Tribo-Dependent Photoluminescent Behavior of Oleylamine-Modified AgInS2 and AgInS2-ZnS Nanoparticles as Lubricant Additives
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of AIS NPs
2.3. Synthesis of ZAIS NPs
2.4. Characterizations
2.5. Tribological Test
3. Results and Discussion
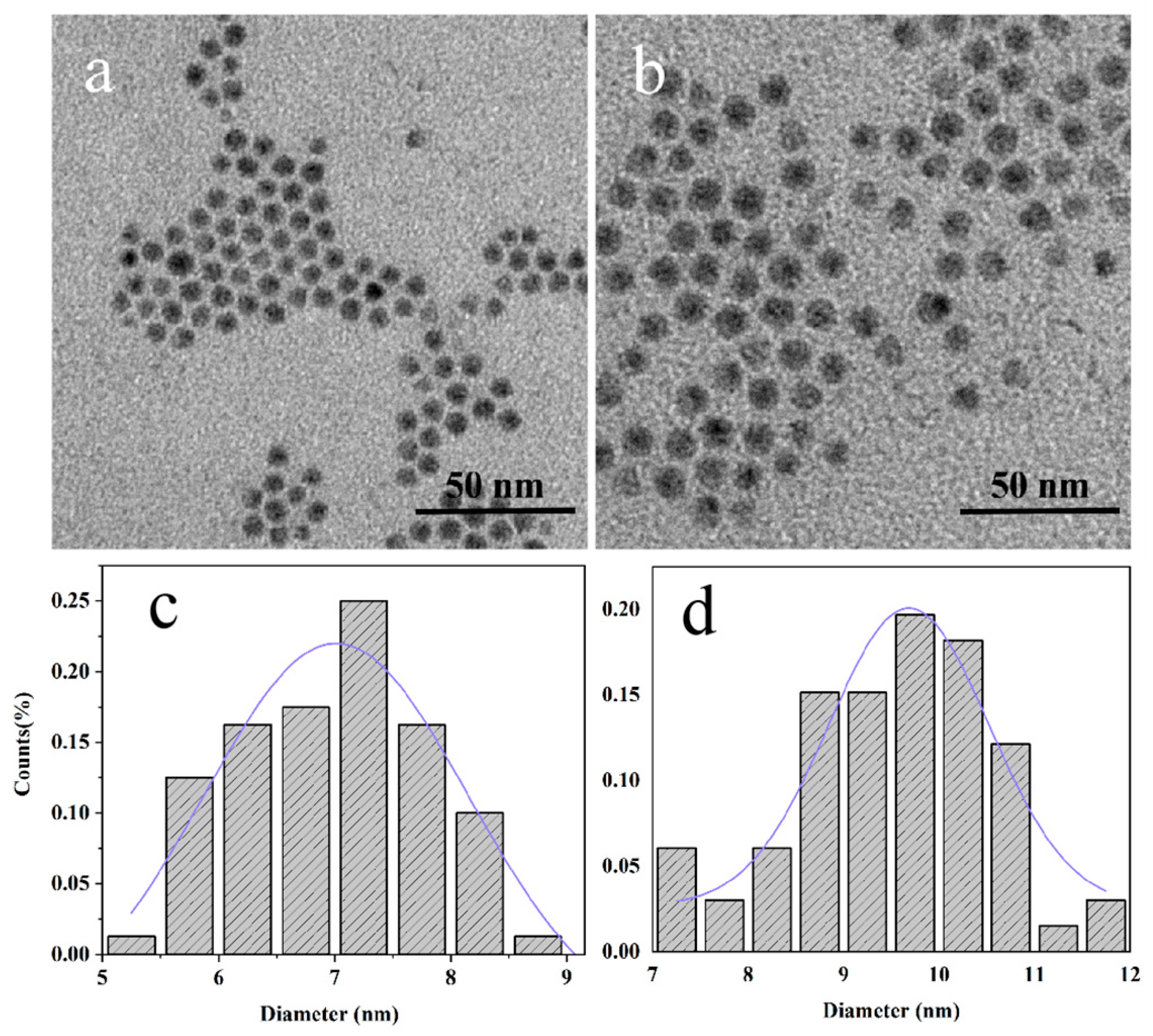
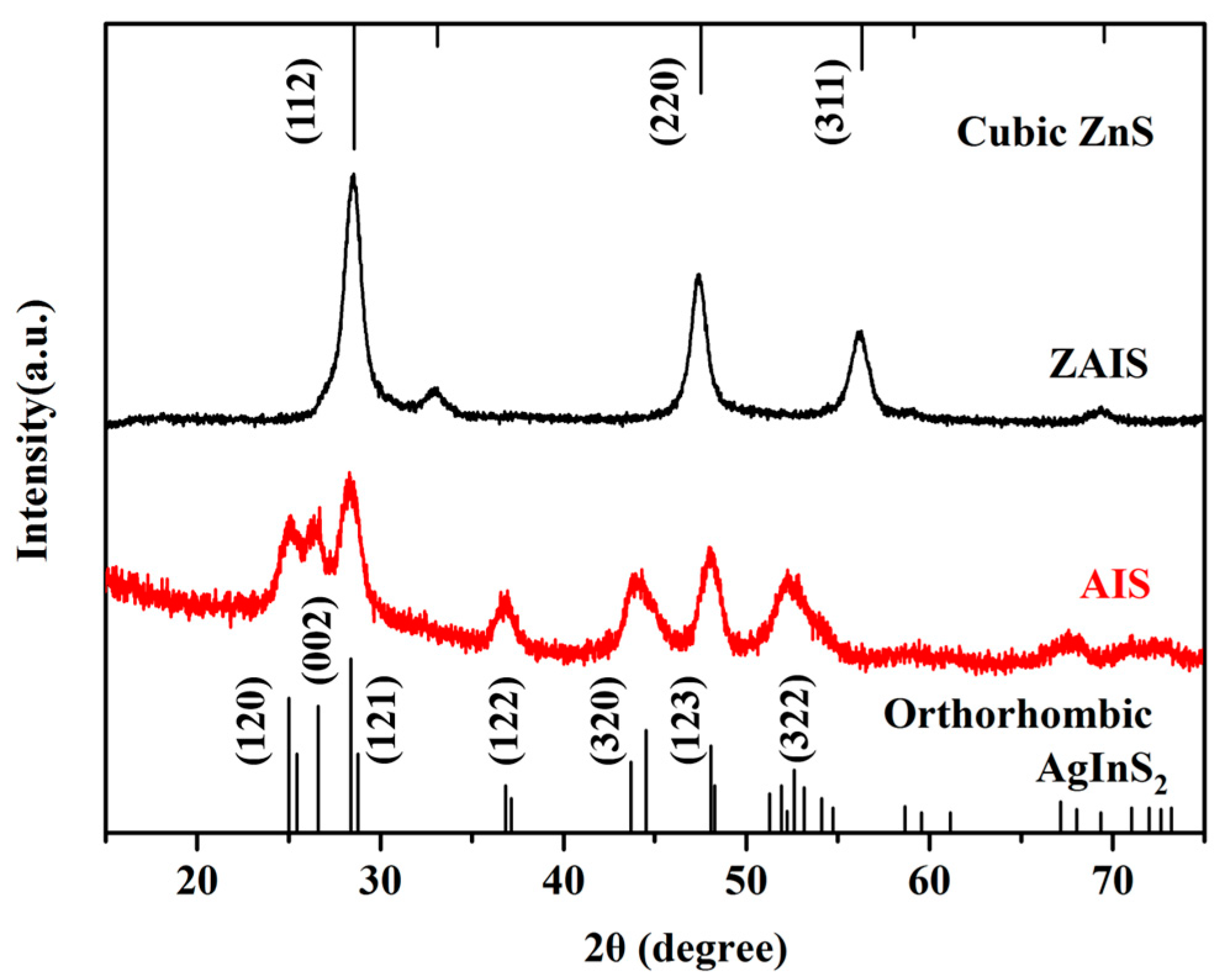
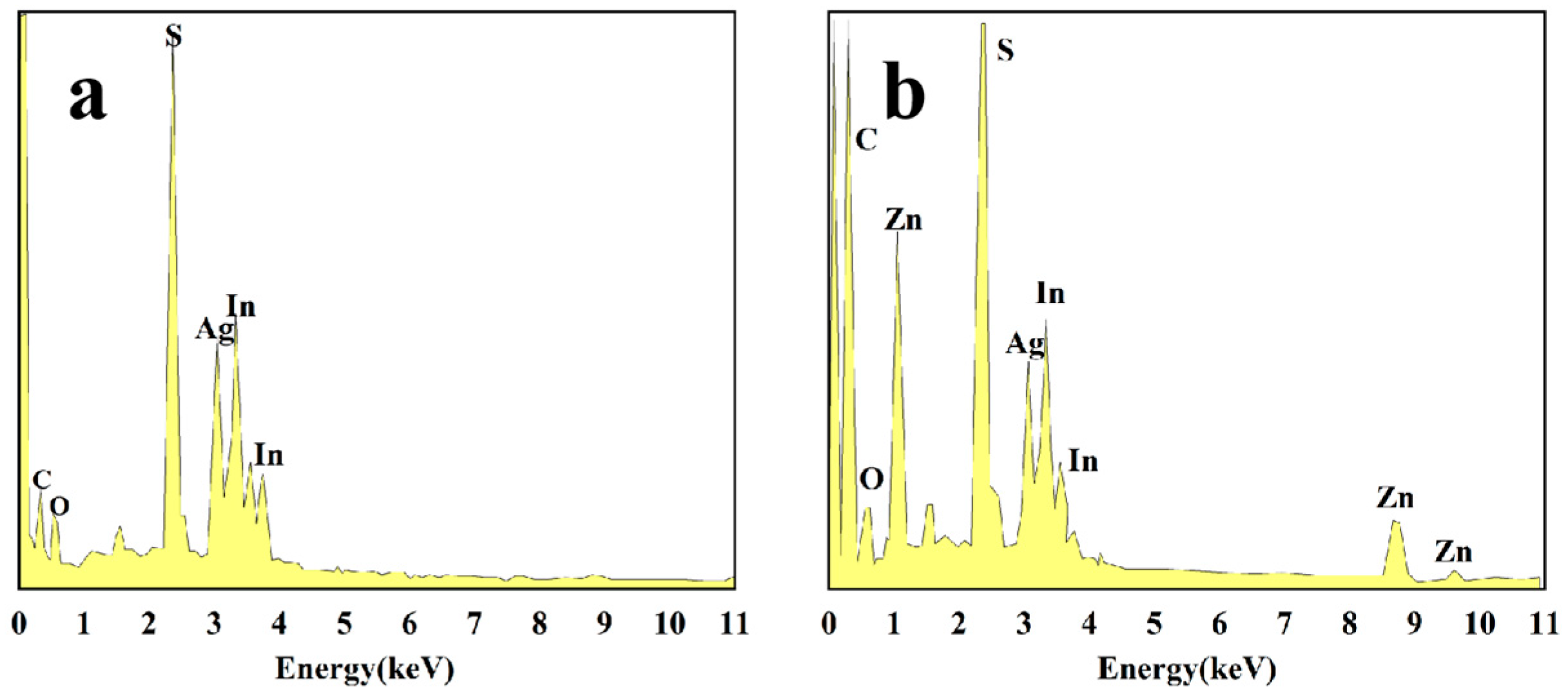
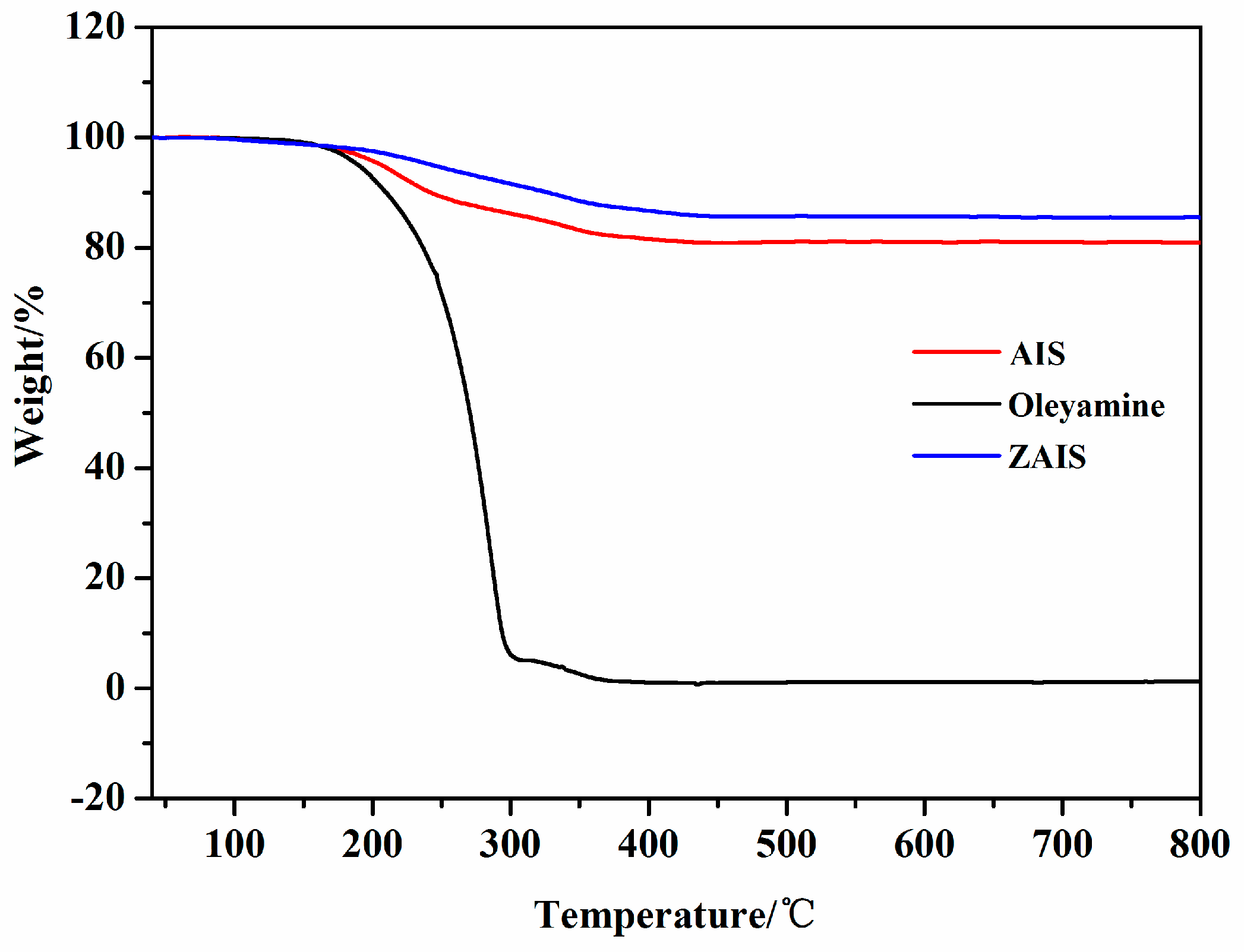
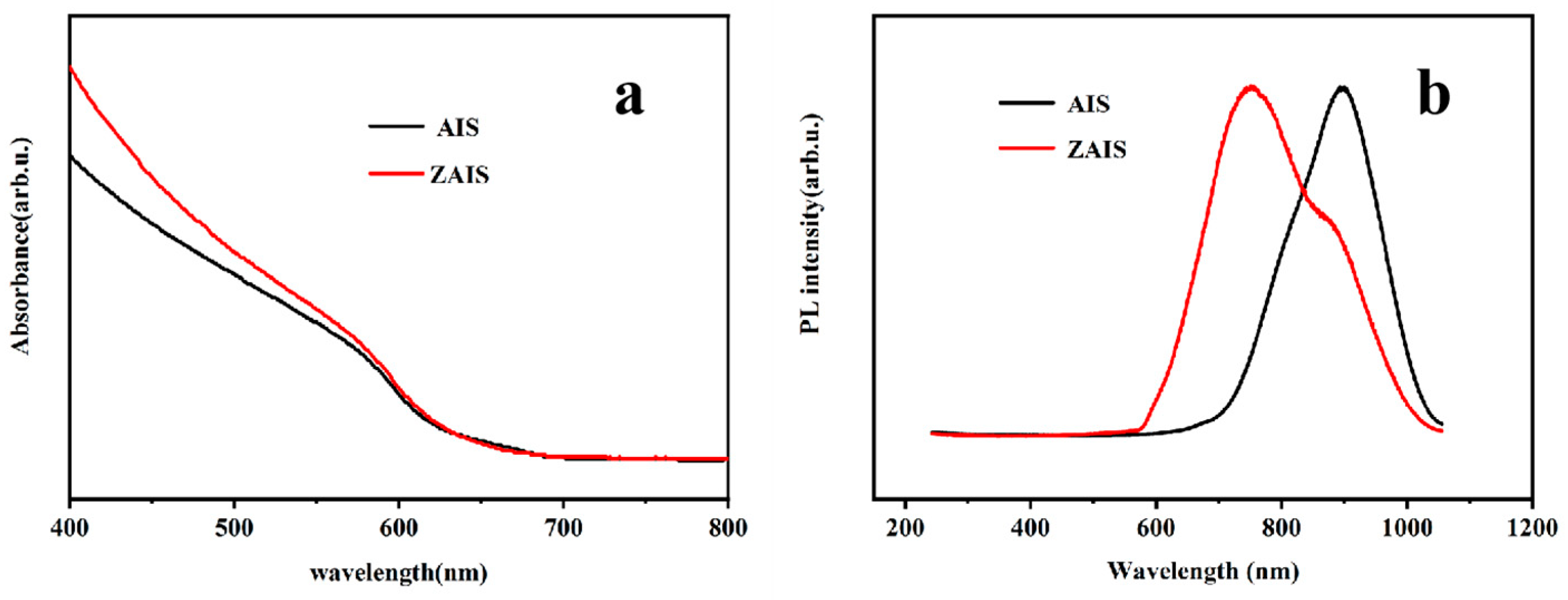
3.1. Characterization of AIS NPs
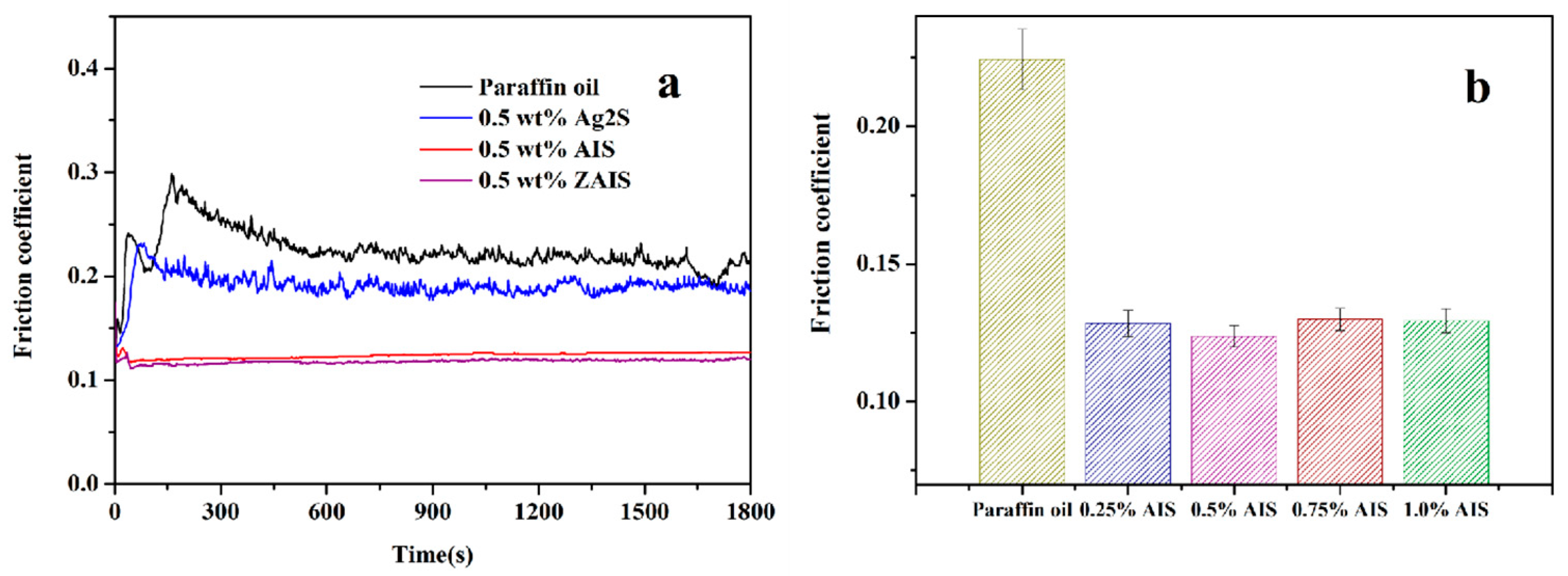
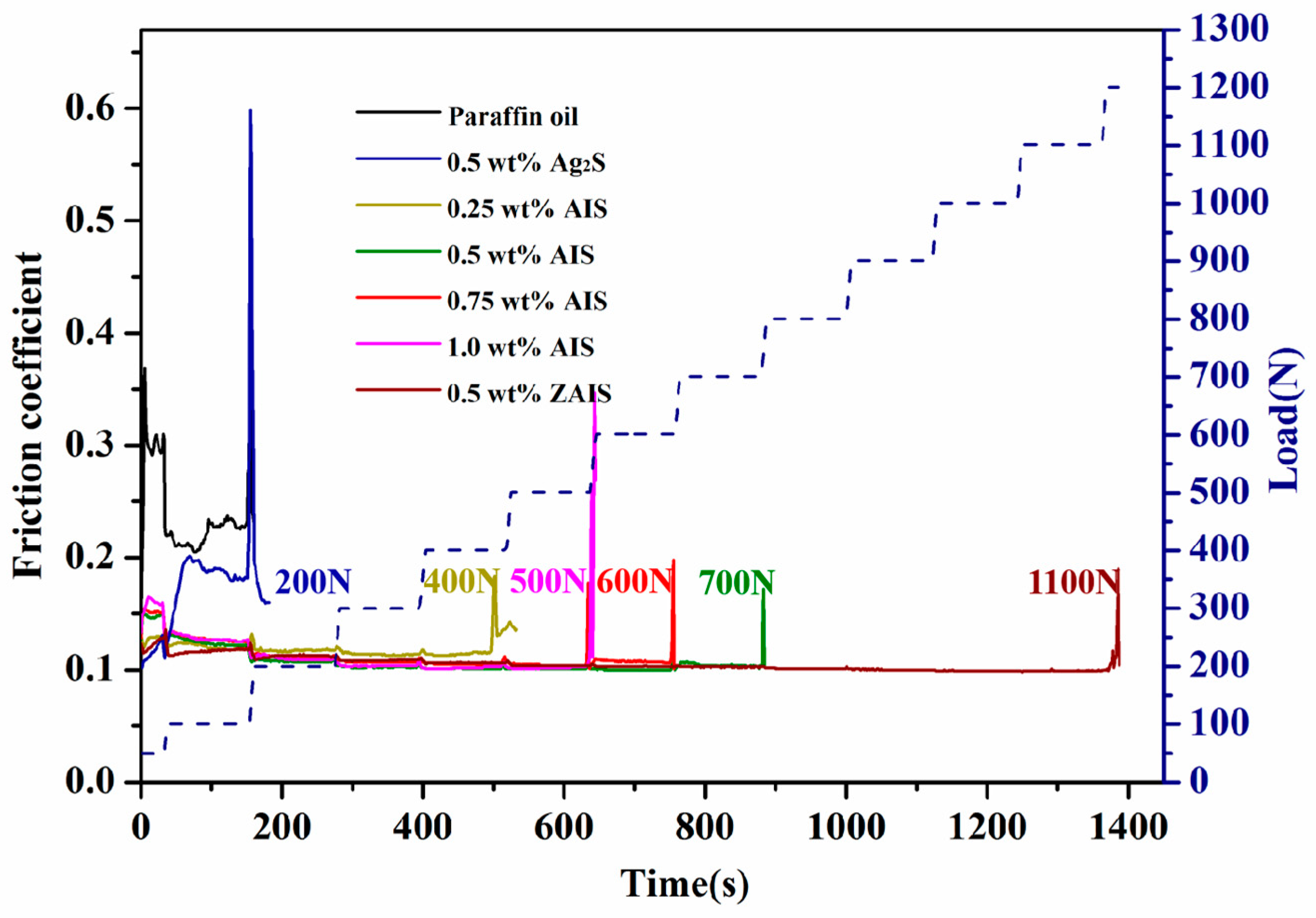
3.2. Tribological Behaviors
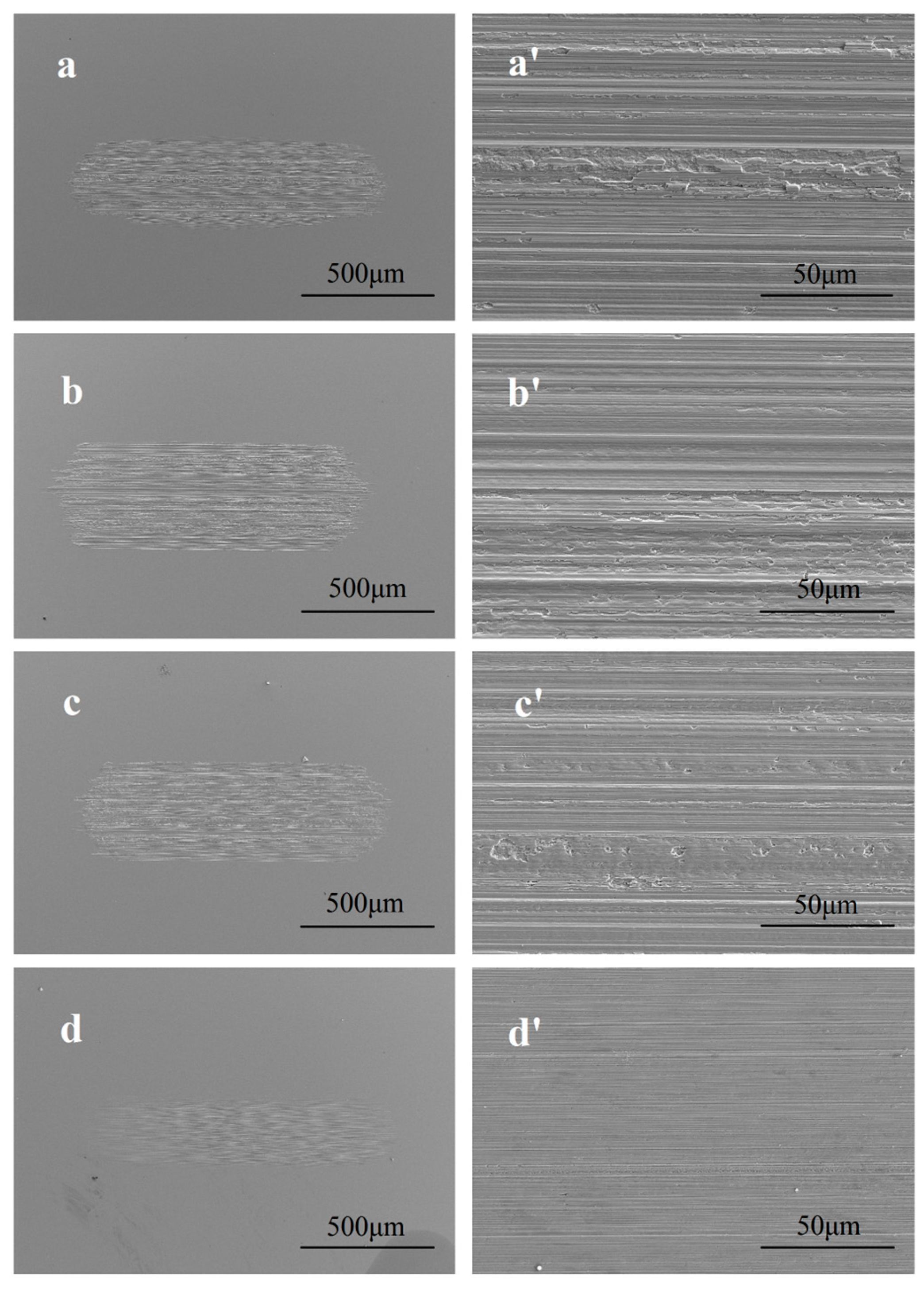
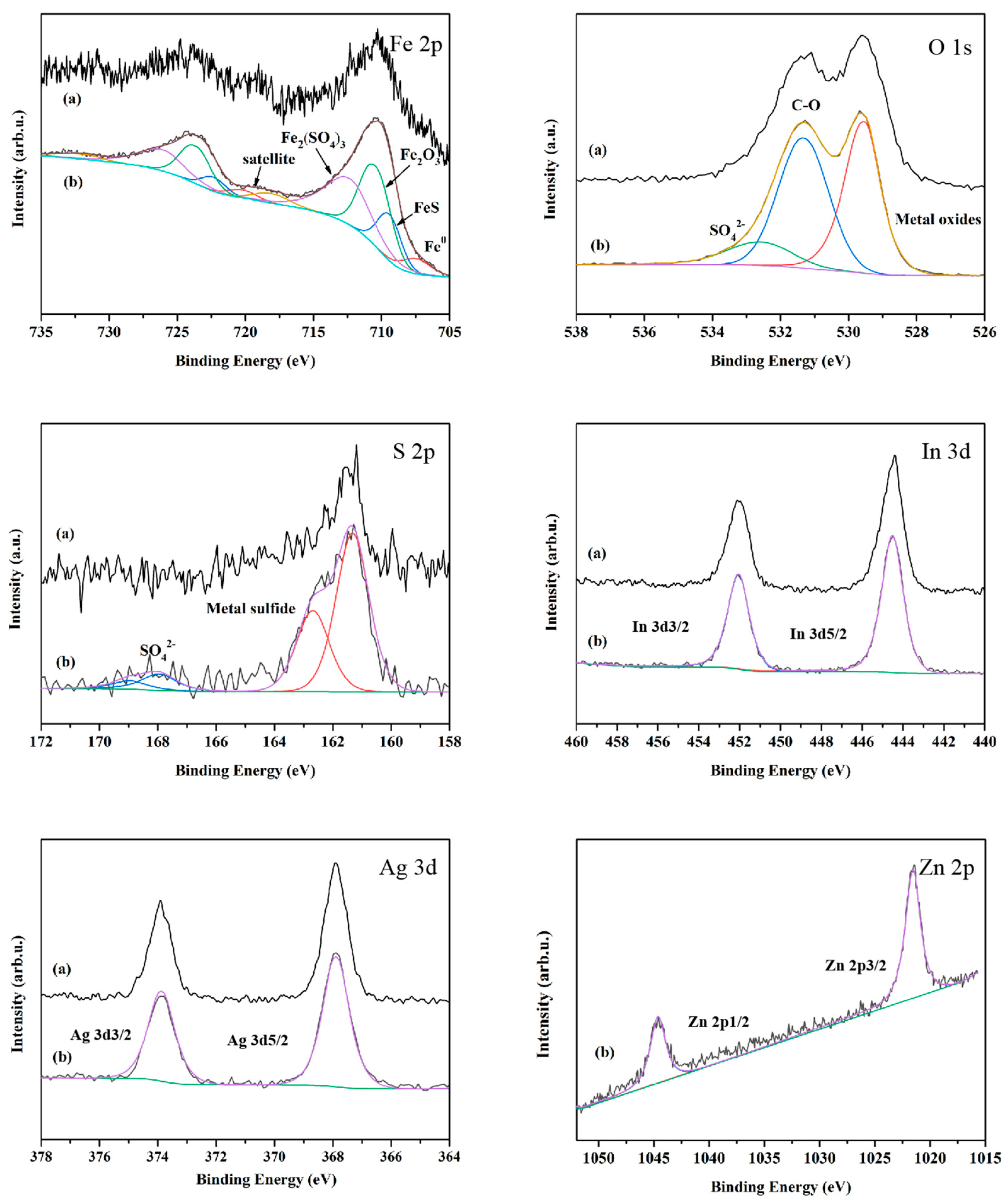
3.3. Worn Surface Analyses
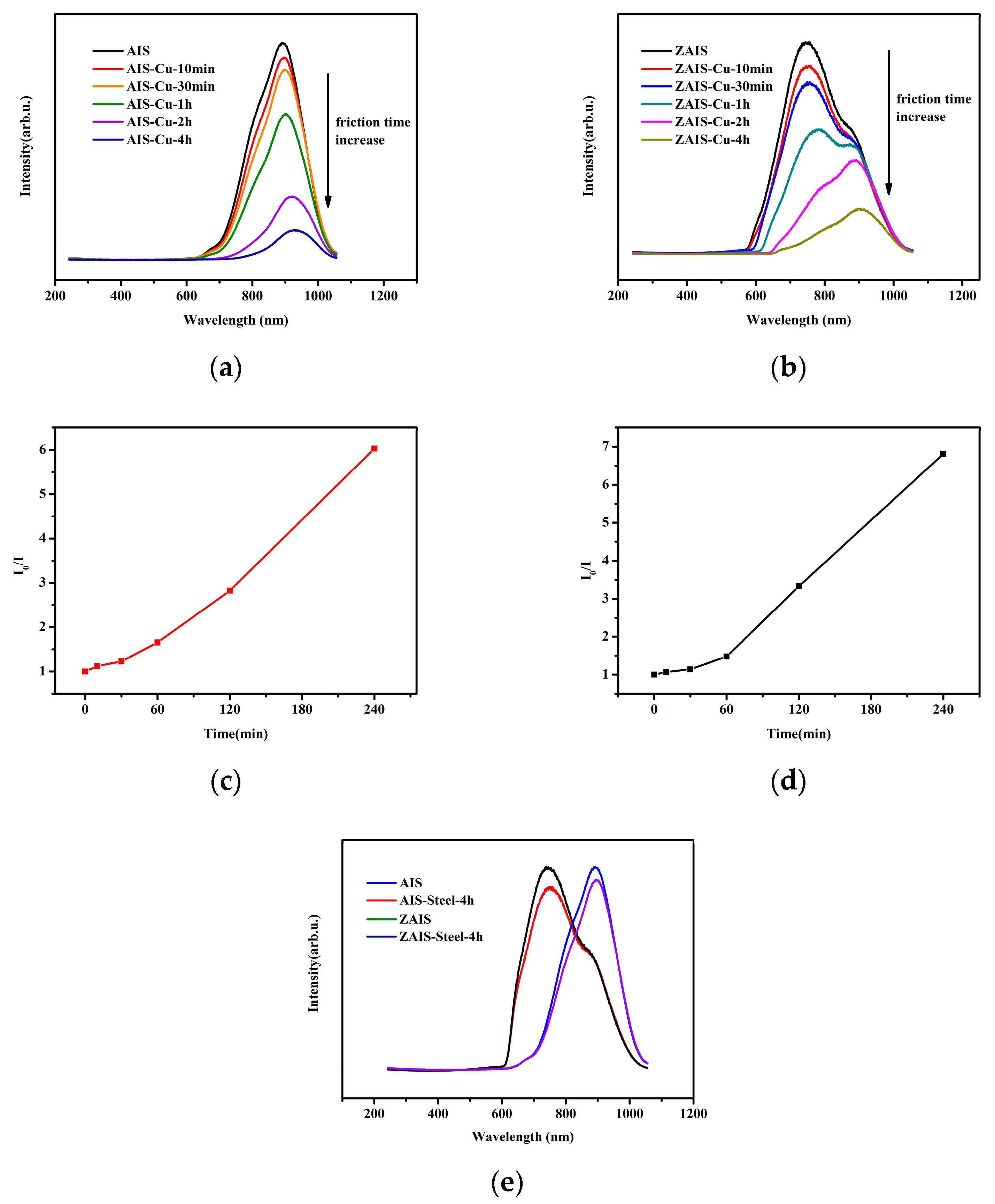
3.4. Fluorescence Dependence on Tribo-Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dai, W.; Kheireddin, B.; Gaob, H.; Liang, H. Roles of nanoparticles in oil lubrication. Tribol. Int. 2016, 102, 88–98. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, W.; Liang, W.; Yan, T.; Li, T.; Zhang, L.; Li, S. Inorganic nanomaterial lubricant additives for base fluids, to improve tribological performance: Recent developments. Friction 2021, 10, 645–676. [Google Scholar] [CrossRef]
