Assessing Antioxidant and Pour Point Depressant Capacity of Turmeric Rhizome Extract in Biolubricants
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material and Processing
2.2. Determination of Functional Groups and Compounds
2.2.1. Fourier-Transform Infrared (FT-IR) Spectroscopy
2.2.2. Gas Chromatography-Mass Spectrometry (GC-MS)
2.3. Blending of Biolubricant with Antioxidant
2.4. Determination of Peroxide Value (PV) and Pour Point
2.5. Statistical Analysis
3. Results and Discussion
3.1. Fourier-Transform Infrared Spectroscopy (FTIR) of Crude Turmeric Extract
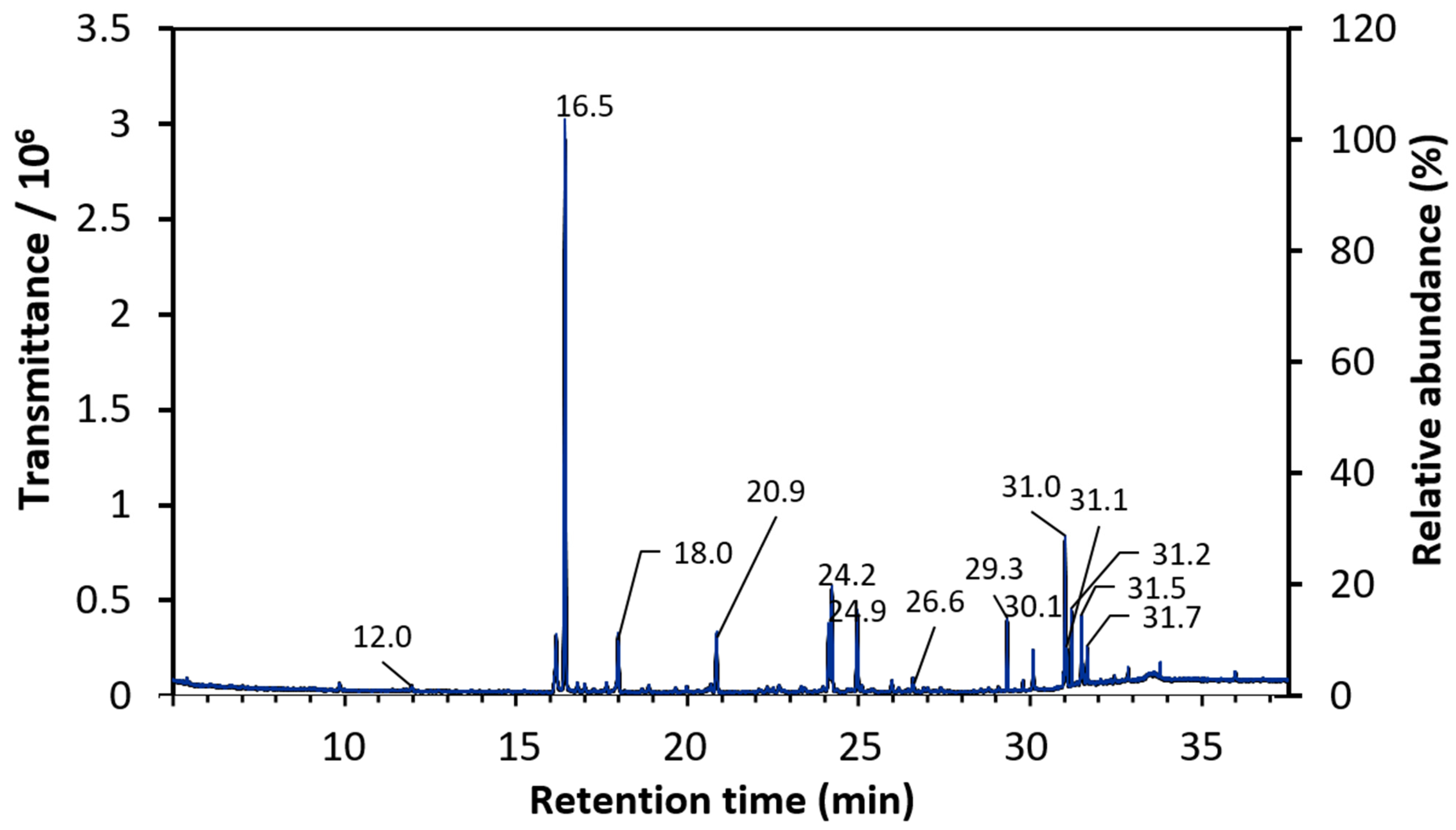
3.2. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
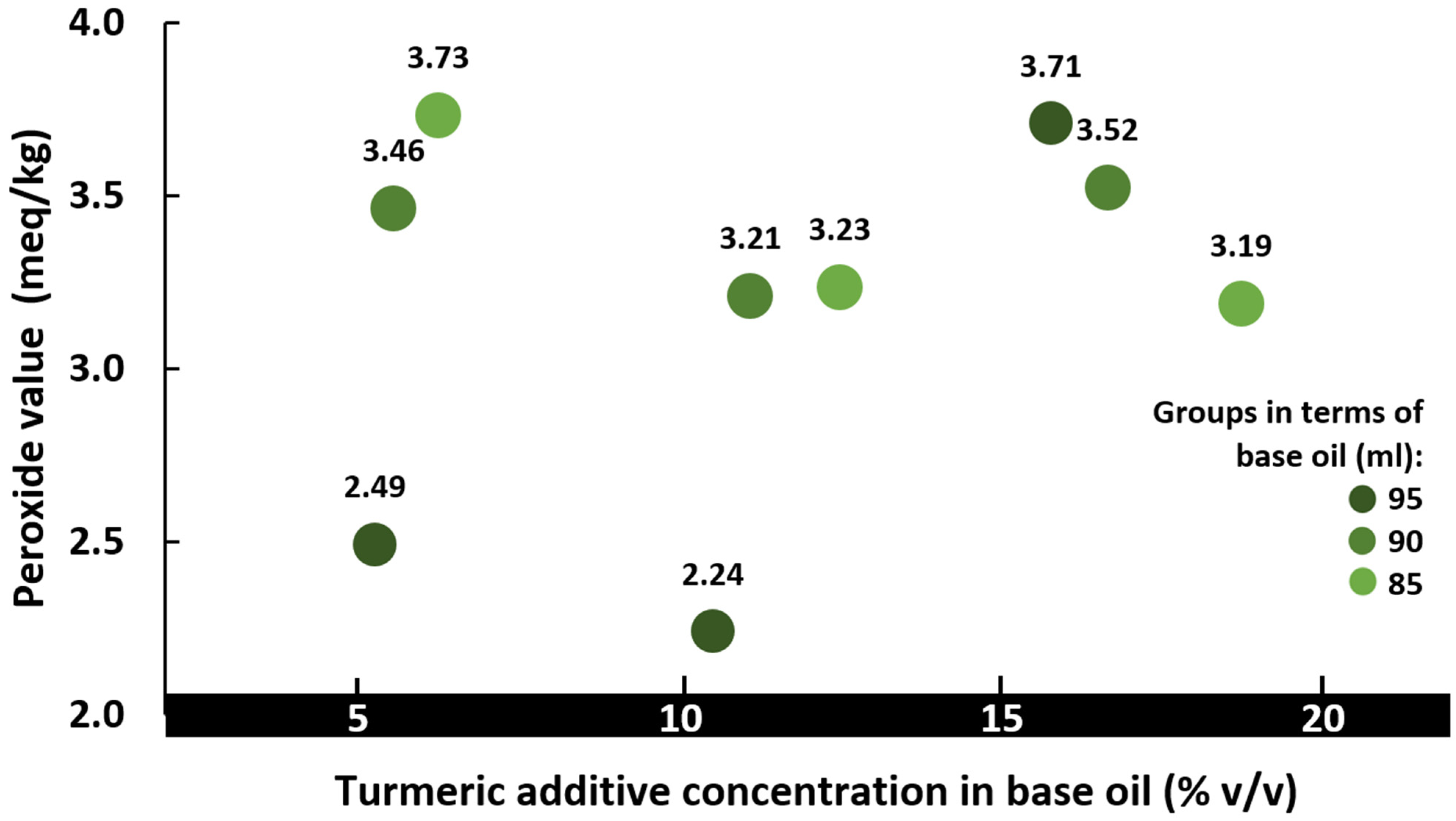
3.3. Peroxide Value of Biolubricant Blended with Turmeric Extract as Additive
