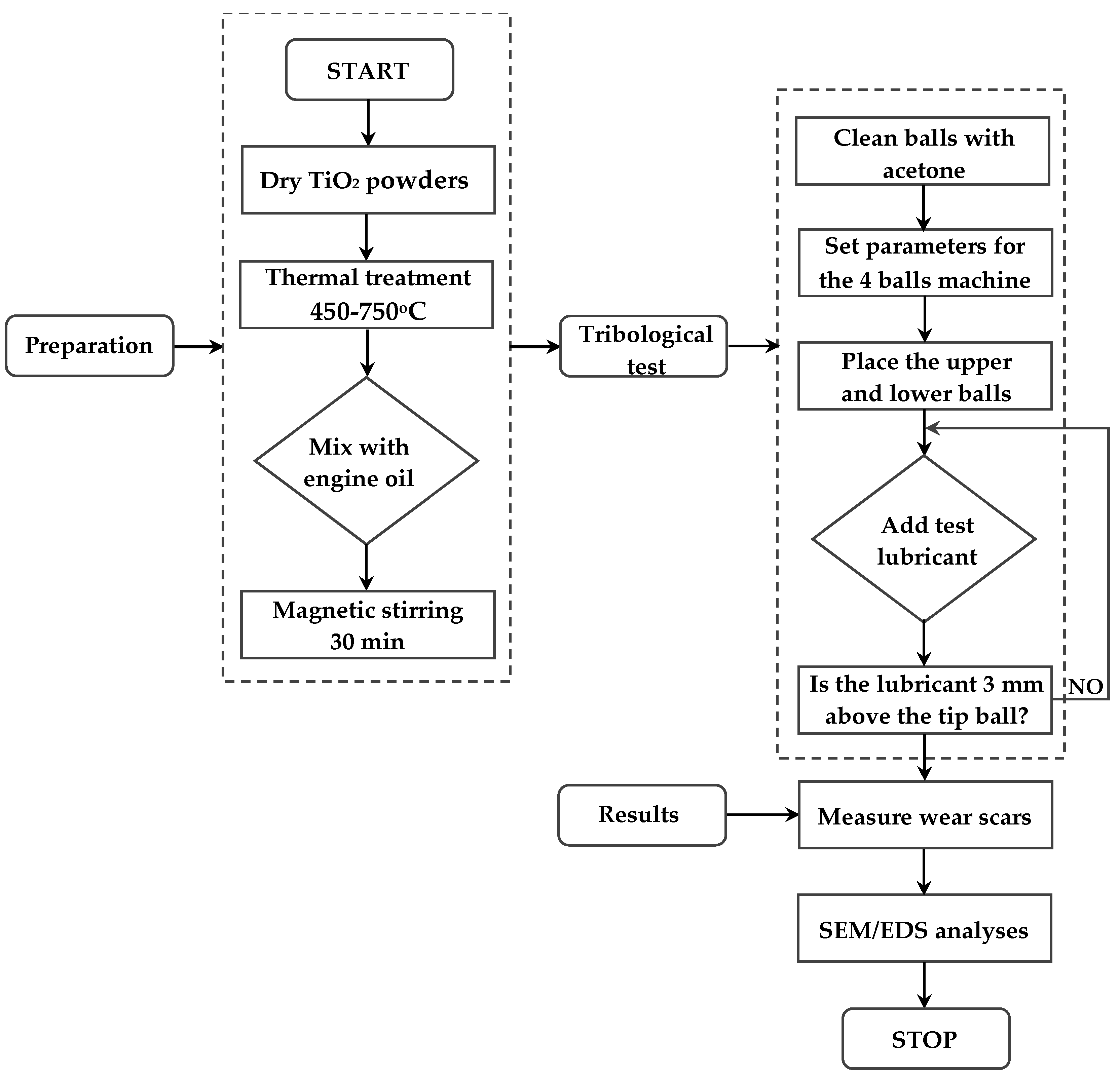
3.1. Coefficient of Friction Behavior
The tribological tests conducted using the four-ball configuration revealed clear differences in the coefficient of friction (COF) depending on both the thermal treatment temperature of the TiO
2 nanoparticles and the lubrication temperature (23 °C vs. 75 °C). As shown in
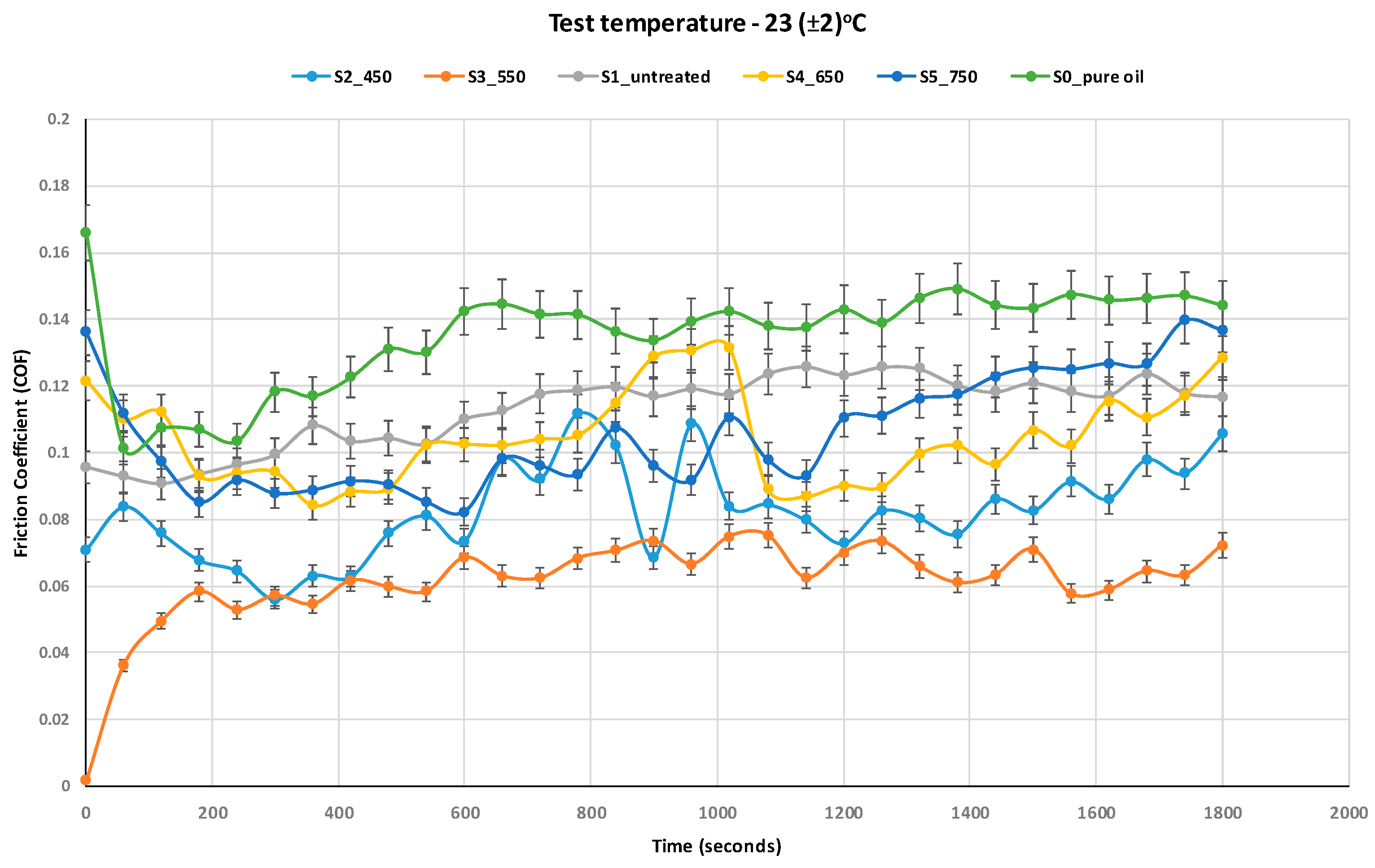
Figure 6 and
Figure 7 (COF curves) and
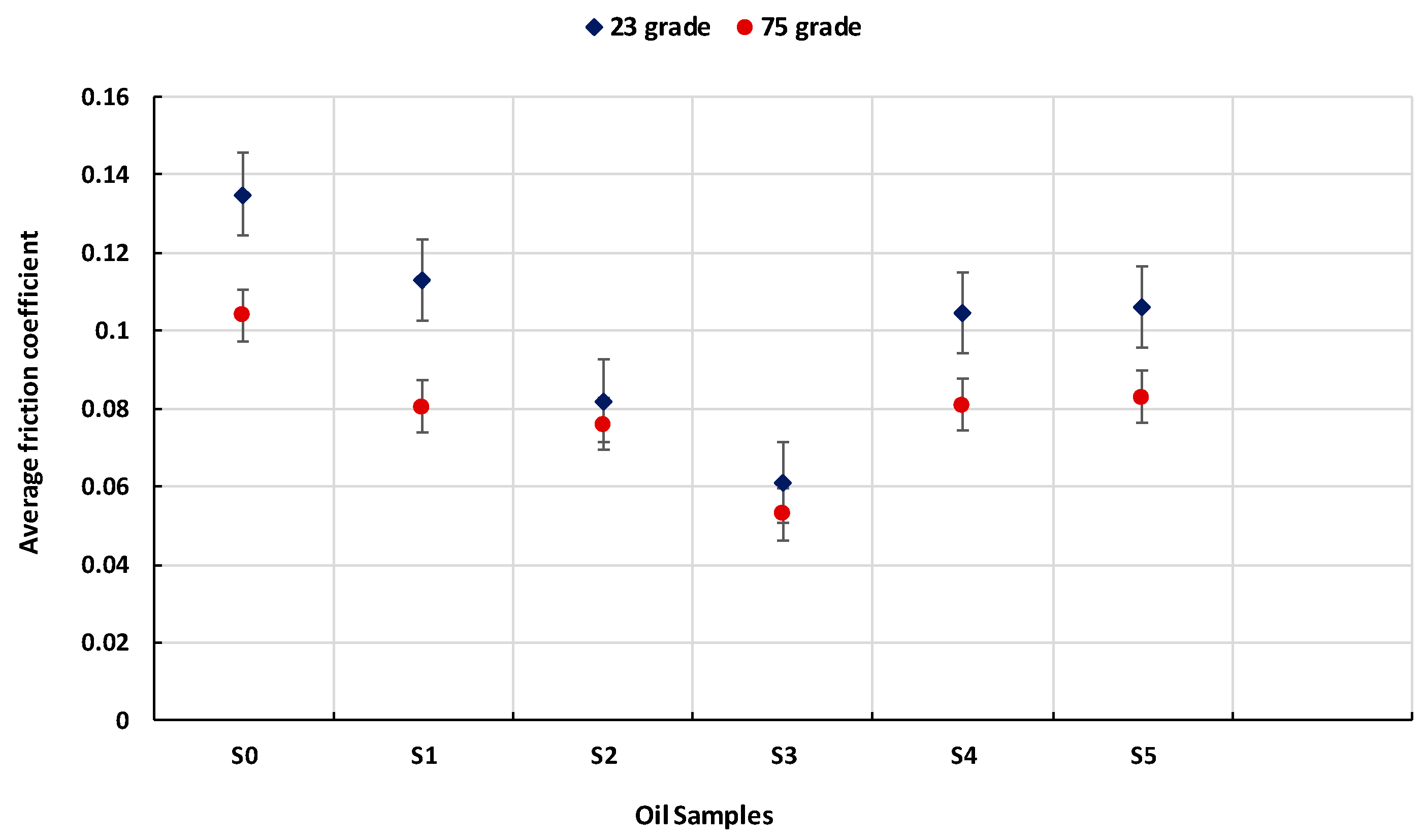
Figure 8 (average COF), the base oil (S0) exhibited the highest COF values under both temperature conditions, due to the absence of solid additives capable of reducing metal-to-metal contact.
The addition of untreated TiO2 nanoparticles (S1) resulted in a moderate reduction in COF, confirming the basic antifriction properties of TiO2 as a solid lubricant. However, the performance of the additive significantly improved when the nanoparticles were thermally treated, particularly at 550 °C (S3) and 650 °C (S4), which produced the lowest average COF values at both 23 °C and 75 °C. The improved performance