Research on Surface Wear Characteristics and Adsorption Mechanism of Biodiesel Engine
Abstract
1. Introduction
2. Experimental Methods and Materials
3. Molecular Structure Characteristics and Molecular Surface Adsorption Simulation of Fuel Systems
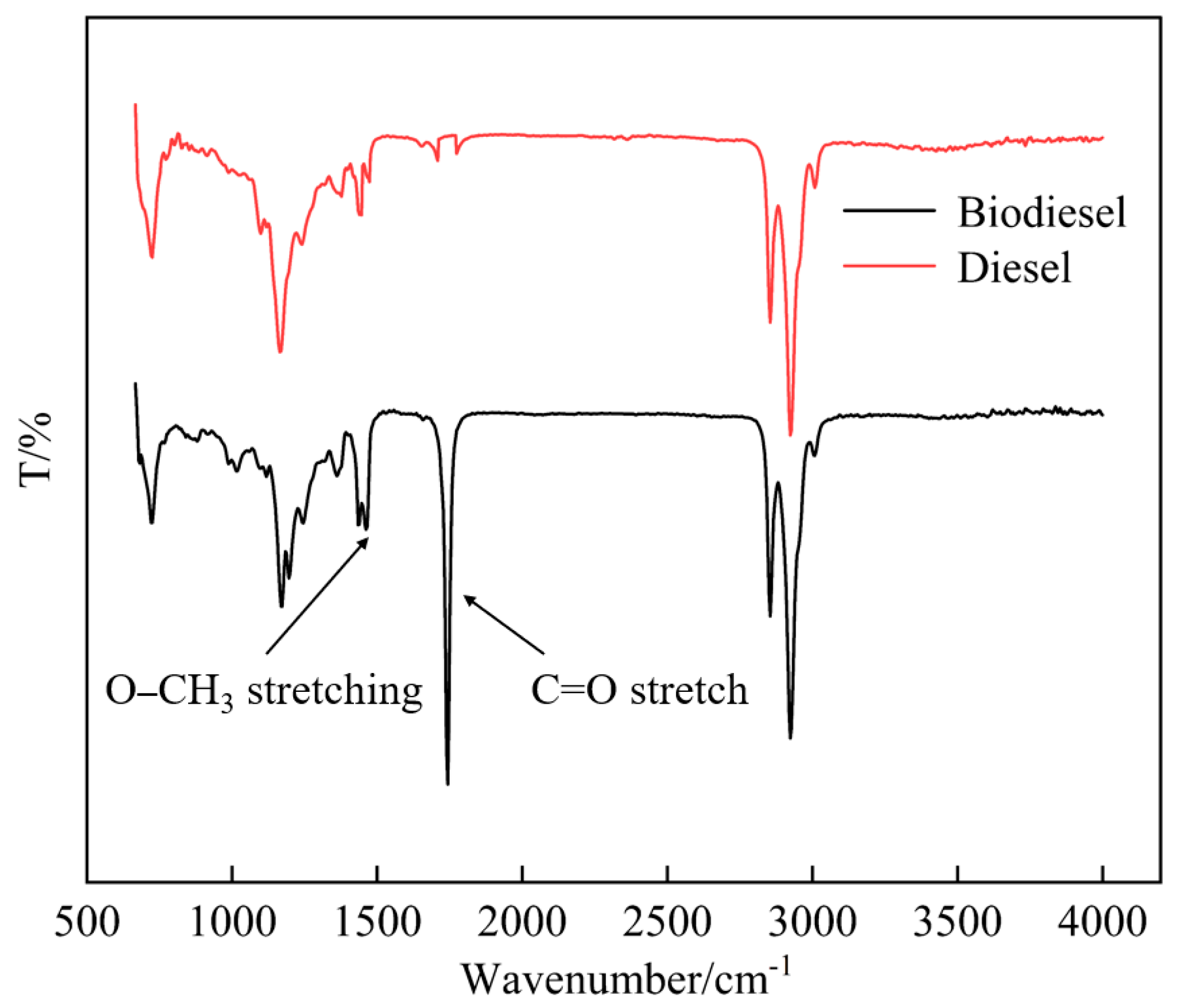
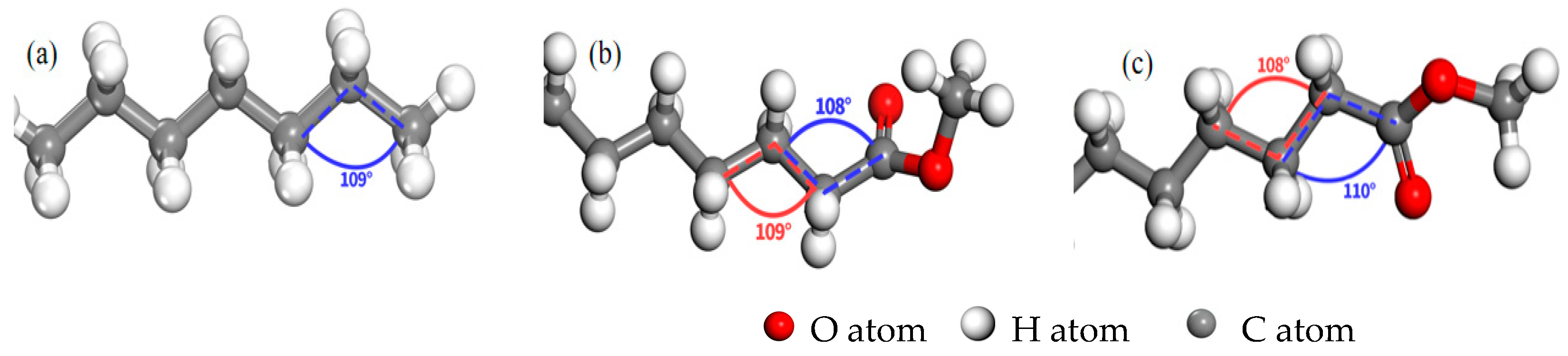
3.1. Molecular Structure Characteristics
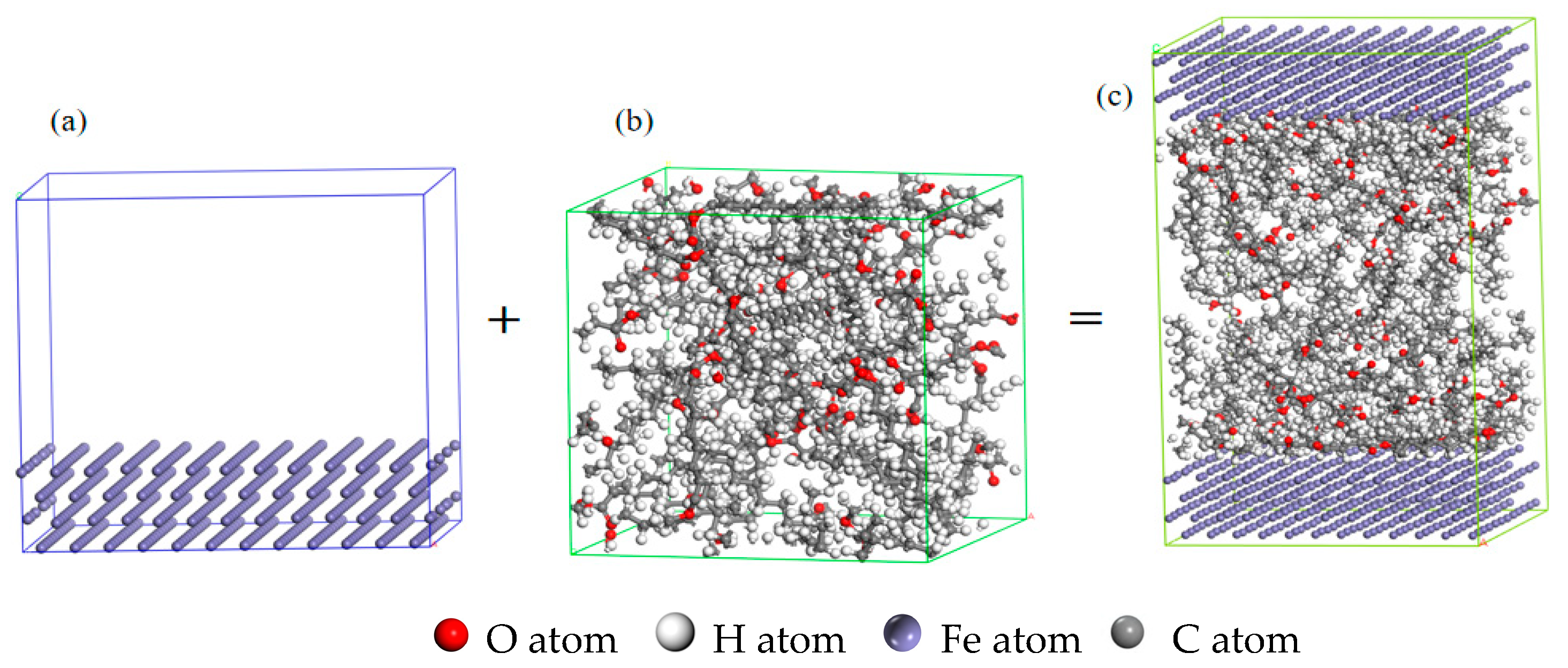
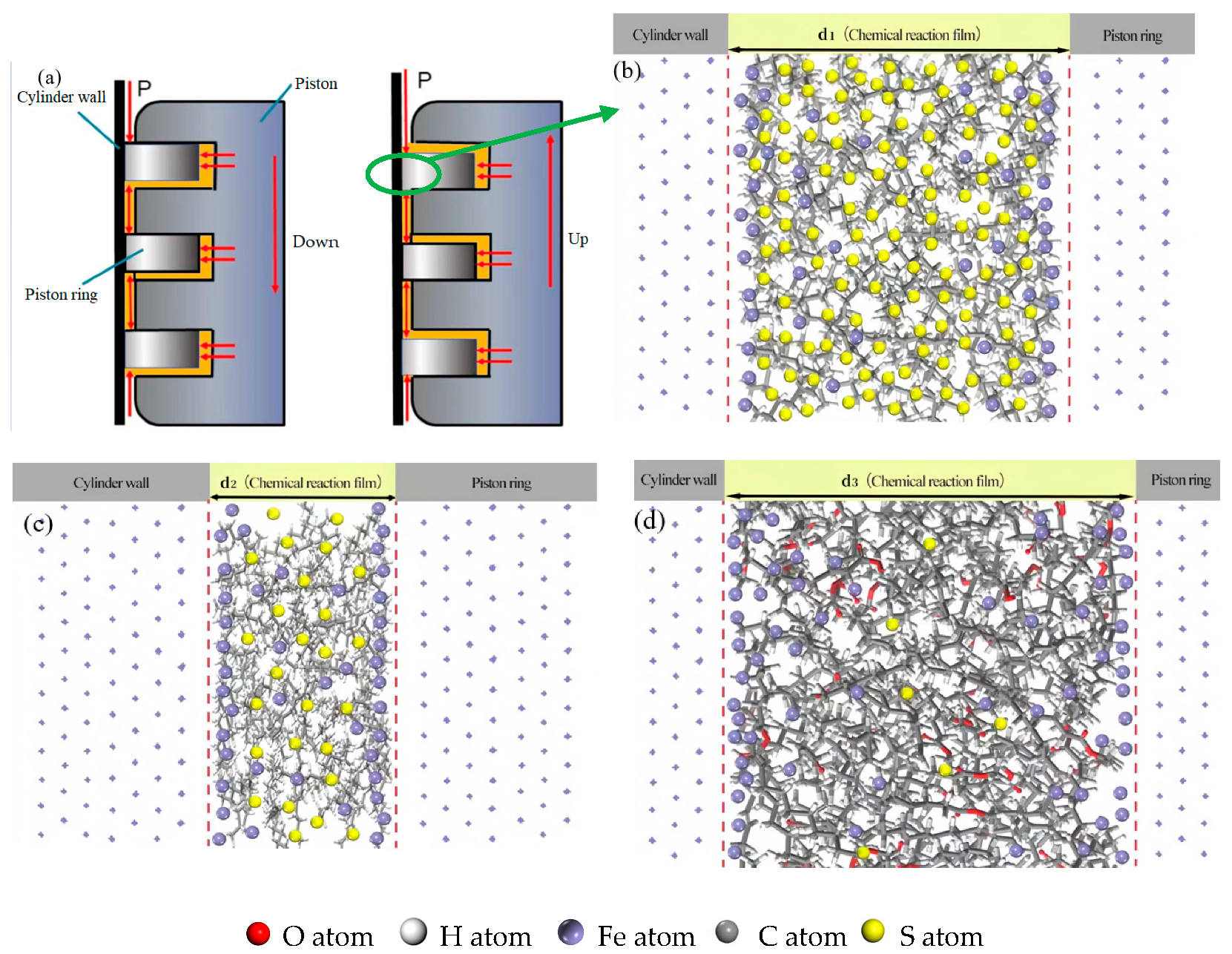