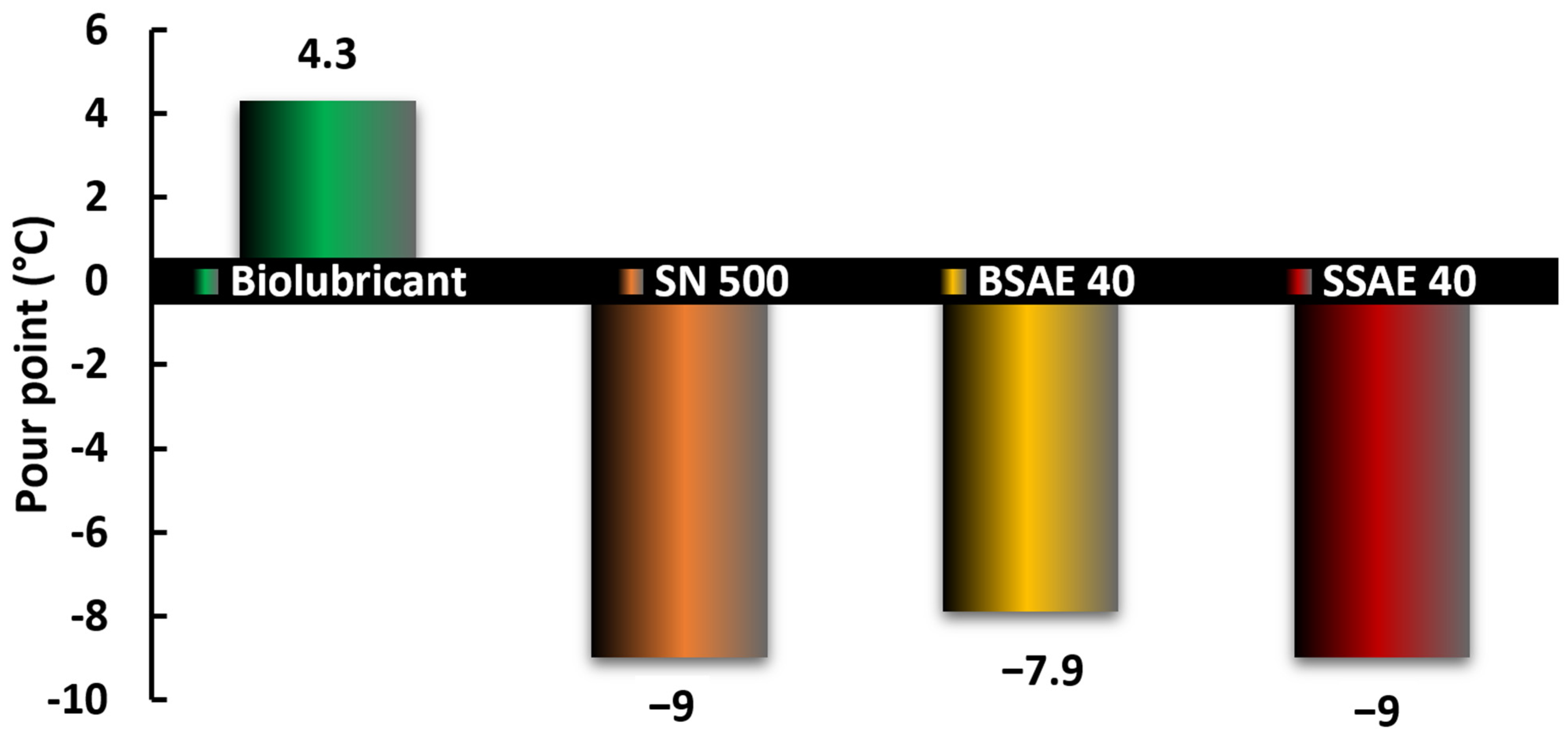
3.4. Pour Point Response of Different Blends with Turmeric Additive
4. Conclusions
- The GC-MS technique detected phenol, 2 -methoxy-3-(2-propenyl) and Phenol, 2-methoxy-4-(2-propenyl)-, acetate having 36.3% and 3.8% area, respectively. The phenols observed in this study are not in their pure state, but rather in combination with other atoms.
