Synergistic Effects of Layered Double Hydroxide and MoS2 on the Performance of Lubricants
Abstract
1. Introduction
2. Experimental
2.1. Materials and Preparation
2.2. Test Instrument and Methods
- (1)
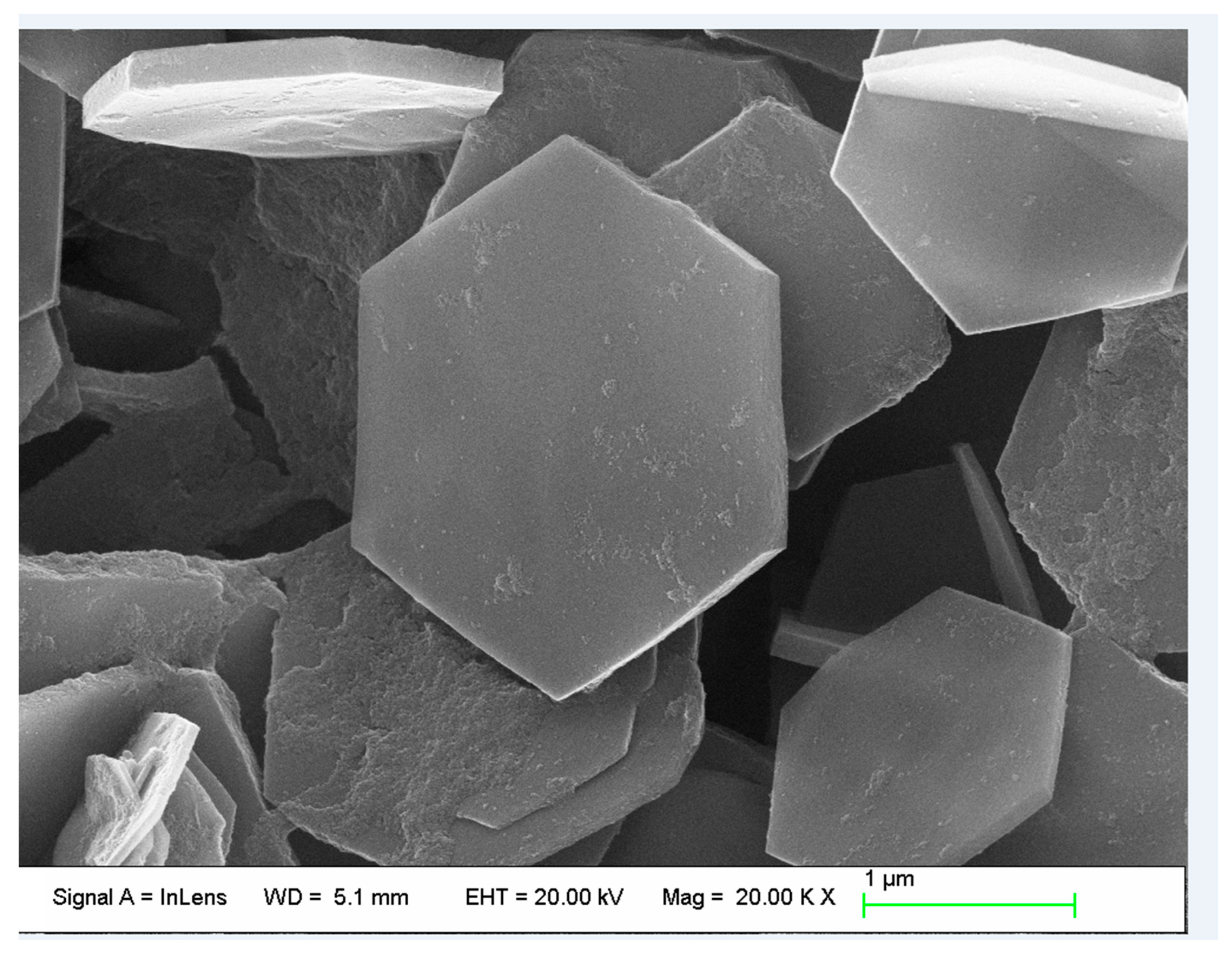
- A scanning electron microscope (SEM) from ZEISS Corporation (Oberkohen, Germany) was used to observe the microstructures of the LDH and MoS2. The crystal structure of the LDH was characterized by X-ray diffraction (XRD) with an instrument from Shimadzu Corporation (Kyoto, Japan) using Cu Kα radiation (λ = 0.15406 nm).
- (2)
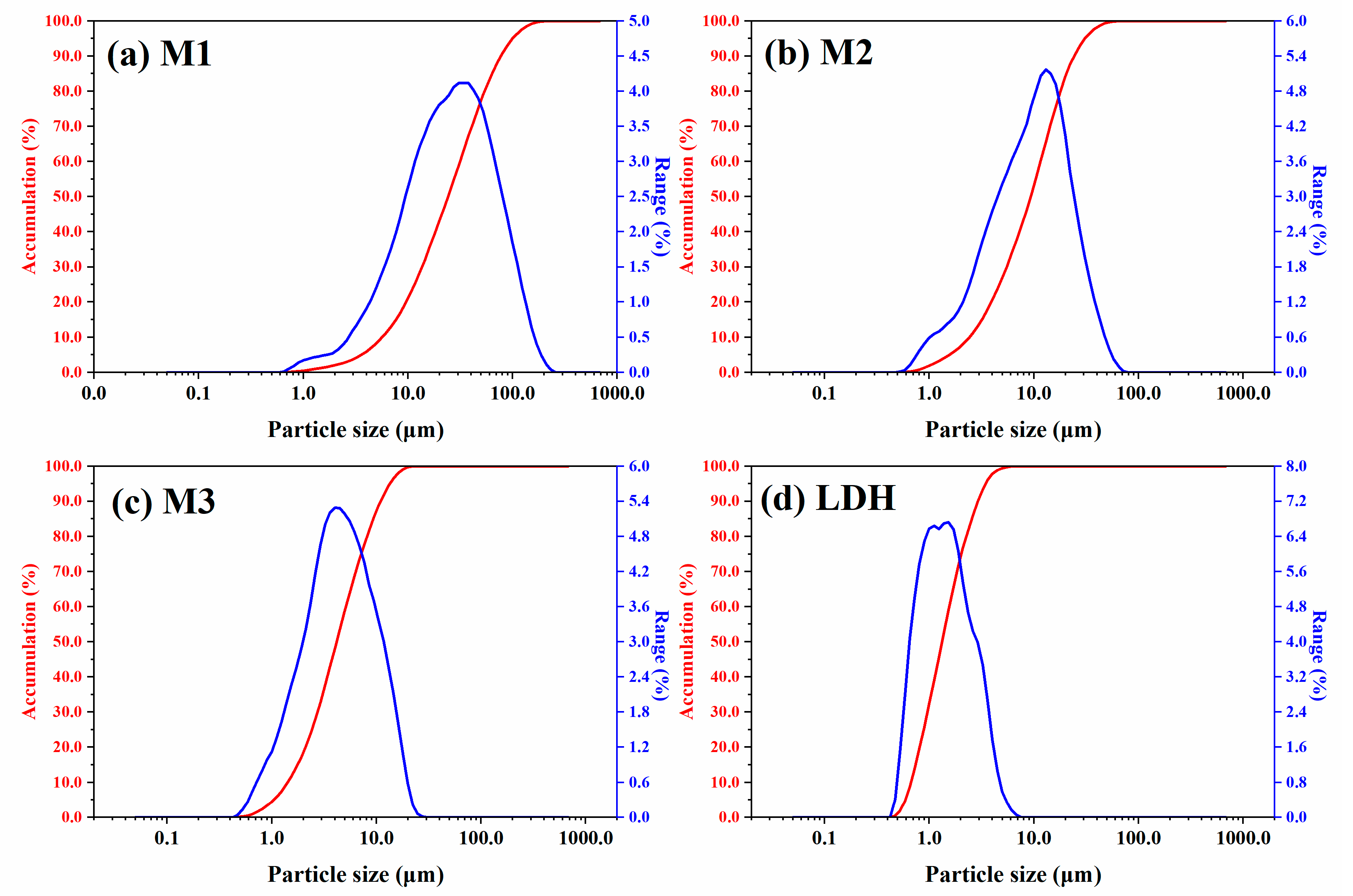
- The particle sizes of the LDH and MoS2 were measured using a Laser Particle Size Analyzer BT-9300S from Dandong Bettersize Instruments Ltd. (Dandong, China), with water as the medium and a refractive index of 1.333, in accordance with ISO 13320 [34].