- Uflyand, I.E.; Zhinzhilo, V.A.; Burlakova, V.E. Metal-containing nanomaterials as lubricant additives: State-of-the-art and future development. Friction 2019, 7, 93–116. [Google Scholar] [CrossRef]
- Kong, L.; Sun, J.; Bao, Y. Preparation, characterization and tribological mechanism of nanofluids. RSC Adv. 2017, 7, 12599–12609. [Google Scholar] [CrossRef]
- Rosenkranz, A.; Costa, H.L.; Baykara, M.Z.; Martini, A. Synergetic effects of surface texturing and solid lubricants to tailor friction and wear—A review. Tribol. Int. 2021, 155, 106792–106812. [Google Scholar] [CrossRef]
- Chen, Y.; Renner, P.; Liang, H. A review of current understanding in tribochemical reactions involving lubricant additives. Friction 2022, 11, 489–512. [Google Scholar] [CrossRef]
- Jia, X.H.; Huang, J.; Li, Y.; Yang, J.; Song, H.J. Monodisperse Cu nanoparticles@MoS2 nanosheets as a lubricant additive for improved tribological properties. Appl. Surf. Sci. 2019, 494, 430–439. [Google Scholar] [CrossRef]
- Yi, M.; Zhang, C. The synthesis of MoS2 particles with different morphologies for tribological applications. Tribol. Int. 2017, 116, 285–294. [Google Scholar] [CrossRef]
- Ripoll, M.R.; Tomala, A.M.; Pirker, L.; Remskar, M. In-situ formation of MoS2 and WS2 tribofilms by the synergy between transition metal oxide nanoparticles and sulphur-containing oil additives. Tribol. Lett. 2020, 68, 41–53. [Google Scholar] [CrossRef]
- Jiang, Z.Q.; Zhang, Y.J.; Yang, G.B.; Gao, C.P.; Yu, L.G.; Zhang, S.M.; Zhang, P.Y. Synthesis of oil-soluble WS2 nanosheets under mild condition and study of their effect on tribological properties of poly-alpha olefin under evaluated temperatures. Tribol. Int. 2019, 138, 68–78. [Google Scholar] [CrossRef]
- Gong, K.L.; Lou, W.J.; Zhao, G.Q.; Wu, X.H.; Wang, X.B. Investigation on tribological behaviors of MoS2 and WS2 quantum dots as lubricant additives in ionic liquids under severe conditions. Friction 2020, 8, 674–683. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, S.; Zhang, Y.; Yu, L.; Yang, G.; Zhang, P.; Yang, K. Tribological properties of oleylamine-modified ultrathin WS2 nanosheets as the additive in polyalpha olefin over a wide temperature range. Tribol. Lett. 2016, 61, 24–37. [Google Scholar] [CrossRef]
- Kang, X.H.; Wang, B.; Zhu, L.; Zhu, H. Synthesis and tribological property study of oleic acid-modified copper sulfide nanoparticles. Wear 2008, 265, 150–154. [Google Scholar] [CrossRef]
- Zhao, J.H.; Yang, G.B.; Zhang, Y.J.; Zhang, S.M.; Zhang, P.Y. A simple preparation of HDA-CuS nanoparticles and their tribological properties as a water-Based lubrication additive. Tribol. Lett. 2019, 67, 67–88. [Google Scholar] [CrossRef]
- Zhou, L.H.; Wei, X.C.; Ma, Z.J.; Mei, B. Anti-friction performance of FeS nanoparticle synthesized by biological method. Appl. Surf. Sci. 2017, 407, 21–28. [Google Scholar] [CrossRef]
- Liu, W.M.; Chen, S. An investigation of the tribological behaviour of surface-modified ZnS nanoparticles in liquid paraffin. Wear 2000, 238, 120–124. [Google Scholar] [CrossRef]
- Wang, L.; Gao, Y.; Li, Z.; Zhou, A.; Li, P. Preparation and tribological properties of surface-modified ZnS nanoparticles. Lubr. Sci. 2015, 27, 241–250. [Google Scholar] [CrossRef]
