Tribological Characteristics of Biolubricant Obtained by Transesterification of Grape Seed Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
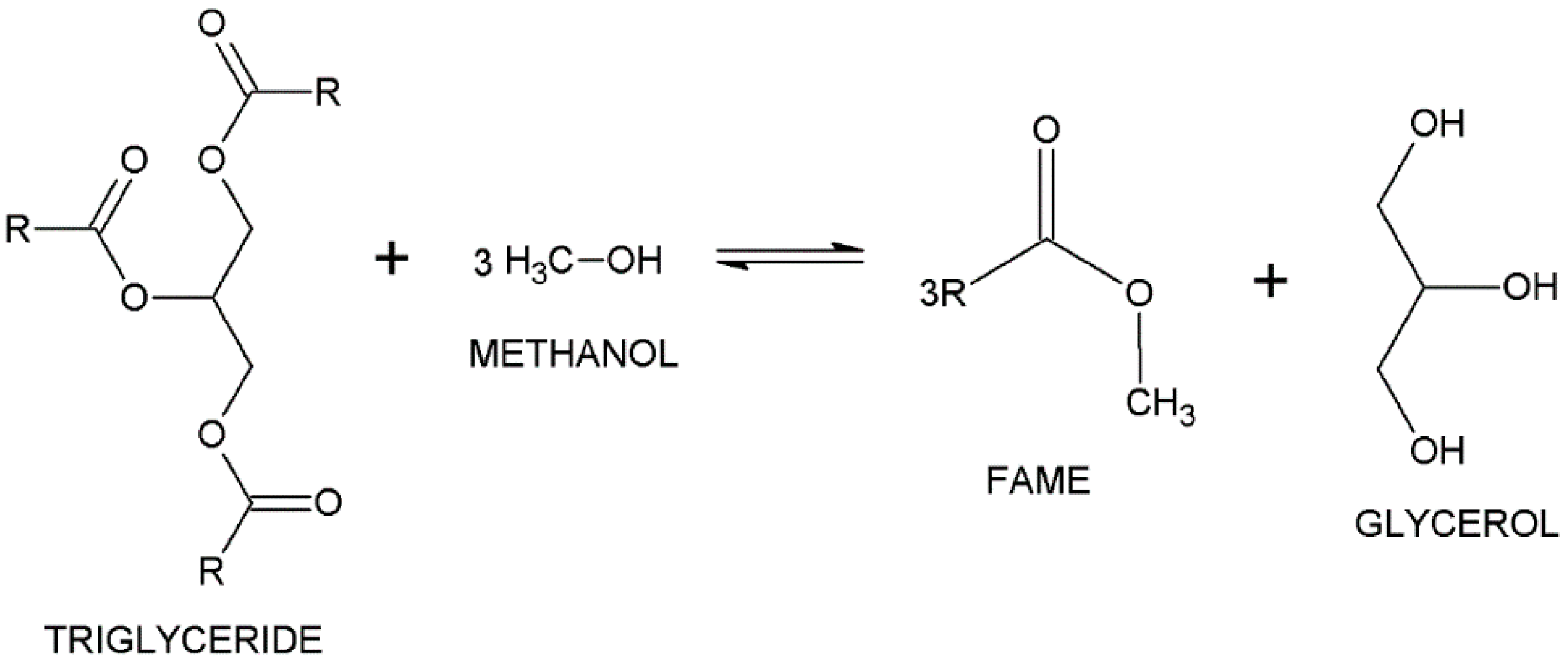
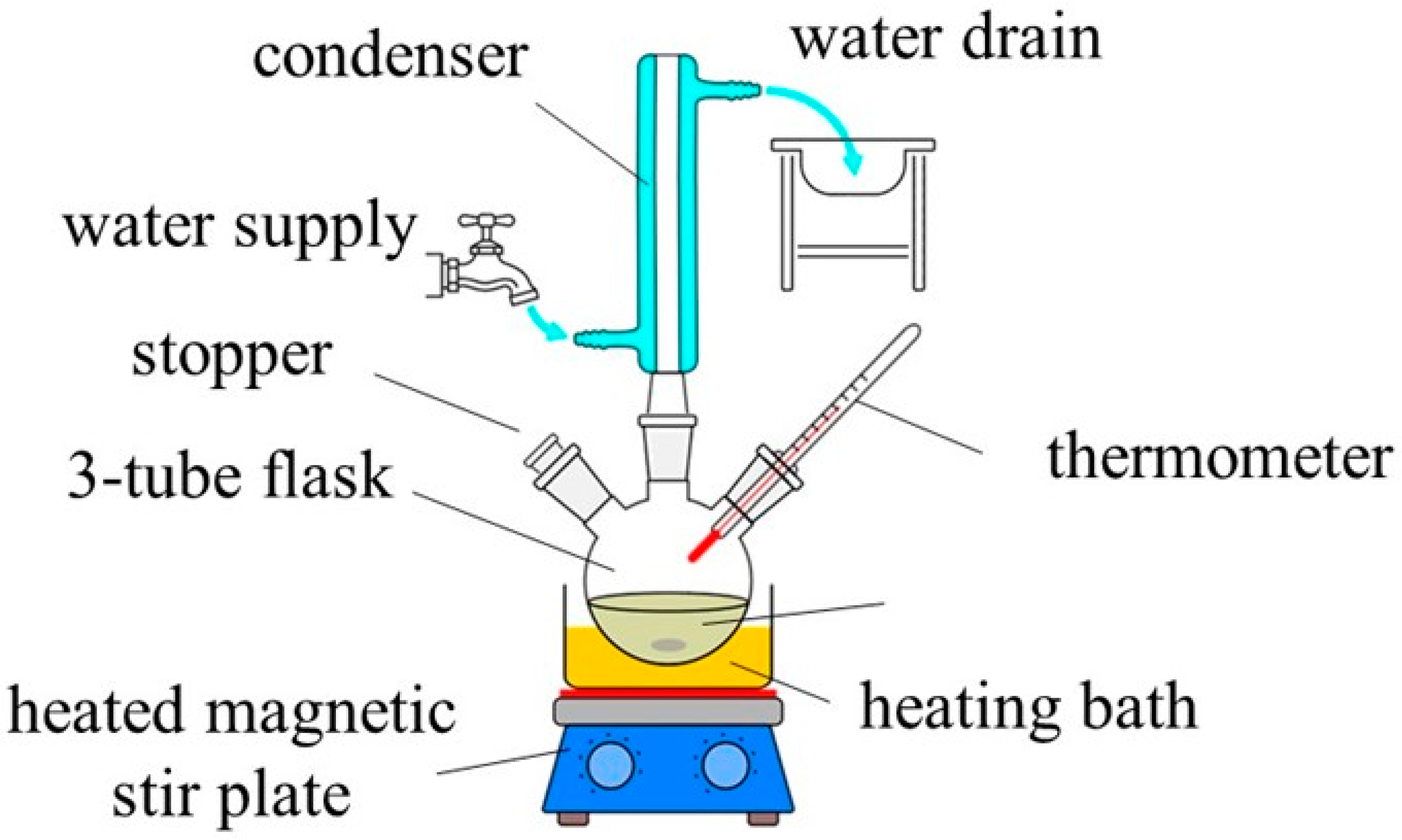
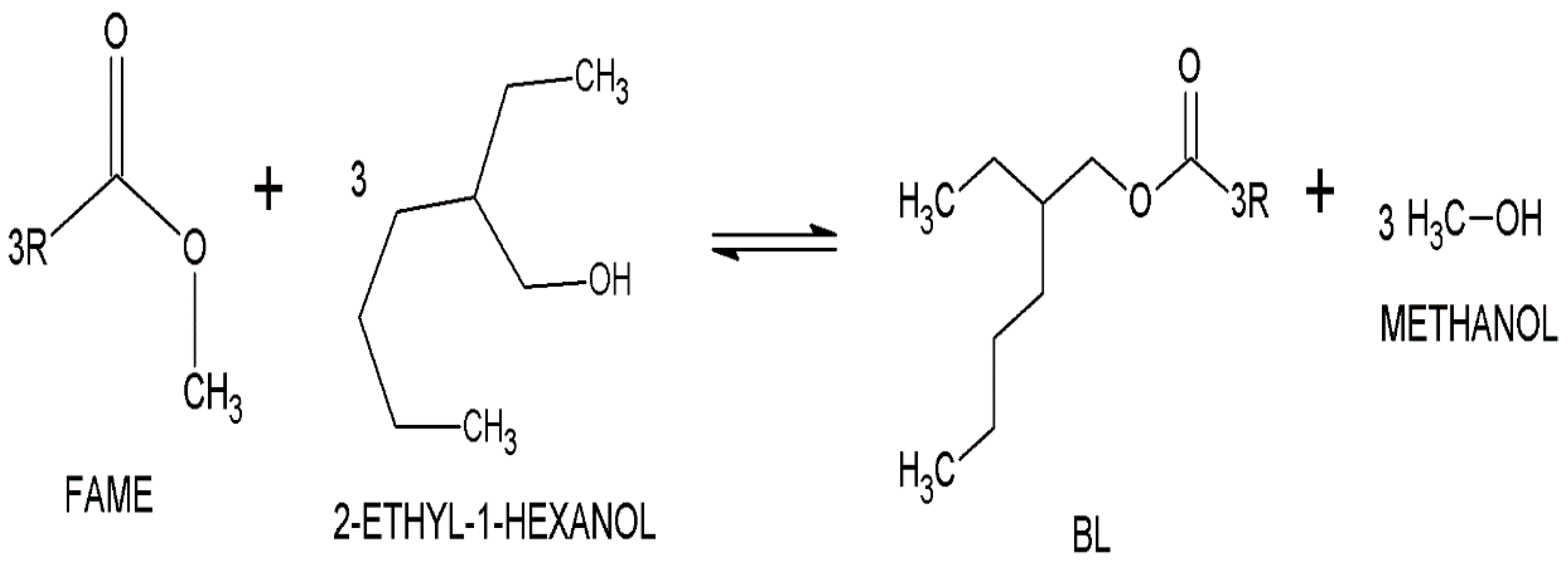
2.2. Synthetic Procedure
2.3. Physicochemical Characterization
2.4. Tribological Evaluation
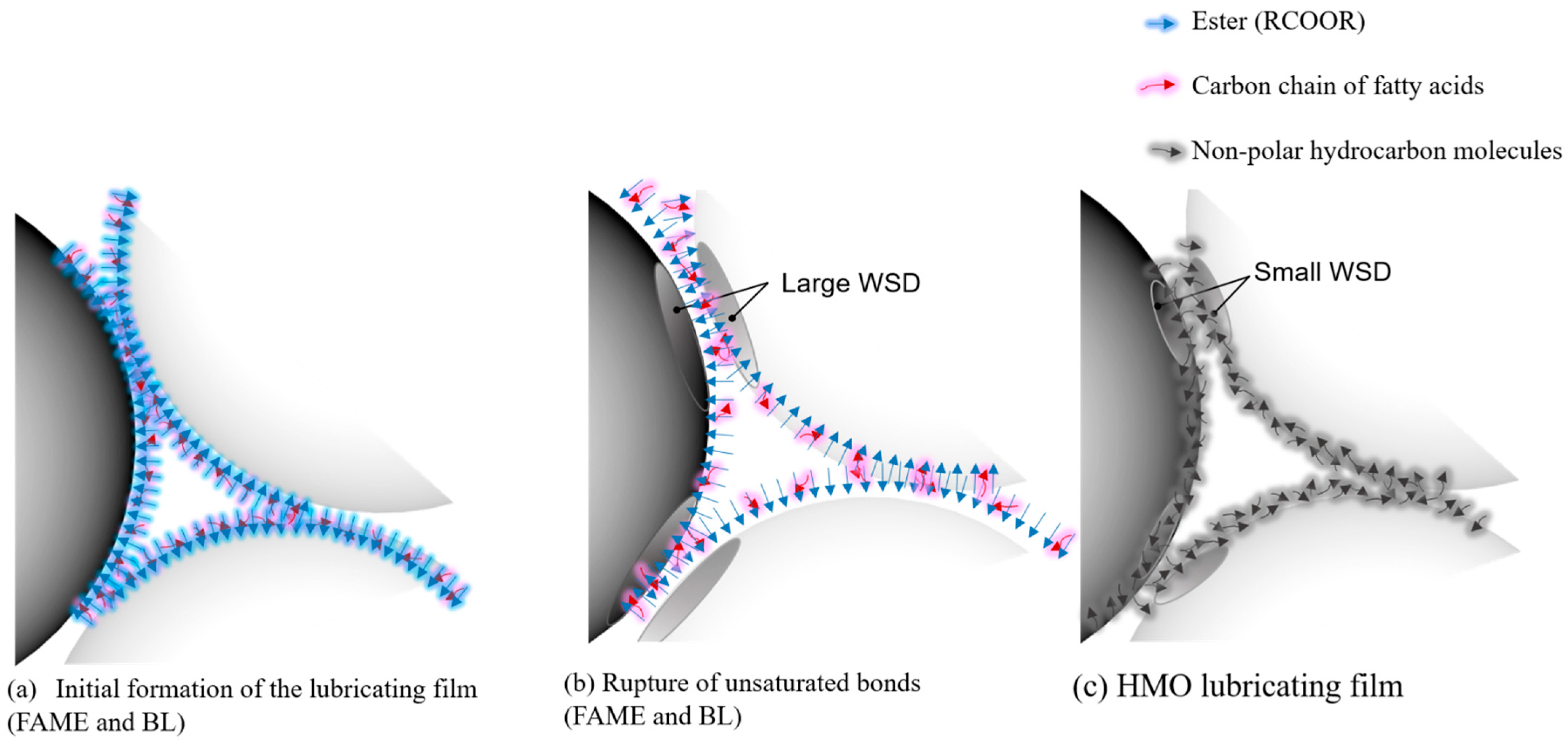
3. Results and Discussion
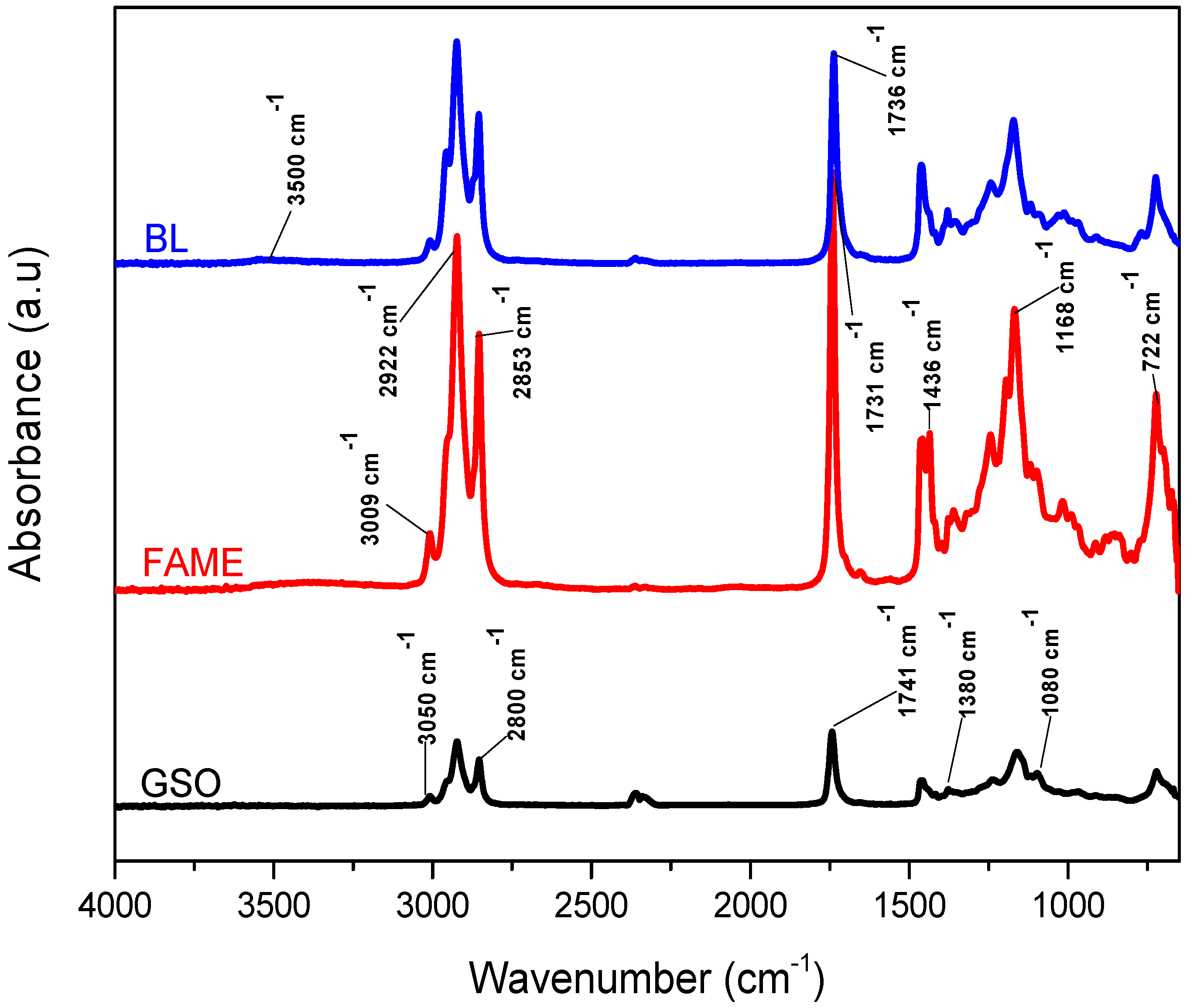
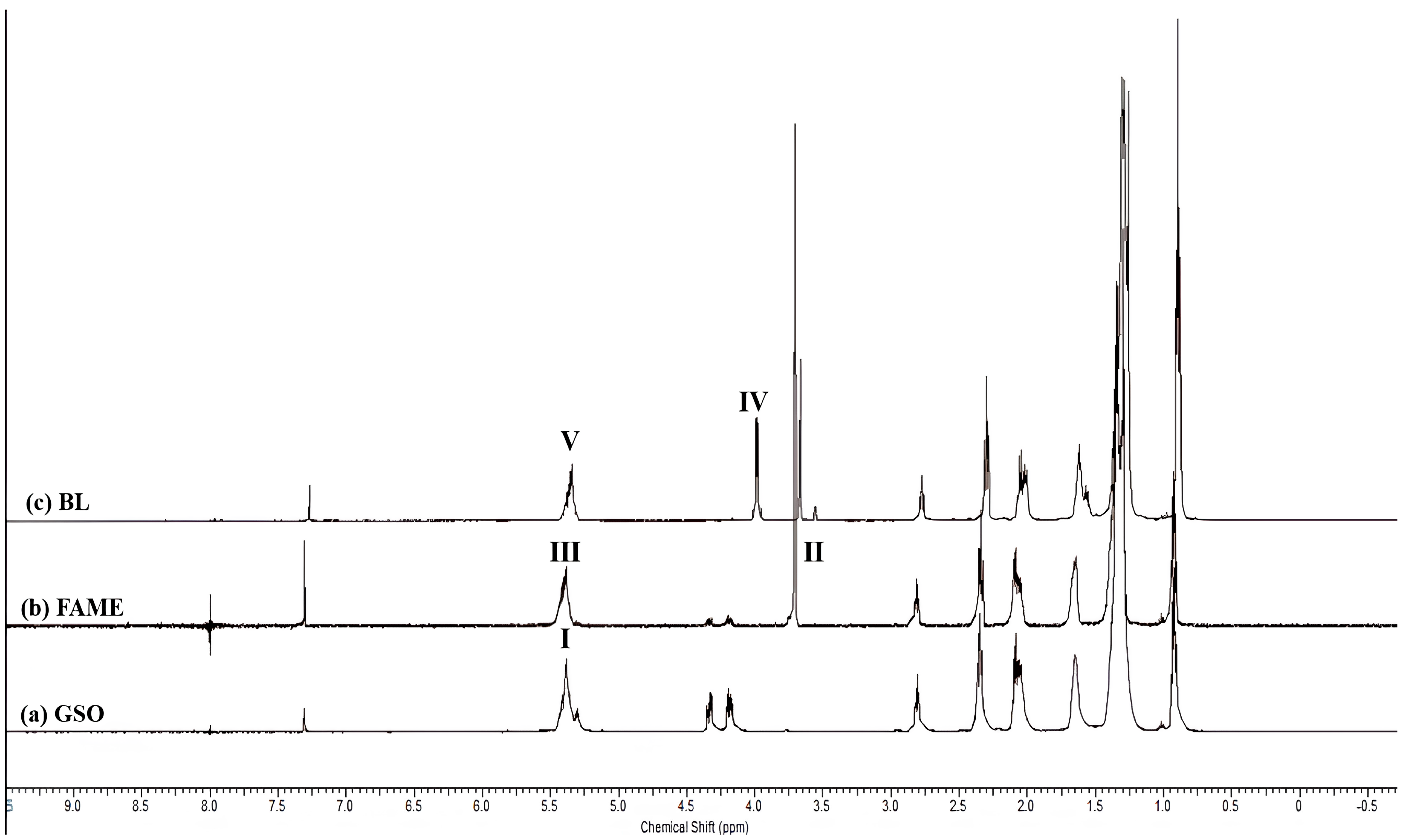
Physicochemical Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nanayakkara, N.K.B.M.P.; Narayanan, R.G. Friction and lubrication in sustainable metal forming. In Sustainable Material Forming and Joining; CRC Press: Boca Raton, FL, USA, 2019; pp. 59–76. [Google Scholar]