- (3)
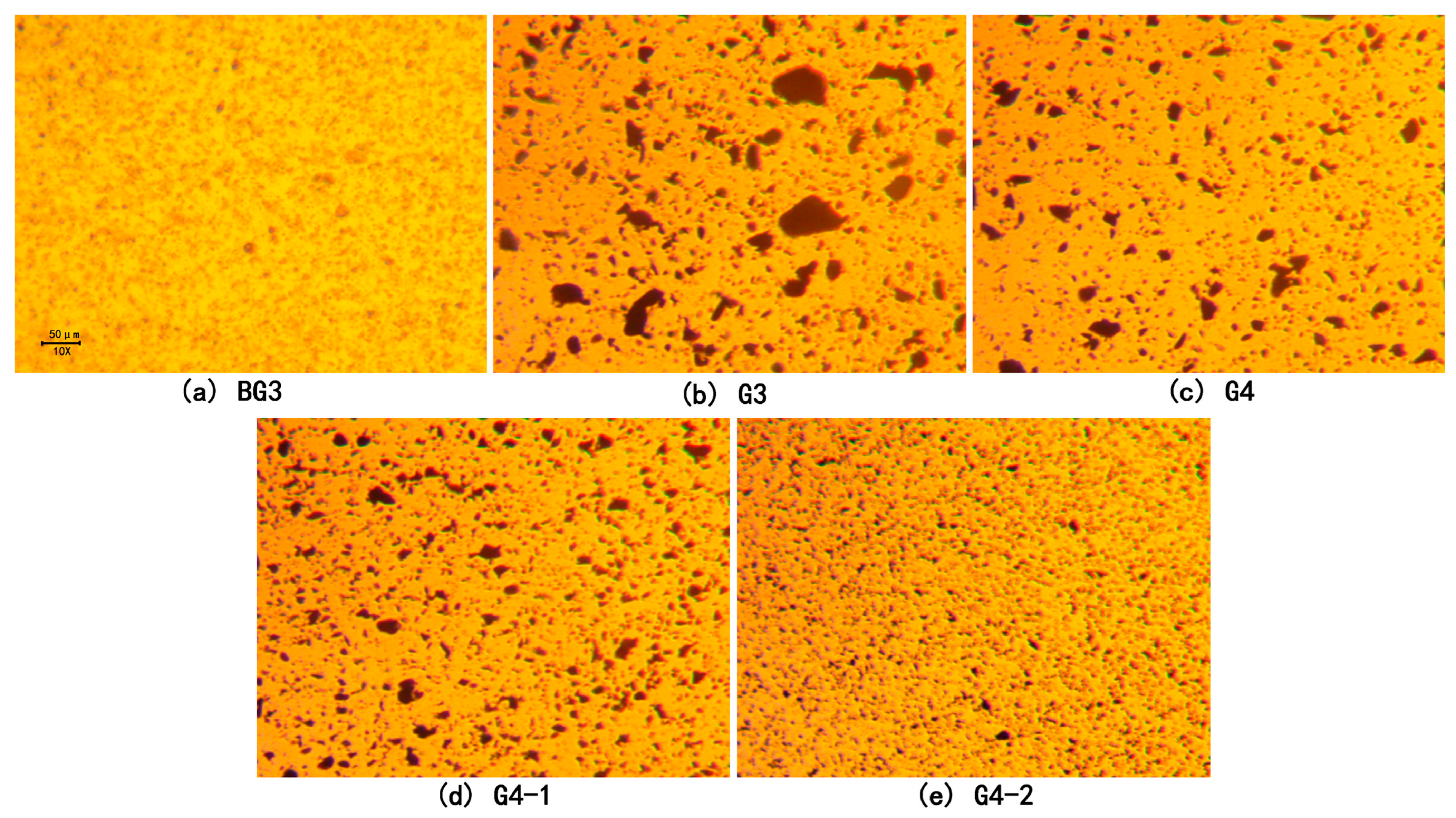
- The distributions of the LDH and MoS2 in the lubricants were examined using a Trinocular Upright Metallurgical Microscope 55XA from Shanghai Optical Instrument Factory No. 6 (Shanghai, China).
- (4)
- The penetration of the different lubricant samples was tested using a Grease Penetration Tester BF-38 from North Dalian Analytical Instrument Co., Ltd. (Dalian, China), with s sensitivity of 0.01 mm, following ASTM D217 [35].
- (5)
- The dropping point of the different lubricant samples was tested using a Wide-Temperature Range Grease Dropping Point Tester SYP4111 from Weiyou Petroleum Instrument Manufacturing Co., Ltd. (Shanghai, China), following ASTM D2265 [36].
- (6)
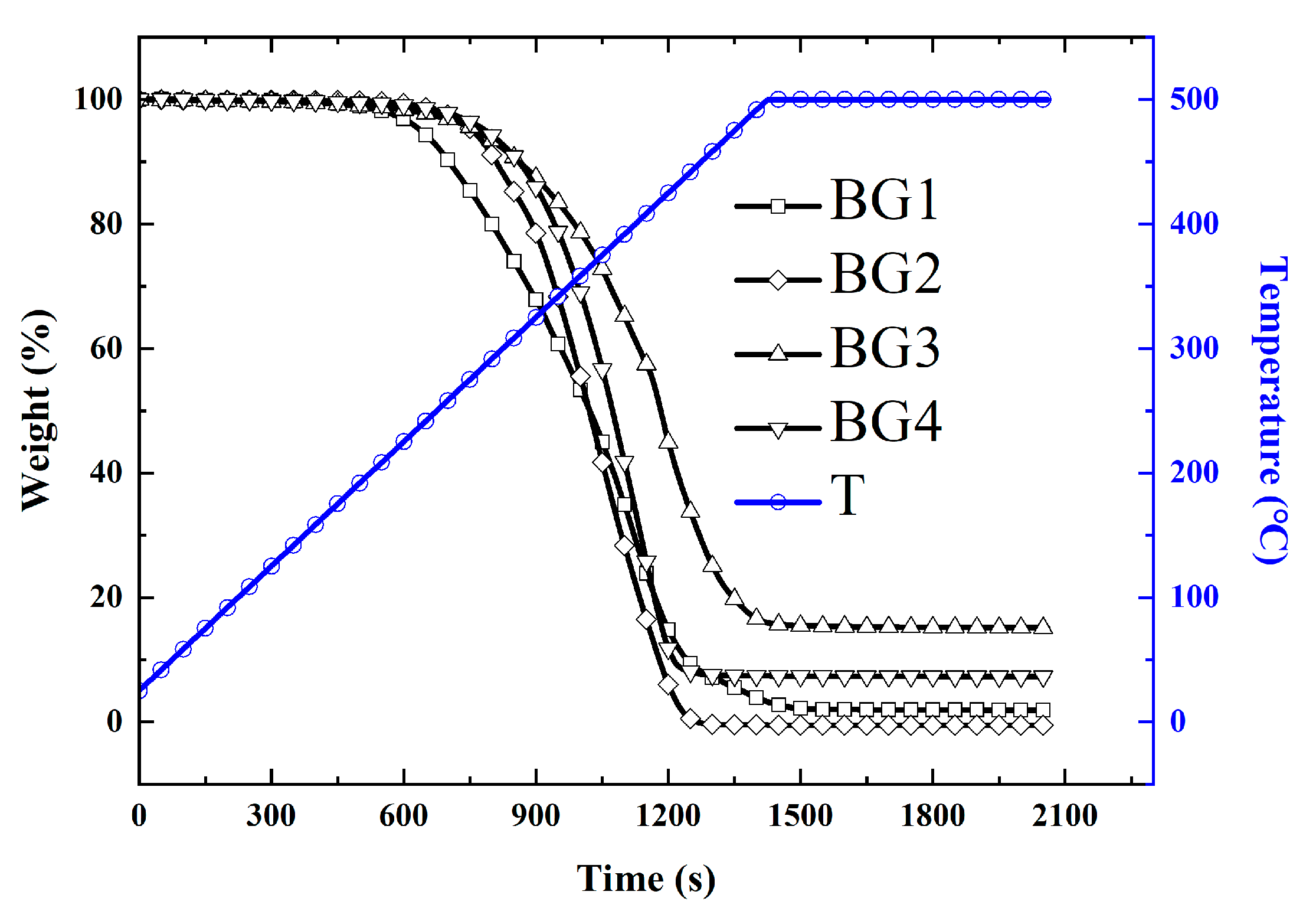