3.2. Molecular Surface Adsorption Simulation in Fuel System
4. Results and Discussion
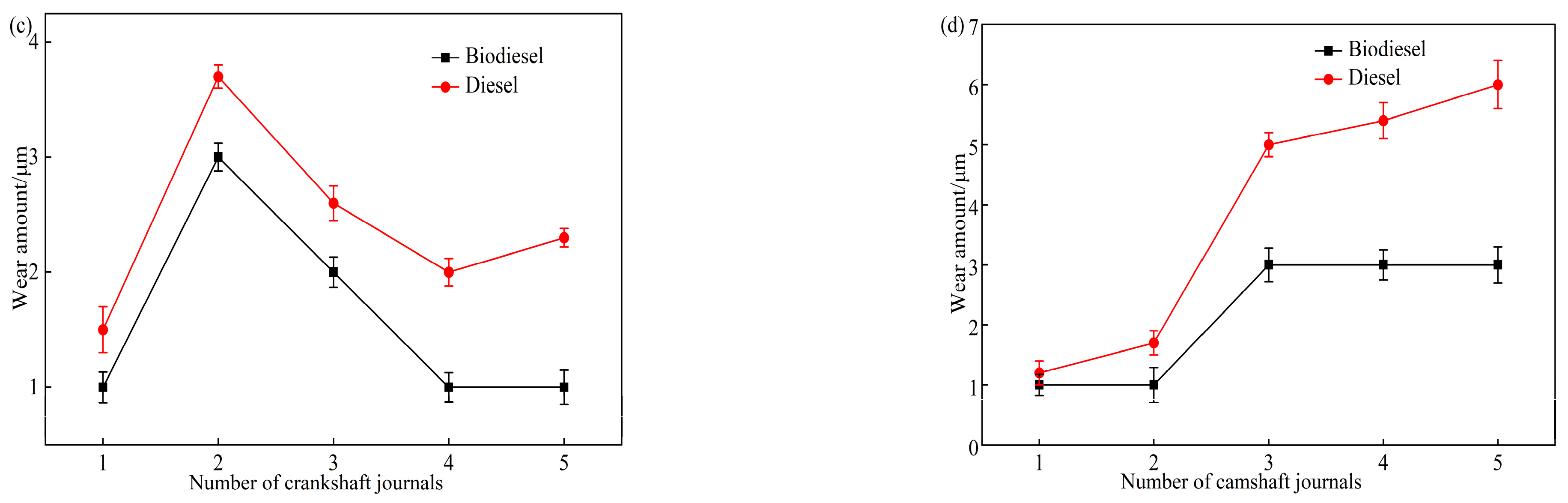
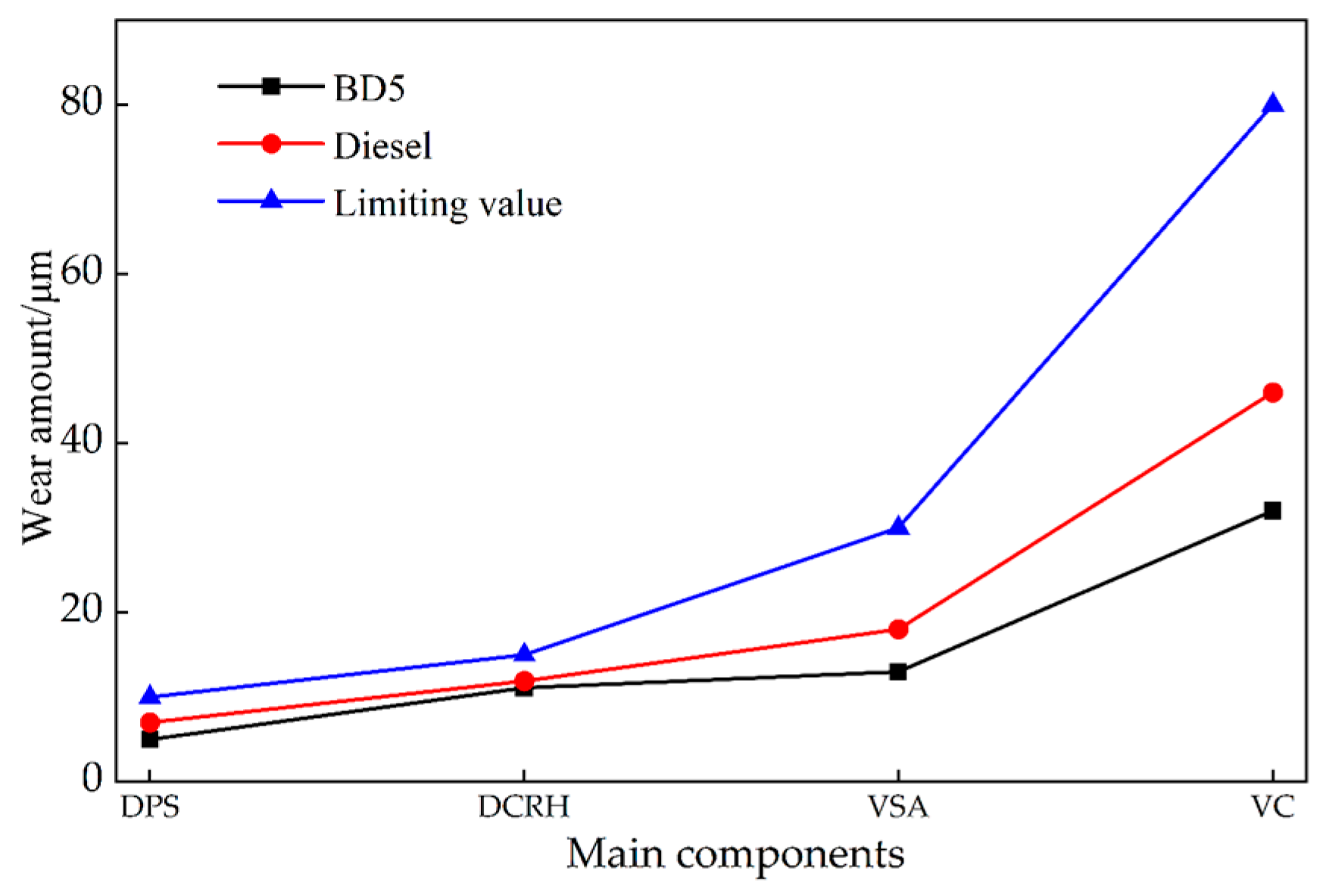
4.1. Tribological Properties