of TiO2 nanoparticles treated at 550 °C can be attributed to thermally induced structural changes, including partial anatase-to-rutile transformation and surface purification, which increase crystallinity and enhance surface activity under sliding contact.
These results suggest that calcination at intermediate temperatures enhances the tribological behavior of TiO
2 by promoting partial phase transformation (anatase to rutile) and removal of surface-bound impurities, while still maintaining a high enough surface area and active sites for tribofilm formation. X-ray diffraction results (
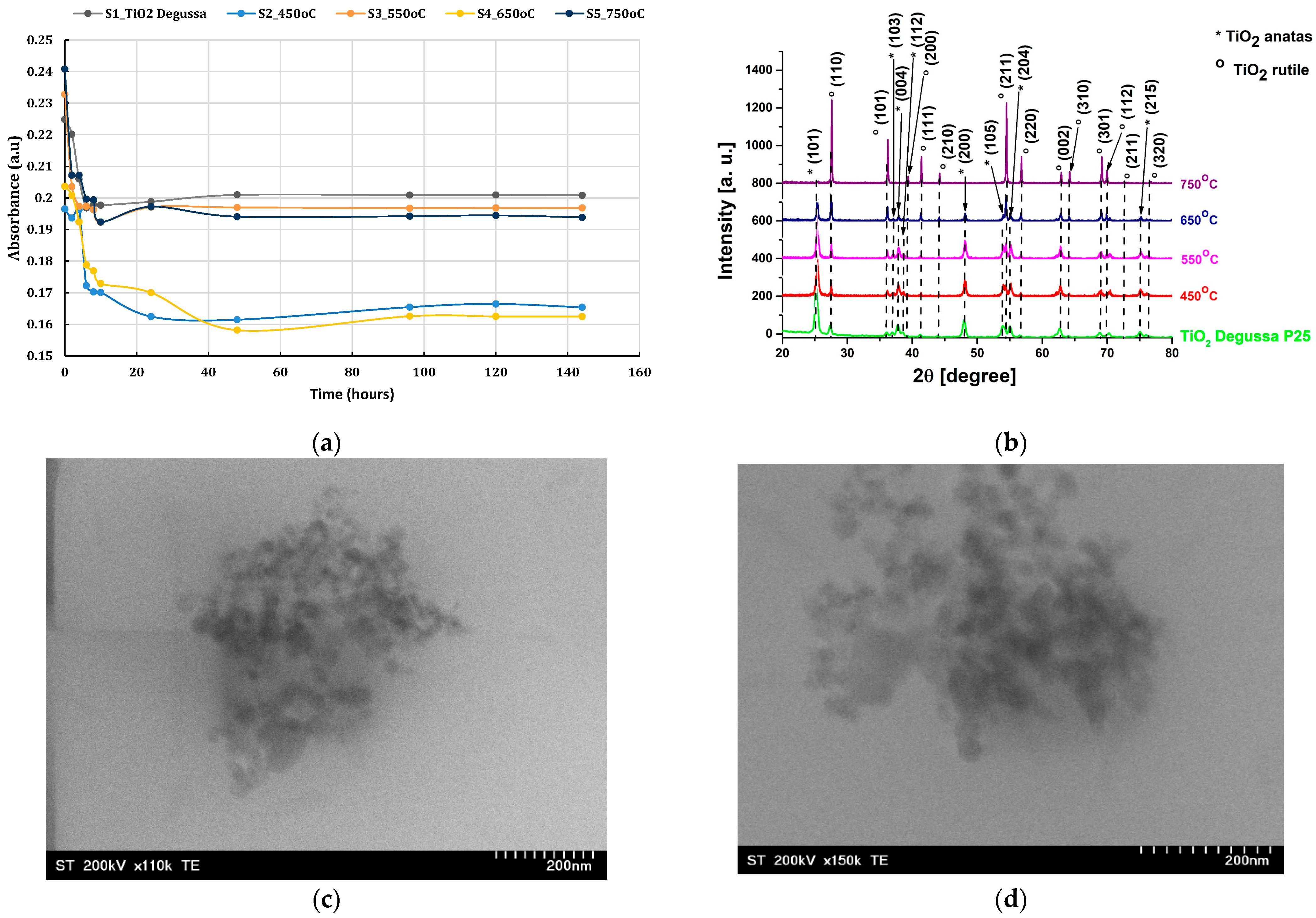
Figure 2b) confirmed the coexistence of anatase and rutile phases in the TiO
2 powders, supporting the hypothesis that thermal treatment at intermediate temperatures (around 550 °C) enhances surface activity through partial phase transformation and removal of surface-bound impurities. This supports findings in the literature, where thermally treated TiO
2 at ~550 °C shows optimal performance in reducing friction and wear [
2,
3,
6].