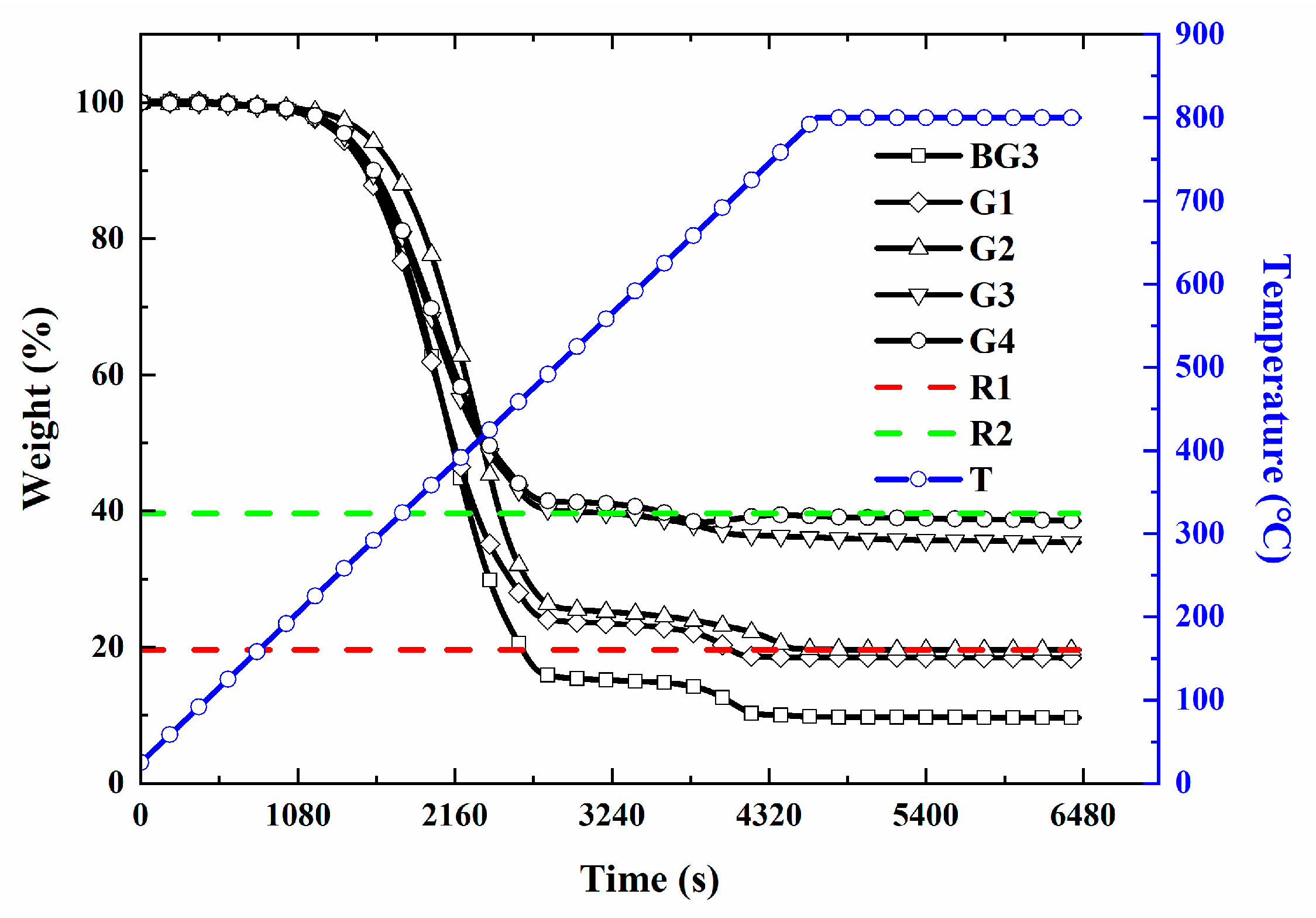
- A Thermal Gravimetric Analyzer (TGA) 2LF from METTLER-TOLEDO Measurement Technology Ltd. (Zurich, Switzerland) was used to measure the thermal weight loss of the different lubricants at high temperatures ranging from 500 to 800 °C, with a heating rate of 10–20 K/min, an argon flow rate of 50 mL/min, and a scale sensitivity of 0.1 μg, following ASTM E1868 [37].
- (7)
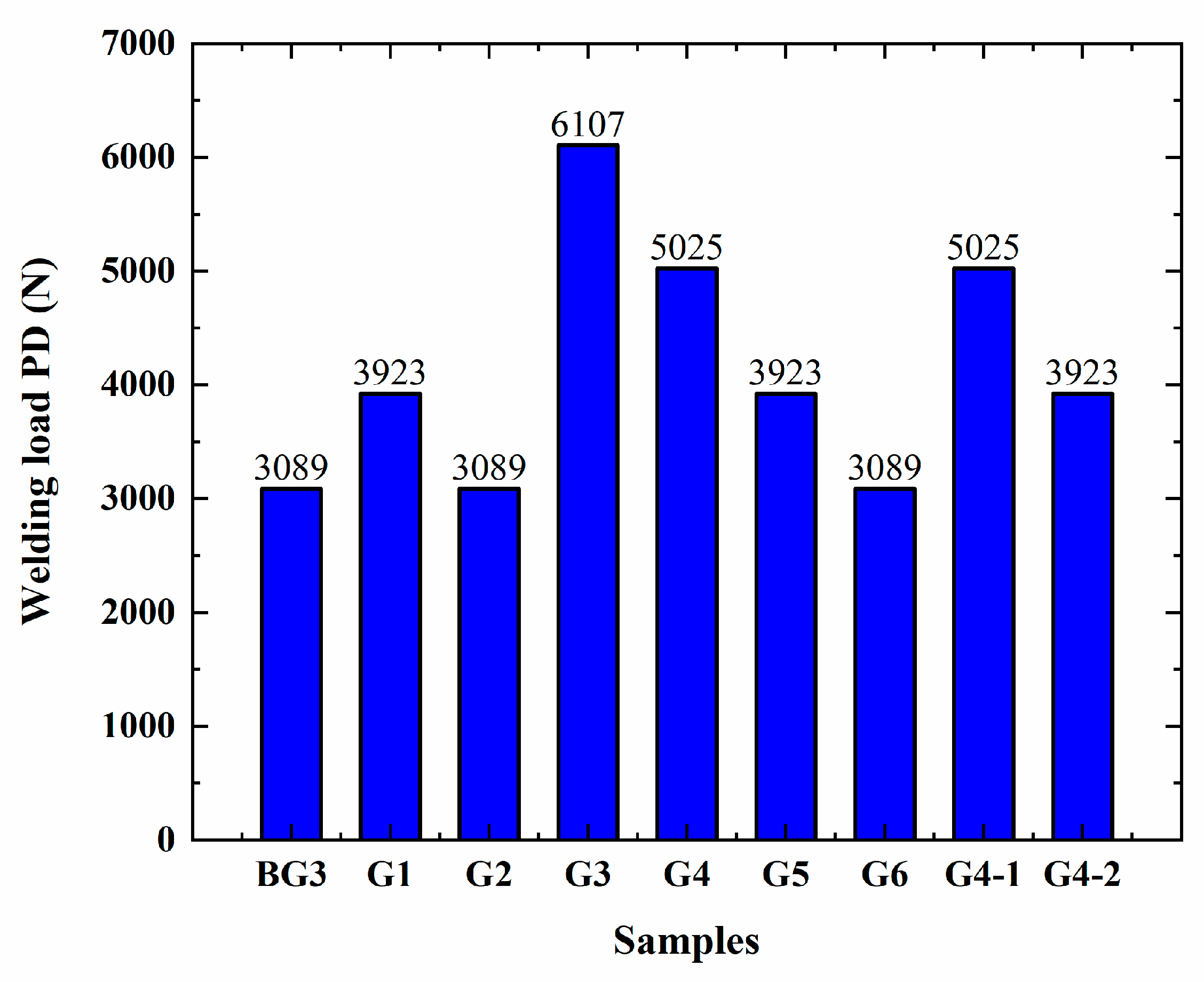
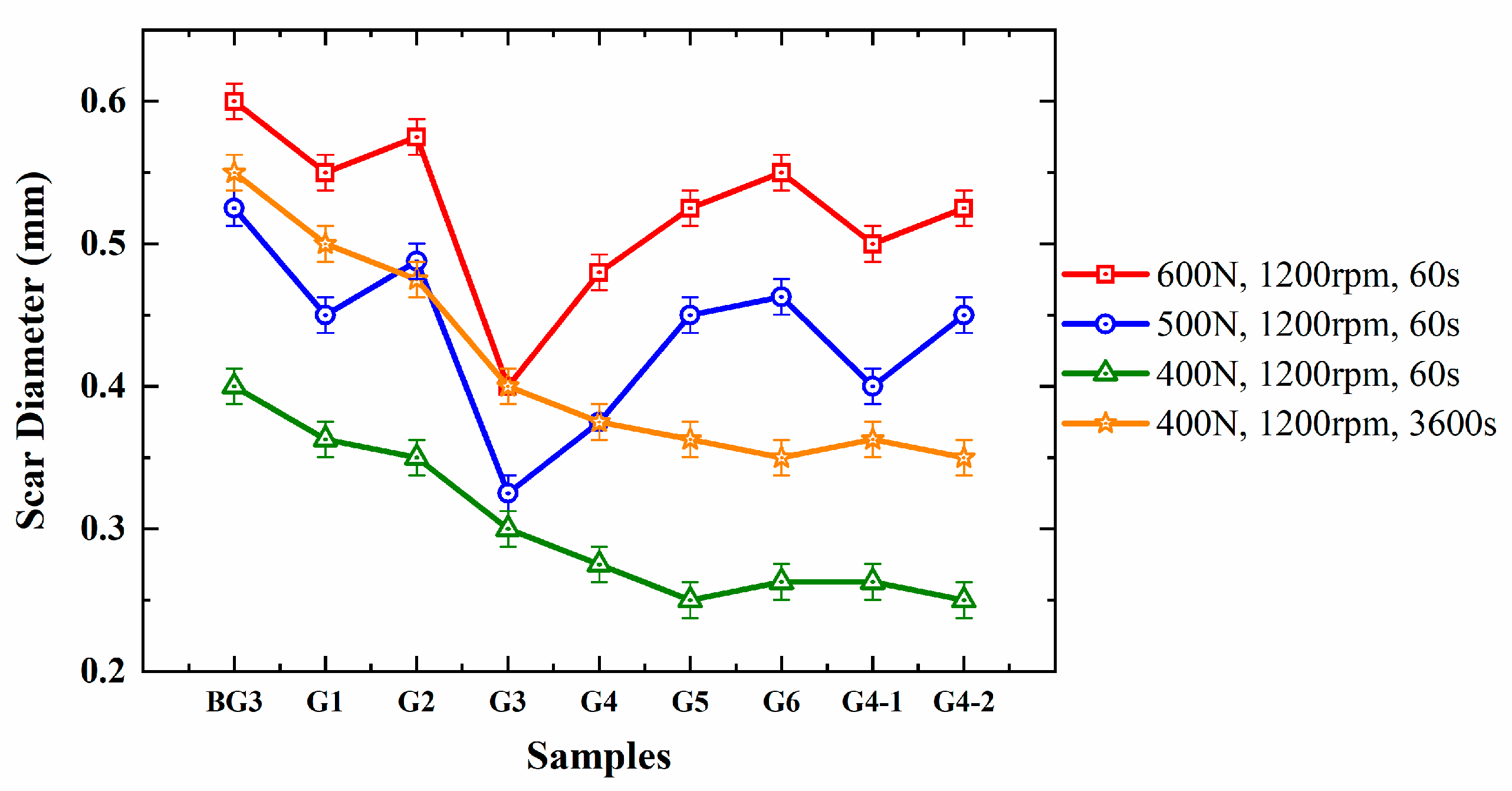
- An Automatic High Load Extreme Pressure Friction Tester STD081 from Falex Corporation (Chicago, IL, USA) was used to test the sintering load PD value of the different grease samples, following ASTM D2596 [38], and the wear scars on the steel balls under different conditions, following ASTM D2266 [39].
