Composition (wt%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | C | S | P | Si | Mn | Mo | Ni | Cr | Fe | Others | |
| AISI 304 SS | 0.04 | 0.005 | 0.052 | 0.5 | 1.36 | 0.138 | 7.7 | 18.12 | 68.2 | 3.88 | |
| AISI 316L SS | 0.015 | 0.014 | 0.044 | 0.24 | 1.68 | 1.76 | 9.56 | 18.04 | 68.0 | 0.64 | |
| Chemical Composition by EDS, wt% | 316L by SNP | 304 by SNP | ||
|---|---|---|---|---|
| Layer 1 | Layer 2 | Layer 1 | Layer 2 | |
| Fe | 23.19 | 7.31 | 22.01 | 48.2 |
| Cr | 15.48 | 26.31 | 13.47 | 14.37 |
| O | 50.29 | 9.61 | 48.98 | 1.62 |
| Ni | 6.24 | 1.06 | 3.34 | 5.08 |
| N | 3.58 | 24.15 | 2.56 | 13.37 |
| C | 28.11 | 8.79 | 16.51 | |
| Mo | 0.83 | 2.95 | ||
| Other | 0.39 | 0.5 | 0.84 | 0.86 |
| Chemical Composition by EDS, wt% | 316L by IPN | 304 by IPN | ||
|---|---|---|---|---|
| Layer 1 | Layer 2 | Layer 1 | Layer 2 | |
| Fe | 60.93 | 61.88 | ||
| Cr | 15.39 | 17.71 | ||
| O | ||||
| Ni | 8.07 | 6.35 | ||
| N | 13.96 | 12.98 | ||
| C | ||||
| Mo | 1.05 | |||
| Other | 0.6 | 1.08 | ||
| Sample | Nanohardness, H (GPa) | Elastic Modulus, E (GPa) | Plastic Factor, Ƞp (%) | Plastic Deformation, Wp (nJ) | Total Deformation, Wt (nJ) | H/E |
|---|---|---|---|---|---|---|
| Untreated 316L SS | 1.46 | 92.6 | 87.44 | 336.53 | 384.84 | 0.015 |
| 316L SS by SPN | 6.83 | 140.98 | 58.61 | 96.85 | 165.23 | 0.048 |
| 316L SS by IPN | 3.41 | 130.76 | 82.52 | 240.62 | 291.58 | 0.026 |
| Untreated 304 SS | 1.77 | 115.01 | 87.67 | 288.4 | 328.94 | 0.015 |
| 304 SS by SPN | 2.23 | 43.11 | 64.68 | 235.97 | 364.82 | 0.051 |
| 304 SS by IPN | 0.92 | 38.75 | 83.47 | 480.82 | 576 | 0.024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Baltodano, F.; Díaz-Guillén, J.C.; López-Ojeda, L.; Vargas-Gutiérrez, G.; Pech-Rodríguez, W. Study of Surface Treatment by Ionic Plasma and Self-Protective Pastes of AISI 304 and 316L Stainless Steels: Chemical, Microstructural, and Nanohardness Evaluation. Lubricants 2025, 13, 195. https://doi.org/10.3390/lubricants13050195
Martínez-Baltodano F, Díaz-Guillén JC, López-Ojeda L, Vargas-Gutiérrez G, Pech-Rodríguez W. Study of Surface Treatment by Ionic Plasma and Self-Protective Pastes of AISI 304 and 316L Stainless Steels: Chemical, Microstructural, and Nanohardness Evaluation. Lubricants. 2025; 13(5):195. https://doi.org/10.3390/lubricants13050195
Chicago/Turabian StyleMartínez-Baltodano, Francisco, Juan C. Díaz-Guillén, Lizsandra López-Ojeda, Gregorio Vargas-Gutiérrez, and Wilian Pech-Rodríguez. 2025. "Study of Surface Treatment by Ionic Plasma and Self-Protective Pastes of AISI 304 and 316L Stainless Steels: Chemical, Microstructural, and Nanohardness Evaluation" Lubricants 13, no. 5: 195. https://doi.org/10.3390/lubricants13050195
APA StyleMartínez-Baltodano, F., Díaz-Guillén, J. C., López-Ojeda, L., Vargas-Gutiérrez, G., & Pech-Rodríguez, W. (2025). Study of Surface Treatment by Ionic Plasma and Self-Protective Pastes of AISI 304 and 316L Stainless Steels: Chemical, Microstructural, and Nanohardness Evaluation. Lubricants, 13(5), 195. https://doi.org/10.3390/lubricants13050195