At 75 °C, all samples demonstrated lower COF values compared to ambient conditions, attributed to the decrease in oil viscosity at higher temperature, enabling better dispersion and penetration of nanoparticles, improved activation of tribochemical processes, such as tribofilm formation and surface passivation.
However, for the sample containing TiO
2 calcined at 750 °C (S5), the COF increased again, approaching the values seen for the untreated sample. This behavior is likely due to the dominance of the rutile phase, which is less reactive tribologically, combined with agglomeration of nanoparticles due to sintering during thermal treatment. Similar trends were reported by Wozniak et al. [
6] and Patil et al. [
5], where excessive thermal exposure reduced the additive’s efficacy.
In the steady-state regime (after ~15–20 min), COF values stabilized across all samples, indicating the establishment of a stable tribolayer. The fastest stabilization was observed in S3 and S4 samples, further supporting their superior frictional performance. The reduction in the running-in time observed with treated TiO2 formulations is beneficial for minimizing wear at startup and extending lubricant life.
To provide a concise overview of the tribological performance of the nanoadditized lubricants,
Table 4 summarizes the average coefficient of friction (COF) values obtained for each tested sample at both ambient temperature (23 °C) and elevated temperature (75 °C). The samples differ by the thermal treatment temperature applied to the TiO
2 nanoparticles prior to dispersion in the base oil.
The data clearly indicate that the sample containing TiO2 treated at 550 °C (S3) achieved the lowest COF values across both test conditions, confirming this temperature as optimal for tribological enhancement. In contrast, the untreated sample (S1) and the one treated at 750 °C (S5) performed less effectively, highlighting the importance of appropriate thermal processing in activating nanoparticle functionality.
The addition of titanium dioxide (TiO2) nanoparticles to lubricating oil can significantly improve its friction-reducing performance, especially under boundary lubrication conditions. However, the tribological effectiveness of TiO2 is not solely determined by its chemical composition or concentration, but also by the physical and structural characteristics of the nanoparticles, which can be tailored through thermal treatment.
In the present study, untreated TiO2 nanoparticles exhibited a moderate reduction in the coefficient of friction (COF) compared to the base oil. This is expected, as the untreated particles can partially contribute to friction reduction by limiting direct metal-to-metal contact and initiating early-stage tribofilm formation. However, the performance of the untreated additive was limited by several factors, including residual surface contaminants, possible amorphous content, and a tendency for agglomeration, all of which reduce dispersion stability and surface reactivity.
When the TiO2 nanoparticles were thermally treated at 450 °C, their tribological performance improved substantially. At this temperature, volatile impurities and surface hydroxyl groups are effectively removed, while the anatase crystalline phase is preserved. The result is a cleaner, more reactive particle surface with a high specific area, which enhances the interaction with the sliding surfaces and promotes the formation of a protective tribofilm. This leads to a noticeable decrease in COF.
The lowest coefficient of friction observed in the tests corresponded to the sample containing TiO2 thermally treated at 550 °C. This condition appears to provide an optimal balance between phase composition, crystallinity, and surface energy. At 550 °C, a partial transformation of anatase to rutile occurs, resulting in a mixed-phase structure. Such a structure has been shown to improve tribological behavior by combining the high reactivity of anatase with the thermal stability of rutile. Moreover, the particle surface becomes more uniform, and the agglomeration tendency is reduced, improving the overall dispersion in the lubricant and ensuring consistent film formation at the interface.
As the calcination temperature increases beyond 650 °C, a gradual decline in tribological performance is observed. This is primarily due to the increasing dominance of the rutile phase, which is more stable but less tribochemically active than anatase. At the same time, particle sintering and crystallite growth lead to a significant reduction in specific surface area and possibly to the formation of larger aggregates, which can act as abrasives rather than protective agents. Consequently, the sample treated at 750 °C showed a higher COF compared to those treated at lower temperatures, approaching the performance of the untreated nanoparticle formulation.
These findings suggest that thermal treatment of TiO2 at intermediate temperatures—particularly around 550 °C—is essential to activate its beneficial properties as a nanoadditive in lubricants. The treated nanoparticles offer enhanced surface reactivity, improved dispersion stability, and optimal tribochemical activity, all of which contribute to a more effective reduction in friction during sliding contact. In contrast, insufficient or excessive thermal treatment results in suboptimal performance due to either incomplete surface activation or degradation of the nanoparticle structure.
These observations regarding COF behavior are further supported by the wear scar diameter measurements and surface analyses, which offer complementary insight into the effectiveness of thermally treated TiO2 nanoparticles as lubricant additives.
3.2. Wear Scar Area of Test Balls for Different TiO2-Treated Oil Formulations
In the present experiments, the upper rotating ball developed a ring-shaped wear pattern due to its continuous motion under high axial load in contact with the three fixed lower balls. However, the wear scars observed on the lower balls were not perfectly circular, but rather elliptical in shape. This deviation from circularity is likely caused by the non-uniform distribution of sliding and normal forces acting on each lower ball during the test. Although the lower balls remain stationary within the ball holder, slight misalignments, asymmetries in loading, or minor variations in contact geometry may lead to anisotropic material removal, resulting in the formation of elliptical wear areas. The elliptical shape of the wear scars on the lower balls may also reflect the influence of tangential forces induced by the upper ball’s rotation, which create a directional component in the frictional contact. Combined with the fixed positioning of the lower balls, this can produce uneven stress distribution and directional wear propagation, leading to ellipsoidal scar geometry.