- (8)
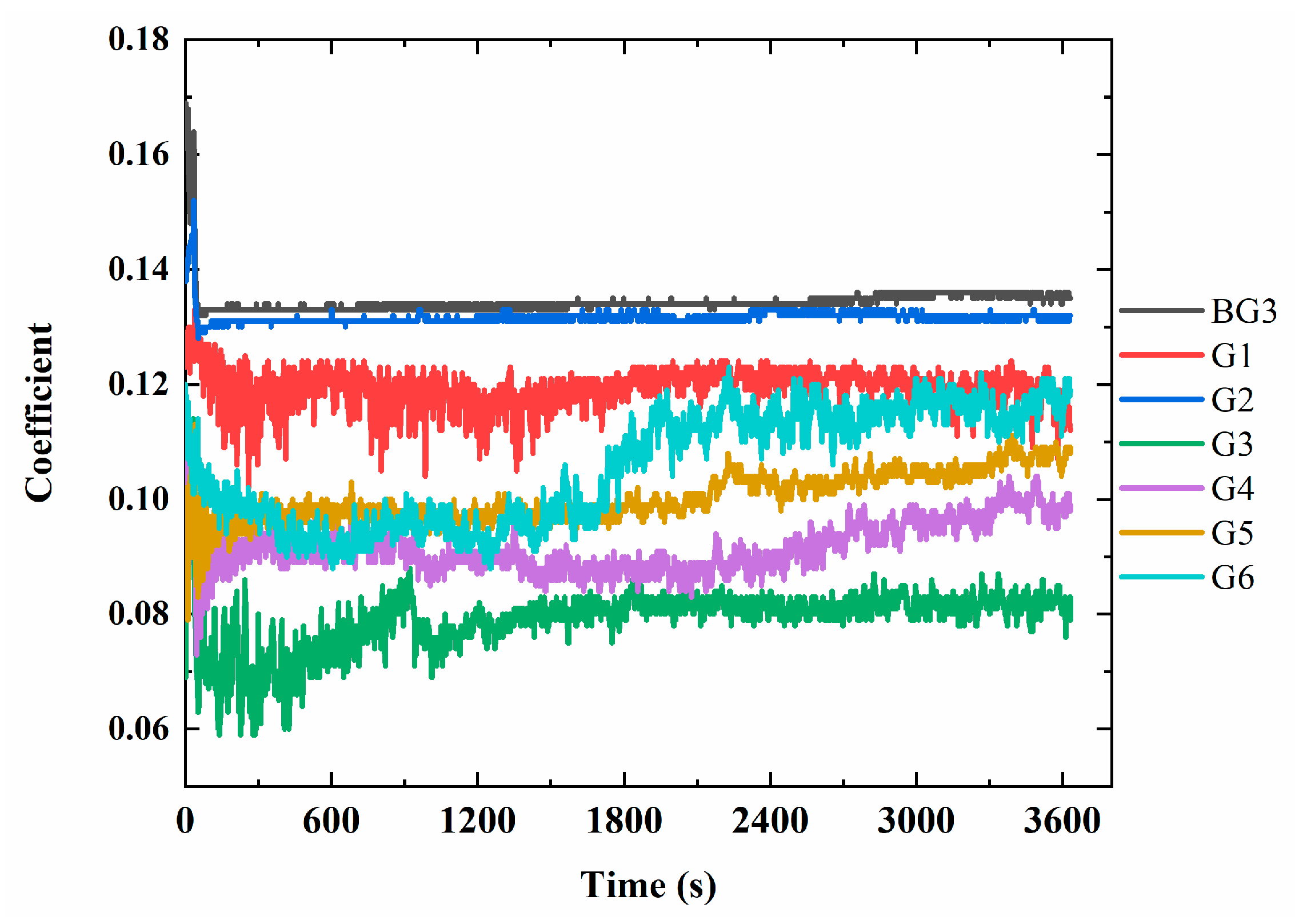
- A High-Frequency Linear Vibration Rig SRV5 from Optimol Instruments Pruftechnik GmbH (Munich, Germany) was used to test the friction coefficient of the different lubricant samples, with a sensitivity of 0.001, referring to ASTM D5707 [40]. The steel ball used in the SRV testing was made of 52,100 bearing steel with a hardness of 60 ± 2 HRC, a diameter of 10 mm, and a roughness (Ra) of 0.025 ± 0.005 μm. The steel disc was made of 52,100 bearing steel with a hardness of 60 ± 2 HRC, a diameter of 24 mm, a height of 7.85 mm, and a roughness (Ra) of 0.040 ± 0.005 μm. The testing conditions were as follows: load of 400 N, temperature of 80 °C, amplitude of 1 mm, frequency of 10 Hz, and duration of 1 h.
