Abstract
In order to cope with the shortage of non-renewable energy and the increasingly environmental pollution, sustainable vegetable oils, as competitive alternatives, have widely been held in the good graces of the researchers. Vegetable oils are suitable for a wide range of applications such as biofuels and biodiesel. However, the development of vegetable oils is limited due to the characteristics of unsatisfactory oxidation stability and poor cold-flow properties. Chemical modification is considered as an effective solution to enhance the performance. The research progress of the chemical modification methods and applications of vegetable oils in recent years are summarized in this review. Reducing the content of carbon–carbon double bonds and increasing the degree of saturation are the keys to improve the physicochemical properties of vegetable oils. The prospects for the development direction and challenges of vegetable oils are proposed. Future research may focus on the use of multifunctional catalysts to optimize reaction conditions or to introduce active groups with lubricating properties in epoxidation reactions and explore the combination of chemical and auxiliary methods.
1. Introduction
The rising population and limited availability of natural energy sources have accelerated the demand for energy [1]. The development of renewable energy sources has become one of the urgent tasks of researchers. As a substitute for petroleum fuel, vegetable-based oil has been applied in different fields because of its excellent physicochemical properties and environmental friendliness [2]. High biodegradability is one of the most desirable advantages of vegetable oils [3]. At the beginning of the 21st century, a mixture of 20% palm oil and 80% diesel has been successfully used as diesel engine oil [4]. The addition of vegetable oils reduces the risks of machine failures and maintains optimal machine operation [5]. The performance of vegetable-based diesel is similar to conventional fuels while the emissions of CO, NOx, and smoke are significantly reduced which can be attributed to the lower production of harmful gases [6]. Vegetable oil molecules formed an adsorption film on the metal surface, and then fatty acids react with the metal surface to form a monolayer film of metal soap which play a vital role in anti-wear and anti-friction. Sajeeb and Rajendrakumar [7] studied the tribological performance of mineral oil (SAE20W-40) and the blend of coconut and mustard oil (the latter ratio is 10–50%). It can be found that the wear diameter of the mixed oil decreased by 18.45% which demonstrates the excellent lubricating performance. The advantages and disadvantages of vegetable oils and mineral oils are summarized in Table 1. At present, mineral oils hold an unshakable dominant share because of the mature technique and sufficient feedstocks. From a long-term perspective, green vegetable oils are available to replace mineral oils when their physical and chemical properties can be improved and the synthesis process can be continuously optimized.

Table 1.
Summary of the advantages and disadvantages of vegetable oils and mineral oils.
In recent years, waste vegetable oil or used cooking oil products have also been utilized in biofuel [8], biodiesel [9], lubrication [10], and other fields after green processing. Due to the low economic benefit and complicated secondary processing, there is still a long way to go. Therefore, the modification of vegetable oil to improve the physicochemical properties is regarded as a potential solution [11]. Chemical modification is the most widely used modification method at present. Among various modified conversion methods, selective hydrogenation, transesterification, and epoxidation addition are currently the preferred choices for the production of biodiesel and biofuels. There is a lack of summary of the methods and applications of chemically modified vegetable oils in recent years. It is necessary to understand and summarize the effects of chemical modification methods. Therefore, this paper aims to review recent applications of vegetable oils obtained via chemical modification in different fields. The intention of this review is related to the emergent necessity of promoting the use of vegetable oils in bio-production. The use of auxiliary methods to further obtain the multi-circulating and high-performance vegetable oils is also discussed.
2. Chemical Modification
The fundamental constituents of vegetable oils are higher fatty acid glycerides, and the types of fatty acids vary from source to source. The structure of saturated fatty acids is generally stable. There are unsaturated double bonds in the molecular structure of vegetable oils. The existence of double bonds leads to poor thermal oxidation stability [12]. The oxidation of vegetable oils is prone to acidification during storage, which leads to corrosion. Therefore, vegetable oils often need to be modified to improve their oxidative stability [13]. The content of carbon–carbon double bonds and allyl carbons in vegetable oil is reduced by chemical modification, and the oxidative stabilities of vegetable oils are improved [14]. Commonly used chemical modification methods include selective hydrogenation [15], transesterification [16], epoxidation [17], and so on.
2.1. Selective Hydrogenation
Unsaturated fatty acids in vegetable oils are converted into higher stable saturated fatty acids by reducing single or multiple carbon–carbon double bonds via selective hydrogenation. As shown in Figure 1, the four-step hydrogenation mechanism [18], including diffusion, adsorption, reaction, and desorption, is a commonly recognized hydrogenation model. In this method, a suitable high-efficiency catalyst is selected to adjust the oxidation stability and low temperature properties.
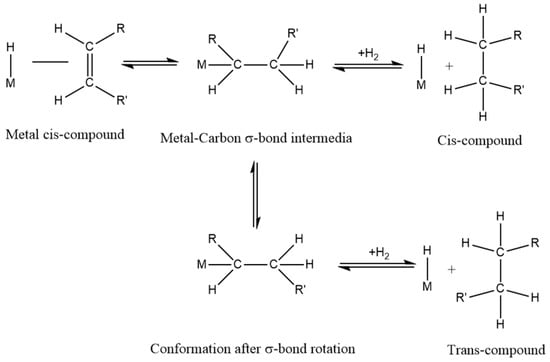
Figure 1.
The four-step hydrogenation mechanism.
2.1.1. Metal Catalysis
The hydrogenation activity of the catalysts depends on both the type of metal and support. Metal catalysts such as Pt [19], Pd [20] and Ni [21] are commonly used as supported catalysts in selective hydrogenation. Pt was supported on TiO2 and ZrO2 by impregnation to evaluate its catalytic behavior in the selective hydrogenation [22]. In the process of the partial hydrogenation of soybean oil, the catalysts exhibited better activity and lower trans-isomer selectivity. Compared with the catalytic behavior of Raney Ni, the Pt/ZrO2 catalyst could be a potential substitute for reducing the formation of trans-fatty acids, while maintaining the physicochemical properties of hydrogenated soybean oil. The Pd supported catalysts were used to prepare soybean oil-based biodiesel through CTH in supercritical methanol [23]. Under the optimum condition with 0.5 mg Pd/g oil for 30 min, the FAMEs yield for Pd/ZSM-5 was 97.1%. Numwong [24] investigated the effect of Pd particle size on catalytic activity and cis-trans selectivity with 0.5 and 1 wt% Pd loadings. The C18:1 FAMEs were more easily selected by the smaller Pd/MCM-41 catalyst. The C18:2 and C18:3 FAMEs could be adsorbed on the metal surface by the Pd/SiO2 catalyst, and the original TOF was improved. The performance of different Al2O3 supported metal catalysts was evaluated in the selective hydrogenation of sunflower oil [25]. It was shown that Pt/Al2O3 was more active in selective hydrogenation than Ni/SiO2. The activity and selectivity of Pd/Al2O3 could be effectively balanced and the outstanding comprehensive performance was obtained. Papageridis et al. [26] investigated the influence on the selective catalysis of palm oil over Al2O3, ZrO2 and SiO2 supported Ni catalysts. The characterization revealed that the materials were evenly dispersed on the Ni/ZrO2 and Ni/Al2O3 catalyst, while the best reduction effect was obtained by Ni/SiO2 samples due to that part of Ni participated in the reaction. The effect of Pd, Pt and Ni catalysts on the partial hydrogenation of FAMEs in soybean oil was investigated [27]. The presence of sulfur influenced the activity of the as-prepared catalyst. Among the sulfur-free compounds, the best hydrogenation was obtained using a Pd/SiO2 catalyst. This is mainly due to the formation of more conjugated dienes that decreased the rate of isomerization, thereby increasing the hydrogenation efficiency. Regardless of the influence of sulfur compounds, the priority of catalytic activity was Pd/SiO2 > Pt/SiO2 > Ni/SiO2. The oxidation stability of biodiesel was increased by 30.4 h, 5 h, and 3.6 h, respectively.
