Effect of Hydrogen Pressure on the Fretting Behavior of Rubber Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
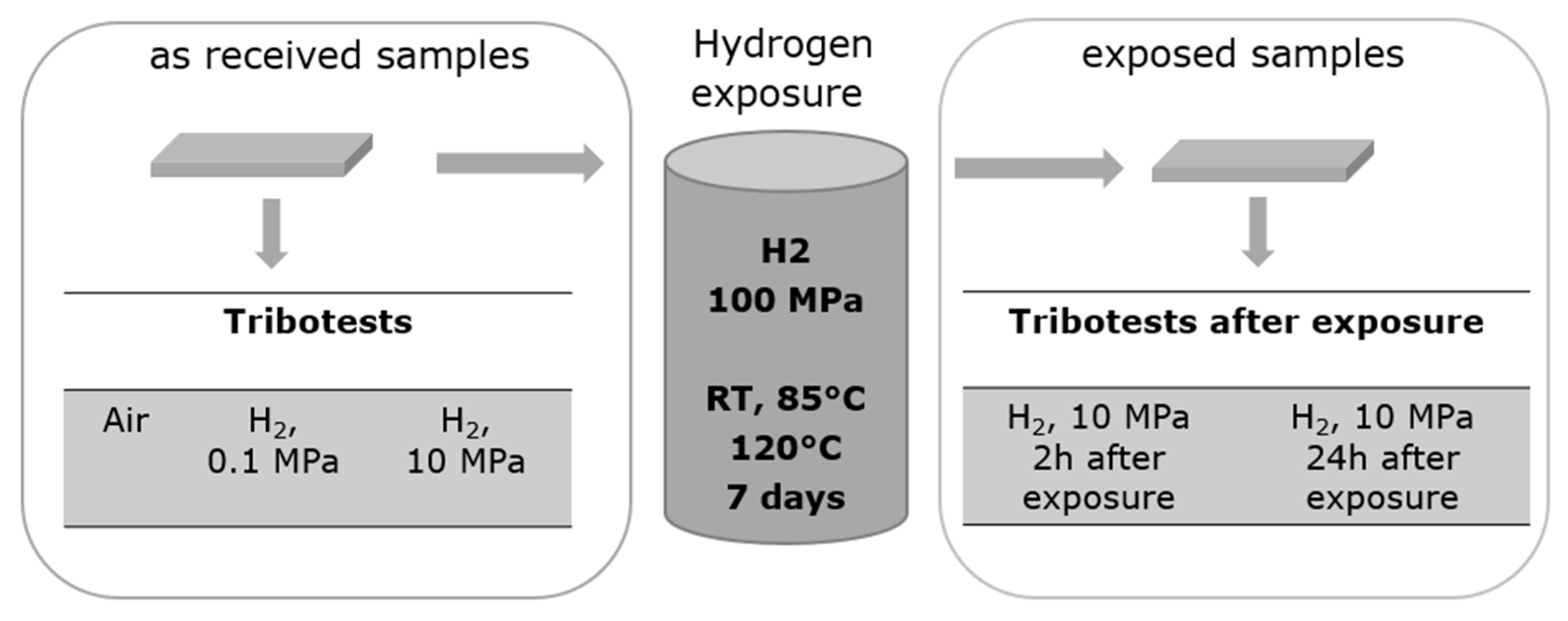
2.2. Test Method
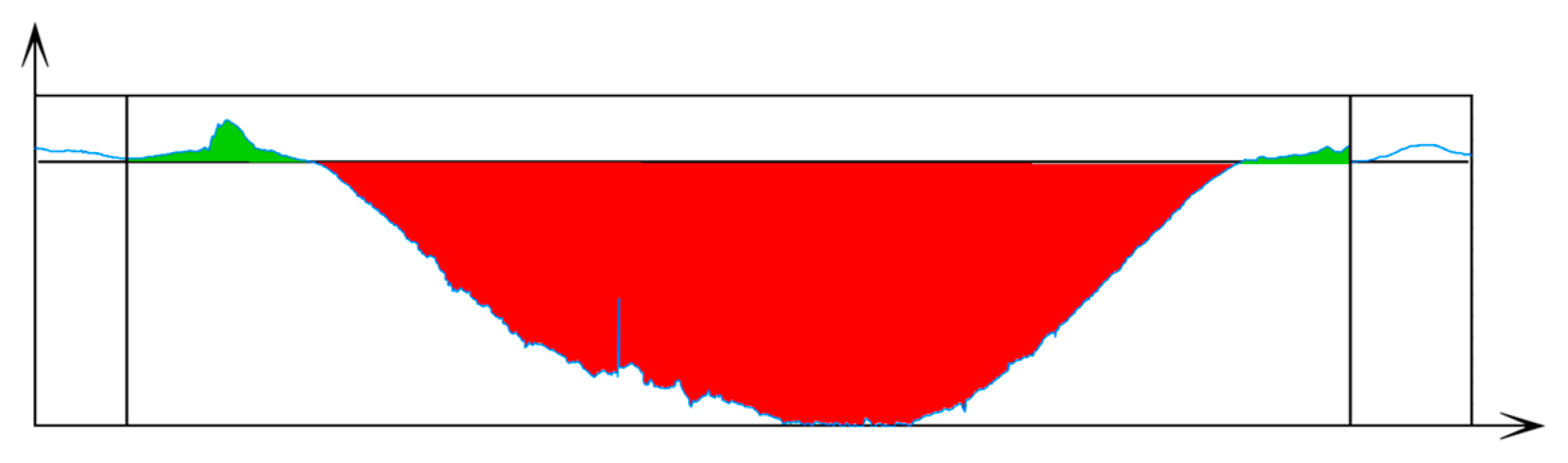
2.3. Friction Measurement
2.4. Surface Analyses and Wear Measurement
3. Results and Discussion
3.1. Friction Tests on as Received Samples: Effect of Hydrogen Environment
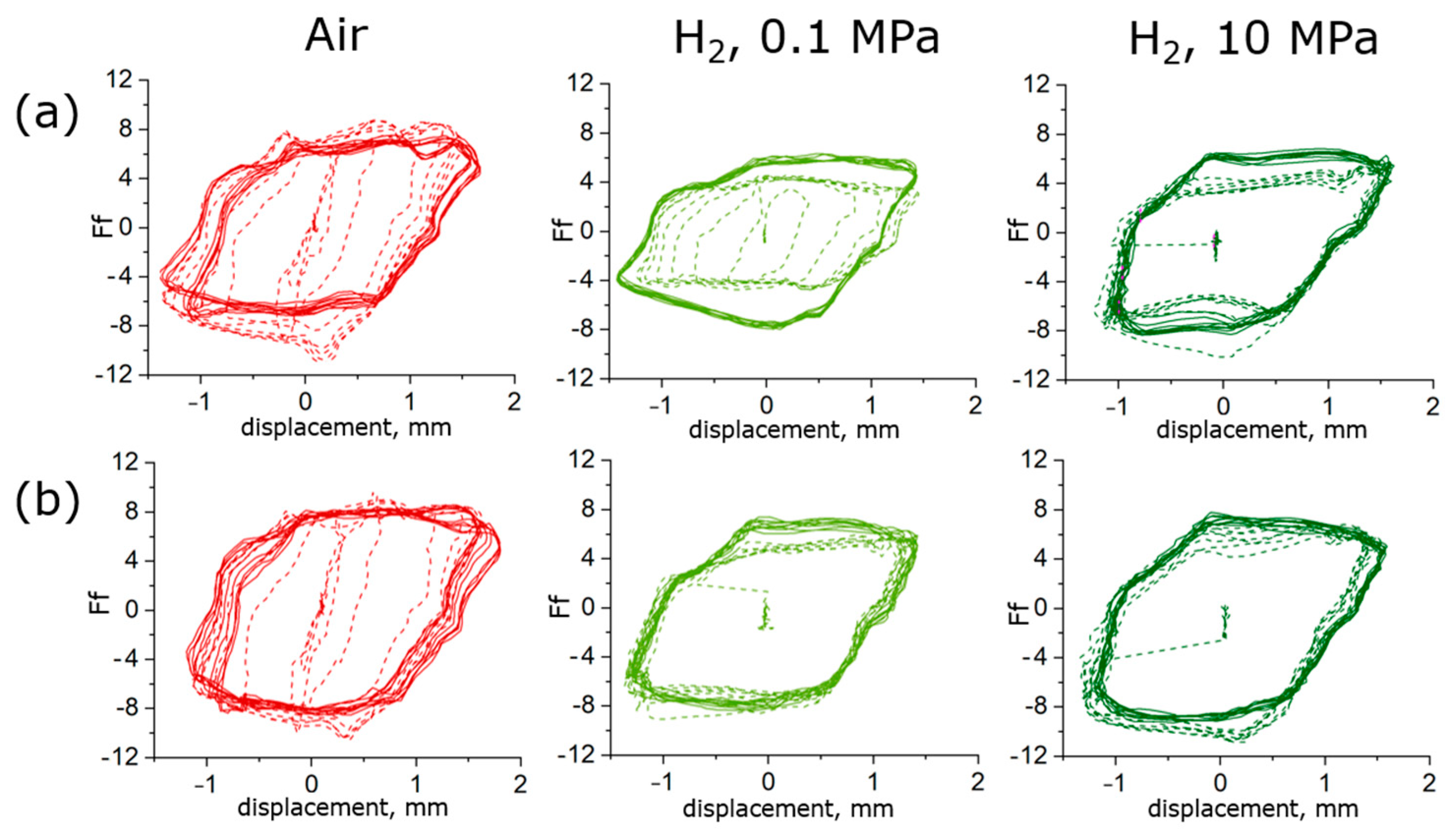
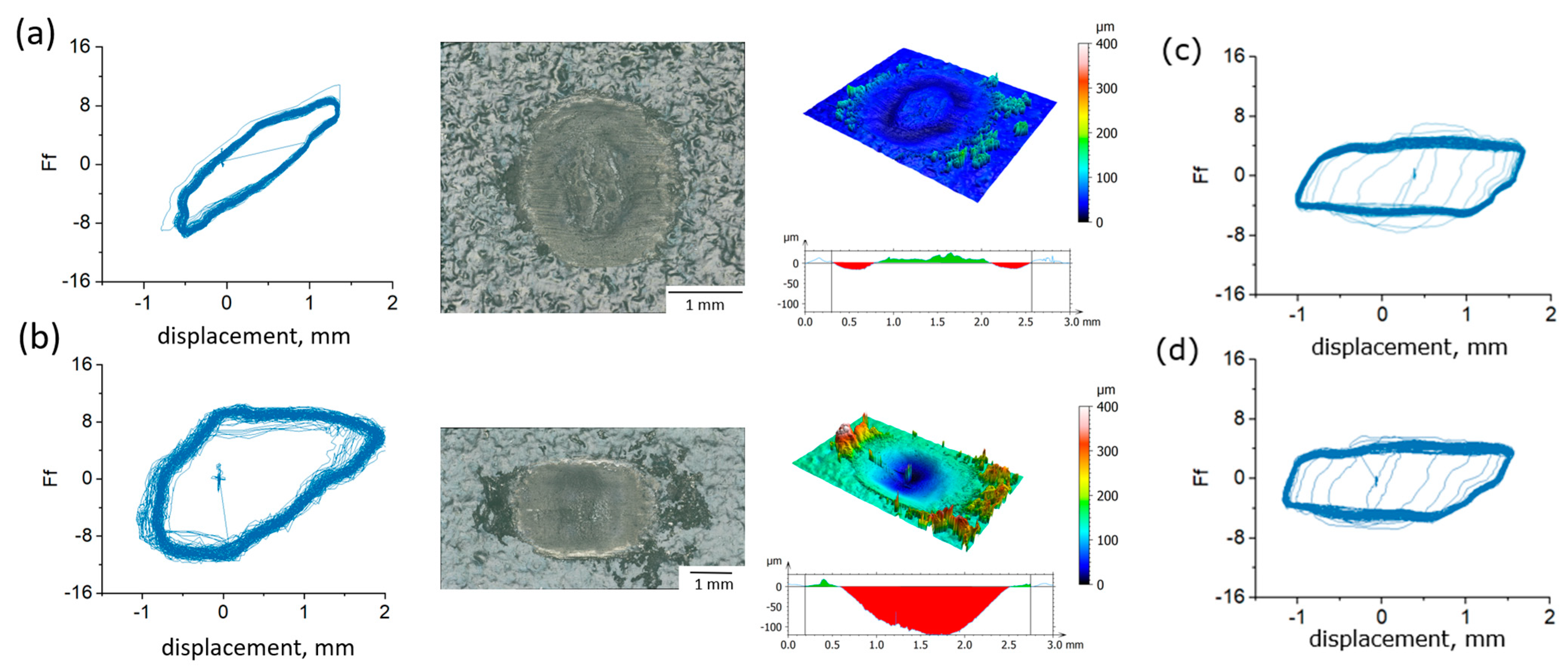