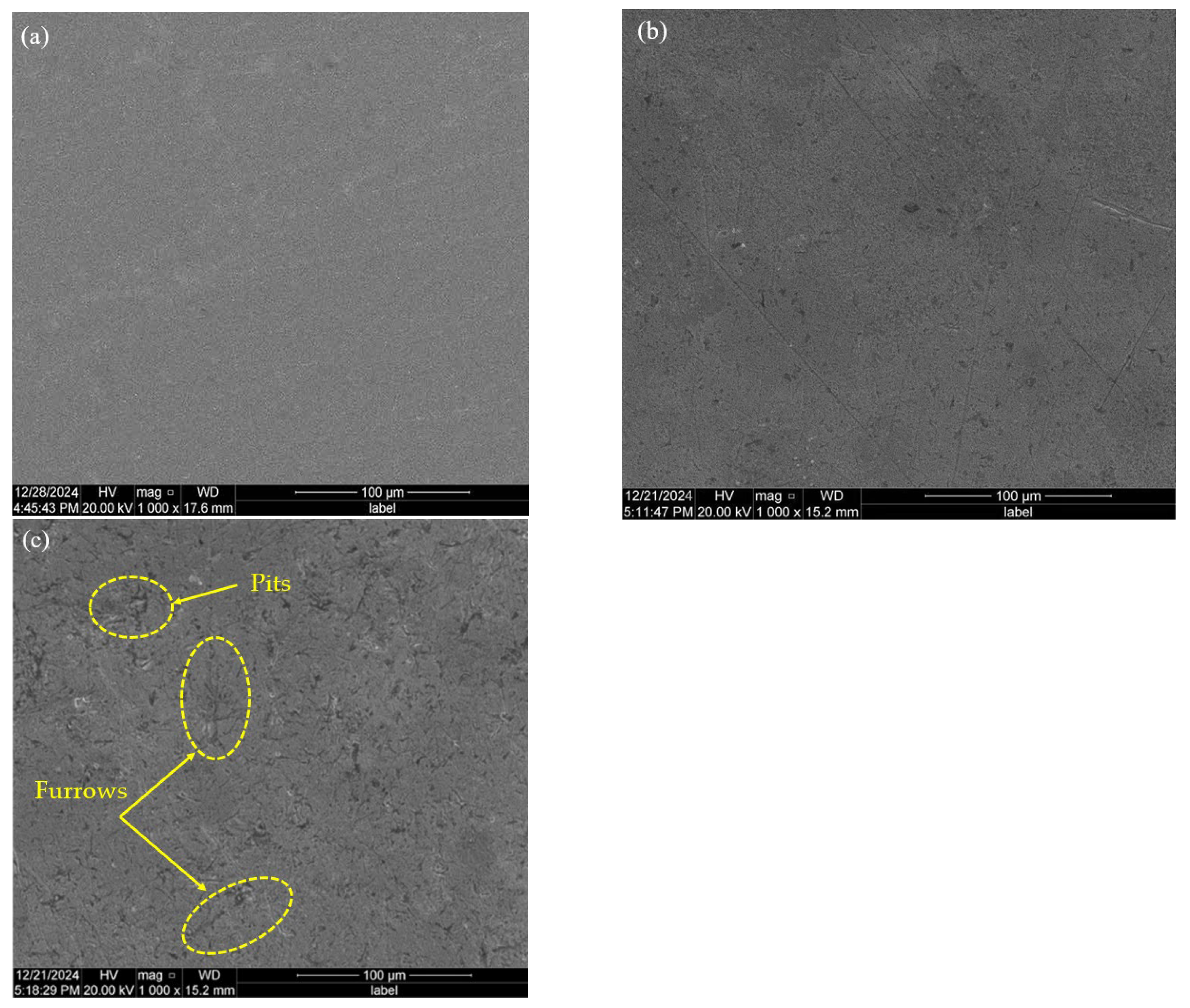
4.2. Friction Morphology and Mechanism Analysis
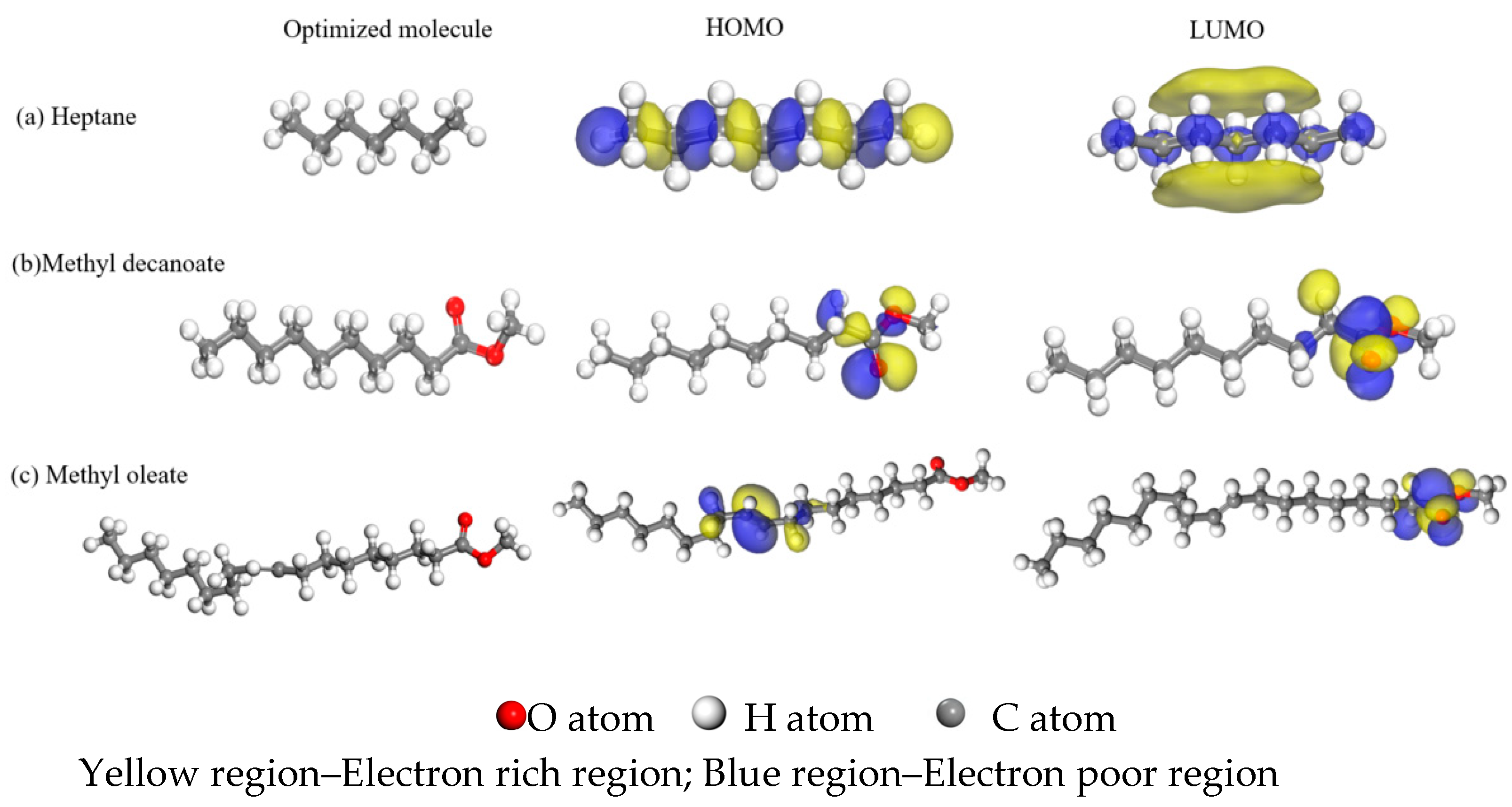
4.3. Molecular Reactivity
4.4. Adsorption Stability
5. Conclusions
- (1)
- During and after the 1000 h endurance test, no significant abnormalities were observed in the main friction pair. Wear amounts were within allowable limits. The results show that biodiesel addition reduces wear in low-sulfur diesel engine components.