It was shown that noble metals (Pt and Pd) are able to be supported on oxides such as Al2O3 [25,27] and give high hydrogenation efficiency. Base metals (Ni) are more suitable for producing hydrogenated oils with fewer trans isomers [26].
2.1.2. Catalyst Supports
The support materials, as the framework of the active components of catalysts, provide a suitable surface area and pore structure which can meet the requirement of mechanical stability. Al2O3, as one of the well-known support materials, possessed a variety of crystal forms (α, β, γ, δ, θ, etc.) [28]. Among them, γ-Al2O3 has been widely used in the field of catalysis owing to its rich pore structure, excellent adsorption performance, and high surface area. Toshtay et al. [29] investigated the influence of Pt/γ-Al2O3 prepared by adsorption on the formation of trans isomers in the hydrogenation of sunflower oil. The content of trans isomer was decreased from 5.6% to 3.5% at a low concentration of 0.2 wt% as-prepared catalyst. The unsupported and γ-Al2O3 supported Ni-Mo catalysts on palm oil hydrogenation were compared [30]. The H-NiMoS2/γ-Al2O3 supported catalyst exhibited higher dispersibility than the unsupported catalyst. When the reaction was performed at 300 °C for 3 h and the catalyst/palm oil ratio was 0.1, 67.0% of C14-C18 alkanes was yielded.
SiO2 can be easily available and ordered mesoporous silica with good extensibility is generally used as a catalyst carrier; therefore, it has been verified as an effective catalyst support in selective hydrogenation reaction [31]. A mesoporous silica-supported nano catalyst offers key advantages over homogeneous catalysts for in vivo applications of parahydrogen-based hyperpolarization. Rungsi et al. [32] reported the influence of a mesoporous MCM-41 silica support on the Pd particle catalyst for the partial hydrogenation of vegetable oil. The results showed that the adsorption of polyunsaturated FAMEs was enhanced on the metal surface due to the incorporation of Pd. When 2 wt% of Pd was loaded on the catalyst carrier, the Pd/MCM-41-SiO2 catalyst system obtained the best TOF performance.
It is undoubtable that tunable carbon-based materials with different physical forms are novel catalyst supports that cannot be ignored, especially Pt-based catalysts. The influence of diverse catalysts such as Pt/C, Pd/C, Co-Mo, Pd/Al2O3 and Pd/ZSM-5 was studied in rapeseed oil [33]. When using noble metal catalysts (Pd, Pt) on active carbon, the highest heating value and the lowest acid value of the products can be achieved, and Pt/C was the most potential catalyst for the production of hydrogenation cracking oil after process optimizations.
In recent years, mixed oxide supports with synergistic effects have also attracted the attention of researchers. Ni/ZnO/Al2O3 and Ni/Al2O3 composite catalysts were prepared by co-precipitation, and their performance in the hydrogenation of sunflower oil was evaluated [34]. The pore size of the Ni/Al2O3 catalyst was promoted by the addition of ZnO. The pore characteristics of the catalyst were changed and the diffusion limit of the large triglyceride molecules was reduced, and then the hydrogenation activity of the catalyst was improved. Furthermore, the catalytic functions of vegetable oil with Ni-Cu catalysts supported by different oxide materials were studied [35]. The outstanding qualities were obtained when the Ni-Cu/ZrO2-SBA-15 catalyst was used, and the supported catalyst retained a hydrodeoxygenation reactivity of 87.3% after reusing for five reaction cycles.
To summarize, efficient catalysts and green reaction systems are involved in the hydrogenation modification method of vegetable oils. However, during the hydrogenation of vegetable oil molecules, the positional isomerization generated trans-fatty acids in the molecules, thereby reducing biodegradability.
2.2. Transesterification
Transesterification is an effective method to improve the oxidative stability of vegetable oils by eliminating glycerol groups and introducing short-chain alcohols in order to reduce molecular viscosity, thereby improving the cold flow properties [14]. The factors that need to be considered in the transesterification reaction are alcohol-to-oil molar ratio, reaction temperature, catalyst type (basic or acidic), catalyst concentration, reactant purity, and FFA content. The transesterification of FAME is shown in Figure 2.
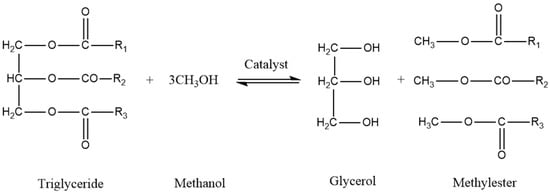
Figure 2.
Transesterification of FAME.
2.2.1. Acid Catalysis
Conventional catalysts are liquid acid catalysts, and the drawbacks of liquid acid catalysts can be equipment corrosion, environmental pollution, complex purification and separation, and catalyst recycling. Therefore, investigators are focused on environmentally friendly and efficient solid acid catalysts, such as ion-exchange resins with sulfonic acid and aluminate groups [36], heteroatoms mesoporous molecular sieves [37], heteropoly acids [38], metal-organic frameworks-based solid acids [39], zeolites [40] and so on. Among them, eco-friendly sulfonic and aluminate acid-modified solid acid catalysts are widely used in transesterification reactions due to their superior acid strength, higher surface area, advantageous pore space, and advanced thermal stability. Para-toluenesulfonic acid was considered to be a talented lubricant which can be utilized in the transesterification of sunflower oil, linseed oil, and jatropha oil [41]. The synthesis and application of sulfonic acid functionalized solid acid catalysts for transesterification reactions have been integrated over past 20 years [42].
2.2.2. Base Catalysis
Compared with acid catalysts, base catalysts, such as NaOH, and KOH, are more widely adopted by researchers owing to their high reaction conversion rate (>90%), high catalytic activity, and wide availability [11]. The transesterification of blended soybean oil and palm oil can be catalyzed by natural diatomite and the NaOH system [43]. It was found that the catalyst performed similar to zeolite with 98% and 98.4% conversion peaks at 63 °C and 70 °C. The temperature-dependent kinetics of the transesterification reaction in excess methanol could be well fitted to the first-order kinetics model, and the first-order rate constant had a good correlation with the Arrhenius equation. Kumar, S. et al. [44] investigated the successful transesterification of Jatropha oil with methanol using potassium hydroxide (0.5 wt%) as the alkaline catalyst at 70 °C.
However, the base is sensitive to water and then soap can be formed in the reaction system. Therefore, a solid base catalyst replaces the traditional liquid base catalyst and plays a critical role in the transesterification [45]. Solid base catalysts based on metal oxides are popular due to their low cost, high efficiency and wide variety [46]. The yield of biodiesel prepared using the transesterification reaction under UV light was 96.67% when employing palm oil as the raw material and TiO2-modified CaO as a catalyst [47]. A similar study had been reported that the as-prepared CaO catalyst with a porous hollow cage-like structure was employed to the transesterification [48]. The yield was 97.80% under the optimal reaction conditions and can be 90.30% after the fifth recycle. Yadav and Sharma [49] revealed that the conversion of methyl ester was 93.28% by the catalyst of BaAl2O4 in transesterification, and the conversion can be maintained as high as 80% after five reaction cycles.
The metal oxides can be also loaded on some support and an active K2CO3 with a Al2O3/SiO2 support has been reported [50]. The production of biodiesel based on sunflower oil at 120 °C and a 15:1 molar ratio of methanol to oil were the optimal conditions. It was shown that the conversion rate reached 93% after 15 min of reaction. Furthermore, a composite base catalyst or so-called multifunctional catalysts were developed. The CaO-MgO/Al2O3 catalyst synthesized by co-precipitation achieved a cottonseed oil conversion rate of 97.62% at 95.63 °C when the molar ratio of ethanol to cottonseed oil was 12.24 and the CaO-MgO loading was 14.4 wt% [51]. A favorable interaction between Al and other metals was recognized to improve the defects of the catalysts. The active sites on the surfaces are enhanced by the interaction of Sr, Al and Zn oxides [52]. The SrO-CaO oxide was loaded on the Al2O3 support to prepare the SrO-CaO-Al2O3 trifunctional catalyst. The yield of biodiesel prepared from palm oil remained at 92.61% after five cycles [53]. The base catalysts used in the transesterification of various vegetable oils are summarized in Table 2.