3.1.1. Friction Force Curves
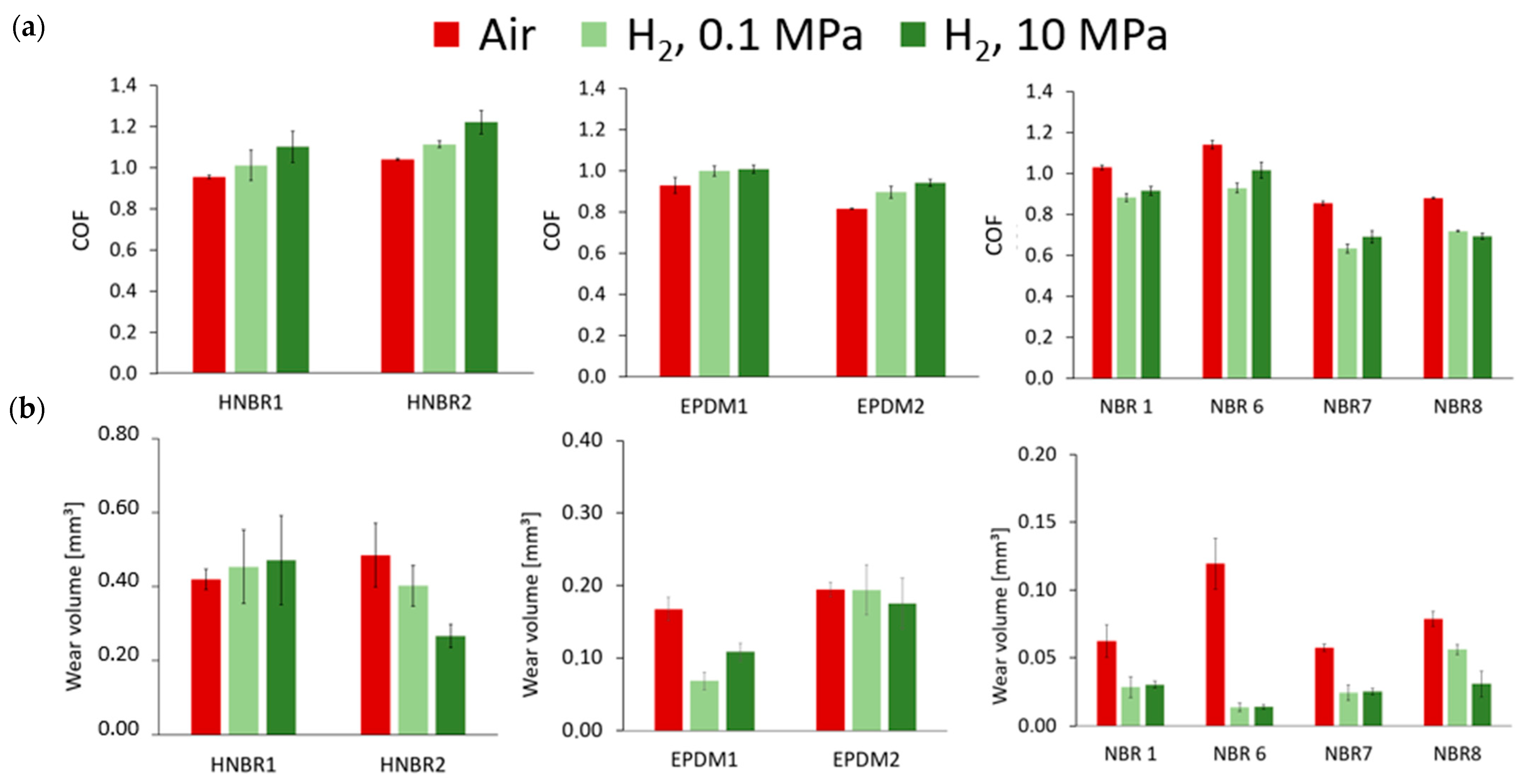
3.1.2. Average Friction Coefficient
3.1.3. Effect of Hydrogen on the Wear Volume
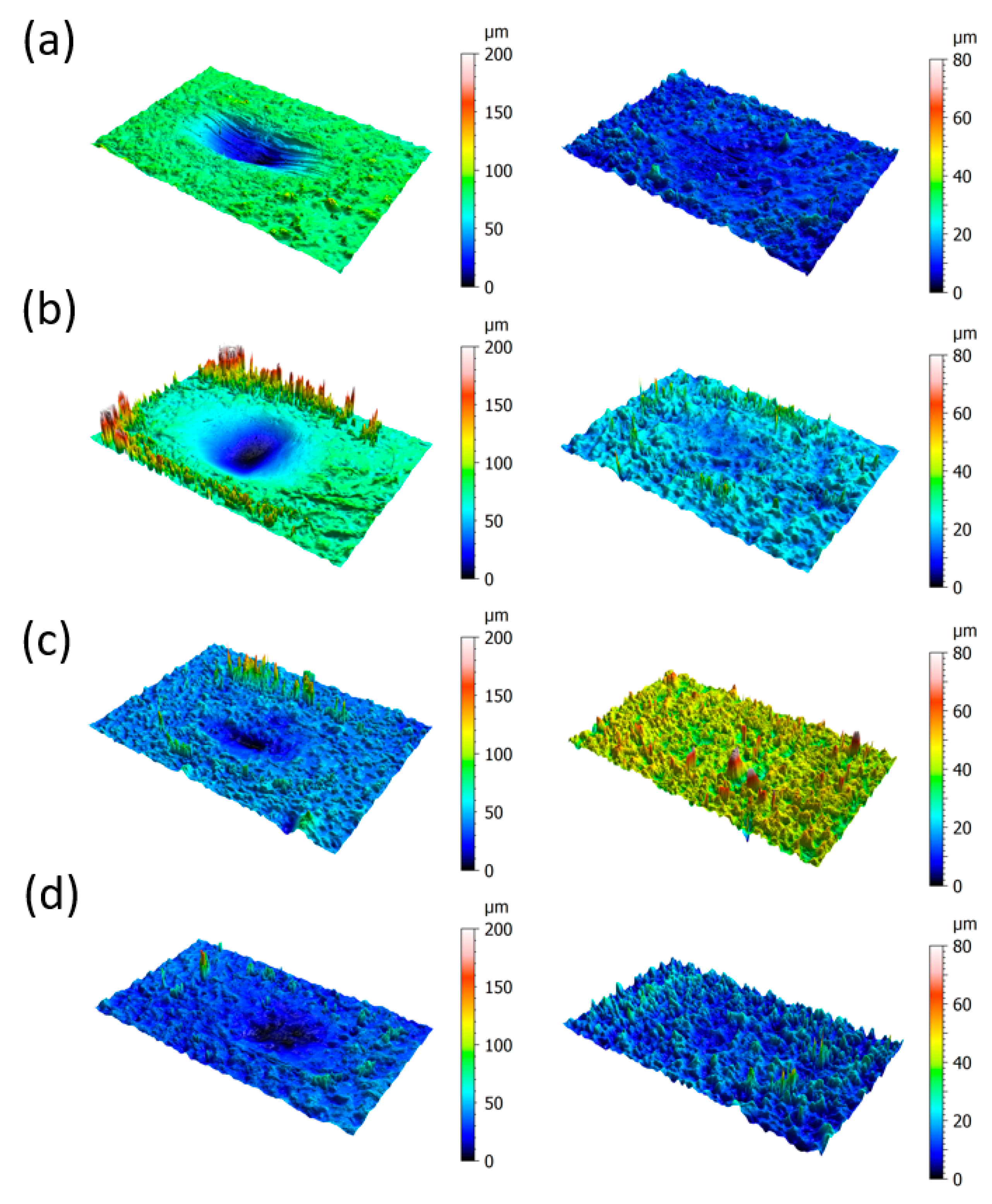
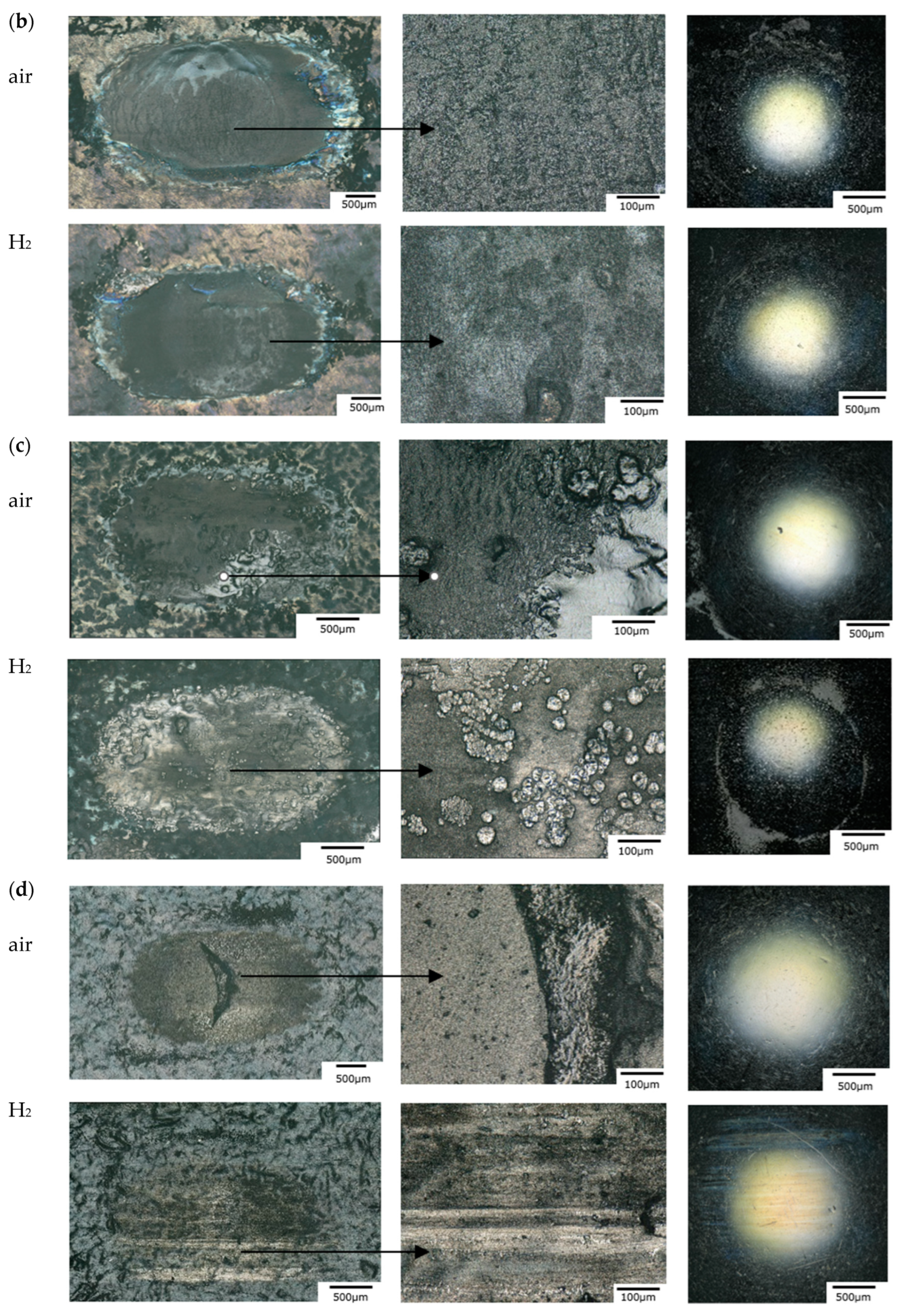
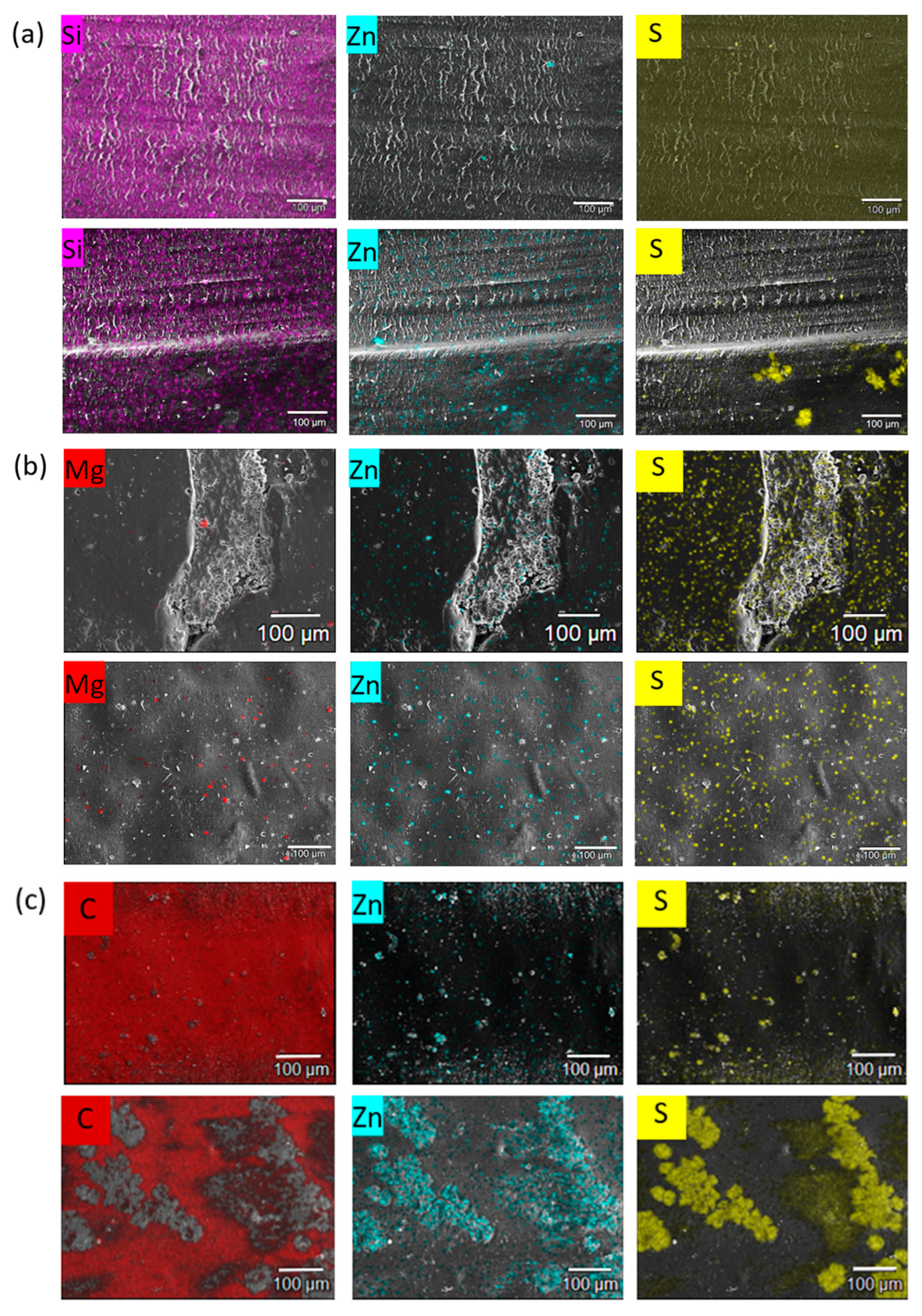
3.1.4. Surface Morphology and Wear Mechanism
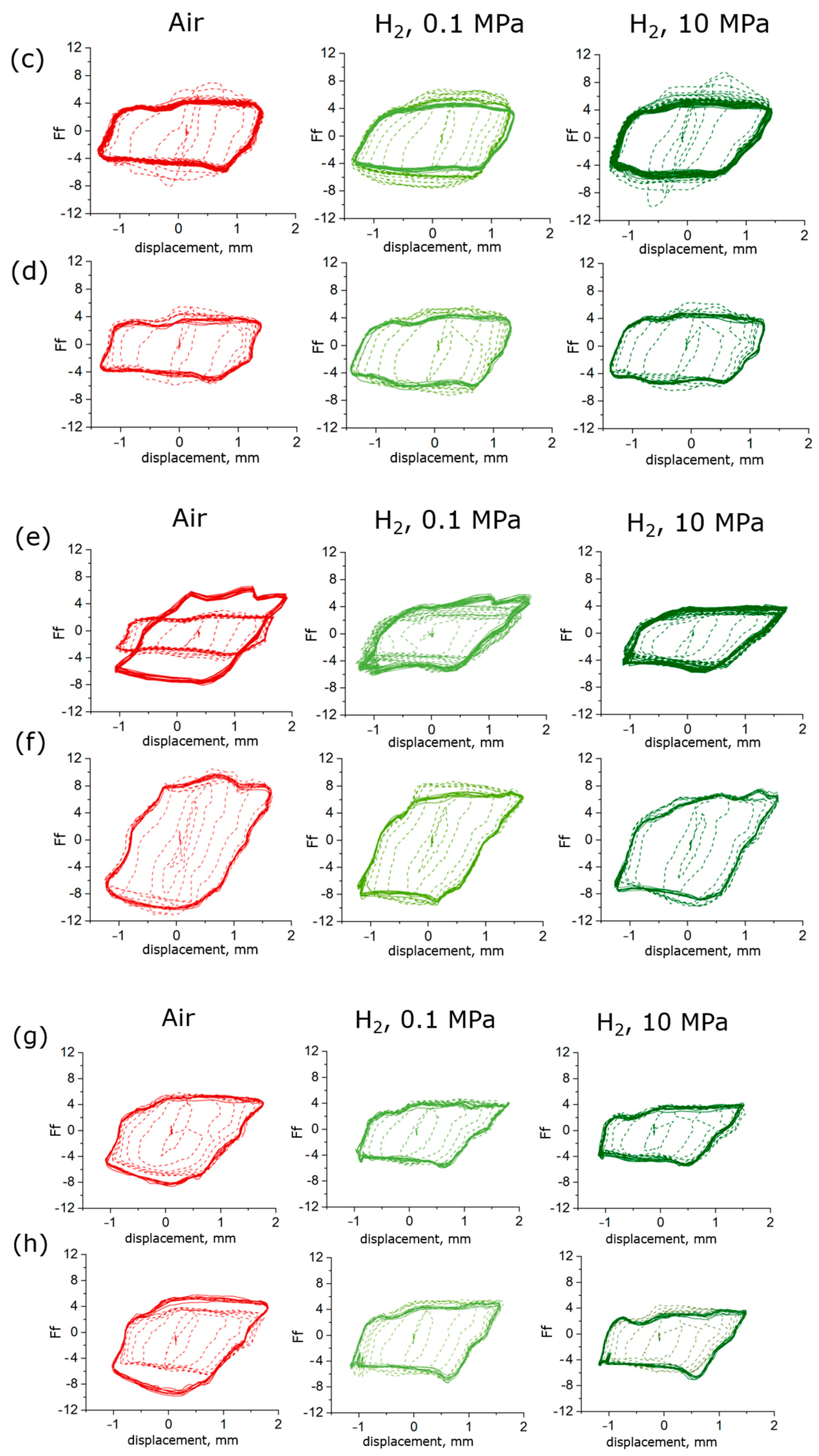
3.2. Friction Tests on Exposed Samples: Effect of High-Pressure Exposure Followed by Rapid Gas Decompression
4. Conclusions
- -
- Under the testing conditions (a normal load of 5 N, frequency of 10 Hz, a 2 mm stroke), the fretting behavior was in the gross slip regime for all rubber materials. The shape of the loop was similar in hydrogen compared to air, and the influence of hydrogen pressure was relatively small, although some effects were seen on the HNBR and EPDM grades. The average friction coefficient and the wear volume, however, were affected differently by the hydrogen conditions depending on the materials. This suggests that the cooling effect of the hydrogen environment is not the only influencing factor.
- -
- The friction of the HNBR and EPDM grades increased with hydrogen pressure. Adhesive wear is predominant in hydrogen in CB filled HNBR, while the addition of PA fillers reduced wear.
- -
- Concerning NBR grades, reduced friction and wear were measured for all grades in hydrogen. It was found that the curing process and the additive have a major influence on the wear properties of these rubber materials in hydrogen. Most significant effects were obtained with the peroxide-cured rubber, having the highest wear value among the NBR materials in air and the lowest one in hydrogen. Among the sulfur-cured NBR grades, lower friction was achieved with CB compared to SiO2 fillers.