- (2)
- Materials Studio simulation results revealed that on the α-Fe (110) crystal plane, the reactivity of C19H36O2, C11H22O2, and C7H16 decreased sequentially (based on density functional theory and Fukui index analysis). Frontier orbital analysis demonstrated that the LUMOs of C11H22O2 and C19H36O2 were localized at their terminal carbonyl groups, which significantly reduced steric hindrance for adsorption. This promoted their effective adsorption on the metal surface, facilitating the formation of more ordered, compact, and stable molecular films. Consequently, higher adsorption energy and absolute cohesive energy values were achieved, ultimately enhancing the anti-wear and friction-reduction performance of biodiesel at friction pair interfaces.
- (3)
- The addition of biodiesel to low-sulfur diesel systems effectively enhances the lubrication performance of diesel engine friction pairs. This improvement primarily originates from the unsaturated bonds and carbonyl functional groups in biodiesel molecules, which significantly strengthen the chemical adsorption capacity of biodiesel components on metal surfaces. Compared to low-sulfur diesel, this stronger adsorption not only promotes the formation of a thicker tribochemical reaction film but also establishes more robust bonding between the film and the metal surface, thereby improving adsorption stability. This firmly bonded thick tribochemical reaction film can form a stable lubricating layer on friction pair surfaces, consequently effectively reducing wear.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FAME | Fatty Acid Methyl Esters |
| ULSD | Ultra-Low Sulfur Diesel |
| DPS | Diameter of piston skirt |
| DCRH | Diameter of connecting rod big end hole |
| VSA | Valve sinking amount |
| VC | Valve clearance |
| NVT | Number of particles (N), volume (V), temperature (T) |
| SEM | Scanning Electron Microscope |
| EHOMO | Energy of the Highest Occupied Molecular Orbital |
| ELUMO | Energy of the Lower Unoccupied Molecular Orbital |
| HOMO | Highest Occupied Molecular Orbital |
| LUMO | Lower Unoccupied Molecular Orbital |
References
- Wang, X.; Li, Y.; Liu, J.S.; Lin, J.M.; Zhang, J.R. Molecular structure design of diesel anti-wear agents. Acta Pet. Sin. 2018, 34, 229–237. [Google Scholar]
- Mateus, J.; Anes, V.; Galvão, I.; Reis, L. Failure mode analysis of a 1.9 turbo diesel engine crankshaft. Eng. Fail. Anal. 2019, 101, 394–406. [Google Scholar] [CrossRef]
- Ding, X.P.; Gu, P.L.; Li, A.W.; Lin, J.M. Formulation and revision of Sinopec enterprise standards for diesel anti-wear additives. Pet. Process. Petrochem. 2018, 49, 64–69. [Google Scholar]
- Li, Y.; Long, J.; Zhao, Y.; Lin, J.M.; Zhou, H. Effect of polar groups of diesel anti-wear agent of anti-wear performance. Pet. Process. Petrochem. 2019, 50, 1005–2399. [Google Scholar]
- Liu, T.X.; Qin, J.; Kang, K.; Wang, J. Effects of lanthanum compounds on tribological properties of diesel soot particles synthesized by Fischer-Tropsch process. Surf. Technol. 2021, 50, 246–254. [Google Scholar]
- Li, L.L.; Wang, Z.; Qin, X.F.; Xu, G.J.; Chen, L. Simulation Study on the Benzene Generated of Composition and Structure of Biodiesel; Zhengzhou University (Engineering Science): Zhengzhou, China, 2011; Volume 32, pp. 67–70. [Google Scholar]