- Yang, G.; Ma, H.; Wu, Z.; Zhang, P. Tribological behavior of ZnS-filled polyelectrolyte multilayers. Wear 2007, 262, 471–476. [Google Scholar] [CrossRef]
- Zhao, F.; Li, G.; Zhang, G.; Wang, T.; Wang, Q. Hybrid effect of ZnS sub-micrometer particles and reinforcing fibers on tribological performance of polyimide under oil lubrication conditions. Wear 2017, 380–381, 86–95. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, J.; Chen, B.; Guo, S.; Li, J.; Li, C. One-step hydrothermal synthesis of reduced graphene oxide/zinc sulfide hybrids for enhanced tribological properties of epoxy coatings. Surf. Coat. Technol. 2017, 326, 87–95. [Google Scholar] [CrossRef]
- Li, X.; Chen, B.; Jia, Y.; Li, X.; Yang, J.; Li, C.; Yan, F. Enhanced tribological properties of epoxy-based lubricating coatings using carbon nanotubes-ZnS hybrid. Surf. Coat. Technol. 2018, 344, 154–162. [Google Scholar] [CrossRef]
- Gulzar, M.; Masjuki, H.H.; Kalam, M.A.; Varman, M.; Zulkifli, N.W.M.; Mufti, R.A.; Zahid, R. Tribological performance of nanoparticles as lubricating oil additives. J. Nanopart. Res. 2016, 18, 223. [Google Scholar] [CrossRef]