- -
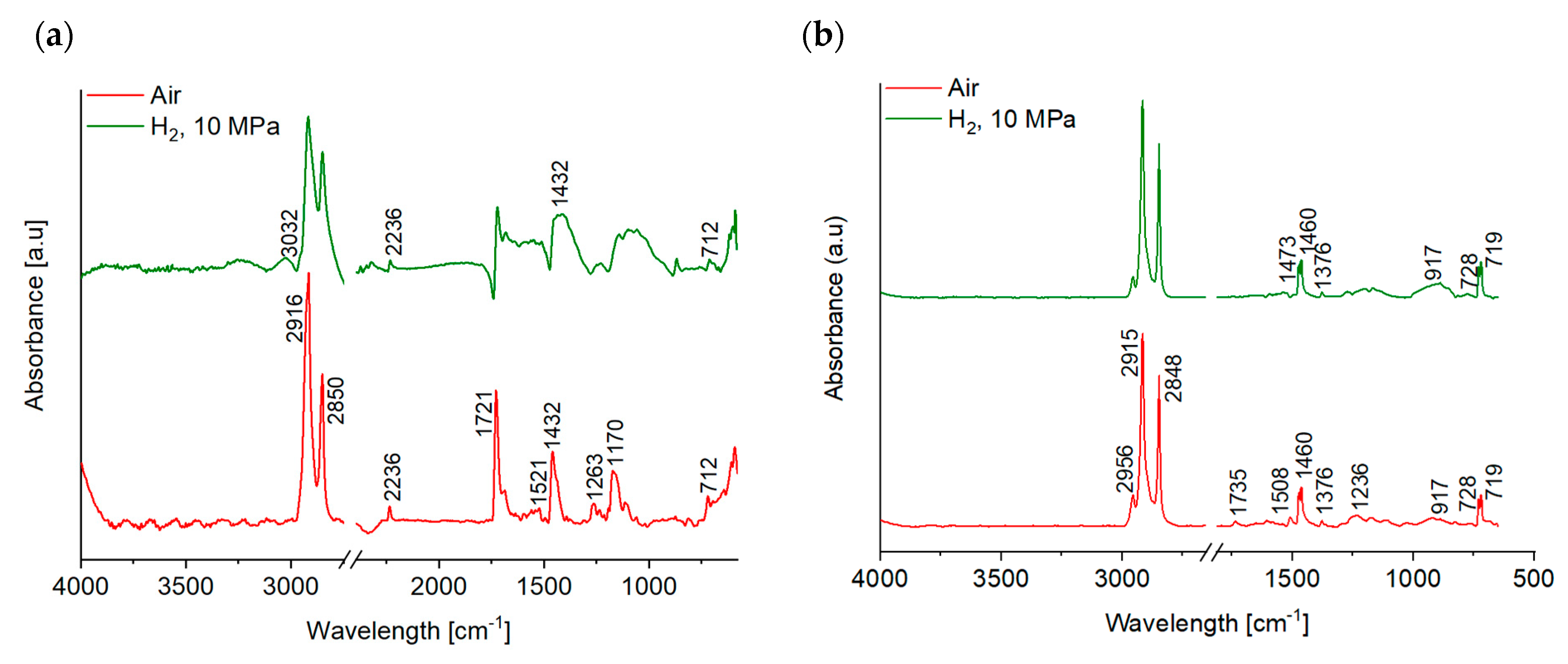
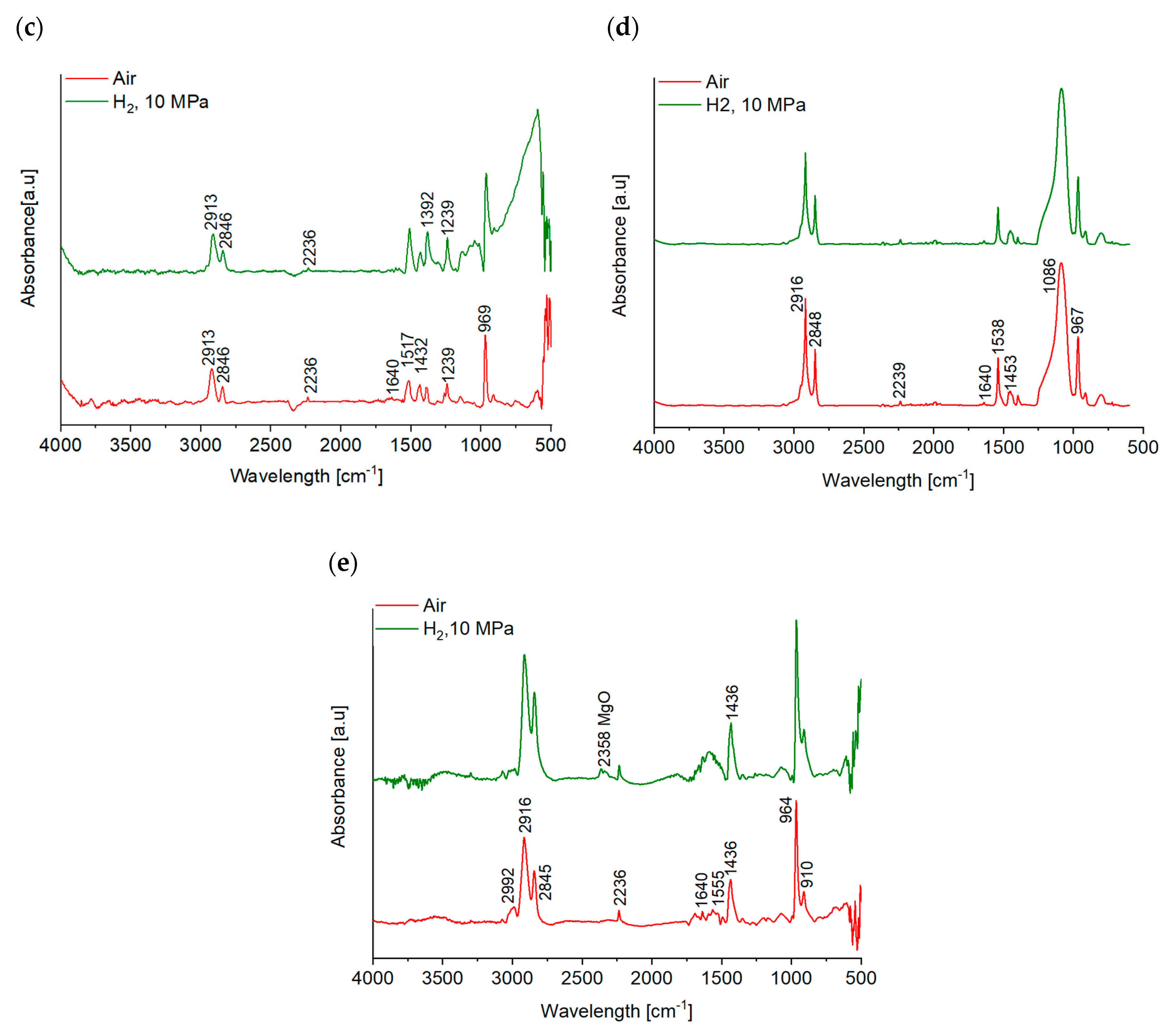
- No significant chemical reactions were detected by means of ATR-IR, apart from a possible oxidation on the worn EPDM surface tested in air. However, both EDX and ATR-IR analyses revealed migration and agglomeration of the additives at the friction contact of the NBR grades in hydrogen, acting favorably on the friction and wear resistance of the rubbers.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balasooriya, W.; Clute, C.; Schrittesser, B.; Pinter, G. A review on applicability, limitations, and improvements of polymeric materials in high-pressure hydrogen gas atmospheres. Polym. Rev. 2021, 175–209. [Google Scholar] [CrossRef]
- Zheng, Y.; Tan, Y.; Zhou, C.; Chen, G.; Li, J.; Liu, Y.; Liao, B.; Zhang, G. A review on effect of hydrogen on rubber seals used in the high-pressure hydrogen infrastructure. Int. J. Hydrogen Energy 2020, 45, 23721–23738. [Google Scholar] [CrossRef]
- Nishimura, S. Rubbers and elastomers for high-pressure hydrogen seal. Soc. Polym. Sci. 2015, 64, 356–357. [Google Scholar]
- Yamabe, J.; Nishimura, S. Influence of fillers on hydrogen penetration properties and blister fracture of rubber composites for O-ring exposed to high-pressure hydrogen gas. Int. J. Hydrogen Energy 2009, 34, 1977–1989. [Google Scholar] [CrossRef]
- Yamabe, J.; Nishimura, S.; Koga, A. A study on sealing behavior of rubber O-ring in high pressure hydrogen gas. SAE Int. J. Mater. Manuf. 2009, 2, 452–460. [Google Scholar] [CrossRef]
- Yamabe, J.; Nishimura, S.; Nakao, M.; Fujiwara, H. Blister fracture of rubbers for O-ring exposed to high pressure hydrogen gas. In Proceedings of the 2008 International Hydrogen Conference—Effects of Hydrogen on Materials, Grand Teton, WY, USA, 7–10 September 2009; pp. 389–396. [Google Scholar]
- Yamabe, J.; Nishimura, S. Nanoscale fracture analysis by atomic force microscopy of EPDM rubber due to high pressure hydrogen decompression. J. Mater. Sci. 2011, 46, 2300–2307. [Google Scholar] [CrossRef]
- Yamabe, J.; Koga, A.; Nishimura, S. Failure behavior of rubber Oring under cyclic exposure to high-pressure hydrogen gas. Eng. Fail. Anal. 2013, 35, 193–205. [Google Scholar] [CrossRef]
- Castagnet, S.; Mellier, D.; Nait-Ali, A.; Benoit, G. In-situ X-ray computed tomography of decompression failure in a rubber exposed to high-pressure gas. Polym. Test. 2018, 70, 255–262. [Google Scholar] [CrossRef]
- Castagnet, S.; Ono, H.; Benoit, G.; Fujiwara, H.; Nishimura, S. Swelling measurement during sorption and decompression in a NBR exposed to high-pressure hydrogen. Int. J. Hydrogen Energy 2017, 42, 19359–19366. [Google Scholar] [CrossRef]
- Simmons, K.L.; Kuang, W.; Burton, S.D.; Arey, B.W.; Shin, Y.; Menon, N.C.; Smith, D.B. H-Mat hydrogen compatibility of polymers and elastomers. Int. J. Hydrogen Energy 2021, 46, 12300–12310. [Google Scholar] [CrossRef]
- Kulkani, S.; Choi, K.; Kuang, W.; Menon, N.; Mills, B.; Soulami, A.; Simmons, K. Damage evolution in polymer due to exposure to high-pressure hydrogen gas. Int. J. Hydrogen Energy 2021, 46, 19001–19022. [Google Scholar] [CrossRef]
- Theiler, G.; Cano Murillo, N.; Halder, K.; Balasooriya, W.; Hausberger, A.; Kaiser, A. Effect of high-pressure hydrogen environment on the physical and mechanical properties of elastomers. Int. J. Hydrogen Energy 2024, 58, 389–399. [Google Scholar] [CrossRef]
- Menon, N.; Alvine, K.; Kruizenga, A.; Nissen, A.; San Marchi, C.; Brooks, K. Behaviour of polymers in high pressure environments as applicable to the hydrogen infrastructure. In Proceedings of the Pressure Vessels and Piping Conference—American Society of Mechanical Engineers, Vancouver, BC, Canada, 17–21 July 2016. [Google Scholar]
- Zhou, C.; Zheng, J.; Gu, C.; Zhao, Y.; Liu, P. Sealing performance analysis of rubber O-ring in high-pressure gaseous hydrogen based on finite element method. Int. J. Hydrogen Energy 2017, 42, 11996–12004. [Google Scholar] [CrossRef]