- The addition of the turmeric rhizome was observed to have a substantial impact on decreasing the pour point, and also decreasing the peroxide values for a proportion range between 5 to 12.5% turmeric additive content (5:95 to 1:8) with biobased shea oil.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abbas, M.; Saeed, F.; Anjum, F.M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef]
- Adeniyi, A.G.; Abdulkareem, S.A.; Ighalo, J.O.; Amosa, M.K.; Papoola, A.O.; Ogunniyi, S.; Abdulkareem, M.T. Usage of Biomass-based Carbon Materials as Lubricant Additive: Effects on Rheological and Tribological Properties. Lett. Appl. NanobioScience 2021, 10, 2861–2868. [Google Scholar] [CrossRef]
- Aluyor, E.O.; Audu TO, K. Effect of lubrication additives on the physical and chemical properties of soyabean oil. Adv. Mater. Res. 2009, 64, 374–379. [Google Scholar] [CrossRef]
- Bouaziz, M.; Fki, I.; Jemai, H.; Ayadi, M.; Sayadi, S. Food Chemistry Effect of storage on refined and husk olive oils composition: Stabilization by addition of natural antioxidants from Chemlali olive leaves. Food Chem. 2008, 108, 253–262. [Google Scholar] [CrossRef]
- Chamorro, S.; Gon, I.; Hervert-herna, D. Changes in polyphenolic content and antioxidant activity after thermal treatments of grape seed extract and grape pomace. Eur. Food Res. Technol. 2012, 234, 147–155. [Google Scholar] [CrossRef]
- Ebrahimzadeh, M.A.; Gharekhani, M.; Ghorbani, M. Effect of Extract of Aerial Parts of Urtica dioica (Urticaceae) on the Stability of Soybean Oil. Trop. J. Pharm. Res. 2015, 14, 125–131. [Google Scholar] [CrossRef]
- Farag, R.S.; El-Baroty, G.S.; Basuny, A.M. The influence of phenolic extracts obtained from the olive plant (cvs. Picual and Kronakii), on the stability of sunflower oil. Int. J. Food Sci. Technol. 2003, 38, 81–87. [Google Scholar] [CrossRef]
- Ishaya, Z. Chemical compound transformation of canarium schweinfurtii using ftir & gcms techniques in biodiesel production. FUW Trends Sci. Technol. J. 2022, 7, 352–356. [Google Scholar]
- Ishaya, Z. Original Research Article Biodiesel Production from Thevetia peruviana Seed Oil using FTIR and GC-MS Techniques to Investigate the Conversion Process. Niger. Res. J. Eng. Environ. Sci. 2022, 7, 519–528. [Google Scholar]
- Jesbin, K.S.; Mahipal, D. Evaluation of tribological characteristics of natural garlic oil as an additive in rubber seed oil. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1114, 012052. [Google Scholar] [CrossRef]