Table 2.
Enhancement of vegetable oil properties via base catalysis.
2.2.3. Enzyme Catalysis
Enzyme catalysis is known as having a high specificity, high efficiency, having mild reaction conditions and being able to easily purify by-products, which plays an indispensable role in the transesterification process. It was found that the addition of methanol could effectively maintain the activity of lipase [54]. The recombinant Rhizopus oryzae lipase was used in the transesterification of olive oil and palm oil and resulted in a product loss of less than 30% under six reuses. A similar enzyme catalysis method was reported by Yu et al. [55], Pseudomonas cepacia lipase was applied to the biodiesel, and the enzyme can be recycled and reused by a magnetic field, retaining 80% of the initial FAME conversion rate.
To summarize, the catalyst for the esterification process can be selected from inorganic salts, metal oxides, ion exchange resins, and lipase. Restaurant waste oil can be also used as the vegetable oil, and the esterification method has been widely used in the preparation of biodiesel. The elimination of glycerol groups in vegetable oil molecules can improve the oxidative stability of vegetable oil, but the double bonds still remain in the molecules, and therefore the activity of double bonds cannot be neglected.
2.3. Epoxidation
The carbon–carbon double bonds in vegetable oils can be reduced by epoxidation with peroxycarboxylic acid to generate epoxy vegetable oils, so as to improve the oxidation stability. The double bond in the molecule is vulnerable to attack from the epoxidation reagent to form epoxy groups [28]. The introduction of functional groups with lubricating or antioxidant properties to the vegetable oil through epoxidation and the ring-opening method is proved to be an effective method to prepare vegetable lubricant with excellent performance. The schematic diagram of the peroxidation and epoxidation reaction is shown in Figure 3.
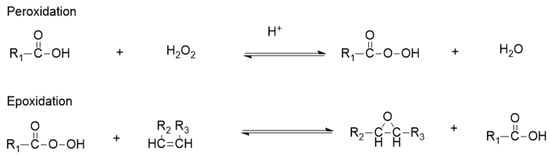
Figure 3.
Schematic diagram of peroxidation and epoxidation reaction.
The Prileshajev oxidation method is the most popular method for vegetable oil epoxidation [56]. Peroxycarboxylic acid formed in situ is considered to be superior because it is more environmentally friendly than the use of halogenated hydrocarbons, safer than oxygen molecules, and more effective than inorganic peroxides. The catalysts used in the epoxidation of vegetable oils is summarized in Table 3.
Bashiri et al. [57] studied the influence of acetic acid on the physicochemical properties of sunflower oil in epoxide reaction. The response surface methodology was applied to conduct statistical modeling, and the reaction yield reached 82.9% under optimal conditions. Afifah et al. [58] investigated acetic acid and hydrogen peroxide on the modification of the unsaturated bond of methyl palmitate. The plant-based environmentally friendly lubricants were successfully synthesized by epoxidation with a conversion rate of 98.9%. Cai et al. [59] prepared a series of xCd/TS-1 catalysts to produce epoxidized soybean oil through the acid-free catalytic epoxidation of soybean oil with H2O2. The five-membered intermediates formed by the coordination of highly dispersed Cd with tetrahedral Ti through oxygen atoms were confirmed to facilitate the epoxidation process of soybean oil.
In terms of the production process, the one-pot method is the main method for preparing epoxy vegetable oils, which has the characteristics of a simple production process, suitable reaction conditions, fewer by-products and a simple post-treatment process. In the study by Somidi et al. [60], the one-pot method was the best method to produce epoxy rapeseed oils. In total, 10 wt% of MoO3 was impregnated on alumina to obtain the excellent catalytic effect on the epoxidation reaction of rapeseed oil with a 98.3% conversion rate. Furthermore, depositing metal oxide nanocomposites on bio-waste-derived materials is also an effective environmentally friendly method. Sahu and Mohanty [61] proposed a method by depositing zinc oxide on fish bones to improve its surface and ion exchange performance. It was applied to the epoxidation reaction of neem oil with an 84.3% conversion rate after 8 h of reaction time. The catalyst maintained catalytic activity after four reaction cycles, providing a new direction for the combination of heterogeneous catalysis and waste utilization.

Table 3.
The catalysts used in the epoxidation of vegetable oils.
Table 3.
The catalysts used in the epoxidation of vegetable oils.
| Type | Catalyst | References |
|---|---|---|
| Inorganic acid | Sulfuric, phosphoric, nitric, hydrochloric acid | [56] |
| Organic acid | Formic, acetic, acrylic acid | [57] |
| Heteropoly acid | Peroxyphosphotungstic, peroxyformic acid | [58] |
| Ion exchange resin | Amberlite IR-120 cation exchange resin | [60] |
| Alumina | Acros, Fluka, Sol-Gel | [62] |
| Titanium silicate molecular sieve | Ti-MCM-41, TS-1 | [59] |
The epoxidation is of great significance in the treatment of waste oil to prepare environmentally friendly lubricants and biodiesel. Kurańska et al. [62] studied the in situ reaction of CH3COOH, H2SO4, H3PO4 and H2O2 to generate peroxyacetic acid in the epoxidation reaction. The conversion was found to be associated with the acid strength of the catalyst used. The H2SO4 catalysis achieved the highest reaction conversions (81%), while the highest selectivity was obtained by using H3PO4. Solving waste oil pollution by recycling and reusing UCO is an important method, which is in accordance with the development philosophy of the clean energy strategy.
The epoxidation modification of vegetable oil as a lubricating oil base oil is a practical modification method, but the epoxide group itself has a high activity, and therefore epoxidized vegetable oil is not the final product. Therefore, the ring can be further opened by nucleophiles that include alcohols, carboxylic acids, acid anhydrides, and amines, although numerous others can also lead to valuable functionalized fatty derivatives [63]. Biolubricants obtained from fatty acids of castor oil were synthesized by epoxidation and oxirane ring opening reactions using 2-ethylhexanol as nucleophilic agents, and it has a lower friction coefficient when compared to the commercial mineral oil [64]. The oleic acid was esterified with 1-octanol, followed by epoxidation. The oxirane ring opening reaction was performed using different alcohol structures (linear, branched, and cyclic), in order to evaluate their influence on the final physicochemical properties with the synthesized samples [65]. The chemical modification of pure rice bran oil via epoxidation the and ring opening process can also be helpful for the improvement of lubricating properties [66]. Soybean and jojoba oil were chemically transformed by epoxidation followed by the transesterification, ring-opening and acetylation of the hydroxylated obtained products. The rheological and tribological characteristics of the compounds demonstrate the possibility of an environmentally sustainable method for producing bio-lubricants [67].
To summarize, epoxidation is commonly carried out using peracetic acid, peroxymosulfuric acid, hydrogen peroxide, etc. as oxidants, with the temperature at 60–80 °C. Inorganic acids, organic acids, heteropoly acids, ion exchange resins, alumina, phase transfer agents, and silica molecular sieves have been used as catalysts for the epoxidation. Modifying epoxidized plant oil through an epoxide-opening reaction is a more effective modification method.
2.4. Estolide Formation
The estolide formation method can be also used in the modification of vegetable oil. Chemical modification in the form of estolide synthesis from oleic acid can lead to increased oxidative and hydrolytic stability and better cold flow properties [68]. Estolides were synthesized and showed similar tribological performance to epoxidized Jatropha oil [69]. A comparative physicochemical property assessment of biolubricants produced using chemical modification was performed, and it was found that the oxidative stabilities of chemically modified biolubricants followed the order of epoxidation > triesterification > estolide probably due to the content of C=C bonds in the molecules [70].
3. Auxiliary Methods
3.1. Modeling
In recent years, modeling was regarded as a more effective method, and it can be used to compare with the experiments, to determine the data conditions consistent with the experimental data, and to verify the accuracy of the dynamic model through continuous optimization [71]. Jalil [72] analyzed the optimal conditions for the catalytic epoxidation reaction optimization of palmitic oleic acid and other oleic acids, formic acid and mixed acid with different proportions, and verified the accuracy of the model through modeling data simulation experiments.