- Mohan, S.; Mohan, A. Wear, friction and prevention of tribo-surfaces by coatings/nanocoatings. In Anti-Abrasive Nanocoatings; Woodhead Publishing: Cambridge, UK, 2015; pp. 3–22. [Google Scholar] [CrossRef]
- Adetunla, A.; Afolalu, S.; Jen, T.C.; Ogundana, A. The Development of Tribology in Lubrication Systems of Industrial Applications: Now and future impact. E3S Web Conf. 2023, 391, 01013. [Google Scholar] [CrossRef]
- Kamyab, B.; Beims, R.; Chambers, D.W.; Bassi, A.S.; Xu, C. Sustainable production of high-performance bio-based hydraulic fluids from vegetable oils: Recent advances, current challenges, and future perspectives. Biomass Bioenergy 2024, 183, 107160. [Google Scholar] [CrossRef]
- Basiron, J.; Abdollah, M.F.B.; Abdullah, M.I.C.; Amiruddin, H. Lubricant mechanisms of eco-friendly lubricant blended with mineral oil for steel-steel contact. Tribol. Int. 2023, 186, 108653. [Google Scholar] [CrossRef]
- Singh, Y.; Farooq, A.; Raza, A.; Mahmood, M.A.; Jain, S. Sustainability of a non-edible vegetable oil based bio-lubricant for automotive applications: A review. Process Saf. Environ. Prot. 2017, 111, 701–713. [Google Scholar] [CrossRef]
- Ahmad, U.; Naqvi, S.R.; Ali, I.; Naqvi, M.; Asif, S.; Bokhari, A.; Juchelková, D.; Klemeš, J.J. A review on properties, challenges and commercial aspects of eco-friendly biolubricants productions. Chemosphere 2022, 309, 136622. [Google Scholar] [CrossRef]
- Reeves, C.J.; Siddaiah, A.; Menezes, P.L. A review on the science and technology of natural and synthetic biolubricants. J. Bio-Tribo-Corros. 2017, 3, 11. [Google Scholar] [CrossRef]
- Bolina, I.C.; Gomes, R.A.; Mendes, A.A. Biolubricant production from several oleaginous feedstocks using lipases as catalysts: Current scenario and future perspectives. BioEnergy Res. 2021, 14, 1039–1057. [Google Scholar] [CrossRef]
- Data Bridge Market Research. Relatório Global de Análise do Tamanho, Quota e Tendências do Mercado de Lubrificantes—Visão Geral do Setor e Previsão Para 2031. DataBridge Market Research. 1 October 2024. Available online: https://www.databridgemarketresearch.com/pt/reports/global-lubricants-market#:~:text=A%20dimens%C3%A3o%20do%20mercado%20global,previs%C3%A3o%20de%202024%20a%202031 (accessed on 10 October 2024).
- DataBridge Market Research. Mercado Global de Biolubrificantes—Tendências da Indústria e Previsão para 2031. DataBridge Market Research. 1 July 2024. Available online: https://www.databridgemarketresearch.com/pt/reports/global-bio-lubricant-market?srsltid=AfmBOoovYaLPykPIT4g5WDrGwKeVqsVglkdLLNmWR0OfUtewSG0hZRiW (accessed on 10 October 2024).