- Kaisan, M.U.; Abubakar, S.; Ashok, B.; Balasubramanian, D.; Narayan, S.; Grujic, I.; Stojanovic, N. Comparative analyses of biodiesel produced from jatropha and neem seed oil using a gas chromatography–mass spectroscopy technique. Biofuels 2018, 12, 757–768. [Google Scholar] [CrossRef]
- Karmakar, G.; Dey, K.; Ghosh, P.; Sharma, B.K. A Short Review on Polymeric Biomaterials as Additives for Lubricants. Polymers 2021, 13, 1333. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Li, S.; Zhang, Y.; Xu, X.; Chen, Y.; Li, H. Resources and Biological Activities of Natural Polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef] [PubMed]
- Malik, P.; Mukherjee, T.K. Structure-Function Elucidation of Antioxidative and Prooxidative Activities of the Polyphenolic Compound Curcumin. Chin. J. Biol. 2014, 2014, 396708. [Google Scholar] [CrossRef]
- Naser, J.A.; Ahmed, Z.W.; Ali, E.H. Plant Leaves Extracts as Green Inhibitors for Corrosion of Carbon Steel; a Review. Ann. Rom. Soc. Cell Biol. 2021, 25, 5332–5340. [Google Scholar]
- Nasibi, M.; Mohammady, M.; Ashrafi, A.; Dehno, A.A.; Moshrefifar, M.; Rafiee, E.; Iranian, N.; Refining, O.; Region, Y. Nanosized scale roughness and corrosion protection of mild steel in hydrochloric acid solution and in the presence of Turmeric (Curcuma longa) Extract as a green corrosion inhibitor: FTIR, polarization, EIS, SEM, EDS, AFM studies, and neural network modeling. J. Adhes. Sci. Technol. 2015, 28, 2001–2015. [Google Scholar] [CrossRef]
- Ogwuche, C.E.; Edema, M.O. GC-MS and FTIR characterization of essential oil from the fresh leaves of Pandanus candalabrum obtained from Bayelsa Ofoegbu and Kelle, Nigeria. Niger. J. Chem. Res. 2020, 25, 1–10. [Google Scholar]
- Ogunleye, O.O.; Arinkoola, A.O.; Eletta, O.A.; Agbede, O.O.; Osho, Y.A.; Morakinyo, A.F.; Hamed, J.O. Green corrosion inhibition and adsorption characteristics of Luffa cylindrica leaf extract on mild steel in hydrochloric acid environment. Heliyon 2020, 6, e03205. [Google Scholar] [CrossRef]
- Özcan, M.M.; Arslan, D. Antioxidant effect of essential oils of rosemary, clove and cinnamon on hazelnut and poppy oils. Food Chem. 2011, 129, 171–174. [Google Scholar] [CrossRef]
- Delgado, M.A.; García-Rico, C.; Franco, J.M. The use of rosemary extracts in vegetable oil-based lubricants. Ind. Crops Prod. 2014, 62, 474–480. [Google Scholar] [CrossRef]