3.2. Thermal Induction
On account of the high content of FFAs and other impurities, the use of acid-base transesterification to prepare biodiesel from BHO is technically and economically challenging in terms of yield and economics. Under high acidity conditions, the in situ transesterification of FFAs and glycerides can also be carried out in porous media without slowing down the reaction rate [73]. Jung et al. [74] reported the feasibility of thermally induced transesterification from BHO to biodiesel. When the molar ratio of methanol to BHO reached 200, the reaction optimally yielded 58 wt%. Without changing the components of C6-22, the yield was proportional to the reaction temperature, and this method provided a new direction for biomass recovery.
3.3. Microalgae
Photosynthesis can be used as a carbon storage product to achieve lipid accumulation. Triacylglycerol could be transformed into biodiesel via transesterification technology, where the utilization rate of light energy of microalgae was 18% higher than that of ordinary crops [75]. Qu et al. [76] discussed the distinction of the microwave-assisted in situ transesterification of various algae with ethanol. The catalytic performance evaluation results showed that the highest yield of biodiesel could reach 95.8% under the optimum reaction condition.
3.4. Ultrasonic
Ultrasound can be used in hydrogenation to improve the selectivity. Sancheti and Gogate [77] discussed the benefits of ultrasound assistance in the synthesis of hydrogenated soybean oil. In total, 5 wt% of Pd/C was applied to the soybean oil, and the hydrogenation reaction effect was the best under the condition of 22 kHz frequency and 100 W irradiation power. In comparison to the traditional hydrogenation reaction under high pressure and high temperature, the ultrasonic-assisted reaction obtained high selectivity, and the ultrasonic-assisted soybean oil CTH reaction generated less trans-isomers at normal temperature. Tan et al. [78] summarized the influencing factors of the ultrasonic-catalyzed transesterification reaction. Under the control of the alcohol–oil ratio, catalyst dosage, reaction time and reaction temperature under certain conditions, ultrasound as an auxiliary method could not only improve the conversion rate and yield of the reaction, but also strengthen the production process of biodiesel.
4. Applications
4.1. Surfactant
Surfactant, as an effective additive for IFT reduction, is able to significantly refine the reservoir rock wettability and emulsion formation. Contamination could be produced after using traditional sulfur- and phosphorus-containing surfactants [79]. Therefore, the preparation of eco-friendly surfactants from vegetable oils has attracted research attention. Surfactants are widely applied in chemical enhanced oil recovery as it reduces the IFT to an ultra-low value and also alters the wettability of oleophilic rocks. The effectiveness of surfactants could generally be assessed by calculating the IFT between the crude oil and the surfactant solution. Saxena et al. [80] investigated alpha-sulfonated ethyl ester surfactants via transesterification. The wettability of oil-wet carbonate and quartz surfaces were changed to water-wet by the surfactants with outstanding thermal stability.
4.2. Hydraulic Fluid
Hydraulic fluid transmits power and energy, and acts in the role of lubricating, cooling, and sealing parts. Due to the good lubricating property, extreme pressure abrasion-resistant properties, and an eco-friendly performance, vegetable oil-based hydraulic fluid is widely applied to the agriculture field. Fanigliulo et al. [81] prepared semi-refined crambe abyssinica oil as a bio-hydraulic fluid and applied it in agriculture so as to relieve food contamination. Nogales-Delgado, Encinar, and Cortés [82] proposed a hydraulic oil prepared from high oleic safflower oil, and the hydraulic oil showed higher oxidative stability than rapeseed or cardoon. By avoiding the use of antioxidant additions, it revealed a cleaner and more sustainable production. Olszak et al. [83] studied the working fluids which are generated form vegetable oils. Compared to raspberry seed oil, blackcurrant seed oil was considered as a better option for hydraulic couplings.
4.3. Cutting Fluid
Traditional cutting fluids are non-biodegradable mineral oil-based fluids, and they are detrimental to workers’ health and the natural environment. Compared to oil-based cutting fluids, Debnath, Reddy, and Yi [84] proposed the potential feasibility of plant-based cutting fluids in green machining. The bio-cutting fluids exhibited excellent biodegradability and similar lubricating properties. The rice bran oil-based cutting fluid formulated by Edla et al. [85] exhibited lower cutting force, higher thermal stability, and similar lubricating properties in the tests, and was considered as an alternative solution for commercial mineral oil cutting fluids. The applicable scope of different types of vegetable oils is summarized in Table 4.

Table 4.
Application scope of vegetable oils.
4.4. Biodiesel
As a fuel for combustion engines, the consumption of diesel increases year by year because of its high operating power and economic efficiency [86]. For decades, researchers put emphasis on the production of biofuels through the addition, modification and doping of vegetable oils. Vegetable oils have been updated to the second generation (non-edible oil) as a feedstock in order to reduce dependence on food crops up to now [87]. The function of vegetable oils on engine performance and emissions was investigated compared to the results without vegetable oil [88]. It is shown that the blend oil with 10% wild mustard oil maintained similar engine performance while decreasing the CO, CO2, O2, and SO2 emissions in a continuous 200 hours experiment.
Biofuels, prepared from chemically modified CPO blended with diesel, also demonstrated similar results to commercial diesel [89]. It was shown that biofuel blended with 30% CPO was the best to balance diesel performance and reduce emissions. HVO is a popular method to prepare ester-type biodiesel. The potential of HVO blends as promising biofuels on fuel consumption reduction in contemporary automotive diesel engines was evaluated and confirmed [90]. Dobrzyönska et al. [91] further analyzed the effect of HVO on commercial B7 fuel. It was reported that HVO can be a promising alternative to conventional diesel engine vehicles and it can meet the requirements of upcoming emission standards (EURO 7). Emissions reductions by fuel modifications were more practical and effective than abandoning higher-emitting engine vehicles.
4.5. Flame Retardant Plasticizer
The majority of the traditional flame retardants in the market are still based on halogen-containing compounds. Bio-based flame-retardant plasticizers have become more and more popular over the past years. A method for preparing flame retardant PVC material with a castor oil-based flame-retardant plasticizer was developed [92]. The as-prepared flame-retardant PVC materials possessed high thermal stability and flame-retardant performance. Epoxidation is one of the most proficient ways for synthesizing plasticizers from vegetable oils. Jia et al. [93] investigated the progress of epoxidized soybean oil on PVC plasticizers. It was shown that soybean oil-based polyol ester plasticizers were beneficial to strengthen the stability of PVC blends. Chang et al. [94] studied the performance of a sustainable flame-retardant plasticizer prepared via the epoxidation of corn oil. The blends maintained good plasticizing properties and effectively retarded flame propagation, which revealed that the sustainable bio-based flame-retardant plasticizers could be a desirable alternative to halogen-based flame-retardant plasticizers.
5. Conclusions and the Outlook
The research progress of the chemical modification methods and applications of vegetable oils in recent years are summarized in this review. Hydrogenation modification has been widely used and regarded as an effective method when efficient catalysts and green reaction systems are involved in the method. However, during the hydrogenation of vegetable oil molecules, the positional isomerization generated trans-fatty acids in the molecules, thereby reducing the biodegradability. Transesterification is used to eliminate glycerol groups in vegetable oil molecules, and low-chain alcohols are introduced to reduce viscosity and strengthen cold flow properties. However, the double bonds still remain in the molecules after transesterification, and therefore the activity of double bonds cannot be neglected. The number of double bonds is reduced by the epoxidation reagent attacking the double bonds to form epoxy groups, improving the oxidation stability of vegetable oil in the epoxidation process. However, the epoxide group itself has a high activity, and therefore epoxidized vegetable oil is not the final product. Modifying epoxidized plant oil through an epoxide-opening reaction is a more effective modification method. It is not difficult to see that reducing the content of carbon–carbon double bonds and increasing the degree of saturation are fundamental to improve the physicochemical properties of vegetable oil. The comparison of the modification methods is shown in Table 5.

Table 5.
Comparison of the modification methods.