- Singh, Y.; Sharma, A.; Singla, A. Non-edible vegetable oil–based feedstocks capable of bio-lubricant production for automotive sector applications—A review. Environ. Sci. Pollut. Res. 2019, 26, 14867–14882. [Google Scholar] [CrossRef]
- Singh, Y.; Sharma, A.; Singh, N.; Singla, A.; Rastogi, P.M. Prospects of inedible plant oil-driven bio-lubricants for tribological characteristics–a review. Int. J. Ambient Energy 2020, 41, 1534–1547. [Google Scholar] [CrossRef]
- Niculescu, V.C.; Ionete, R.E. An overview on management and valorisation of winery wastes. Appl. Sci. 2023, 13, 5063. [Google Scholar] [CrossRef]
- Evtuguin, D.; Aniceto, J.P.; Marques, R.; Portugal, I.; Silva, C.M.; Serafim, L.S.; Xavier, A.M. Obtaining Value from Wine Wastes: Paving the Way for Sustainable Development. Fermentation 2023, 10, 24. [Google Scholar] [CrossRef]
- Fadda, A.; Sanna, D.; Sakar, E.H.; Gharby, S.; Mulas, M.; Medda, S.; Yesilcubuk, N.S.; Karaca, A.C.; Gozukirmizi, C.K.; Lucarini, M.; et al. Innovative and Sustainable Technologies to Enhance the Oxidative Stability of Vegetable Oils. Sustainability 2022, 14, 849. [Google Scholar] [CrossRef]
- Kim, J.; Kim, D.N.; Lee, S.H.; Yoo, S.H.; Lee, S. Correlation of fatty acid composition of vegetable oils with rheological behaviour and oil uptake. Food Chem. 2010, 118, 398–402. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; Rodrigues, E.; Gonzaga, L.V.; Fett, R. Composição de ácidos graxos de óleo de semente de uva (Vitis vinifera L. e Vitis labrusca L.). Braz. J. Food Technol. 2010, 13, 23–26. [Google Scholar] [CrossRef]
- Sharma, B.K.; Karmakar, G.; Erhan, S.Z. Modified vegetable oils for environmentally friendly lubricant applications. In Synthetics, Mineral Oils, and Bio-Based Lubricants; CRC Press: Boca Raton, FL, USA, 2020; pp. 399–430. [Google Scholar]
- Cecilia, J.A.; Ballesteros Plata, D.; Alves Saboya, R.M.; Tavares de Luna, F.M.; Cavalcante, C.L., Jr.; Rodríguez-Castellón, E. An overview of the biolubricant production process: Challenges and future perspectives. Processes 2020, 8, 257. [Google Scholar] [CrossRef]
- Sanjurjo, C.; Rodríguez, E.; Viesca, J.L.; Battez, A.H. Influence of molecular structure on the physicochemical and tribological properties of biolubricants: A review. Lubricants 2023, 11, 380. [Google Scholar] [CrossRef]
- Vitiello, R.; Taddeo, F.; Russo, V.; Turco, R.; Buonerba, A.; Grassi, A.; Di Serio, M.; Tesser, R. Production of Sustainable Biochemicals by Means of Esterification Reaction and Heterogeneous Acid Catalysts. ChemEngineering 2021, 5, 46. [Google Scholar] [CrossRef]
- Salaheldeen, M.; Mariod, A.A.; Aroua, M.K.; Rahman, S.A.; Soudagar, M.E.M.; Fattah, I.R. Current state and perspectives on transesterification of triglycerides for biodiesel production. Catalysts 2021, 11, 1121. [Google Scholar] [CrossRef]
- Chen, J.; Bian, X.; Rapp, G.; Lang, J.; Montoya, A.; Trethowan, R.; Bouyssiere, B.; Portha, J.F.; Jaubert, J.N.; Pratt, P.; et al. From ethyl biodiesel to biolubricants: Options for an Indian mustard integrated biorefinery toward a green and circular economy. Ind. Crops Prod. 2019, 137, 597–614. [Google Scholar] [CrossRef]
- Al-Quraan, T.M.A.; Alfaqs, F.; Haddad, J.; Vojtov, V.; Voitov, A.; Kravtsov, A.; Miroshnyk, O.; Kondratiev, A. A Methodological Approach to Assessing the Tribological Properties of Lubricants Using a Four-Ball Tribometer. Lubricants 2023, 11, 457. [Google Scholar] [CrossRef]
- Chan, C.H.; Tang, S.W.; Mohd, N.K.; Lim, W.H.; Yeong, S.K.; Idris, Z. Tribological behavior of biolubricant base stocks and additives. Renew. Sustain. Energy Rev. 2018, 93, 145–157. [Google Scholar] [CrossRef]
- Yunus, R.; Fakhru’l-Razi, A.; Ooi, T.L.; Iyuke, S.E.; Perez, J.M. Lubrication properties of trimethylolpropane esters based on palm oil and palm kernel oils. Eur. J. Lipid Sci. Technol. 2004, 106, 52–60. [Google Scholar] [CrossRef]
- Zulkifli, N.W.M.; Kalam, M.A.; Masjuki, H.H.; Shahabuddin, M.; Yunus, R. Wear prevention characteristics of a palm oil-based TMP (trimethylolpropane) ester as an engine lubricant. Energy 2013, 54, 167–173. [Google Scholar] [CrossRef]
- Durango-Giraldo, G.; Zapata-Hernandez, C.; Santa, J.F.; Buitrago-Sierra, R. Palm oil as a biolubricant: Literature review of processing parameters and tribological performance. J. Ind. Eng. Chem. 2022, 107, 31–44. [Google Scholar] [CrossRef]
- ASTM D1298; Standard Test Method for Density, Relative Density, or API Gravity of Crude Petroleum and Liquid Petroleum Products by Hydrometer Method. ASTM International: West Conshohocken, PA, USA, 2012.
- ASTM D445; Standard Test Method for Kinematic Viscosity of Transparent and Opaque Liquids: (and Calculation of Dynamic Viscosity). ASTM International: West Conshohocken, PA, USA, 2006.
- ASTM D2270; Standard Practice for Calculating Viscosity Index from Kinematic Viscosity at 40 and 100 °C. ASTM International: West Conshohocken, PA, USA, 2016.
- ASTM D97; Standard Test Method for Pour Point of Petroleum Products. ASTM International: West Conshohocken, PA, USA, 2022.
- ASTM D92; Standard Test Method for Flash and Fire Points by Cleveland Open Cup Tester. ASTM International: West Conshohocken, PA, USA, 2024.