- Rafiee, Z.; Jafari, S.M.; Alami, M.; Khomeiri, M. Antioxidant effect of microwave-assisted extracts of olive leaves on sunflower oil. J. Agric. Sci. Technol. 2012, 14, 1497–1509. [Google Scholar]
- Xia, Y.; Xu, X.; Feng, X.; Chen, G. Leaf-surface wax of desert plants as a potential lubricant additive. Friction 2015, 3, 208–213. [Google Scholar] [CrossRef]
- Gavalás-Olea, A.; Siol, A.; Sakka, Y.; Köser, J.; Nentwig, N.; Hauser, T.; Filser, J.; Thöming, J.; Lang, I. Potential of the Red Alga Dixoniella grisea for the Production of Additives for Lubricants. Plants 2021, 10, 1836. [Google Scholar] [CrossRef] [PubMed]
- Oshunsanya, S.O.; Yu, H.; Ojeade, D.E.; Odebode, A.M. Soil transportation due to harvesting of ginger and turmeric under tillage management practices. Soil Tillage Res. 2024, 242, 106154. [Google Scholar] [CrossRef]
- Taghvaei, M.; Jafari, S.M. Application and stability of natural antioxidants in edible oils in order to substitute synthetic additives. J. Food Sci. Technol. 2015, 52, 1272–1282. [Google Scholar] [CrossRef] [PubMed]
- Lučan Čolić, M.; Antunović, M.; Jukić, M.; Popović, I.; Lukinac, J. Sensory Acceptance and Characterisation of Turmeric- and Black-Pepper-Enriched Ice Cream. Appl. Sci. 2023, 13, 11802. [Google Scholar] [CrossRef]
- Augustyńska-Prejsnar, A.; Topczewska, J.; Ormian, M.; Saletnik, A.; Sokołowicz, Z.; Lechowska, J. The Effect of the Addition Turmeric on Selected Quality Characteristics of Duck Burgers Stored under Refrigeration. Appl. Sci. 2022, 12, 805. [Google Scholar] [CrossRef]
- Tanvir, E.M.; Hossen, S.; Hossain, F.; Afroz, R.; Gan, S.H. Antioxidant Properties of Popular Turmeric (Curcuma longa) Varieties Bangladesh. J. Food Qual. 2017, 2017, 8471785. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, Z.; Chen, F.; Su, P.; Chen, D.; Pan, W.; Fang, Y.; Dong, C.; Zheng, X.; Du, Z. Composition and bioactivity assessment of essential oils of Curcuma longa L. collected in China. Ind. Crops Prod. 2017, 109, 60–73. [Google Scholar] [CrossRef]
- Calovi, M.; Rossi, S. Exploiting Turmeric’s Coloring Capability to Develop a Functional Pigment for Wood Paints: Sustainable Coloring Process of Polyamide 11 Powders and Their Strengthening Performance. Coatings 2024, 14, 858. [Google Scholar] [CrossRef]