- Zuin, A.; Cousseau, T.; Sinatora, A.; Toma, H.E. Lipophilic magnetite nanoparticles coated with stearic acid: A potential green and low cost way to improve thermal stability and tribological properties of fully formulated low viscosity engine oils. Tribol. Int. 2020, 146, 106209–106215. [Google Scholar] [CrossRef]
- Wei, J.; Hu, Z.; Zhou, W.; Lu, H.; Zhang, W.; Guo, R. Color-converted white light-emitting diodes based on I-III-VI quantum dots: Package strategies and stability promotion. Appl. Mater. Today 2022, 29, 101585–101599. [Google Scholar] [CrossRef]
- May, B.M.; Bambo, M.F.; Hosseini, S.S.; Sidwaba, U.; Nxumalo, E.N.; Mishra, A.K. A review on I-III-VI ternary quantum dots for fluorescence detection of heavy metals ions in water: Optical properties, synthesis and application. RSC Adv. 2022, 12, 11216–11232. [Google Scholar] [CrossRef] [PubMed]
- Moodelly, D.; Kowalik, P.; Bujak, P.; Pron, A.; Reiss, P. Synthesis, photophysical properties and surface chemistry of chalcopyrite-type semiconductor nanocrystals. J. Mater. Chem. C 2019, 7, 11665–11709. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, T.; Deng, M.; Tang, X.; Han, S.; Liu, A.; Bai, Y.; Qu, D.; Huang, X.; Qiu, F. Selective and sensitive detection of copper(II) based on fluorescent zincdoped AgInS2 quantum dots. J. Lumin. 2018, 201, 182–188. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, M.; Zhu, T.; Tang, X.; Han, S.; Huang, W.; Shi, Y.; Liu, A. The synthesis of water-dispersible zinc doped AgInS2 quantum dots and their application in Cu2+ detection. J. Lumin. 2017, 192, 547–554. [Google Scholar] [CrossRef]
- Xiong, W.W.; Yang, G.H.; Wu, X.C.; Zhu, J.J. Microwave-assisted synthesis of highly luminescent AgInS(2)/ZnS nanocrystals for dynamic intracellular Cu(ii) detection. J. Mater. Chem. B 2013, 1, 4160–4165. [Google Scholar] [CrossRef]
- Bujak, P.; Wrobel, Z.; Penkala, M.; Kotwica, K.; Kmita, A.; Gajewska, M.; Ostrowski, A.; Kowalik, P.; Pron, A. Highly luminescent Ag-In-Zn-S quaternary nanocrystals: Growth mechanism and surface chemistry elucidation. Inorg. Chem. 2019, 58, 1358–1370. [Google Scholar] [CrossRef]