- ASTM D3339; Standard Test Method for Acid Number of Petroleum Products by Semi-Micro Color Indicator Titration. ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM D7042; Standard Test Method for Dynamic Viscosity and Density of Liquids by Stabinger Viscometer (and the Calculation of Kinematic Viscosity). ASTM International: West Conshohocken, PA, USA, 2014.
- Ruggiero, A.; Valášek, P.; Müller, M.; D’Amato, R. Tribological investigation of epoxy/seed particle composite obtained from residues of processing Jatropha curcas L. fruits. Compos. Part B Eng. 2019, 167, 654–667. [Google Scholar] [CrossRef]
- TA Instruments. Available online: https://www.tainstruments.com/tribo-rheometry-accessory/ (accessed on 30 September 2024).
- API. Appendix E—API Base Oil Interchangeability Guidelines for Passenger Car Motor Oils and Diesel Engine Oils. 1509. Available online: https://www.api.org/~/media/files/certification/engine-oil-diesel/publications/annerev043019%20rev043019.pdf (accessed on 30 June 2024).
- Lin, L.; Kedzierski, M.A. Density and viscosity of a polyol ester lubricant: Measurement and molecular dynamics simulation. Int. J. Refrig. 2020, 118, 188–201. [Google Scholar] [CrossRef]
- Ewen, J.P.; Gattinoni, C.; Thakkar, F.M.; Morgan, N.; Spikes, H.A.; Dini, D. A comparison of classical force-fields for molecular dynamics simulations of lubricants. Materials 2016, 9, 651. [Google Scholar] [CrossRef]
- Ribeiro Filho, P.R.; do Nascimento, M.R.; Cavalcante, C.L., Jr.; de Luna, F.M. Synthesis and tribological properties of bio-based lubricants from soybean oil. Biomass Convers. Biorefinery 2024, 14, 20509–20521. [Google Scholar] [CrossRef]
- Campos Flexa Ribeiro Filho, P.R.; Rocha do Nascimento, M.; Otaviano da Silva, S.S.; Tavares de Luna, F.M.; Rodríguez-Castellón, E.; Loureiro Cavalcante, C., Jr. Synthesis and frictional characteristics of bio-based lubricants obtained from fatty acids of castor oil. Lubricants 2023, 11, 57. [Google Scholar] [CrossRef]
- Salimon, J.; Salih, N. Epoxidized Malaysian Elaeis guineensis palm kernel oil trimethylolpropane polyol ester as green renewable biolubricants. Biomass Bioenergy 2023, 175, 106883. [Google Scholar] [CrossRef]
- ISO 3448; Industrial Liquid Lubricants—ISO Viscosity Classification. ISO: Geneva, Switzerland, 1992.
- API. Engine Oil Licensing and Certification System (Annex E); American Petroleum Institute: Washington, DC, USA, 2022; Available online: https://www.api.org/-/media/Files/Certification/Engine-Oil-Diesel/Publications/API%201509-%2021st%20Edition.pdf (accessed on 2 November 2024).
- Oliveira, A.A.; Santos, R.P.; Rocha, W.S.; de Luna, F.M.; Fernandez-Lafuente, R.; Monteiro, R.R.; Vieira, R.S. Design of a biolubricant by the enzymatic esterification of the free fatty acids from castor oil with neopentylglycol. Process Biochem. 2024, 147, 318–331. [Google Scholar] [CrossRef]
- Monteiro, R.R.; de Melo Neta, M.M.; Rocha, W.S.; Soares, J.B.; de Luna, F.M.T.; Fernandez-Lafuente, R.; Vieira, R.S. Optimizing the enzymatic production of biolubricants by the Taguchi method: Esterification of the free fatty acids from castor oil with 2-ethyl-1-hexanol catalyzed by Eversa Transform 2.0. Enzym. Microb. Technol. 2024, 175, 110409. [Google Scholar] [CrossRef] [PubMed]
- Lopes, E.D.L. Estratégias para a caracterização de amostras brasileiras de óleo de soja empregando ATR-FTIR e HLPC-ELSD aliadas a ferramentas quimiométricas. Master’s Thesis, Universidade Federal do Rio Grande do Sul, Farroupilha, Brazil, 2015. [Google Scholar]
- Ferreira, E.N.; Arruda, T.B.M.G.; Rodrigues, F.E.A.; Arruda, D.T.D.; da Silva Júnior, J.H.; Porto, D.L.; Ricardo, N.M.P.S. Investigation of the thermal degradation of the biolubricant through TG-FTIR and characterization of the biodiesel-Pequi (Caryocar brasiliensis) as energetic raw material. Fuel 2019, 245, 398–405. [Google Scholar] [CrossRef]