- (9)
- A Contour GT-K0 Optical Profilometer from Bruker GmbH (Saarbrucken, Germany) was used to measure the wear volume of the steel disc. The Vertical Scanning Interferometry (VSI) mode was used, with an adjacent pixel height difference greater than 135 nm and a maximum scanning length of 10 mm.
- (10)
- A High-Temperature Anti-Seize Device from Tianjin University was used to test the anti-seize performance of the different lubricant samples at high temperature, referring to the standard MIL-PRF-907H [41]. The fasteners used were M10, with bolts made of B16 alloy steel according to ASTM A193 [42] and nuts made of 2H according to ASTM A194 [43].
3. Results and Discussion
3.1. Characterization of MoS2 and LDH
3.2. Distributions of MoS2 and LDH in Lubricants
3.3. Thermogravimetric Analysis (TGA)
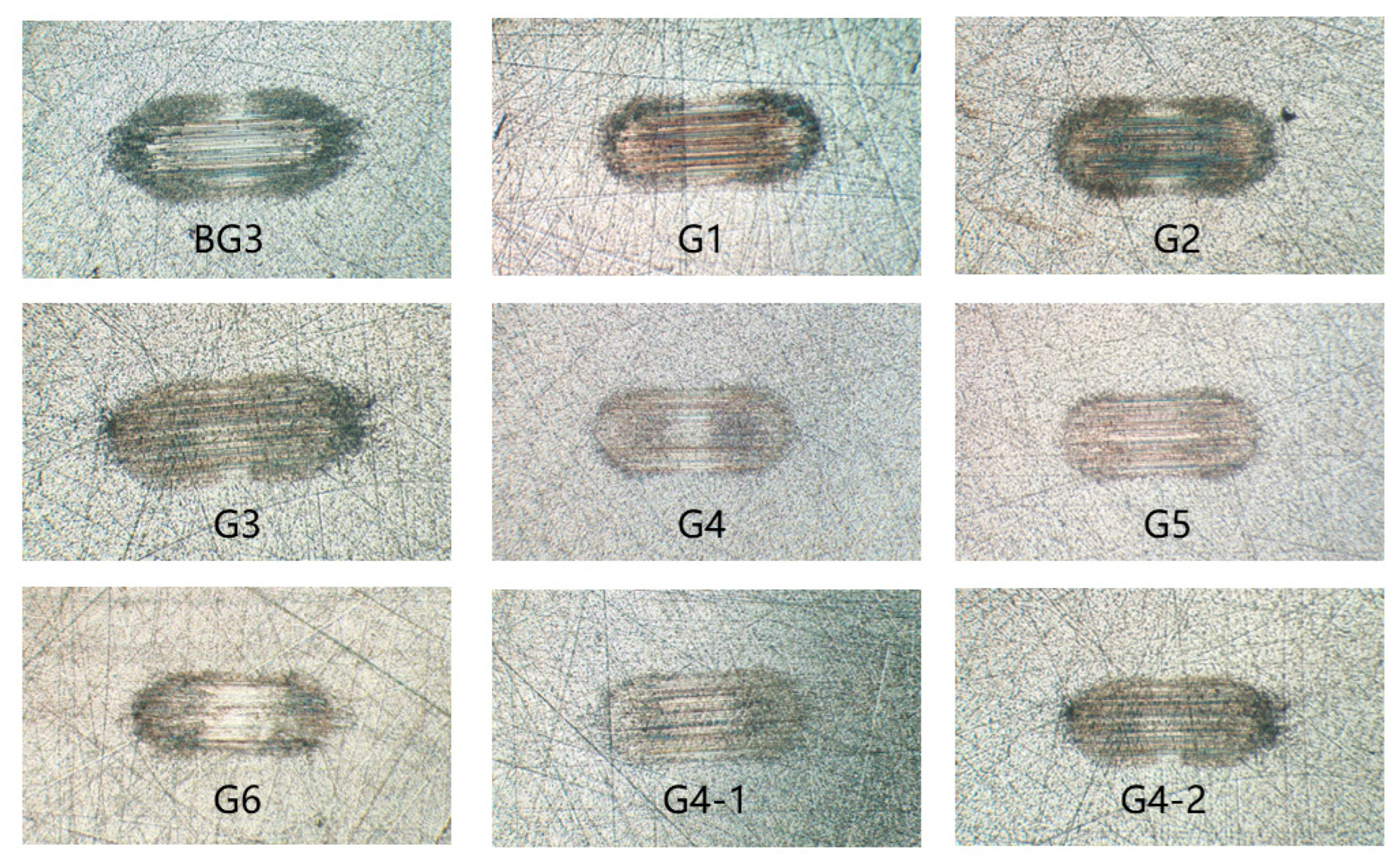
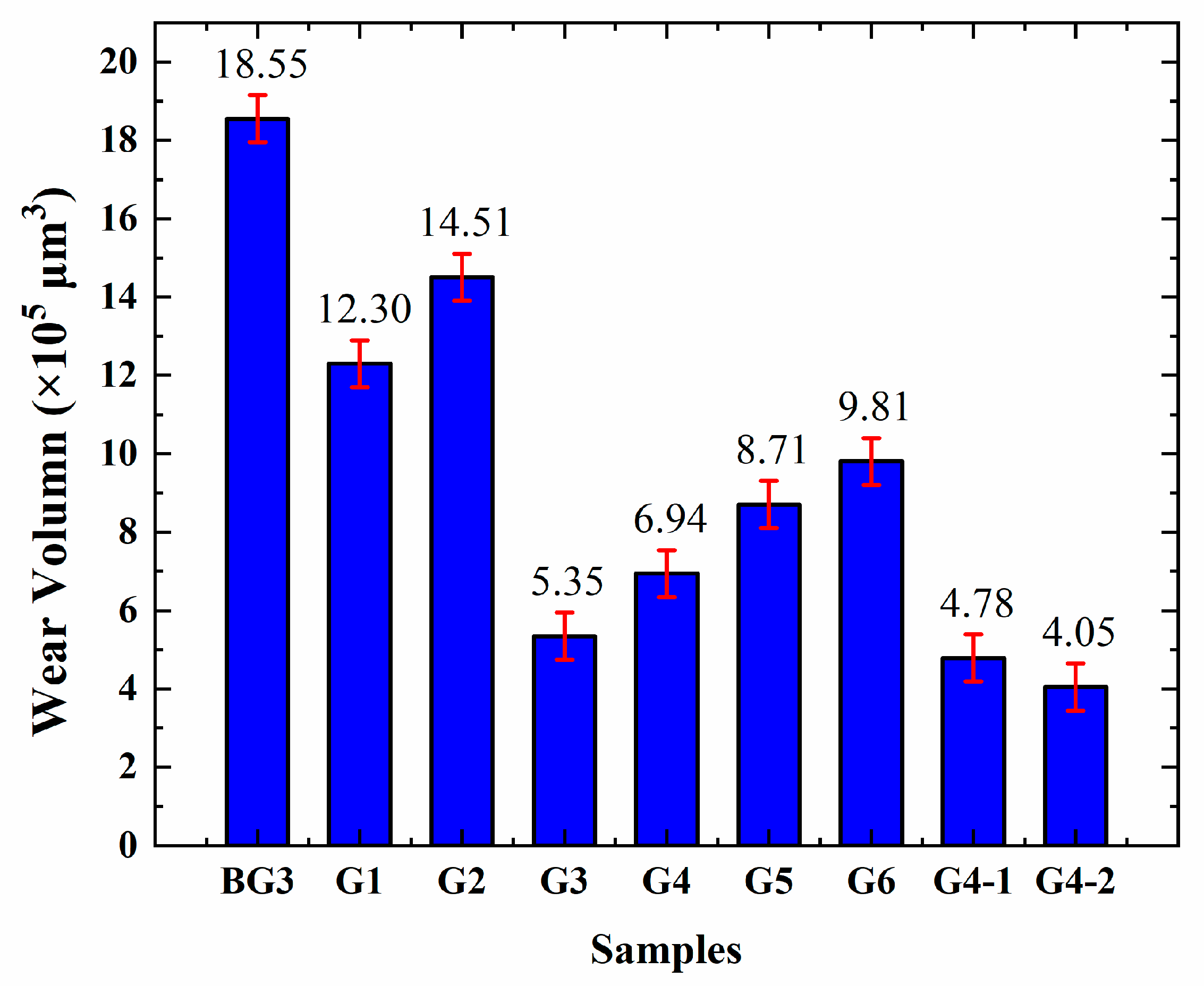
3.4. Analysis of the Extreme Pressure and Anti-Wear Performance of Lubricants
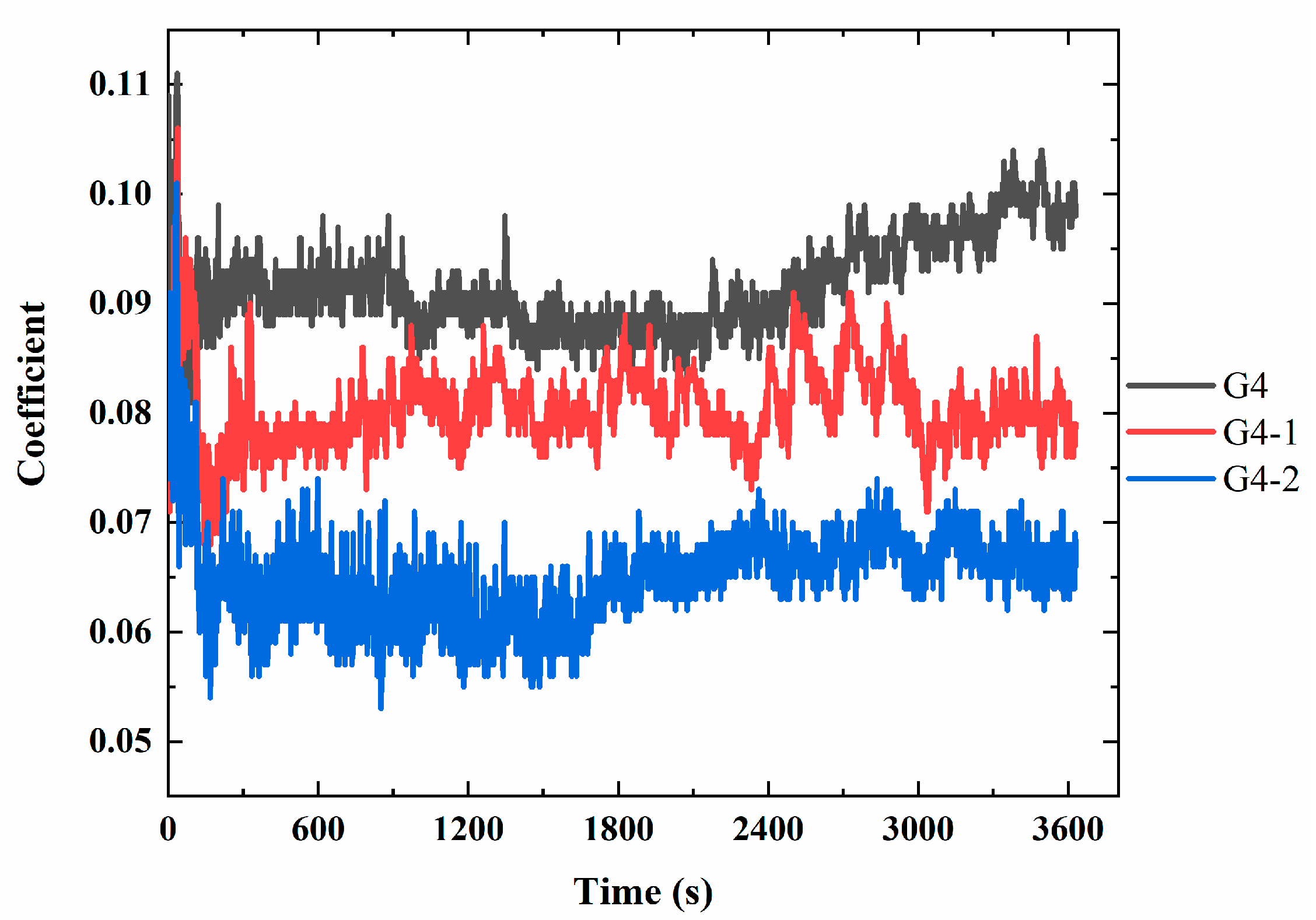
3.5. Analysis of Fretting-Wear Performance of Lubricants
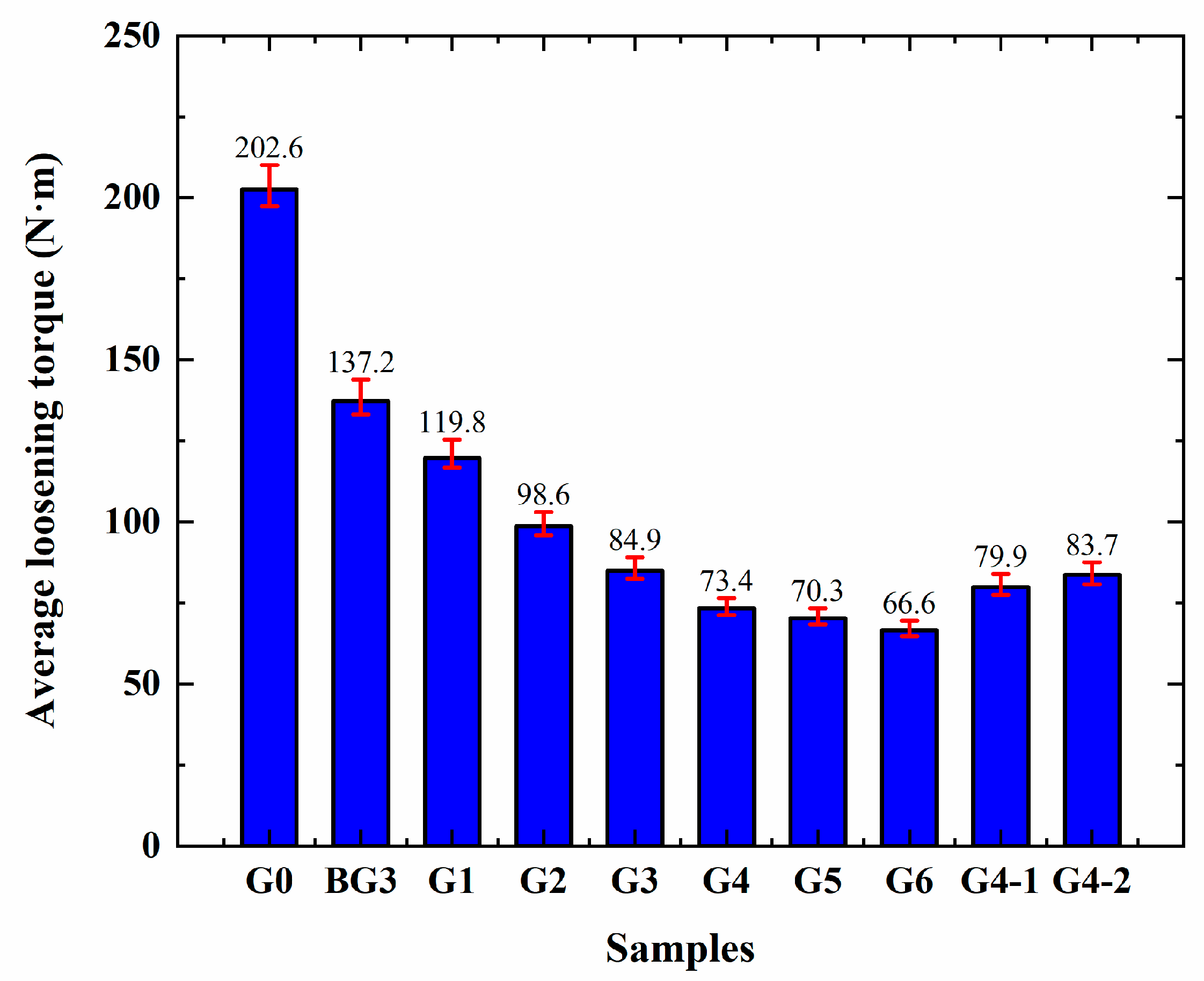
3.6. Analysis of High-Temperature Anti-Seize Performance of Lubricants
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cho, M.H.; Ju, J.; Kim, S.J.; Jang, H. Tribological properties of solid lubricants (graphite, Sb2S3, MoS2) for automotive brake friction materials. Wear 2006, 260, 855–860. [Google Scholar] [CrossRef]
- Gulzar, M.; Masjuki, H.H.; Kalam, M.A.; Varman, M.; Zulkifli, N.W.M.; Mufti, R.A.; Zahid, R. Tribological performance of nanoparticles as lubricating oil additives. J. Nanoparticle Res. 2016, 18, 223–247. [Google Scholar] [CrossRef]
- Dai, W.; Kheireddin, B.; Gao, H.; Liang, H. Roles of nanoparticles in oil lubrication. Tribol. Int. 2016, 102, 88–98. [Google Scholar] [CrossRef]
- Bukvić, M.; Gajević, S.; Skulić, A.; Savić, S.; Ašonja, A.; Stojanović, B. Tribological Application of Nanocomposite Additives in Industrial Oils. Lubricants 2024, 12, 6–26. [Google Scholar] [CrossRef]
- Farag, H.; Al-Megren, H. Textural characterizations and catalytic properties of quasi spherical nanosized molybdenum disulfide. J. Colloid Interface Sci. 2009, 332, 425–431. [Google Scholar] [CrossRef]
- Hu, K.H.; Hu, X.G.; Xu, Y.F.; Huang, F.; Liu, J.S. The effect of morphology on the tribological properties of MoS2 in liquid paraffin. Tribol. Lett. 2010, 40, 155–165. [Google Scholar] [CrossRef]
- Sahoo, R.R.; Biswas, S.K. Deformation and friction of MoS2 particles in liquid suspensions used to lubricate sliding contact. Thin Solid Films 2010, 518, 5995–6005. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, D.; Wang, Y.; Sun, A. Preparation and tribological properties of MoS2 nanosheets. Adv. Eng. Mater. 2010, 12, 534–538. [Google Scholar] [CrossRef]
- Dai, H.D.; Qu, J.J.; Zhuang, Q.X. Ultra lubrication tribological characteristic of MoS2/PTFE composite versus quartz glass under water lubrication. J. Jilin Univ. 2010, 40, 1015–1018. [Google Scholar]