The improvement of the oxidative stability and cold flow properties of vegetable oils by chemical modification is the main direction of future work. Looking back on the past few years, modification with a single method is not satisfactory; therefore, the combination of different methods can improve the performance of vegetable oil. Auxiliary methods combined with chemical modification are beneficial to obtain better performance from both experiments and simulations. This multidisciplinary approach may allow UCO to be used as a latent feedstock in various areas.
In terms of application, the use of modified vegetable oil can achieve stable production capacity, high economic efficiency, and environmental friendliness [95,96]. Biofuel and bio-lubricants can be the main applications for vegetable oil. Biofuel is used for combustion with high heat density but almost zero SO2 and low CO2 emission. Bio-lubricants can be directly used to reduce friction and wear, and then the energy loss and consumption can be reduced. Although waste oil is still generated after the use of bio-lubricants, it can be easily degraded in the environment. Therefore, biofuel and bio-lubricants still have a prospect in the future.
It is extremely urgent to speed up basic research on vegetable oils and cease the dependence on crude oil. Unfortunately, the price and regulations can be a challenge of the application of vegetable oil. Therefore, conducting research on the degradation mechanism of modified vegetable oil, establishing experimental methods for biodegradability testing, and formulating a set of evaluation standards for biodegradable lubricants are of a great practical significance, as they can promote the development of biodegradable lubricants and their application in equipment.
Author Contributions
Conceptualization, S.H. and J.Y.; methodology, Y.Z., Z.S. and J.Y.; formal analysis, S.H. and J.Y.; investigation, Y.Z., Z.S., Z.Z., N.S. and B.Y.; writing—original draft preparation, Y.Z., Z.S. and Z.Z.; writing—review and editing, B.Y. and J.Y.; project administration, S.H. and J.Y.; funding acquisition, S.H. and J.Y. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful for the financial support of the National Natural Science Foundation of China (Project Number 22075183, 22278269 and 21975161), Joint Fund of the Ministry of Education (Project Number 8091B02052304), Foundation of Science and Technology Commission of Shanghai Municipality (Grant No. 22010503900), Industrial Collaborative Innovation Project of Shanghai (Project Number 2021-cyxt1-kj37 and XTCX-KJ-2022-70), Research and Innovation Project of Shanghai Municipal Education Commission (Project Number 2023ZKZD54), Natural Science Foundation Project of Shanghai (Project Number 23ZR1425300 and 22ZR1426100), Technical Standard Project of Shanghai (Project Number 23DZ2201100), High-end Special oils Technical Innovation Center of Shandong Province and State Key Laboratory of Heavy Oil Processing.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
List of Abbreviations
| UCO | Used Cooking Oil |
| CTH | Catalytic Transfer Hydrogenation |
| TOF | Turnover Frequency |
| FAME | Fatty Acid Methyl Ester |
| BHO | Bio-Heavy Oil |
| IFT | Interfacial Tension |
| CPO | Crude Palm Oil |
| HVO | Hydrogenated Vegetable Oil |
| PVC | Polyvinyl Chloride |
| FFAs | Free Fatty Acids |
References
- Ramamurthy, P.C.; Singh, S.; Kapoor, D.; Parihar, P.; Samuel, J.; Prasad, R.; Kumar, A.; Singh, J. Microbial biotechnological approaches: Renewable bioprocessing for the future energy systems. Microb. Cell Factories 2021, 20, 55. [Google Scholar] [CrossRef]
- Hwang, J.; Bae, C.; Gupta, T. Application of waste cooking oil (WCO) biodiesel in a compression ignition engine. Fuel 2016, 176, 20–31. [Google Scholar] [CrossRef]
- Breiing, V.; Hillmer, J.; Schmidt, C.; Petry, M.; Behrends, B.; Steiner, U.; Kraska, T.; Pude, R. Fungicidal Efficacy of Drying Plant Oils in Green Beans against Bean Rust (Uromyces appendiculatus). Plants 2021, 10, 143. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, S.C.A.; Belchior, C.R.; Nascimento, M.V.G.; Vieira, L.D.S.R.; Fleury, G. Performance of a diesel generator fuelled with palm oil. Fuel 2002, 81, 2097–2102. [Google Scholar] [CrossRef]
- Thomas, J.J.; Manojkumar, C.V.; Sabu, V.R.; Nagarajan, G. Development and validation of a reduced chemical kinetic model for used vegetable oil biodiesel/1-Hexanol blend for engine application. Fuel 2020, 273, 117780. [Google Scholar] [CrossRef]
- No, S.-Y. Inedible vegetable oils and their derivatives for alternative diesel fuels in CI engines: A review. Renew. Sustain. Energy Rev. 2011, 15, 131–149. [Google Scholar] [CrossRef]
- Sajeeb, A.; Rajendrakumar, P.K. Comparative evaluation of lubricant properties of biodegradable blend of coconut and mustard oil. J. Clean. Prod. 2019, 240, 118255. [Google Scholar] [CrossRef]
- Jiang, X.; Long, F.; Cao, X.; Zhao, J.; Liu, P.; Xu, J. Catalytic cracking of waste cooking oil followed with hydro-isomerization for high-quality biofuel production. J. Clean. Prod. 2022, 345, 131027. [Google Scholar] [CrossRef]
- Muhbat, S.; Tufail, M.; Hashmi, S. Production of diesel-like fuel by co-pyrolysis of waste lubricating oil and waste cooking oil. Biomass Convers. Biorefinery 2021. [Google Scholar] [CrossRef]
- Milano, J.; Shamsuddin, A.H.; Silitonga, A.S.; Sebayang, A.H.; Siregar, M.A.; Masjuki, H.H.; Pulungan, M.A.; Chia, S.R.; Zamri, M.F.M.A. Tribological study on the biodiesel produced from waste cooking oil, waste cooking oil blend with Calophyllum inophyllum and its diesel blends on lubricant oil. Energy Rep. 2022, 8, 1578–1590. [Google Scholar] [CrossRef]
- Baskar, G.; Aiswarya, R. Trends in catalytic production of biodiesel from various feedstocks. Renew. Sustain. Energy Rev. 2016, 57, 496–504. [Google Scholar] [CrossRef]
- Barbera, E.; Hirayama, K.; Maglinao, R.L.; Davis, R.W.; Kumar, S. Recent developments in synthesizing biolubricants—A review. Biomass Convers. Biorefinery 2024, 14, 2867–2887. [Google Scholar] [CrossRef]
- Naji, S.Z.; Tye, C.T.; Abd, A.A. State of the art of vegetable oil transformation into biofuels using catalytic cracking technology: Recent trends and future perspectives. Process Biochem. 2021, 109, 148–168. [Google Scholar] [CrossRef]
- Na, H.; Mok, C.; Lee, J. Effects of plasma treatment on the oxidative stability of vegetable oil containing antioxidants. Food Chem. 2020, 302, 125306. [Google Scholar] [CrossRef]
- Sonthalia, A.; Kumar, N. Hydroprocessed vegetable oil as a fuel for transportation sector: A review. J. Energy Inst. 2019, 92, 1–17. [Google Scholar] [CrossRef]