- LI, L.L.; LIU, J.Y. Numerical simulation on the impact of NOx emissions with initial inlet conditions of biodiesel engine. Mach. Des. Manuf. 2015, 3, 221–224. [Google Scholar]
- Reddy, S.M.; Sharma, N.; Gupta, N.; Agarwal, A.K. Effect of non-edible oil and its biodiesel on wear of fuel injection equipment components of a genset engine. Fuel 2018, 222, 841–851. [Google Scholar] [CrossRef]
- Gupta, J.G.; Agarwal, A.K. Engine durability and lubricating oil tribology study of a biodiesel fuelled common rail direct injection medium-duty transportation diesel engine. Wear 2021, 487, 204104. [Google Scholar] [CrossRef]
- Awang, M.S.N.; Zulkifli, N.W.M.; Abbas, M.M.; Zulkifli, S.A.; Kalam, M.A.; Yusoff, M.N.A.M.; Daud, W.M.A.W.; Ahmad, M.H. Effect of diesel-palm biodiesel fuel with plastic pyrolysis oil and waste cooking biodiesel on tribological characteristics of lubricating oil. Alex. Eng. J. 2022, 61, 7221–7231. [Google Scholar] [CrossRef]
- Li, F.S.; Liu, Z.W.; Ni, Z.H.; Wang, H. Effect of biodiesel components on its lubrication performance. J. Mater. Res. Technol. 2019, 8, 3681–3687. [Google Scholar] [CrossRef]
- Xu, Y.F.; Zheng, Q.; Geng, J.; Dong, Y.H.; Tian, M.; Yao, L.L.; Dearn, K.D. Synergistic effects of electroless piston ring coatings and nano-additives in oil on the friction and wear of a piston ring/cylinder liner pair. Wear 2019, 422, 201–211. [Google Scholar] [CrossRef]
- Li, B.; Dearn, D.K.; Lui, Y.M.; Hu, E.Z.; Xu, Y.F.; Hu, X.G. Effect of emulsified biomass fuel on the film formation behavior of diesel engine lubricating oil. Acta Pet. Sin. 2016, 32, 1142–1149. [Google Scholar]
- Zhou, D.P.; Wei, H.J.; Wang, Z.C.; Wu, G.; Qiu, Y. Analysis on oxidation mechanism and lubricity evolution of biodiesel. J. Shanghai Marit. Univ. 2020, 41, 105–109. [Google Scholar]
- Mei, D.Q.; Luo, Y.Q.; Shen, X.F.; Lu, L.D.; Yuan, Y.Y. Lubrication properties of fatty acid methyl esters as low-sulfur diesel enhancers. Trans. Chin. Soc. Agric. Eng. 2016, 32, 193–197. [Google Scholar]
- Mei, D.; Wang, H.; Dai, P.; Li, X. A statistical analysis of emission features in non-road small SI engines with the same displacement. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2017, 232, 7–14. [Google Scholar] [CrossRef]
- Li, Z.; Niu, S.; Han, K.; Li, Y.; Wang, Y.; Lu, C.; Cheng, S. Investigation into influences of methanol pre-adsorption on Ca0(100) surface in transesterification for biodiesel production with molecular simulation. Appl. Catal. A Gen. 2021, 609, 117908. [Google Scholar] [CrossRef]
- Canterelli, C.; Darenne, B.; Fortunato, M.A.; Bruin, T.; Costa, D. DFT screening of adsorption of biodiesel molecules on aluminum and stainless steel surfaces. Results Surf. Interfaces 2022, 6, 100050. [Google Scholar] [CrossRef]
- Curran, H.J.; Gaffuri, P.; Pitz, W.J.; Westbrook, C.K. A comprehensive Modeling Study of n-Heptane Oxidation. Combust. Flame 1998, 114, 149–177. [Google Scholar] [CrossRef]
- Marchal, C.; Delfau, J.L.; Vovelle, C.; Moréac, G.; Mounaı, C.; Mauss, F. Modelling of aromatics and soot formation from large fuel molecules. Proc. Combust. Inst. 2009, 32, 753–759. [Google Scholar] [CrossRef]