- Tannous, J.; Dassenoy, F.; Lahouij, I.; Le Mogne, T.; Vacher, B.; Bruhács, A.; Tremel, W. Understanding the tribochemical mechanisms of IF-MoS2 nanoparticles under boundary lubrication. Tribol. Lett. 2011, 41, 55–64. [Google Scholar] [CrossRef]
- Alves, S.M.; Barros, B.S.; Trajano, M.F.; Ribeiro, K.S.B.; Moura, E. Tribological behavior of vegetable oil-based lubricants with nanoparticles of oxides in boundary lubrication conditions. Tribol. Int. 2013, 65, 28–36. [Google Scholar] [CrossRef]
- Wang, Y.; Du, Y.; Deng, J.; Wang, Z. Friction reduction of water based lubricant with highly dispersed functional MoS2 nanosheets. Colloids Surf. A Phys.-Chem. Eng. Asp. 2019, 562, 321–328. [Google Scholar] [CrossRef]
- Rabaso, P.; Ville, F.; Dassenoy, F.; Diaby, M.; Afanasiev, P.; Cavoret, J.; Vacher, B.; Le Mogne, T. Boundary lubrication: Influence of the size and structure of inorganic fullerene-like MoS2 nanoparticles on friction and wear reduction. Wear 2014, 320, 161–178. [Google Scholar] [CrossRef]
- Sahoo, R.R.; Biswas, S.K. Effect of layered MoS2 nanoparticles on the frictional behavior and microstructure of lubricating greases. Tribol. Lett. 2014, 53, 157–171. [Google Scholar] [CrossRef]
- Tontini, G.; Semione, G.D.L.; Bernardi, C.; Binder, R.; de Mello, J.D.B.; Drago, V. Synthesis of nanostructured flower-like MoS2 and its friction properties as additive in lubricating oils. Ind. Lubr. Tribol. 2016, 68, 658–664. [Google Scholar] [CrossRef]
- Wu, H.; Wang, L.; Dong, G.; Yang, S.; Zhang, J.; Zhou, B.; Tang, Z. Lubrication effectiveness investigation on the friendly capped MoS2 nanoparticles. Lubr. Sci. 2017, 29, 115–129. [Google Scholar] [CrossRef]
- Farsadi, M.; Bagheri, S.; Ismail, N.A. Nanocomposite of functionalized graphene and molybdenum disulfide as friction modifier additive for lubricant. J. Mol. Liq. 2017, 244, 304–308. [Google Scholar] [CrossRef]
- Jung, D.; Kim, D.; Yang, W.J.; Cho, E.S.; Kwon, S.J.; Han, J.H. Surface Functionalization of Liquid-Phase Exfoliated, Two-Dimensional MoS2 and WS2 Nanosheets with 2-Mercaptoethanol. J. Nanosci. Nanotechnol. 2018, 18, 6265–6269. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, P.; Cai, Z.; Di, Y.; Hou, Y. Preparation, microstructure and tribological properties of CrN/MoS2 solid self-lubricant composite coating. Rare Met. Mater. Eng. 2013, 42, 2384–2388. [Google Scholar]
- Takeda, S.; Miki, H.; Takeishi, H.; Takagi, T. Cu-based MoS2-dispersed composite material formed by the compression shearing method at room temperature. Tribol. Online 2017, 12, 29–36. [Google Scholar] [CrossRef]
- Han, J.; Dou, Y.; Wei, M.; Evans, D.G.; Duan, X. Erasable nanoporous antireflection coatings based on the reconstruction effect of layered double hydroxides. Angew. Chem. Int. Ed. 2010, 49, 2171. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Pan, T.; Xu, S.; Yan, H.; Han, J.; Wei, M.; Evans, D.G.; Duan, X. Transparent, ultrahigh-gas-barrier films with a brick-mortar-sand structure. Angew. Chem. Int. Ed. 2015, 54, 9673. [Google Scholar] [CrossRef]