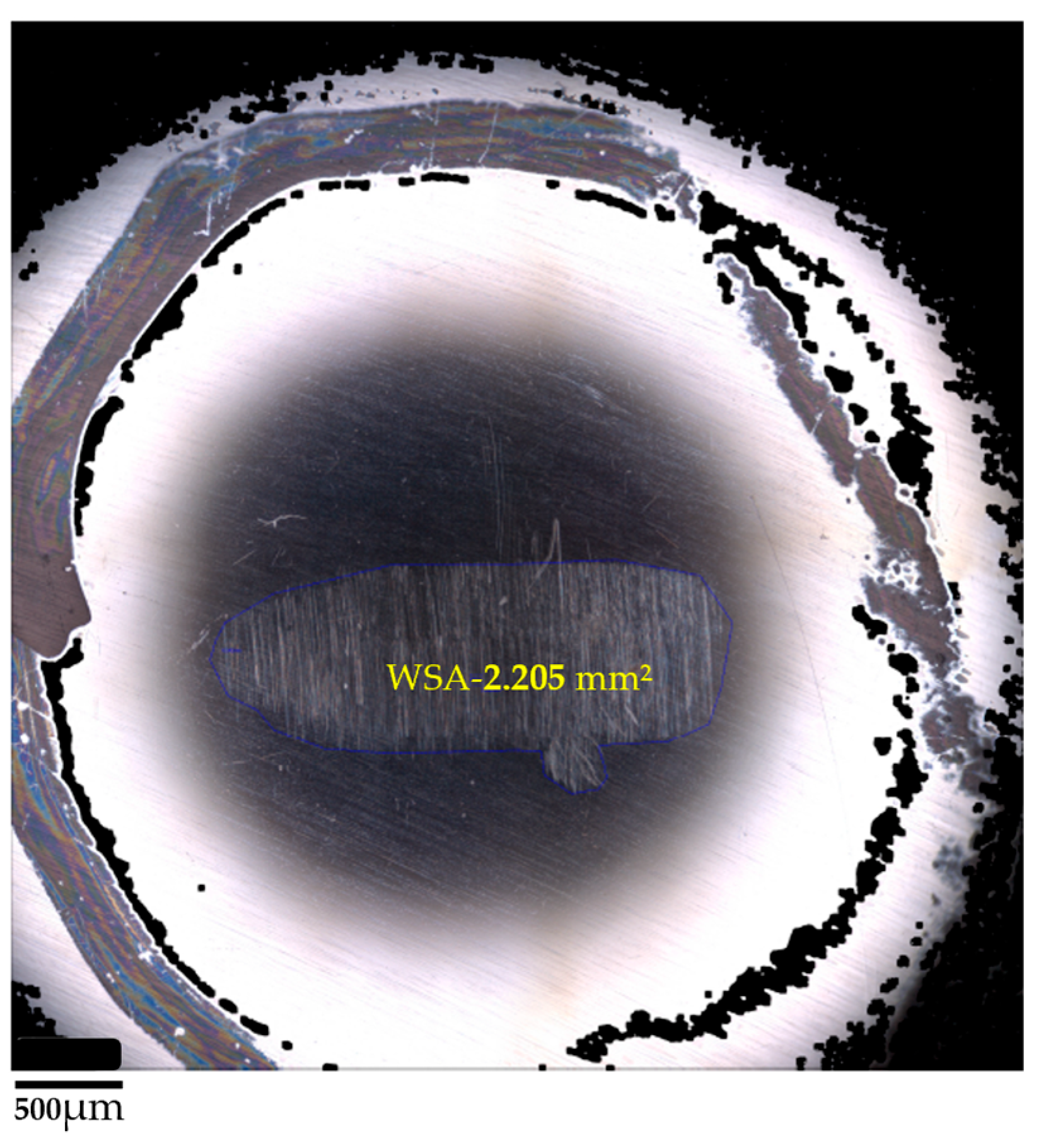
The wear scar area (WSA) was used as a key metric to evaluate the anti-wear performance of the tested lubricants. Given the extremely shallow nature of the wear track in some samples, the depth of material loss could not be measured reliably. Therefore, 3D optical profilometry (Alicona Infinite Focus) was used to determine the projected area of the wear scar on the surface of each steel ball.
The results, presented in
Table 5 show a clear reduction in wear scar area with the addition of TiO₂ nanoparticles to the base oil. The unmodified oil (S0) produced the largest wear scar of 5.34 mm
2, confirming the absence of anti-wear protection. The addition of untreated TiO
2 (S1) significantly reduced wear to 4.83 mm
2, while thermally treated TiO
2 samples showed even greater improvement. Notably, the sample treated at 550 °C (S3) achieved the lowest wear scar area of 2.205 mm
2, followed closely by the 750 °C sample (S5), which also performed well (2.25 mm
2).
Figure 9.
Surface morphology of the wear scar on the test ball (sample S1) as revealed by 3D optical microscopy (untreated TiO2, 23 °C).
Figure 9.
Surface morphology of the wear scar on the test ball (sample S1) as revealed by 3D optical microscopy (untreated TiO2, 23 °C).
Figure 10.
Surface morphology of the wear scar on the test ball (sample S3) as revealed by 3D optical microscopy (TiO2 treated at 550 °C, 23 °C).
Figure 10.
Surface morphology of the wear scar on the test ball (sample S3) as revealed by 3D optical microscopy (TiO2 treated at 550 °C, 23 °C).
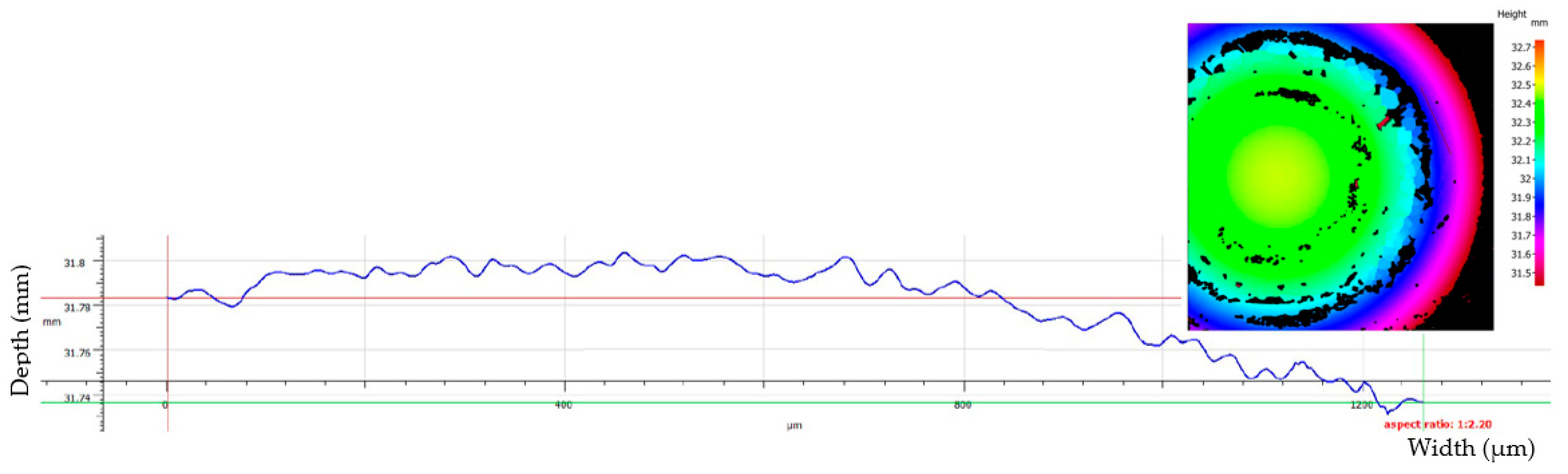
Figure 11.