- Encinar, J.M.; Nogales-Delgado, S.; Álvez-Medina, C.M. High oleic safflower biolubricant through double transesterification with methanol and pentaerythritol: Production, characterization, and antioxidant addition. Arab. J. Chem. 2022, 15, 103796. [Google Scholar] [CrossRef]
- do Valle, C.P.; Rodrigues, J.S.; Fechine, L.M.U.D.; Cunha, A.P.; Malveira, J.Q.; Luna, F.M.T.; Ricardo, N.M.P.S. Chemical modification of Tilapia oil for biolubricant applications. J. Clean. Prod. 2018, 191, 158–166. [Google Scholar] [CrossRef]
- Kamalakar, K.; Rajak, A.K.; Prasad, R.B.N.; Karuna, M.S.L. Rubber seed oil-based biolubricant base stocks: A potential source for hydraulic oils. Ind. Crops Prod. 2013, 51, 249–257. [Google Scholar] [CrossRef]
- Padmaja, K.V.; Rao, B.V.; Reddy, R.K.; Bhaskar, P.S.; Singh, A.K.; Prasad, R.B. 10-Undecenoic acid-based polyol esters as potential lubricant base stocks. Ind. Crops Prod. 2012, 35, 237–240. [Google Scholar] [CrossRef]
- Ruggiero, A.; De Stefano, M. Evaluation of the real contact area of rough surfaces by using a finite element model. In Advances in Italian Mechanism Science: Proceedings of the 3rd International Conference of IFToMM Italy; Springer International Publishing: Cham, Switzerland, 2021; pp. 679–686. [Google Scholar] [CrossRef]
- Liu, Z.; Neville, A.; Reuben, R.L. A numerical calculation of the contact area and pressure of real surfaces in sliding wear. J. Trib. 2001, 123, 27–35. [Google Scholar] [CrossRef]
- Ing, T.C.; Rafiq, A.K.M.; Azli, Y.; Syahrullail, S. Tribological behaviour of refined bleached and deodorized palm olein in different loads using a four-ball tribotester. Sci. Iran. 2012, 19, 1487–1492. [Google Scholar] [CrossRef]
- Rani, S.; Joy, M.L.; Nair, K.P. Evaluation of physiochemical and tribological properties of rice bran oil–biodegradable and potential base stoke for industrial lubricants. Ind. Crops Prod. 2015, 65, 328–333. [Google Scholar] [CrossRef]
- Zulkifli, N.W.M.; Azman, S.S.N.; Kalam, M.A.; Masjuki, H.H.; Yunus, R.; Gulzar, M. Lubricity of bio-based lubricant derived from different chemically modified fatty acid methyl ester. Tribol. Int. 2016, 93, 555–562. [Google Scholar] [CrossRef]
- Barriga, J.; Kalin, M.; Van Acker, K.; Vercammen, K.; Ortega, A.; Leiaristi, L. Tribological performance of titanium doped and pure DLC coatings combined with a synthetic bio-lubricant. Wear 2006, 261, 9–14. [Google Scholar] [CrossRef]
- Martins, R.C.; Cardoso, N.F.R.; Bock, H.; Igartua, A.; Seabra, J.H.O. Power loss performance of high pressure nitrided steel gears. Tribol. Int. 2009, 42, 1807–1815. [Google Scholar] [CrossRef]
- Martins, R.; Seabra, J.; Brito, A.; Seyfert, C.; Luther, R.; Igartua, A. Friction coefficient in FZG gears lubricated with industrial gear oils: Biodegradable ester vs. mineral oil. Tribol. Int. 2006, 39, 512–521. [Google Scholar] [CrossRef]
- Cardoso, N.F.R.; Martins, R.C.; Seabra, J.H.O.; Igartua, A.; Rodriguez, J.C.; Luther, R. Micropitting performance of nitrided steel gears lubricated with mineral and ester oils. Tribol. Int. 2009, 42, 77–87. [Google Scholar] [CrossRef]
- Afifah, A.N.; Syahrullail, S.; Azlee, N.I.W.; Rohah, A.M. Synthesis and tribological studies of epoxidized palm stearin methyl ester as a green lubricant. J. Clean. Prod. 2021, 280, 124320. [Google Scholar] [CrossRef]
- de Andrade Souza, L.; Moreira, D.R.; Ricardo, N.M.P.S.; Maier, M.E.; Filho, P.R.C.F.R.; Luna, F.M.T.; Petzhold, C.L. Esters from castor oil functionalized with aromatic amines as a potential lubricant. J. Am. Oil Chem. Soc. 2023, 100, 175–184. [Google Scholar] [CrossRef]
- Moreira, D.R.; Worman, M.; Ferreira, E.N.; Ribeiro Filho, P.R.; da Silva, L.M.; Arruda, T.B.; Rodrigues, F.E.; de Luna, F.M.; Petzhold, C.L.; Maier, M.E.; et al. Development of polyols analogous to neopentyl glycol and trimethylolpropane for the production of oleic acid-based biolubricants. Fuel 2025, 381, 133156. [Google Scholar] [CrossRef]