- Orege, J.I.; Oderinde, O.; Kifle, G.A.; Ibikunle, A.A.; Raheem, S.A.; Ejeromedoghene, O.; Okeke, E.S.; Olukowi, O.M.; Orege, O.B.; Fagbohun, E.O. Recent advances in heterogeneous catalysis for green biodiesel production by transesterification. Energy Convers. Manag. 2022, 258, 115406. [Google Scholar] [CrossRef]
- Mao, Q.; He, C.; Chen, B.; Zhang, X. Enzymatic synthesis of TMP esters based on pelargonic acid from the cleavage of oleic acid: Evaluation of synthetic process, physicochemical properties and lubrication performance. Biochem. Eng. J. 2024, 204, 109238. [Google Scholar] [CrossRef]
- Streitwieser, A.; Heathcock, C.; Kosower, E. Introduction to Organic Chemistry; Macmillan: New York, NY, USA, 1992; pp. 676–677. [Google Scholar]
- Tian, S.; Wang, B.; Gong, W.; He, Z.; Xu, Q.; Chen, W.; Zhang, Q.; Zhu, Y.; Yang, J.; Fu, Q.; et al. Dual-atom Pt heterogeneous catalyst with excellent catalytic performances for the selective hydrogenation and epoxidation. Nat. Commun. 2021, 12, 3181. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Jayakumar, S.; Luo, M.; Kong, X.; Li, C.; Li, H.; Chen, J.; Yang, Q. The promotion effect of π-π interactions in Pd NPs catalysed selective hydrogenation. Nat. Commun. 2022, 13, 1770. [Google Scholar] [CrossRef]
- Gousi, M.; Kordouli, E.; Bourikas, K.; Simianakis, E.; Ladas, S.; Panagiotou, G.D.; Kordulis, C.; Lycourghiotis, A. Green diesel production over nickel-alumina nanostructured catalysts promoted by zinc. Catal. Today 2020, 355, 903–909. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Chen, Y.; Nie, S.; Xie, M. Characteristics and catalytic behavior of different platinum supported catalysts in the selective hydrogenation of soybean oil. React. Kinet. Mech. Catal. 2017, 122, 915–930. [Google Scholar] [CrossRef]
- Lee, H.-S.; Lee, J.; Seo, H.; Kang, H.; Kim, D.H.; Lee, Y.-W. Evaluation of Pd/ZSM-5 catalyst for simultaneous reaction of transesterification and partial catalytic transfer hydrogenation of soybean oil under supercritical methanol. Fuel Process. Technol. 2021, 218, 106870. [Google Scholar] [CrossRef]
- Numwong, N.; Prabnasak, P.; Prayoonpunratn, P.; Triphatthanaphong, P.; Thunyaratchatanon, C.; Mochizuki, T.; Chen, S.-Y.; Luengnaruemitchai, A.; Sooknoi, T. Effect of Pd particle size on activity and cis-trans selectivity in partial hydrogenation of soybean oil-derived FAMEs over Pd/SiO2 catalysts. Fuel Process. Technol. 2020, 203, 106393. [Google Scholar] [CrossRef]
- Cepeda, E.A.; Iriarte-Velasco, U.; Calvo, B.; Sierra, I. Hydrogenation of Sunflower Oil over M/SiO2 and M/Al2O3 (M = Ni, Pd, Pt, Co, Cu) Catalysts. J. Am. Oil Chem. Soc. 2016, 93, 731–741. [Google Scholar] [CrossRef]
- Papageridis, K.N.; Charisiou, N.D.; Douvartzides, S.L.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; AlKhoori, S.; Polychronopoulou, K.; Goula, M.A. Effect of operating parameters on the selective catalytic deoxygenation of palm oil to produce renewable diesel over Ni supported on Al2O3, ZrO2 and SiO2 catalysts. Fuel Process. Technol. 2020, 209, 106547. [Google Scholar] [CrossRef]
- Thunyaratchatanon, C.; Luengnaruemitchai, A.; Chollacoop, N.; Yoshimura, Y. Catalytic upgrading of soybean oil methyl esters by partial hydrogenation using Pd catalysts. Fuel 2016, 163, 8–16. [Google Scholar] [CrossRef]
- Zhang, C.; Garrison, T.F.; Madbouly, S.A.; Kessler, M.R. Recent advances in vegetable oil-based polymers and their composites. Prog. Polym. Sci. 2017, 71, 91–143. [Google Scholar] [CrossRef]
- Toshtay, K.; Auyezov, A.; Korkembay, Z.; Toktassynov, S.; Seytkhan, A.; Nurakyshev, A. Partial hydrogenation of sunflower oil on platinum catalysts: Influence of process conditions on the mass content of geometric isomers. Mol. Catal. 2021, 513, 111819. [Google Scholar] [CrossRef]
- Aiamsiri, P.; Tumnantong, D.; Yoosuk, B.; Ngamcharussrivichai, C.; Prasassarakich, P. Biohydrogenated Diesel from Palm Oil Deoxygenation over Unsupported and γ-Al2O3 Supported Ni–Mo Catalysts. Energy Fuels 2021, 35, 14793–14804. [Google Scholar] [CrossRef]
- Du, Y.; Behera, R.K.; Maligal-Ganesh, R.V.; Chen, M.; Zhao, T.Y.; Huang, W.; Bowers, C.R. Mesoporous Silica Encapsulated Platinum–Tin Intermetallic Nanoparticles Catalyze Hydrogenation with an Unprecedented 20% Pairwise Selectivity for Parahydrogen Enhanced Nuclear Magnetic Resonance. J. Phys. Chem. Lett. 2022, 13, 4125–4132. [Google Scholar] [CrossRef]
- Na Rungsi, A.; Luengnaruemitchai, A.; Wongkasemjit, S.; Chollacoop, N.; Chen, S.-Y.; Yoshimura, Y. Influence of silica sources on structural property and activity of Pd-supported on mesoporous MCM-41 synthesized with an aid of microwave heating for partial hydrogenation of soybean methyl esters. Appl. Catal. A Gen. 2018, 563, 80–90. [Google Scholar] [CrossRef]
- Baldauf, E.; Sievers, A.; Willner, T. Heterogeneous catalysts for the production of hydrotreated cracked vegetable oil. Biofuels 2017, 8, 555–564. [Google Scholar] [CrossRef]
- Wong, F.H.; Tiong, T.J.; Leong, L.K.; Lin, K.-S.; Yap, Y.H. Effects of ZnO on Characteristics and Selectivity of Coprecipitated Ni/ZnO/Al2O3 Catalysts for Partial Hydrogenation of Sunflower Oil. Ind. Eng. Chem. Res. 2018, 57, 3163–3174. [Google Scholar] [CrossRef]
- Laosiripojana, W.; Kiatkittipong, W.; Sakdaronnarong, C.; Assabumrungrat, S.; Laosiripojana, N. Catalytic hydrotreatment of pyrolysis-oil with bimetallic Ni-Cu catalysts supported by several mono-oxide and mixed-oxide materials. Renew. Energy 2019, 135, 1048–1055. [Google Scholar] [CrossRef]
- Deboni, T.M.; Hirata, G.A.M.; Shimamoto, G.G.; Tubino, M.; Meirelles, A.J.d.A. Deacidification and ethyl biodiesel production from acid soybean oil using a strong anion exchange resin. Chem. Eng. J. 2018, 333, 686–696. [Google Scholar] [CrossRef]
- Xin, H.; Ke, T. Preparation and adsorption denitrogenation from model fuel or diesel oil of heteroatoms mesoporous molecular sieve Co-MCM-41. Energy Sources Part A Recovery Util. Environ. Eff. 2016, 38, 2560–2567. [Google Scholar] [CrossRef]
- Jeon, Y.; Chi, W.S.; Hwang, J.; Kim, D.H.; Kim, J.H.; Shul, Y.-G. Core-shell nanostructured heteropoly acid-functionalized metal-organic frameworks: Bifunctional heterogeneous catalyst for efficient biodiesel production. Appl. Catal. B Environ. 2019, 242, 51–59. [Google Scholar] [CrossRef]
- Ghorbani-Choghamarani, A.; Taherinia, Z.; Tyula, Y.A. Efficient biodiesel production from oleic and palmitic acid using a novel molybdenum metal–organic framework as efficient and reusable catalyst. Sci. Rep. 2022, 12, 10338. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Cai, L.; Zhang, L.; Fu, W.; Hong, Y.; Gao, X.; Jiang, Y.; Li, L.; Yan, X.; Wu, G. Transesterification of rice bran oil to biodiesel using mesoporous NaBeta zeolite-supported molybdenum catalyst: Experimental and kinetic studies. Chem. Eng. J. 2020, 382, 122839. [Google Scholar] [CrossRef]