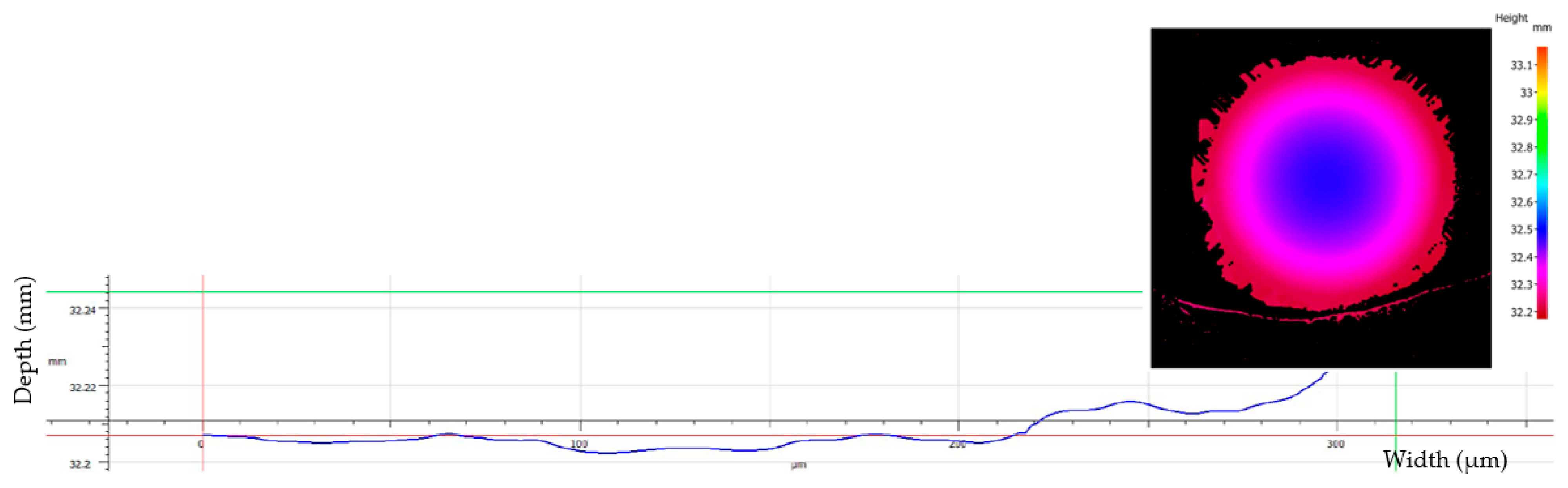
Example of wear depth profile for sample S1 (untreated TiO2) showing minimal material removal and a flat abrasion profile.
Figure 11.
Example of wear depth profile for sample S1 (untreated TiO2) showing minimal material removal and a flat abrasion profile.
Although the wear area slightly increased at 650 °C (S4), it remained much lower than the base oil, indicating that even over-calcined TiO2 maintains partial anti-wear functionality. The sample treated at 450 °C (S2) also performed well (2.752 mm2), suggesting that all thermally treated samples outperformed the untreated condition.
These findings align with the COF results and support the hypothesis that thermal treatment improves the tribochemical interaction of TiO2 at the sliding interface, enhancing its ability to protect the contact surface against material loss.
The projected wear scar area (WSA) was measured using Alicona 3D optical profilometry for all test samples under both ambient (23 °C) and elevated (75 °C) temperature conditions. As shown in
Table 5, the base oil without additives (S0) exhibited the largest wear scars at both temperatures, while nanoadditized samples showed substantial reductions in wear, particularly those containing TiO
2 nanoparticles treated at 550 °C.
The measurements confirm that thermal treatment of TiO2 plays a critical role in enhancing the anti-wear performance of the lubricant. While all treated samples outperformed the untreated oil, the sample calcined at 550 °C (S3) resulted in the smallest wear scar area at both temperatures, indicating the most efficient protection of the contact surfaces.
In the following two figures (
Figure 9 and
Figure 10), representative wear scar images are presented for samples S1 and S3, both tested at 23 °C, using Alicona Infinite Focus 3D optical profilometry. These samples were selected to illustrate the contrast in wear behavior between the untreated TiO
2 formulation (S1) and the thermally optimized formulation at 550 °C (S3). The images reveal distinct differences in the geometry and surface features of the wear scars, consistent with the numerical values discussed earlier.
Wear scar contours were manually traced on the Alicona-acquired images at a measurement resolution of 500,000 µm, enabling high-fidelity capture of the worn surface topography. This approach was applied to all tested balls under all experimental conditions presented in this study. However, it should be noted that, due to the manual delineation of wear scar boundaries, a degree of subjective variation may have been introduced. While every effort was made to ensure consistency and precision in contour definition, minor human-induced error cannot be fully excluded and was considered in the interpretation of the measured wear scar areas.
Although the system is capable of profiling wear depths with nanometer precision, the measured wear tracks exhibited minimal penetration, often at or below the vertical resolution threshold of the system. Consequently, the volumetric wear could not be reliably quantified across all samples. As a result, the wear performance was evaluated based on the projected wear scar area (WSA), traced manually from the Alicona images. While this method introduces a minor degree of subjectivity, the same contouring procedure was consistently applied to all samples to ensure reliable comparison. Two representative cross-sectional profiles are shown in
Figure 11 and
Figure 12, highlighting the extremely shallow depth of wear and the predominance of surface-level abrasion, rather than volumetric material removal.
In conclusion, both the friction and wear analyses confirm that TiO2 nanoparticles thermally treated at 550 °C offer optimal tribological performance under the tested conditions. The combined reduction in COF and wear scar area highlights the potential of such nanoadditized lubricants for high-performance applications. These results are further explored through surface morphology and elemental characterization, as discussed in the following section.
3.3. SEM and EDS Analysis of Worn Ball Surfaces
To better understand the wear mechanisms and surface interactions during tribological testing, scanning electron microscopy (SEM) was used to investigate the morphology of wear scars on the steel balls. The SEM images revealed surface degradation features such as abrasive grooves, fine scratches, and occasional localized deposits possibly resulting from oxidation or carbonaceous build-up. These microstructural observations help distinguish the tribological behavior of different lubricant formulations. The representative SEM images and corresponding EDS spectra presented in
Figure 13,
Figure 14,
Figure 15 illustrate the typical surface morphology of worn steel balls lubricated with the S1–S3 oil formulations at 23 °C. These micrographs highlight the progressive improvement in surface smoothness and reduction in abrasion as the thermal treatment temperature of TiO₂ increases, consistent with the COF and WSA results discussed earlier.