- Ribeiro Filho, P.R.C.F.; da Silva, S.S.O.; do Nascimento, M.R.; de Aguiar Soares, S.; de Luna, F.M.T.; Cavalcante, C.L., Jr. Tribological properties of bio-based lubricant basestock obtained from pequi oil (Caryocar brasiliensis). J. Braz. Soc. Mech. Sci. Eng. 2022, 44, 51. [Google Scholar] [CrossRef]
- Ma, W.; Lu, J. Effect of sliding speed on surface modification and tribological behavior of copper–graphite composite. Tribol. Lett. 2011, 41, 363–370. [Google Scholar] [CrossRef]
- Okonkwo, P.C.; Kelly, G.; Rolfe, B.F.; Pereira, M.P. The effect of sliding speed on the wear of steel–tool steel pairs. Tribol. Int. 2016, 97, 218–227. [Google Scholar] [CrossRef]
- Noorawzi, N.; Samion, S. Tribological effects of vegetable oil as alternative lubricant: A pin-on-disk tribometer and wear study. Tribol. Trans. 2016, 59, 831–837. [Google Scholar] [CrossRef]
- Afifah, A.N.; Syahrullail, S.; Azlee, N.I.W.; Sidik, N.A.C.; Yahya, W.J.; Abd Rahim, E. Biolubricant production from palm stearin through enzymatic transesterification method. Biochem. Eng. J. 2019, 148, 178–184. [Google Scholar] [CrossRef]
- elo Neta, M.M.F.; Lima, G.R.R.; Tavares, P.d.O.; Figueredo, I.d.M.; Rocha, W.d.S.; Ribeiro Filho, P.R.C.F.; Cavalcante, C.L., Jr.; Luna, F.M.T. Thermo-oxidative stability and tribological properties of biolubricants obtained from castor oil fatty acids and isoamyl alcohol. Lubricants 2023, 11, 490. [Google Scholar] [CrossRef]
- Kurre, S.K.; Yadav, J. A review on bio-based feedstock, synthesis, and chemical modification to enhance tribological properties of biolubricants. Ind. Crops Prod. 2023, 193, 116122. [Google Scholar] [CrossRef]
- Syahrullail, S.; Kamitani, S.; Shakirin, A.J.P.E. Performance of vegetable oil as lubricant in extreme pressure condition. Procedia Eng. 2013, 68, 172–177. [Google Scholar] [CrossRef]
- Rudnick, L.R. (Ed.) Synthetics, Mineral Oils, and Bio-Based Lubricants: Chemistry and Technology; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]

| Properties | Results | Methods |
|---|---|---|
| Density at 20 °C (g/cm3) | 0.892 | [30] |
| Viscosity at 40 °C (cSt) | 10.1 | [31] |
| Viscosity at 100 °C (cSt) | 2.38 | [31] |
| Viscosity Index | 22 | [32] |
| Pour point (°C) | −42 | [33] |
| Flash point (°C) | 162 | [34] |
| Total acid number (mg KOH/g) | 0.01 | [35] |
| Properties | GSO | FAME | BL | Methods |
|---|---|---|---|---|
| Density at 20 °C (g/cm3) | 0.9290 | 0.8995 | 0.8846 | [36] |
| Viscosity at 40 °C (cSt) | 49.7 | 8.9 | 9.4 | [31] |
| Viscosity at 100 °C (cSt) | 9.91 | 2.91 | 2.86 | [31] |
| Viscosity Index | 191 | 206 | 168 | [32] |
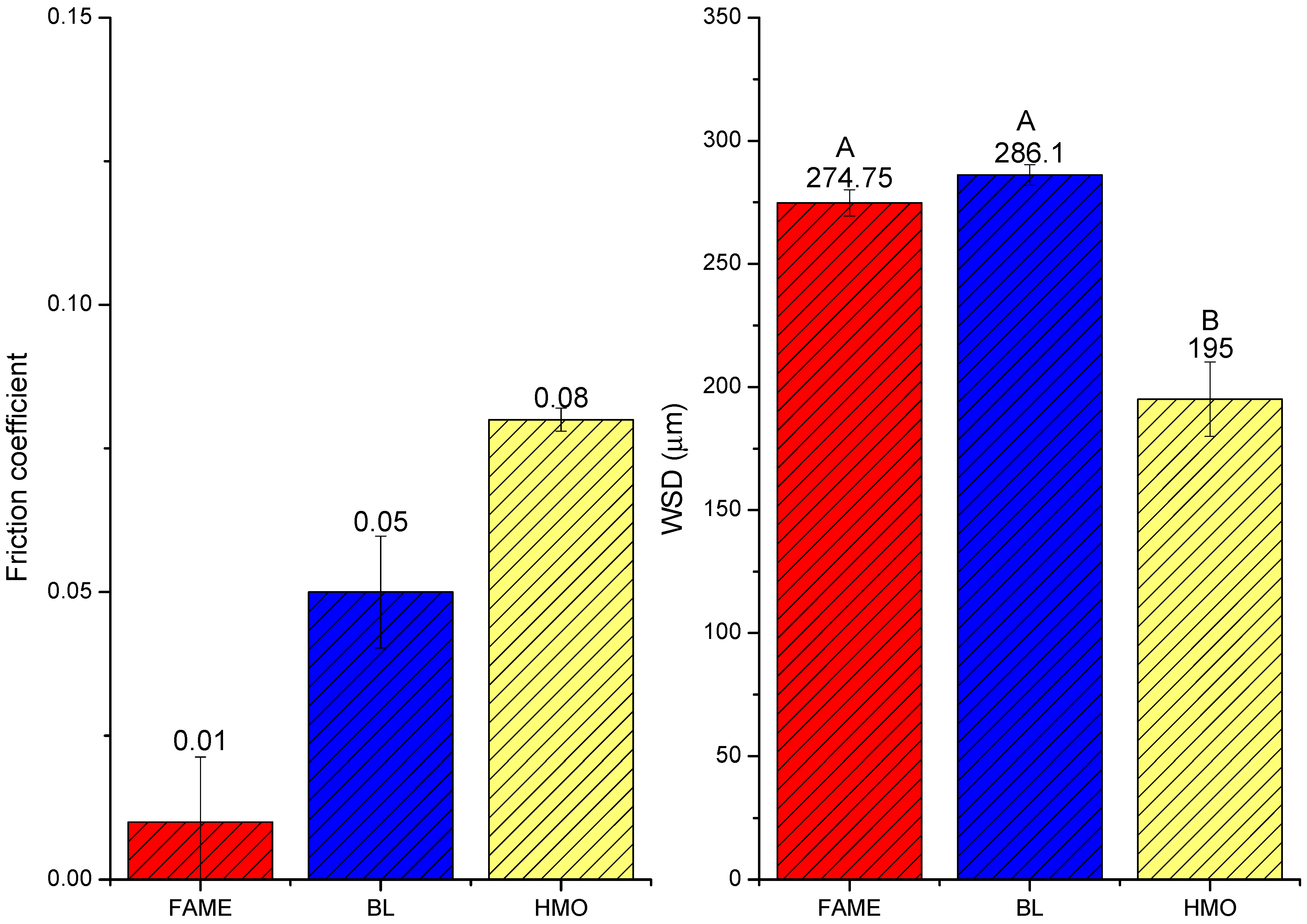
| 1st Ball | 2nd Ball | 3rd Ball | |
|---|---|---|---|
| FAME |  277.95 µm |  268.62 µm |  277.69 µm |
| BL |  287.42 µm |  281.37 µm |  289.52 µm |
| HMO | 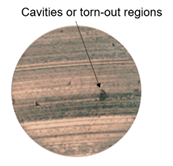 178 µm |  207 µm | 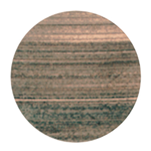 200 µm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, T.F.; Melo Neta, M.M.F.d.; Ribeiro Filho, P.R.C.F.; Luna, F.M.T.d.; Cavalcante, C.L., Jr. Tribological Characteristics of Biolubricant Obtained by Transesterification of Grape Seed Oil. Lubricants 2024, 12, 459. https://doi.org/10.3390/lubricants12120459
Silva TF, Melo Neta MMFd, Ribeiro Filho PRCF, Luna FMTd, Cavalcante CL Jr. Tribological Characteristics of Biolubricant Obtained by Transesterification of Grape Seed Oil. Lubricants. 2024; 12(12):459. https://doi.org/10.3390/lubricants12120459
Chicago/Turabian StyleSilva, Thawan Fonseca, Maria Marliete Fernandes de Melo Neta, Paulo Roberto Campos Flexa Ribeiro Filho, Francisco Murilo Tavares de Luna, and Célio Loureiro Cavalcante, Jr. 2024. "Tribological Characteristics of Biolubricant Obtained by Transesterification of Grape Seed Oil" Lubricants 12, no. 12: 459. https://doi.org/10.3390/lubricants12120459
APA StyleSilva, T. F., Melo Neta, M. M. F. d., Ribeiro Filho, P. R. C. F., Luna, F. M. T. d., & Cavalcante, C. L., Jr. (2024). Tribological Characteristics of Biolubricant Obtained by Transesterification of Grape Seed Oil. Lubricants, 12(12), 459. https://doi.org/10.3390/lubricants12120459