- Xu, W.; Ollevier, T.; Kleitz, F. Iron-Modified Mesoporous Silica as an Efficient Solid Lewis Acid Catalyst for the Mukaiyama Aldol Reaction. ACS Catal. 2018, 8, 1932–1944. [Google Scholar] [CrossRef]
- Shagufta; Ahmad, I.; Dhar, R. Sulfonic Acid-Functionalized Solid Acid Catalyst in Esterification and Transesterification Reactions. Catal. Surv. Asia 2017, 21, 53–69. [Google Scholar] [CrossRef]
- Đặng, T.-H.; Nguyễn, X.-H.; Chou, C.-L.; Chen, B.-H. Preparation of cancrinite-type zeolite from diatomaceous earth as transesterification catalysts for biodiesel production. Renew. Energy 2021, 174, 347–358. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, A.; Mandal, A. Characterizations of surfactant synthesized from Jatropha oil and its application in enhanced oil recovery. AIChE J. 2017, 63, 2731–2741. [Google Scholar] [CrossRef]
- Jambhulkar, D.K.; Ugwekar, R.P.; Bhanvase, B.A.; Barai, D.P. A review on solid base heterogeneous catalysts: Preparation, characterization and applications. Chem. Eng. Commun. 2022, 209, 433–484. [Google Scholar] [CrossRef]
- Marwaha, A.; Dhir, A.; Mahla, S.K.; Mohapatra, S.K. An overview of solid base heterogeneous catalysts for biodiesel production. Catal. Rev. 2018, 60, 594–628. [Google Scholar] [CrossRef]
- Mohamad, M.; Ngadi, N.; Wong, S.L.; Jusoh, M.; Yahya, N.Y. Prediction of biodiesel yield during transesterification process using response surface methodology. Fuel 2017, 190, 104–112. [Google Scholar] [CrossRef]
- Huang, J.; Zou, Y.; Yaseen, M.; Qu, H.; He, R.; Tong, Z. Fabrication of hollow cage-like CaO catalyst for the enhanced biodiesel production via transesterification of soybean oil and methanol. Fuel 2021, 290, 119799. [Google Scholar] [CrossRef]
- Yadav, M.; Sharma, Y.C. Transesterification of used vegetable oil using BaAl2O4 spinel as heterogeneous base catalyst. Energy Convers. Manag. 2019, 198, 111795. [Google Scholar] [CrossRef]
- Lukić, I.; Krstić, J.; Jovanović, D.; Skala, D. Alumina/silica supported K2CO3 as a catalyst for biodiesel synthesis from sunflower oil. Bioresour. Technol. 2009, 100, 4690–4696. [Google Scholar] [CrossRef]
- Mahdavi, V.; Monajemi, A. Optimization of operational conditions for biodiesel production from cottonseed oil on CaO–MgO/Al2O3 solid base catalysts. J. Taiwan Inst. Chem. Eng. 2014, 45, 2286–2292. [Google Scholar] [CrossRef]
- Al-Saadi, A.; Mathan, B.; He, Y. Biodiesel production via simultaneous transesterification and esterification reactions over SrO–ZnO/Al2O3 as a bifunctional catalyst using high acidic waste cooking oil. Chem. Eng. Res. Des. 2020, 162, 238–248. [Google Scholar] [CrossRef]
- Zhang, Y.; Niu, S.; Han, K.; Li, Y.; Lu, C. Synthesis of the SrO–CaO–Al2O3 trimetallic oxide catalyst for transesterification to produce biodiesel. Renew. Energy 2021, 168, 981–990. [Google Scholar] [CrossRef]
- Duarte, S.H.; del Peso Hernández, G.L.; Canet, A.; Benaiges, M.D.; Maugeri, F.; Valero, F. Enzymatic biodiesel synthesis from yeast oil using immobilized recombinant Rhizopus oryzae lipase. Bioresour. Technol. 2015, 183, 175–180. [Google Scholar] [CrossRef]
- Yu, C.Y.; Huang, L.Y.; Kuan, I.C.; Lee, S.L. Optimized Production of Biodiesel from Waste Cooking Oil by Lipase Immobilized on Magnetic Nanoparticles. Int. J. Mol. Sci. 2013, 14, 24074–24086. [Google Scholar] [CrossRef] [PubMed]
- Klaas, M.R.; Warwel, S. Chemoenzymatic epoxidation of alkenes by dimethyl carbonate and hydrogen peroxide. Org. Lett. 1999, 1, 1025–1026. [Google Scholar] [CrossRef]
- Bashiri, S.; Ghobadian, B.; Dehghani Soufi, M.; Gorjian, S. Chemical modification of sunflower waste cooking oil for biolubricant production through epoxidation reaction. Mater. Sci. Energy Technol. 2021, 4, 119–127. [Google Scholar] [CrossRef]
- Afifah, A.N.; Syahrullail, S.; Wan Azlee, N.I.; Rohah, A.M. Synthesis and tribological studies of epoxidized palm stearin methyl ester as a green lubricant. J. Clean. Prod. 2021, 280, 124320. [Google Scholar] [CrossRef]
- Cai, L.; Chen, C.; Wang, W.; Gao, X.; Kuang, X.; Jiang, Y.; Li, L.; Wu, G. Acid-free epoxidation of soybean oil with hydrogen peroxide to epoxidized soybean oil over titanium silicalite-1 zeolite supported cadmium catalysts. J. Ind. Eng. Chem. 2020, 91, 191–200. [Google Scholar] [CrossRef]
- Somidi, A.K.R.; Das, U.; Dalai, A.K. One-pot synthesis of canola oil based biolubricants catalyzed by MoO3/Al2O3 and process optimization study. Chem. Eng. J. 2016, 293, 259–272. [Google Scholar] [CrossRef]
- Sahu, H.; Mohanty, K. One pot peroxidation of oleic acid rich Azadirachta indica oil over bio-waste derived heterogeneous catalyst. Can. J. Chem. Eng. 2017, 95, 1526–1536. [Google Scholar] [CrossRef]
- Kurańska, M.; Beneš, H.; Prociak, A.; Trhlíková, O.; Walterová, Z.; Stochlińska, W. Investigation of epoxidation of used cooking oils with homogeneous and heterogeneous catalysts. J. Clean. Prod. 2019, 236, 117615. [Google Scholar] [CrossRef]
- Moser, B.R.; Germak, S.C.; Doll, K.M.; Kenar, J.A.; Sharma, B.K. A review of fatty epoxide ring opening reactions: Chemistry, recent advances, and applications. J. Am. Oil Chem. Soc. 2022, 99, 801–842. [Google Scholar] [CrossRef]
- Filho, P.; Nascimento, M.; Silva, S.; Luna, F.; Castellón, E.; Cavalcante, C. Synthesis and Frictional Characteristics of Bio-Based Lubricants Obtained from Fatty Acids of Castor Oil. Lubricants 2023, 11, 57. [Google Scholar] [CrossRef]
- Marques, J.; Rios, Í.; Parente, E.; Quintella, S.; Luna, F.; Cavalcante, C. Synthesis and Characterization of Potential Bio-Based Lubricant Basestocks via Epoxidation Process. J. Am. Oil Chem. Soc. 2020, 97, 437–446. [Google Scholar] [CrossRef]
- Thampi, A.; John, A.; Arif, M.; Rani, S. Evaluation of the tribological properties and oxidative stability of epoxidized and ring opened products of pure rice bran oil. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2021, 235, 1093–1100. [Google Scholar] [CrossRef]
- Abdel-Hameed, H.; El-Saeed, S.; Ahmed, N.; Nassar, M.; El-Kafrawy, A.; Hashem, A. Chemical transformation of Jojoba oil and Soybean oil and study of their uses as bio-lubricants. Ind. Crops Prod. 2022, 187, 115256. [Google Scholar] [CrossRef]
- Sanap, P.; Sonawane, D.; Patil, S.; Pratap, A. Optimization of oleic-estolide fatty acid synthesis using response surface methodology and artificial neural networks. Ind. Crops Prod. 2022, 188, 115711. [Google Scholar] [CrossRef]