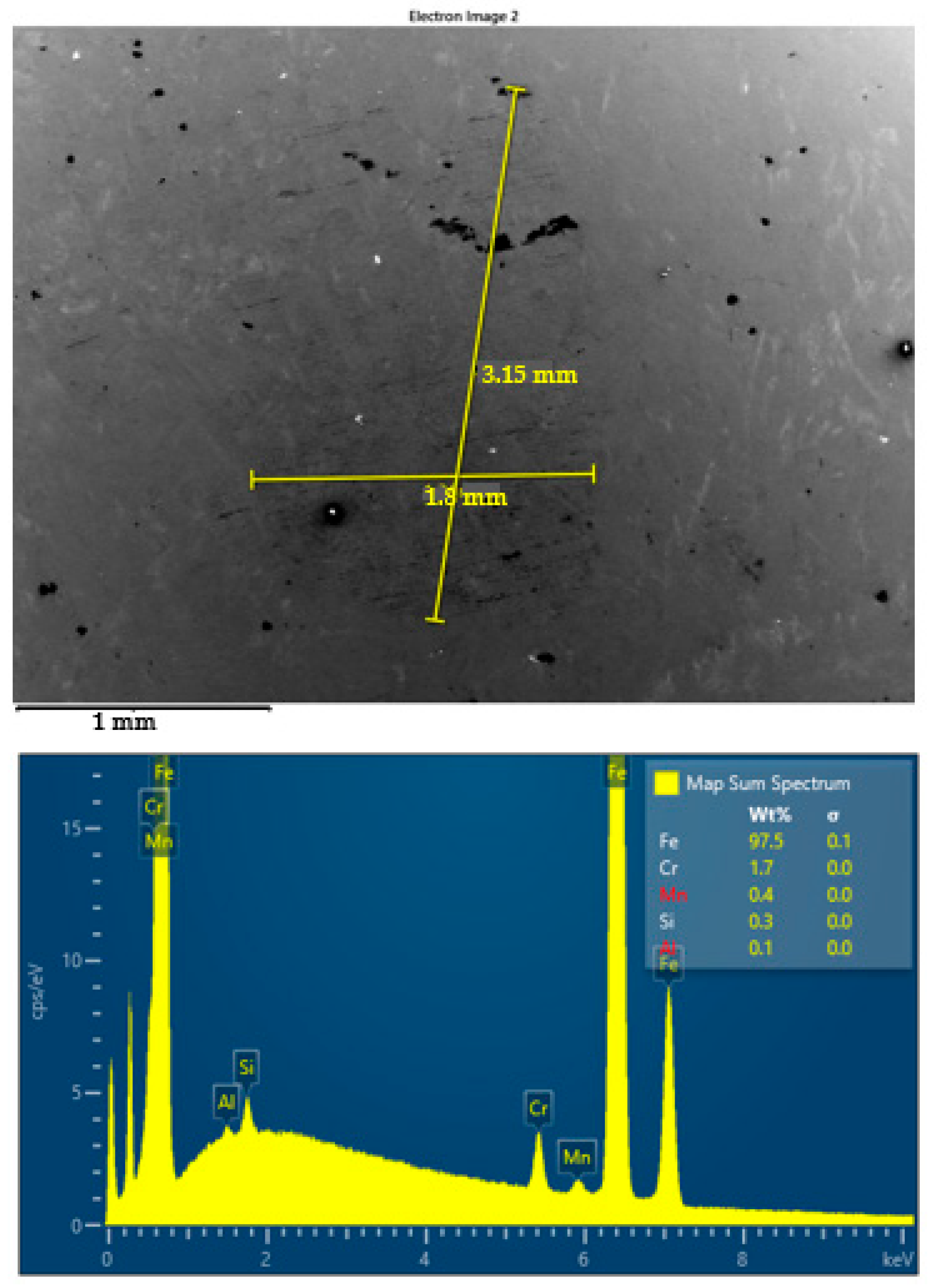
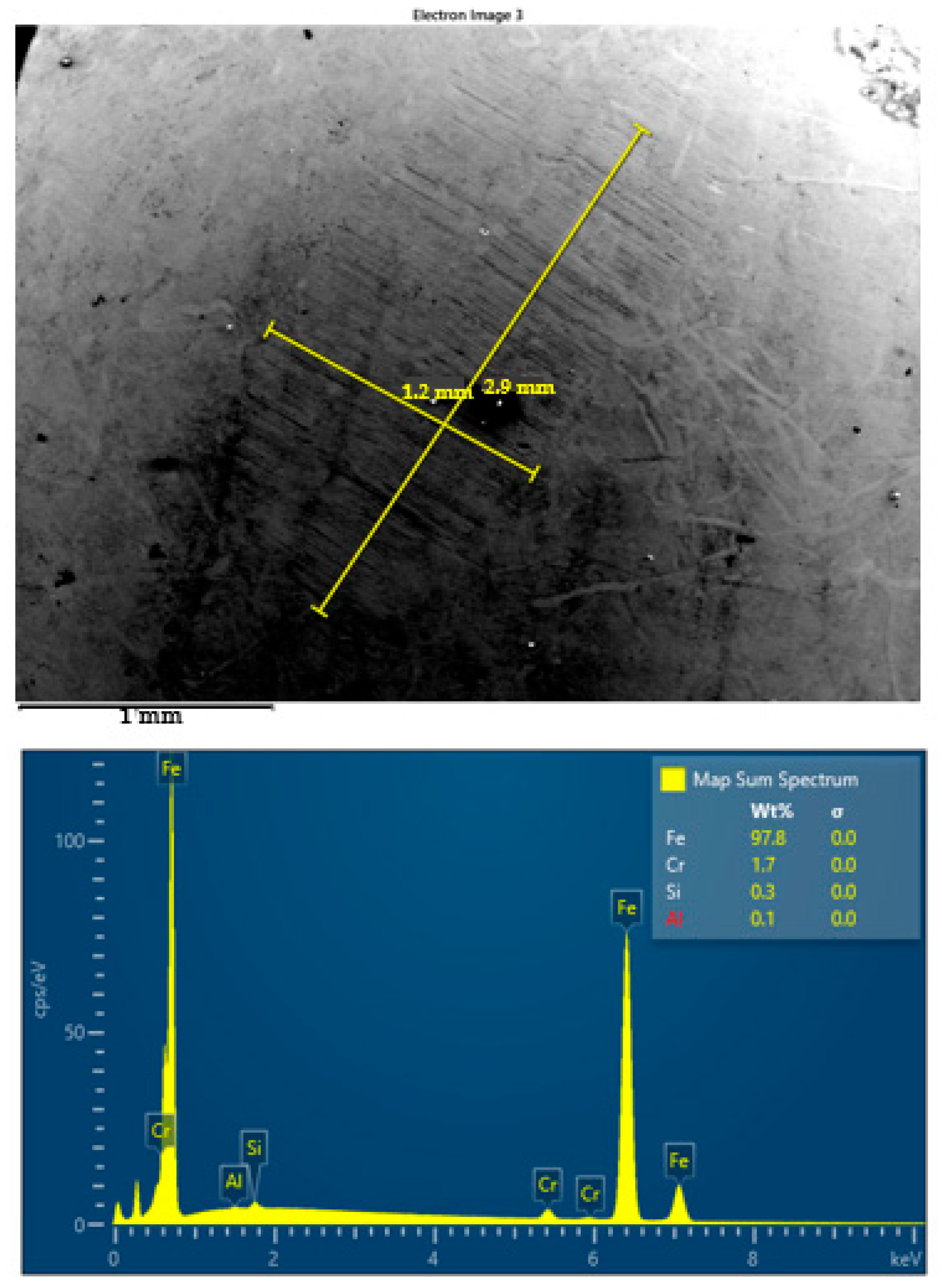
In parallel, energy-dispersive X-ray spectroscopy (EDS) was employed to detect elemental signatures indicative of tribochemical interaction. However, under the conditions applied in this study, no Ti or TiO2 residues were detected on the worn surfaces. The absence of titanium-based signals in the EDS spectra suggests that the nanoparticles primarily influenced tribological behavior through physical mechanisms—such as rolling, spacing, or polishing—rather than forming stable tribochemical films. This lack of elemental transfer suggests that the nanoparticles act primarily through physical mechanisms (e.g., rolling, spacing, or polishing) rather than forming a persistent chemical tribofilm on the steel substrate.