- Valenzuela, M.; Ciudad, G.; Cárdenas, J.P.; Medina, C.; Salas, A.; Oñate, A.; Pincheira, G.; Attia, S.; Tuninetti, V. Towards the development of performance-efficient compressed earth blocks from industrial and agro-industrial by-products. Renew. Sustain. Energy Rev. 2024, 194, 114323. [Google Scholar] [CrossRef]
- Ibrahim IA, A.; Yusuf, A.J. Extraction and physicochemical analysis of Citrus sinesis seed oil (sweet orange). Eur. J. Exp. Biol. 2015, 5, 77–81. [Google Scholar]
- Taylor, P.; Chane-ming, J.; Vera, R.; Chalchat, J.; Cabassu, P.; Chane-ming, J. Chemical Composition of Essential Oils from Rhizomes, Leaves and Flowers of Curcuma longa L. from Reunion Island. J. Essent. Oil Res. 2002, 14, 249–251. [Google Scholar]
- Wealleans, A.L.; Bierinckx, K.; Witters, E.; Wiseman, J. Assessment of the quality, oxidative status and dietary energy value of lipids used in non-ruminant animal nutrition. J. Sci. Food Agric. 2021, 101, 4266–4277. [Google Scholar] [CrossRef] [PubMed]
- Mariod, A.; Matthäus, B.; Hussein, I.H. Antioxidant activities of extracts from Combretum hartmannianum and Guiera senegalensis on the oxidative stability of sunflower oil. Emir. J. Food Agric. 2006, 18, 20–28. [Google Scholar] [CrossRef]
- Syahir, A.Z.; Zulkifli NW, M.; Masjuki, H.H.; Kalam, M.A.; Alabdulkarem, A.; Gulzar, M.; Khuong, L.S.; Harith, M.H. A review on bio-based lubricants and their applications. J. Clean. Prod. 2017, 168, 997–1016. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Z.; Zhang, Y.; Gao, B.; Li, Y.; He, X.; Sun, J.; Choe, U.; Chen, P.; Blaustein, R.A.; et al. Chemical Composition of Turmeric (Curcuma longa L.) Ethanol Extract and Its Antimicrobial Activities and Free Radical Scavenging Capacities. Foods 2024, 13, 1550. [Google Scholar] [CrossRef]
- Kępińska-Pacelik, J.; Biel, W. Turmeric and Curcumin—Health-Promoting Properties in Humans versus Dogs. Int. J. Mol. Sci. 2023, 24, 14561. [Google Scholar] [CrossRef]
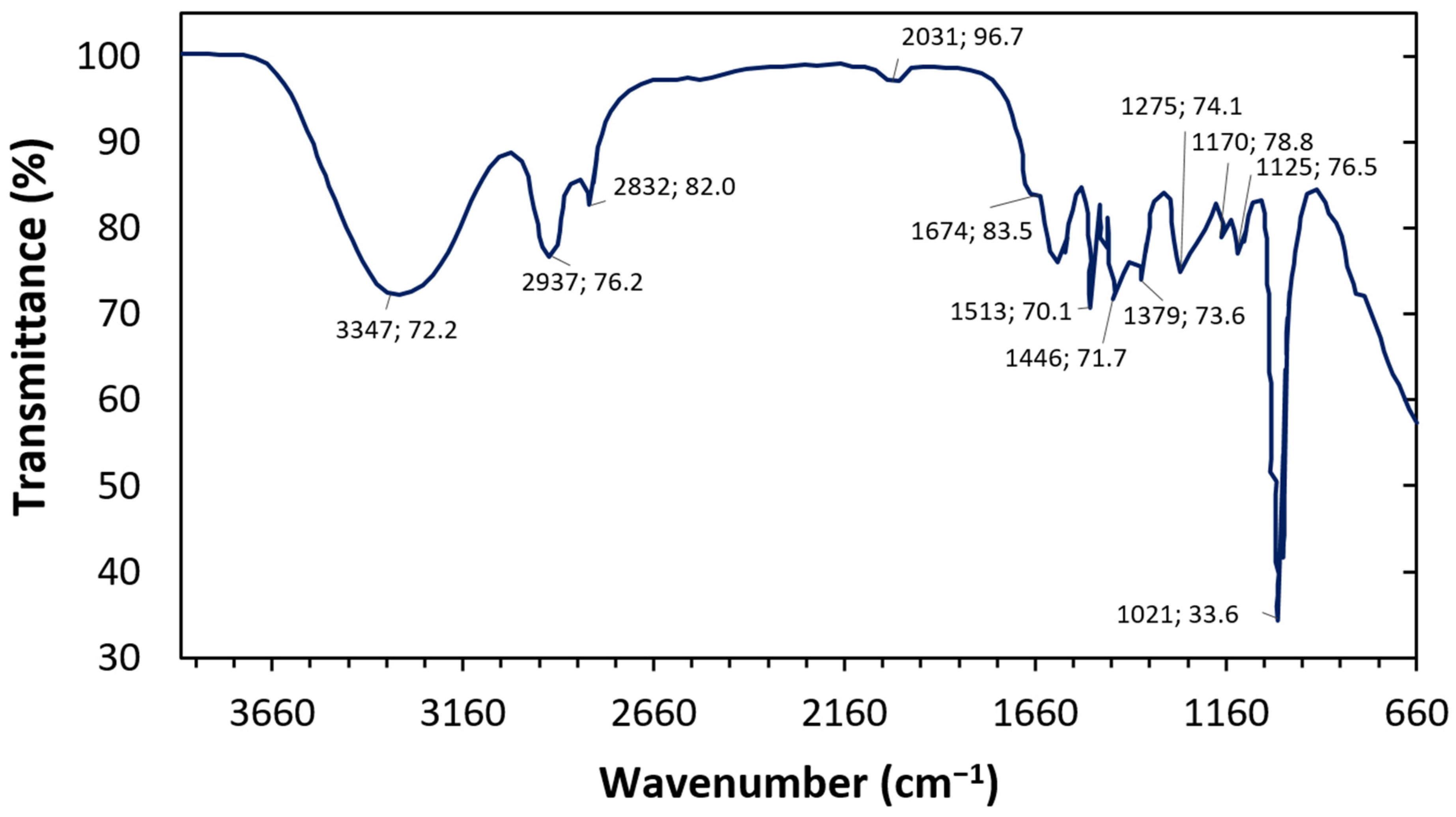

| Wavelength (cm−1) | Functional Group |
|---|---|
| 3335 | O-H medium stretch (alkanols) |
| 2940, 2834 | C-H medium stretch (alkanes) |
| 1680, 1515 | C=C cyclic ring stretch |
| 1342 | C-H medium stretch (terminal alkane bend) |
| 1278, 1126 | C-O medium stretch, (ester) |
| 1020 | =C-H strong stretch (alkene bending) |
| Peak | Retention Time | Area (%) | Library/ID |
|---|---|---|---|
| 5 | 16.5 | 36.3 | phenol, 2 -methoxy-3-(2-propenyl) |
| 19 | 24.2 | 6.98 | Turmerone (α-turmerone) |
| 20 | 24.9 | 5.50 | 2-Methyl-6-(4-methylenecyclohex-2-en-1-yl) hept-2-en-4-one (β-turmerone or Curlone) |