- Jeon, S.K.; Kwon, O.H.; Tak, N.H.; Chung, N.K.; Baek, U.B.; Nahm, S.H. Relationships between properties and rapid gas decompression (RGD) resistance of various filled nitrile butadiene rubber vulcanizates under high-pressure hydrogen. Mater. Today Commun. 2022, 30, 103038. [Google Scholar] [CrossRef]
- Ono, H.; Nait-Ali, A.; Kane Diallo, O.; Benoit, G.; Castagnet, S. Influence of pressure cycling on damage evolution in an unfilled EPDM exposed to high-pressure hydrogen. Int. J. Fract. 2018, 210, 137–152. [Google Scholar] [CrossRef]
- Fujiwara, H.; Ono, H.; Nishimura, S. Effects of fillers on the hydrogen uptake and volume expansion of acrylonitrile butadiene rubber composites exposed to high pressure hydrogen: Property of polymeric materials for high pressure hydrogen devices (3). Int. J. Hydrogen Energy 2022, 47, 4725–4740. [Google Scholar] [CrossRef]
- Kuang, W.; Nickerson, E.K.; Li, D.; Clelland, D.T.; Seffens, R.J.; Ramos, J.L.; Simmons, K.L. An in-situ view cell system for investigating swelling behavior of elastomers upon high-pressure hydrogen exposure. Int. J. Hydrogen Energy 2024, 71, 1317–1325. [Google Scholar] [CrossRef]
- Chen, Q.; Morita, T.; Sawae, Y.; Fukuda, K.; Sugimura, J. Effects of trace moisture content of tribofilm formation, friction and wear of CF-filled PTFE in hydrogen. Tribol. Int. 2023, 188, 108905. [Google Scholar] [CrossRef]
- Theiler, G.; Gradt, T. Tribological characteristics of polyimide composites in hydrogen environment. Tribol. Int. 2015, 92, 162–171. [Google Scholar] [CrossRef]
- Theiler, G.; Gradt, T. Environmental effects on the sliding behaviour of PEEK composites. Wear 2016, 368–369, 278–286. [Google Scholar] [CrossRef]
- Theiler, G.; Gradt, T. Comparison of the Sliding Behavior of Several Polymers in Gaseous and Liquid Hydrogen. Tribol. Online 2023, 18, 217–231. [Google Scholar] [CrossRef]
- Sawae, Y.; Morita, T.; Takeda, K.; Onitsuka, S.; Kaneuti, J.; Yamaguchi, T.; Sugimura, J. Friction and wear of PTFE composites with different filler in high purity hydrogen gas. Tribol. Int. 2021, 157, 106884. [Google Scholar] [CrossRef]
- Sawae, Y.; Fukuda, K.; Miyakoshi, E.; Doi, S.; Watanabe, H.; Nakashima, K.; Sugimura, J. Tribological Characterization of Polymeric Sealing Materials in High-Pressure Hydrogen Gas. In Proceedings of the STLE/ASME 2010 International Joint Tribology Conference, San Francisco, CA, USA, 17–20 October 2010. [Google Scholar]
- Sawae, Y.; Nakashima, K.; Doi, S.; Murakami, T.; Sugimura, J. Effects of High-Pressure Hydrogen on Wear of PTFE and PTFE Composite. In Proceedings of the ASME/STLE 2009 International Joint Tribology Conference, Memphis, Tennessee, USA, 19–21 October 2009; pp. 233–235. [Google Scholar]
- Duranty, E.R.; Roosendaal, T.J.; Pitman, S.G.; Tucker, J.C.; Owsley, S.L., Jr.; Suter, J.D. In situ high pressure hydrogen tribological testing of common polymer materials used in the hydrogen delivery infrastructure. JoVE 2018, 133, e56884. [Google Scholar]
- Kuang, W.; Bennett, W.D.; Roosendaal, T.J.; Arey, B.W.; Dohnalkova, A. In situ friction and wear behavior of rubber materials incorporating various fillers and/or a plasticizer in high-pressure hydrogen. Tribol. Int. 2021, 153, 106627. [Google Scholar] [CrossRef]
- Choi, B.L.; Jung, J.K.; Baek, U.B.; Choi, B.-H. Effect of Functional Fillers on Tribological Characteristics of Acrylonitrile Butadiene Rubber after High-Pressure Hydrogen Exposures. Polymers 2022, 14, 861. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Chen, G.; Xiao, S.; Hua, Z.; Gu, C. Study on fretting behavior of rubber O-ring seal in high-pressure gaseous hydrogen. Int. J. Hydrogen Energy 2019, 44, 22569–22575. [Google Scholar] [CrossRef]
- CSA/ANSI CHMC 2:19; Test Methods for Evaluating Material Compatibility in Compressed Hydrogen Applications—Polymers. CSA Group: Toronto, ON, Canada, 2019.