The SEM and EDS findings indicate that the primary tribological effects of TiO2 addition—such as friction and wear reduction—are likely attributable to physical mechanisms, such as rolling or polishing effects, rather than to tribochemical film formation or strong surface adsorption.
On several wear scars, micro-abrasion tracks were visible, oriented along the direction of sliding, confirming adhesive–abrasive mixed wear as the dominant mechanism. These findings were further supported by SEM micrographs, which revealed micro-abrasion tracks aligned with the sliding direction—indicative of a mixed adhesive–abrasive wear mechanism.
In some regions, light surface discoloration and minor accumulations of carbon-rich compounds were observed, likely resulting from lubricant degradation under load and temperature, rather than from nanoparticle interaction.
The elliptical geometry of the wear scars, particularly on the lower balls, was consistent with Alicona measurements, with less than 10% deviation between the SEM and optical methods—validating the accuracy of both approaches despite possible human error in manual tracing.
Figure 13.
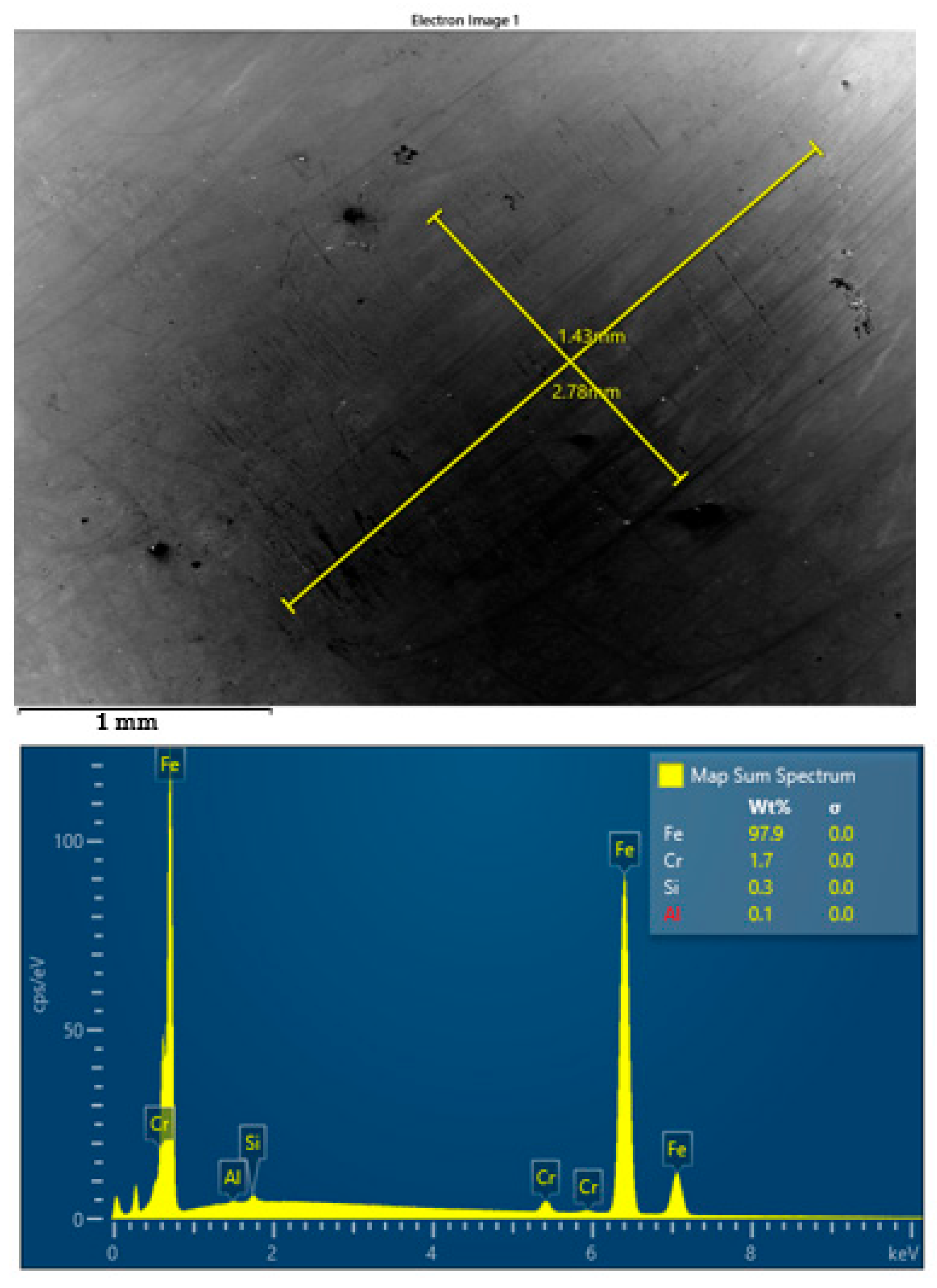
SEM images and corresponding EDS spectra of worn surface of steel ball after four-ball test lubricated by S1 oil at 23 °C.
Figure 13.
SEM images and corresponding EDS spectra of worn surface of steel ball after four-ball test lubricated by S1 oil at 23 °C.
Figure 14.
SEM images and corresponding EDS spectra of worn surface of steel ball after four-ball test lubricated by S2 oil at 23 °C.
Figure 14.
SEM images and corresponding EDS spectra of worn surface of steel ball after four-ball test lubricated by S2 oil at 23 °C.
Figure 15.