| 4 | 12.0 | 5.14 | Trans-Isoeugenol |
| 28 | 31.0 | 4.85 | Cis-13-Octadecenoic acid, methyl ester |
| 10 | 18.0 | 4.42 | (Z,Z)-.alpha.-Farnesene |
| 13 | 20.9 | 3.84 | Phenol, 2-methoxy-4-(2-propenyl)-, acetate |
| 24 | 29.3 | 3.06 | Hexadecanoic acid, methyl ester |
| 30 | 31.2 | 2.42 | Methyl stearate |
| 32 | 31.5 | 2.28 | Ethyl Oleate |
| 26 | 30.1 | 1.66 | Hexadecanoic acid, ethyl ester |
| 29 | 31.1 | 1.62 | 9-Octadecenoic acid (Z)-, methyl ester |
| 31 | 31.7 | 1.45 | Octadecanoic acid, ethyl ester |
| 23 | 26.6 | 1.09 | (E)-Atlantone |
| Turmeric Concentration (% v/v) | Source of Variation | Sum of Squares | Degree of Freedom | Mean of Squares | F | p-Value | F Critic |
|---|---|---|---|---|---|---|---|
| Between 5 to 20 | Between Groups | 0.5247 | 2 | 0.262 | 0.939 | 0.441 | 5.143 |
| Within Groups | 1.676 | 6 | 0.279 | ||||
| Total | 2.201 | 8 | |||||
| Between 5 to 12.5 | Between Groups | 1.479 | 2 | 0.739 | 11.8 | 0.0379 | 9.552 |
| Within Groups | 0.187 | 3 | 0.062 | ||||
| Total | 1.677 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samuel, J.; Kaisan, M.U.; Sanusi, Y.S.; Narayan, S.; Menacer, B.; Valenzuela, M.; Salas, A.; Oñate, A.; Mahroogi, F.O.; Tuninetti, V. Assessing Antioxidant and Pour Point Depressant Capacity of Turmeric Rhizome Extract in Biolubricants. Lubricants 2024, 12, 282. https://doi.org/10.3390/lubricants12080282
Samuel J, Kaisan MU, Sanusi YS, Narayan S, Menacer B, Valenzuela M, Salas A, Oñate A, Mahroogi FO, Tuninetti V. Assessing Antioxidant and Pour Point Depressant Capacity of Turmeric Rhizome Extract in Biolubricants. Lubricants. 2024; 12(8):282. https://doi.org/10.3390/lubricants12080282
Chicago/Turabian StyleSamuel, Joseph, Muhammad U. Kaisan, Yinka S. Sanusi, Sunny Narayan, Brahim Menacer, Marian Valenzuela, Alexis Salas, Angelo Oñate, Faisal O. Mahroogi, and Víctor Tuninetti. 2024. "Assessing Antioxidant and Pour Point Depressant Capacity of Turmeric Rhizome Extract in Biolubricants" Lubricants 12, no. 8: 282. https://doi.org/10.3390/lubricants12080282
APA StyleSamuel, J., Kaisan, M. U., Sanusi, Y. S., Narayan, S., Menacer, B., Valenzuela, M., Salas, A., Oñate, A., Mahroogi, F. O., & Tuninetti, V. (2024). Assessing Antioxidant and Pour Point Depressant Capacity of Turmeric Rhizome Extract in Biolubricants. Lubricants, 12(8), 282. https://doi.org/10.3390/lubricants12080282