- Zhang, T.; Su, J.; Shu, Y.; Shen, F.; Ke, L. Fretting Wear Behavior of Three Kinds of Rubbers under Sphere-On-Flat Contact. Materials 2021, 14, 2153. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Hausberger, A.; Berer, M.; Pinter, G.; Grün, F.; Schwarz, T. An investigation of fretting behavior of thermoplastic polyurethane for mechanical seal application. Polymer Testing 2018, 72, 271–284. [Google Scholar] [CrossRef]
- Karger-Kocsis, J.; Mousa, A.; Major, Z.; Bekesi, N. Dry friction and sliding wear of EPDM rubbers against steel as a function of carbon black content. Wear 2008, 264, 359–367. [Google Scholar] [CrossRef]
- ISO 15113:2015; Rubber—Determination of Frictional Properties. ISO: Geneva, Switzerland, 2015.
- Karger-Kocsis, J.; Felhös, D.; Xu, D.; Schlarb, A.K. Unlubricated sliding and rolling wear of thermoplastic dynamic vulcanizates (Santoprene R) against steel. Wear 2008, 265, 292–300. [Google Scholar] [CrossRef]
- Shen, M.; Peng, X.; Meng, X.; Zheng, J.; Zhu, M. Fretting wear behavior of acrylonitrile–butadiene rubber (NBR) for mechanical seal applications. Tribol. Int. 2016, 93, 419–428. [Google Scholar] [CrossRef]
- Zhou, Z.R.; Nakazawa, K.; Zhu, M.-H.; Maruyama, N.; Kapsa, P.; Vincent, L. Progress in fretting maps. Tribol. Int. 2006, 39, 1068–1073. [Google Scholar] [CrossRef]
- Vingsbo, O.; Soderberg, S. On fretting maps. Wear 1988, 126, 131–147. [Google Scholar] [CrossRef]
- Adam, A.; Paulkowski, D.; Mayer, B. Friction and Deformation Behavior of Elastomers. Mater. Sci. Appl. 2019, 10, 527–542. [Google Scholar] [CrossRef]
- Mokhtari, M.; Schipper, D.J.; Tolpekina, T.V. On the Friction of Carbon Black- and Silica-Reinforced BR and S-SBR Elastomers. Tribol. Lett. 2014, 54, 297–308. [Google Scholar] [CrossRef]
- Clute, C.; Balasooriya, W.; Cano Murillo, N.; Theiler, G.; Kaiser, A.; Fasching, M.; Schwarz, T.; Hausberger, A.; Pinter, G.; Schlögl, S. Morphological investigations on silica and carbon-black filled acrylonitrile butadiene rubber for sealings used in high-pressure H2 applications. Int. J. Hydrogen Energy 2024, 67, 540–552. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Wang, H.; Fan, N.; Yan, F. Experimental investigation on tribological behavior of several polymer materials under reciprocating sliding and fretting wear conditions. Tribol. Int. 2016, 104, 73–82. [Google Scholar] [CrossRef]
- Guo, Q.; Luo, W. Mechanisms of fretting wear resistance in terms of material structures for unfilled engineering polymers. Wear 2002, 249, 924–931. [Google Scholar] [CrossRef]
- Cano Murillo, N.; Kaiser, A.; Balasooriya, W.; Meinel, D.; Theiler, G. Effect of hydrogen environments on the physical and mechanical properties of elastomers. Bundesanstalt für Materialforschung und -prüfung (BAM), Berlin, Germany. 2024; to be published. [Google Scholar]
- Zaghdoudi, M.; Kommling, A.; Bohning, M.; Jaunich, M. Ageing of elastomers in air and in hydrogen environment: A comparative study. Int. J. Hydrogen Energy 2024, 63, 207–216. [Google Scholar] [CrossRef]



| Materials | Curing | Fillers | Hardness (ShA) |
|---|---|---|---|
| HNBR-CB | Peroxide | carbon black (75 phr) | 79 |
| HNBR-CB-PA | Peroxide | carbon black (67 phr) + PA (10 phr) | 82 |
| NBR-Sil | Sulfur | silica (60 phr) + SCA 2 phr | 70 |
| NBR-CB-perox | Peroxide | carbon black (70 phr) (MgO) | 81 |
| NBR-CB | Sulfur | carbon black (75 phr) | 76 |
| NBR-CB-plast | Sulfur | carbon black (95 phr) + plast. (10 phr) | 78 |
| EPDM1 | Sulfur | carbon black (100 phr) | 77 |
| EPDM2 | Sulfur | carbon black (120 phr) | 81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theiler, G.; Cano Murillo, N.; Hausberger, A. Effect of Hydrogen Pressure on the Fretting Behavior of Rubber Materials. Lubricants 2024, 12, 233. https://doi.org/10.3390/lubricants12070233
Theiler G, Cano Murillo N, Hausberger A. Effect of Hydrogen Pressure on the Fretting Behavior of Rubber Materials. Lubricants. 2024; 12(7):233. https://doi.org/10.3390/lubricants12070233
Chicago/Turabian StyleTheiler, Géraldine, Natalia Cano Murillo, and Andreas Hausberger. 2024. "Effect of Hydrogen Pressure on the Fretting Behavior of Rubber Materials" Lubricants 12, no. 7: 233. https://doi.org/10.3390/lubricants12070233
APA StyleTheiler, G., Cano Murillo, N., & Hausberger, A. (2024). Effect of Hydrogen Pressure on the Fretting Behavior of Rubber Materials. Lubricants, 12(7), 233. https://doi.org/10.3390/lubricants12070233