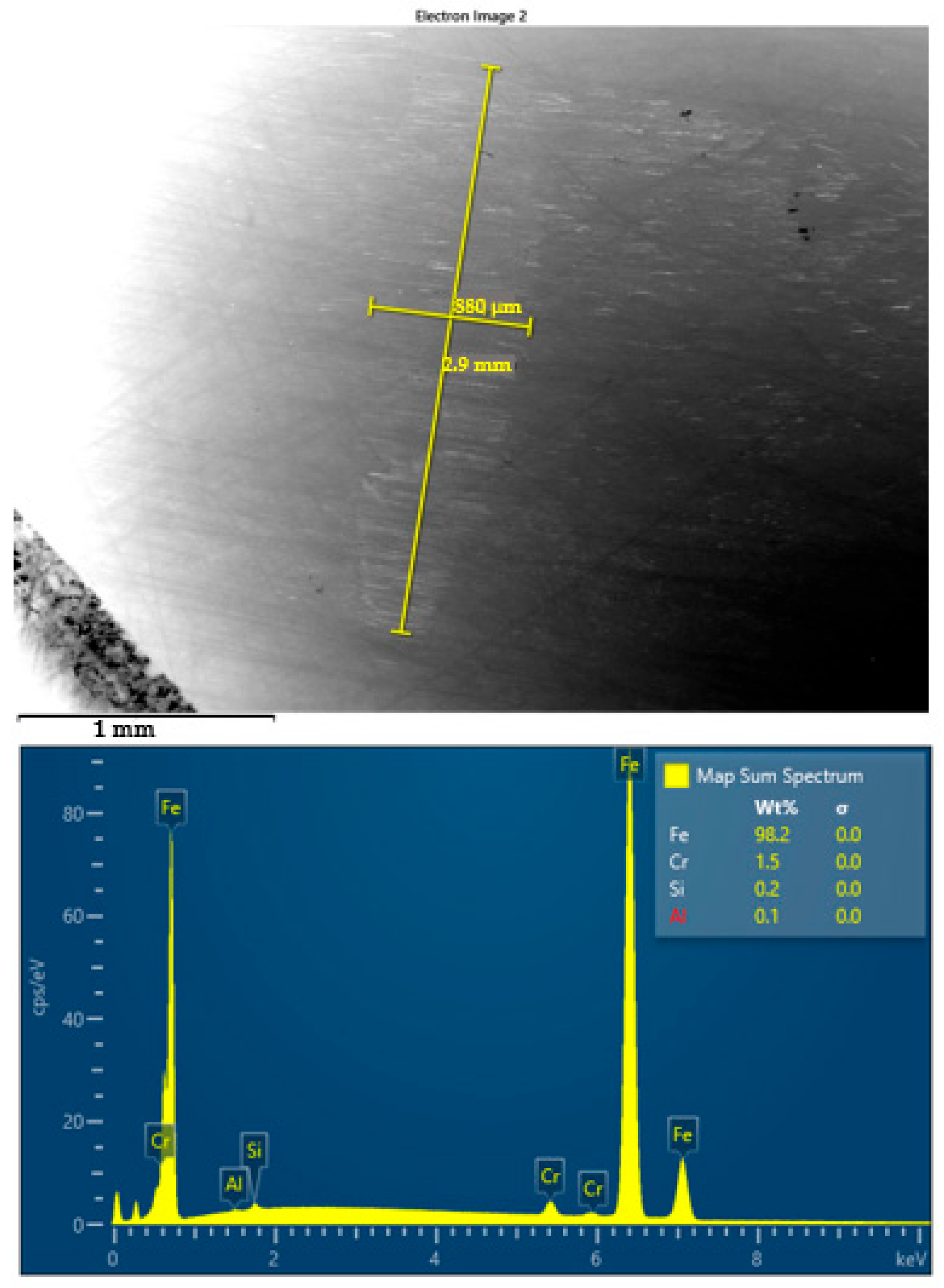
SEM images and corresponding EDS spectra of worn surface of steel ball after four-ball test lubricated by S3 oil at 23 °C.
Figure 15.
SEM images and corresponding EDS spectra of worn surface of steel ball after four-ball test lubricated by S3 oil at 23 °C.
The SEM micrographs presented for samples S1, S2, and S3 tested at 23 °C, as well as for S2 and S3 at 75 °C, reveal distinct differences in surface wear morphology as a function of thermal treatment applied to the TiO2 nanoparticles.
Representative SEM images of samples S1, S2, and S3 tested at 23 °C reveal progressive changes in wear morphology as a function of nanoparticle treatment temperature.
In S1 (untreated), more pronounced abrasive grooves and debris suggest poor surface protection. In contrast, samples S2 and S3 exhibited smoother, more uniform wear scars—consistent with improved friction and wear performance observed previously
For S1 (untreated TiO2), the worn surface shows irregular abrasive grooves, signs of mild plastic deformation, and scattered surface debris, indicative of adhesive–abrasive wear mechanisms. The absence of uniform wear patterns suggests limited protection of the surface due to weak interaction between the untreated nanoparticles and the steel substrate.
The wear scar areas measured via SEM imaging correlated well with the values obtained through Alicona 3D optical profilometry, with deviations remaining within a ±10% margin. This consistency confirms the reliability of the image-based wear area estimation, despite the minor human factor involved in manual scar tracing.
Although no tribochemical film formation was confirmed, the physical action of thermally treated TiO2 nanoparticles appears to contribute to surface protection by reducing the severity of contact, smoothing asperities, and limiting adhesive wear. These findings align with the observed improvements in friction and wear performance, especially for samples treated at intermediate calcination temperatures.
This behavior is consistent with the tribological mechanisms reported in recent studies on oxide-based nanolubricants [
33,
34,
35,
36,
37], where nanoparticles enhance load-carrying capacity and reduce shear stress through synergistic rolling, polishing, and third-body effects. Local temperature and pressure can also promote transient adsorption and surface passivation, minimizing adhesive junctions without forming a stable tribochemical layer. The observed tribological improvements are consistent with the mechanisms previously reported by Birleanu et al. [
3], where TiO
2 nanoparticles act as micro-rolling elements, surface polishers, and third-body spacers that reduce direct asperity contact and friction.
Although quantitative surface energy measurements were not performed, the correlation between improved crystallinity (confirmed by XRD) and smoother wear morphology (observed by SEM) indicates a thermally induced increase in surface energy and activity, consistent with literature findings [
3,
21].
In contrast, S2 (450 °C) and S3 (550 °C) present smoother wear tracks with less pronounced abrasion marks, particularly at 75 °C. The wear scars are more homogenous and reveal parallel micro-scratches, suggesting a more stable lubricating effect. For S3, this behavior is especially evident, correlating with the lowest COF and wear area values previously reported. However, no significant presence of tribofilm structures or particle deposition zones was detected in these areas. The SEM images and corresponding EDS spectra presented in
Figure 16,
Figure 17 illustrate the worn surface morphology of samples S2 and S3 tested at 75 °C. These micrographs further confirm the smoother wear tracks and reduced surface damage observed for thermally treated TiO₂ additives under elevated temperature conditions, supporting the enhanced tribological behavior previously discussed.
Figure 16.
SEM images and corresponding EDS spectra of worn surface of steel ball after four-ball test lubricated by S2 oil at 75 °C.
Figure 16.
SEM images and corresponding EDS spectra of worn surface of steel ball after four-ball test lubricated by S2 oil at 75 °C.
Figure 17.
SEM images and corresponding EDS spectra of worn surface of steel ball after four-ball test lubricated by S3 oil at 75 °C.
Figure 17.
SEM images and corresponding EDS spectra of worn surface of steel ball after four-ball test lubricated by S3 oil at 75 °C.
The EDS spectra acquired from selected wear zones did not reveal elemental Ti, nor did they indicate the formation of titanium-based compounds at the contact surface. This confirms that no detectable tribochemical film formed under the conditions tested. Instead, the improved tribological performance is attributed to physical interactions, such as rolling effects, asperity smoothing, or third body spacing, rather than permanent surface modification.
Slight traces of carbonaceous residues were noted in some regions, likely from thermal degradation of the organic components in the base oil at elevated temperature. These deposits were not uniformly distributed and do not indicate strong film formation, but rather localized residue accumulation.
Overall, the SEM and EDS results confirm that the observed improvements in tribological performance with thermally treated TiO2 additives arise from physical rather than chemical surface interactions. Although no tribofilm formation was detected, the smoother and uniform wear scars observed for samples S2 and S3 confirm the protective role of nanoparticles treated at intermediate calcination temperatures.
These surface-level findings support the overall tribological trends and are further discussed in the Conclusions section. Future studies will employ X-ray photoelectron spectroscopy (XPS) to identify the chemical states of titanium and oxygen on worn surfaces, providing additional insight into tribochemical reactions and possible film formation mechanisms.
Furthermore, the synergy between enhanced crystallinity, increased surface energy, and smoother wear morphology supports a multifactorial lubrication mechanism governed by both physical and interfacial phenomena. While the present work focuses on experimental validation, future investigations combining tribological testing with surface energy quantification and molecular dynamics simulations could provide deeper insight into the atomistic origins of friction and wear reduction in thermally optimized TiO2 nanolubricants.