- Herbinet, O.; Pitz, W.J.; Westbrook, C.K. Detailed chemical kinetic oxidation mechanism for a biodiesel surrogate. Combust. Flame 2008, 154, 507–528. [Google Scholar] [CrossRef]
- Wang, H.; Frenklach, M. A detailed kinetic modeling study of aromatics formation in laminar premixed acetylene and ethylene flames. Combust. Flame 1997, 110, 173–221. [Google Scholar] [CrossRef]
- Pham, T.H.; Lee, W.H.; Byun, J.H.; Kim, J.G. Improving the performance of primary aluminum-air batteries through suppressing water activity by hydrogen bond-rich glycerol solvent additive. Energy Storage Mater. 2023, 55, 406–416. [Google Scholar] [CrossRef]
- Bai, N.; Ni, Z.H.; Zhang, H.C.; Li, F.H.; Wang, H. Influence mechanism of phenolic-amine antioxidant composite on biodiesel-induced metal corrosion: The role of competitive adsorption. Renew. Energy 2025, 239, 122118. [Google Scholar] [CrossRef]
- Ouakki, M.; Rbaa, M.; Benhiba, F.; Galai, M.; Idouhli, R.; Maatallah, M.; Jarid, A.; Warad, I.; Lakhrissi, B.; Zarrouk, A.; et al. Experimental and theoretical investigation of corrosion inhibition effect of two 8-hydroxyquinoline carbonitrile derivatives on mild steel in 1 M HCl solution. J. Phys. Chem. Solids 2022, 169, 110866. [Google Scholar] [CrossRef]
- Han, X.R.; Li, R.Y.; Li, F.S.; Duan, Y.Z. Insights into the inhibitive mechanism of chelators for biodiesel oxidation induced by Cu2+/Fe2+: An experimental and computational study. Fuel 2025, 389, 134607. [Google Scholar] [CrossRef]
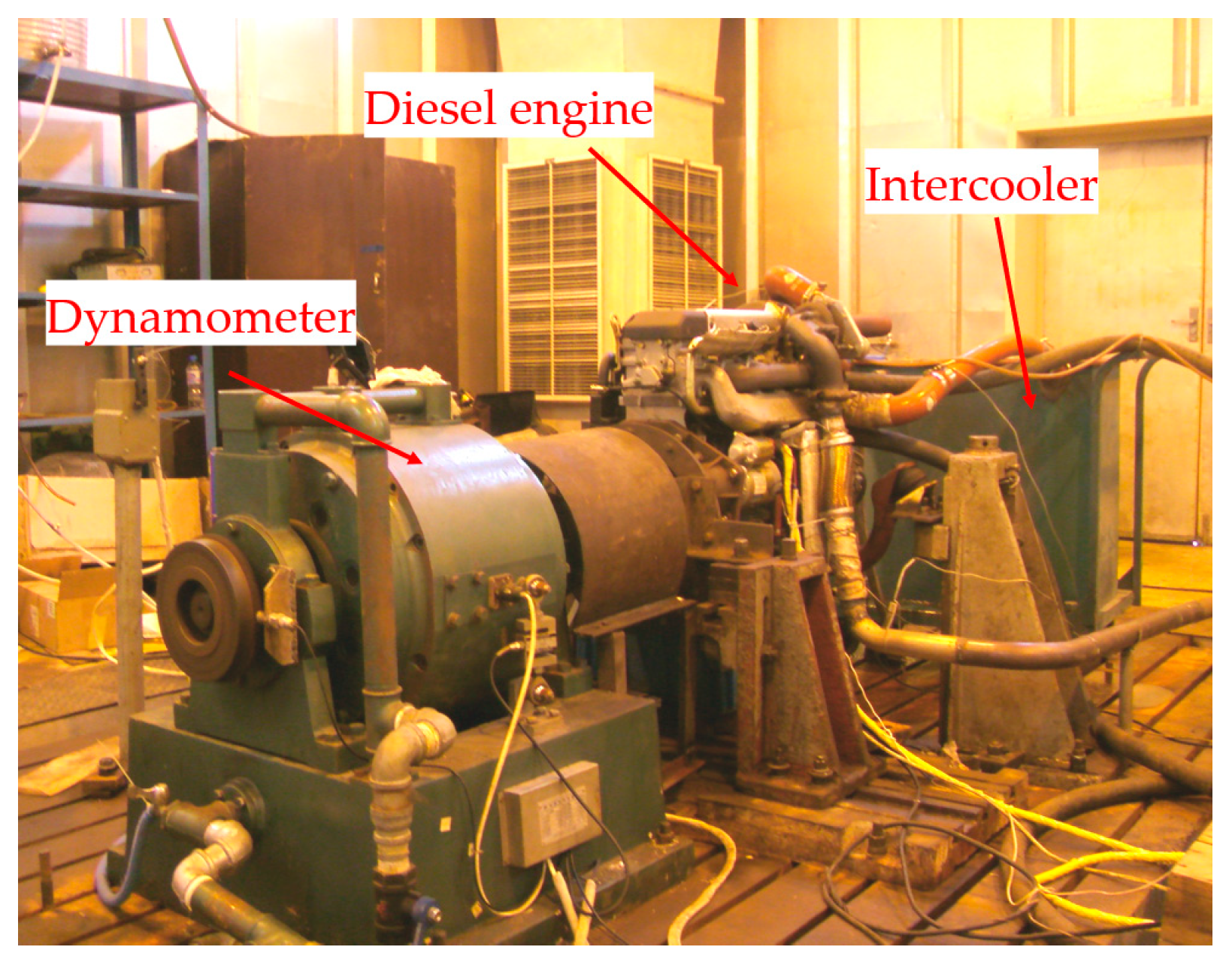






| Engine Type | 4-Stroke, Turbocharged, Inter-Cooled Diesel |
|---|---|
| Number of Cylinders | 4 |
| Cylinder Diameter | 94 mm |
| Cubic Capacity | 2770 cc |
| Max. Power Output | 92@3000 (kW, rpm) |
| Maximum Torque | 285@1800 (Nm, rpm) |
| Compression ratio | 18.5:1 |
| Molecule | EHOMO/eV | ELUMO/eV | ΔE/eV | μ/eV | η/eV | σ/eV−1 | ω/eV |
|---|---|---|---|---|---|---|---|
| C7H16 | −7.088 | 1.511 | 8.600 | −2.788 | 4.300 | 0.232 | 0.904 |
| C11H22O2 | −6.103 | 0.755 | 6.859 | −2.673 | 3.429 | 0.291 | 1.042 |
| C19H36O2 | −5.429 | 0.736 | 6.166 | −2.346 | 3.083 | 0.324 | 0.893 |
| System | Adsorption Energy/kJ·mol−1 | Cohesive Energy/kJ·mol−1 |
|---|---|---|
| C7H16 | −2862.3 | −1570.7 |
| C11H22O2 | −3193.5 | −2286.4 |
| C19H36O2 | −3150.1 | −2104.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Mao, Y.; Chen, D.; Chang, J.; Qin, X.; Qu, X.; Wei, Z.; Ma, R. Research on Surface Wear Characteristics and Adsorption Mechanism of Biodiesel Engine. Lubricants 2025, 13, 434. https://doi.org/10.3390/lubricants13100434
Li L, Mao Y, Chen D, Chang J, Qin X, Qu X, Wei Z, Ma R. Research on Surface Wear Characteristics and Adsorption Mechanism of Biodiesel Engine. Lubricants. 2025; 13(10):434. https://doi.org/10.3390/lubricants13100434
Chicago/Turabian StyleLi, Lilin, Yazhou Mao, Dan Chen, Jingjing Chang, Xianfeng Qin, Xiang Qu, Zhenghan Wei, and Runyi Ma. 2025. "Research on Surface Wear Characteristics and Adsorption Mechanism of Biodiesel Engine" Lubricants 13, no. 10: 434. https://doi.org/10.3390/lubricants13100434
APA StyleLi, L., Mao, Y., Chen, D., Chang, J., Qin, X., Qu, X., Wei, Z., & Ma, R. (2025). Research on Surface Wear Characteristics and Adsorption Mechanism of Biodiesel Engine. Lubricants, 13(10), 434. https://doi.org/10.3390/lubricants13100434