- Kruger, J.S.; Cleveland, N.S.; Zhang, S.; Katahira, R.; Black, B.A.; Chupka, G.M.; Lammens, T.M.; Hamilton, P.G.; Biddy, M.J.; Beckham, G.T. Depolymerization with Nitrate-Intercalated Hydrotalcite Catalysts. ACS Catal. 2016, 6, 1316–1328. [Google Scholar] [CrossRef]
- Yang, C.; Liao, L.; Lv, G.; Wu, L.; Mei, L.; Li, Z. Synthesis and characterization of Mn intercalated Mg-Al hydrotalcite. J. Colloid Interface Sci. 2016, 479, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liang, Y.Q.; Mao, X.M.; Li, H. Efficient removal of Cu(II) from an aqueous solution using a novel chitosan assisted EDTA-intercalated hydrotalcite-like compound composite: Preparation, characterization, and adsorption mechanism. Chem. Eng. J. 2022, 438, 135531. [Google Scholar] [CrossRef]
- Xu, S.; Li, S.-Y.; Zhang, M.; Zeng, H.Y.; Wu, K.; Tian, X.Y.; Chen, C.R.; Pan, Y. Fabrication of green alginate-based and layered double hydroxides flame retardant for enhancing the fire retardancy properties of polypropylene. Carbohydr. Polym. 2020, 234, 115891. [Google Scholar] [CrossRef] [PubMed]
- Du, J.-Z.; Jin, L.; Zeng, H.-Y.; Feng, B.; Xu, S.; Zhou, E.-G.; Shi, X.-K.; Liu, L.; Hu, X. Facile preparation of an efficient flame retardant and its application in ethylene vinyl acetate. Appl. Clay Sci. 2019, 168, 96–105. [Google Scholar] [CrossRef]
- Korkusuz, Ç.; Demir, A.P.T. Evaluation of the thermal stabilization behavior of hydrotalcite against organic stabilizers for plasticized PVC films. Polym. Bull. 2020, 77, 4805–4831. [Google Scholar] [CrossRef]
- Yan, J.; Yang, Z. Intercalated hydrotalcite-like materials and their application as thermal stabilizers in poly(vinyl chloride). J. Appl. Polym. Sci. 2017, 134, 44896. [Google Scholar] [CrossRef]
- Yang, Z.; Li, B.; Li, S.; Dou, Y.; Han, J. Layered double hydroxide/PBAT hybrid films with simultaneously high-barrier and degradable properties. Chem. Eng. Sci. 2023, 280, 119016. [Google Scholar] [CrossRef]
- Yang, Z.; Shi, K.; Jin, Z.; Liu, Z.; Li, Y.; Huang, Y.; Gao, F.; Han, J. Biodegradable Layered Double Hydroxide/Polymer Films for Efficient Oxygen and Water Vapor Barriers. Ind. Eng. Chem. Res. 2022, 61, 1367–1374. [Google Scholar] [CrossRef]
- Ba, Z.; Han, Y.; Qiao, D.; Feng, D.; Huang, G. Composite Nanoparticles Based on Hydrotalcite as High Performance Lubricant Additives. Ind. Eng. Chem. Res. 2018, 57, 15225–15233. [Google Scholar] [CrossRef]
- Wang, K.; Wu, H.; Wang, H.; Liu, Y. Superior extreme pressure properties of different layer LDH nanoplatelets used as boundary lubricants. Appl. Surf. Sci. 2020, 530, 147203.1–147203.14. [Google Scholar] [CrossRef]
- ISO 13320:2020; ISO Particle Size Analysis Laser Diffraction Methods. ISO: Geneva, Switzerland. Available online: https://www.iso.org/standard/69111.html (accessed on 24 April 2024).