- Rajasozhaperumal, G.; Kannan, C. Comparative evaluation of chemically modified Jatropha oils as sustainable biolubricants in boundary lubrication regime. Tribol. Int. 2023, 186, 108594. [Google Scholar] [CrossRef]
- Khan, S.; Das, P.; Quadir, M.; Thaher, M.; Annamalai, S.; Mahata, C.; Hawari, A.; Jabri, H. A comparative physicochemical property assessment and techno-economic analysis of biolubricants produced using chemical modification and additive-based routes. Sci. Total Environ. 2022, 847, 157648. [Google Scholar] [CrossRef]
- Jalil, M.J.; Hadi, A.; Azmi, I.S. Catalytic epoxidation of palm oleic acid using in situ generated performic acid—Optimization and kinetic studies. Mater. Chem. Phys. 2021, 270, 124754. [Google Scholar] [CrossRef]
- Jalil, M.J. Optimization of Palm Oleic Acid Epoxidation via in Situ Generated Performic Acid Using Taguchi Orthogonal Array Design and the Study of Reaction Kinetics. Smart Sci. 2019, 7, 252–259. [Google Scholar] [CrossRef]
- Jung, S.; Kim, H.; Tsang, Y.F.; Lin, K.-Y.A.; Park, Y.-K.; Kwon, E.E. A new biorefinery platform for producing (C2-5) bioalcohols through the biological/chemical hybridization process. Bioresour. Technol. 2020, 311, 123568. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Kim, M.; Lin, K.-Y.A.; Park, Y.-K.; Kwon, E.E. Biodiesel synthesis from bio-heavy oil through thermally induced transesterification. J. Clean. Prod. 2021, 294, 126347. [Google Scholar] [CrossRef]
- Sun, B.; Tian, P.; Ren, F.; Yan, J.; Wang, C.; Lin, H.; Xue, Y.; Han, S. Synthesis and evaluation of alkyl methacrylate-norbornene anhydride copolymers with various pendants as pour point depressants for soybean biodiesel-diesel blends. Fuel 2022, 317, 123542. [Google Scholar] [CrossRef]
- Qu, S.; Chen, C.; Guo, M.; Jiang, W.; Lu, J.; Yi, W.; Ding, J. Microwave-assisted in-situ transesterification of Spirulina platensis to biodiesel using PEG/MgO/ZSM-5 magnetic catalyst. J. Clean. Prod. 2021, 311, 127490. [Google Scholar] [CrossRef]
- Sancheti, S.V.; Gogate, P.R. Ultrasound assisted selective catalytic transfer hydrogenation of soybean oil using 5% Pd/C as catalyst under ambient conditions in water. Ultrason. Sonochemistry 2017, 38, 161–167. [Google Scholar] [CrossRef]
- Tan, S.X.; Lim, S.; Ong, H.C.; Pang, Y.L. State of the art review on development of ultrasound-assisted catalytic transesterification process for biodiesel production. Fuel 2019, 235, 886–907. [Google Scholar] [CrossRef]
- Nowrouzi, I.; Mohammadi, A.H.; Manshad, A.K. Chemical Enhanced Oil Recovery by Different Scenarios of Slug Injection into Carbonate/Sandstone Composite Oil Reservoirs Using an Anionic Surfactant Derived from Rapeseed Oil. Energy Fuels 2021, 35, 1248–1258. [Google Scholar] [CrossRef]
- Saxena, N.; Pal, N.; Dey, S.; Mandal, A. Characterizations of surfactant synthesized from palm oil and its application in enhanced oil recovery. J. Taiwan Inst. Chem. Eng. 2017, 81, 343–355. [Google Scholar] [CrossRef]
- Fanigliulo, R.; Pochi, D.; Bondioli, P.; Grilli, R.; Fornaciari, L.; Folegatti, L.; Malaguti, L.; Matteo, R.; Ugolini, L.; Lazzeri, L. Semi-refined Crambe abyssinica (Hochst. EX R.E.Fr.) oil as a biobased hydraulic fluid for agricultural applications. Biomass Convers. Biorefinery 2023, 13, 1859–1871. [Google Scholar] [CrossRef]
- Nogales-Delgado, S.; Encinar, J.M.; González Cortés, Á. High oleic safflower oil as a feedstock for stable biodiesel and biolubricant production. Ind. Crops Prod. 2021, 170, 113701. [Google Scholar] [CrossRef]
- Olszak, A.; Osowski, K.; Musiałek, I.; Rogoś, E.; Kęsy, A.; Kęsy, Z. Application of Plant Oils as Ecologically Friendly Hydraulic Fluids. Appl. Sci. 2020, 10, 9086. [Google Scholar] [CrossRef]
- Debnath, S.; Reddy, M.M.; Yi, Q.S. Environmental friendly cutting fluids and cooling techniques in machining: A review. J. Clean. Prod. 2014, 83, 33–47. [Google Scholar] [CrossRef]
- Edla, S.; Thampi, A.D.; Pillai, A.B.K.; Sivan, V.V.; Arif, M.M.; Sasidharan, B.; Sasidharan, R. Formulation of rice bran oil-based green cutting fluid with holy basil oil and clove oil as bio-additives. Biomass Convers. Biorefinery 2022, 13, 16877–16886. [Google Scholar] [CrossRef]
- Mitrova, T. Review of the “Global and Russian energy outlook up to 2040”. Energy Strategy Rev. 2014, 2, 323–325. [Google Scholar] [CrossRef]
- Yadav, A.K.; Khan, M.E.; Dubey, A.M.; Pal, A. Performance and emission characteristics of a transportation diesel engine operated with non-edible vegetable oils biodiesel. Case Stud. Therm. Eng. 2016, 8, 236–244. [Google Scholar] [CrossRef]
- Öğüt, H.; Oğuz, H.; Aydın, F.; Ciniviz, M.; Deveci, H. The effects of the use of vegetable oil based as engine lubrication oil on engine performance and emissions in diesel engines. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 42, 2381–2396. [Google Scholar] [CrossRef]
- Ge, J.C.; Yoon, S.K.; Song, J.H. Comparative Evaluation on Combustion and Emission Characteristics of a Diesel Engine Fueled with Crude Palm Oil Blends. Appl. Sci. 2021, 11, 11502. [Google Scholar] [CrossRef]
- Napolitano, P.; Guido, C.; Beatrice, C.; Pellegrini, L. Impact of hydrocracked diesel fuel and Hydrotreated Vegetable Oil blends on the fuel consumption of automotive diesel engines. Fuel 2018, 222, 718–732. [Google Scholar] [CrossRef]
- Dobrzyńska, E.; Szewczyńska, M.; Pośniak, M.; Szczotka, A.; Puchałka, B.; Woodburn, J. Exhaust emissions from diesel engines fueled by different blends with the addition of nanomodifiers and hydrotreated vegetable oil HVO. Environ. Pollut. 2020, 259, 113772. [Google Scholar] [CrossRef]
- Jia, P.; Zhang, M.; Hu, L.; Liu, C.; Feng, G.; Yang, X.; Bo, C.; Zhou, Y. Development of a vegetable oil based plasticizer for preparing flame retardant poly(vinyl chloride) materials. RSC Adv. 2015, 5, 76392–76400. [Google Scholar] [CrossRef]
- Jia, P.; Zhang, M.; Hu, L.; Zhou, Y. Green plasticizers derived from soybean oil for poly(vinyl chloride) as a renewable resource material. Korean J. Chem. Eng. 2016, 33, 1080–1087. [Google Scholar] [CrossRef]
- Chang, B.P.; Thakur, S.; Mohanty, A.K.; Misra, M. Novel sustainable biobased flame retardant from functionalized vegetable oil for enhanced flame retardancy of engineering plastic. Sci. Rep. 2019, 9, 15971. [Google Scholar] [CrossRef] [PubMed]
- Alcock, T.; Salt, D.; Wilson, P.; Ramsden, S. More sustainable vegetable oil: Balancing productivity with carbon storage opportunities. Sci. Total Environ. 2022, 829, 154539. [Google Scholar] [CrossRef]
- You, Y.; Shie, J.; Chang, C.; Huang, S.; Pai, C.; Yu, Y.; Chang, C. Economic cost analysis of biodiesel production: Case in soybean oil. Energy Fuels 2008, 22, 182–189. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).