- ASTM D217-21a; Standard Test Methods for Cone Penetration of Lubricating Grease. ASTM International: West Conshohocken, PA, USA. [CrossRef]
- ASTM D2265-22; Standard Test Method for Dropping Point of Lubricating Grease Over Wide Temperature Range. ASTM International: West Conshohocken, PA, USA. [CrossRef]
- ASTM E1868-10(2021); Standard Test Methods for Loss-On-Drying by Thermogravimetry. ASTM International: West Conshohocken, PA, USA. [CrossRef]
- ASTM D2596-20; Standard Test Method for Measurement of Extreme-Pressure Properties of Lubricating Grease (Four-Ball Method). ASTM International: West Conshohocken, PA, USA. [CrossRef]
- ASTM D2266-23; Standard Test Method for Wear Preventive Characteristics of Lubricating Grease (Four-Ball Method). ASTM International: West Conshohocken, PA, USA. [CrossRef]
- ASTM D5707-23; Standard Test Method for Measuring Friction and Wear Properties of Lubricating Grease Using a High-Frequency, Linear-Oscillation (SRV) Test Machine. ASTM International: West Conshohocken, PA, USA. [CrossRef]
- NSAI MIL-PRF-907 REVISION F: 2004; Antiseize Thread Compound, High Temperature. US Military Specs/Standards/Handbooks. Available online: https://shop.standards.ie/en-ie/standards/mil-prf-907-revision-f-2004-714544_saig_mil_mil_1654206/ (accessed on 20 April 2024).
- ASTM A193/A193M-24; Standard Specification for Alloy-Steel and Stainless Steel Bolting for High Temperature or High Pressure Service and Other Special Purpose Applications. ASTM International: West Conshohocken, PA, USA. [CrossRef]
- ASTM A194/A194M-23; Standard Specification for Carbon Steel, Alloy Steel, and Stainless Steel Nuts for Bolts for High Pressure or High Temperature Service, or Both. ASTM International: West Conshohocken, PA, USA. [CrossRef]
- Sreedhara, M.B.; Matte, H.R.; Govindaraj, A.; Rao, C.N.R. Synthesis, characterization, and properties of few-layer MoO3. Chem.-Asian J. 2013, 8, 2430–2435. [Google Scholar] [CrossRef]
- Balendhran, S.; Walia, S.; Nili, H.; Ou, J.Z.; Zhuiykov, S.; Kaner, R.B.; Sriram, S.; Bhaskaran, M.; Kalantar-Zadeh, K. Two-Dimensional molybdenum trioxide and dichalcogenides. Adv. Funct. Mater. 2013, 23, 3952–3970. [Google Scholar] [CrossRef]



| Chemical Substances | Chemical Grade | Source |
|---|---|---|
| Mg(NO3)2·6H2O | >99.0%, (AR) | Aladdin (Shanghai, China) |
| Al(NO3)3·9H2O | >99.0%, (AR) | Aladdin (Shanghai, China) |
| Urea | >99.5%, (AR) | Aladdin (Shanghai, China) |
| Anhydrous ethanol | >99.7%, (AR) | Fuyu Co., Ltd. (Tianjin, China) |
| Polyalphaolefins oil 6 (PAO6) | >99.0% | ExxonMobil |
| Sample | Thickener | Base Oil | Penetration, 0.1 mm | Dropping Point, °C |
|---|---|---|---|---|
| BG1 | Lithium Complex | PAO6 | 272 | 310 |
| BG2 | Polyurea | PAO6 | 275 | 304 |
| BG3 | Calcium sulfonate | PAO6 | 274 | 328 |
| BG4 | Bentonite | PAO6 | 273 | 338 |
| Sample | Base Grease | M1 Content, % | LDH Content, % | Penetration, mm | Dropping Point, °C |
|---|---|---|---|---|---|
| G1 | BG3 | 10 | 0 | 27.91 | 324 |
| G2 | BG3 | 0 | 10 | 27.06 | 331 |
| G3 | BG3 | 30 | 0 | 28.83 | 317 |
| G4 | BG3 | 20 | 10 | 28.32 | 320 |
| G5 | BG3 | 15 | 15 | 27.64 | 329 |
| G6 | BG3 | 0 | 30 | 27.59 | 335 |
| Sample | Base Grease | MoS2 Type | MoS2 Content, % | LDH Content, % | Penetration, mm | Dropping Point, °C |
|---|---|---|---|---|---|---|
| G4-1 | BG3 | M2 | 20 | 10 | 28.45 | 319 |
| G4-2 | BG3 | M3 | 20 | 10 | 28.36 | 321 |
| Samples | Particle Size Distributions | ||
|---|---|---|---|
| D50, μm | D90, μm | D100, μm | |
| M1 | 25.430 | 81.510 | 239.400 |
| M2 | 9.828 | 25.580 | 71.930 |
| M3 | 4.381 | 11.580 | 26.000 |
| LDH | 1.398 | 3.073 | 7.060 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, W.; Li, Y.; Cheng, S.; He, Y.; Song, J.; Zhang, Q. Synergistic Effects of Layered Double Hydroxide and MoS2 on the Performance of Lubricants. Lubricants 2024, 12, 155. https://doi.org/10.3390/lubricants12050155
Zhou W, Li Y, Cheng S, He Y, Song J, Zhang Q. Synergistic Effects of Layered Double Hydroxide and MoS2 on the Performance of Lubricants. Lubricants. 2024; 12(5):155. https://doi.org/10.3390/lubricants12050155
Chicago/Turabian StyleZhou, Weidong, Yong Li, Shutian Cheng, Yongdi He, Jinou Song, and Qiang Zhang. 2024. "Synergistic Effects of Layered Double Hydroxide and MoS2 on the Performance of Lubricants" Lubricants 12, no. 5: 155. https://doi.org/10.3390/lubricants12050155
APA StyleZhou, W., Li, Y., Cheng, S., He, Y., Song, J., & Zhang, Q. (2024). Synergistic Effects of Layered Double Hydroxide and MoS2 on the Performance of Lubricants. Lubricants, 12(5), 155. https://doi.org/10.3390/lubricants12050155