- Shamirian, A.; Appelbe, O.; Zhang, Q.; Ganesh, B.; Kron, S.J.; Snee, P.T. A toolkit for bioimaging using near-infrared AgInS2/ZnS quantum dots. J. Mater. Chem. B 2015, 3, 8188–8196. [Google Scholar] [CrossRef]
- Mohan, C.N.; Renuga, V. Exploration of dopant and surface passivation on optical and morphological properties of AgInS2 nanocrystals. J. Alloys Compd. 2019, 787, 972–981. [Google Scholar] [CrossRef]
- Hamanaka, Y.; Yukitoki, D.; Kuzuya, T. Structural transformation and photoluminescence modification of AgInS2 nanoparticles induced by ZnS shell formation. Appl. Phys. Express 2015, 8, 095001–095003. [Google Scholar] [CrossRef]
- Yi, M.; Zhang, C. The synthesis of two-dimensional MoS2 nanosheets with enhanced tribological properties as oil additives. RSC Adv. 2018, 8, 9564–9573. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.K.; Franco, A.M.; Gul, S.; Corrado, C.; Zhang, J.Z. Characterization of primary amine capped CdSe, ZnSe, and ZnS quantum dots by FT-IR: Determination of surface bonding interaction and identification of selective desorption. Langmuir 2011, 27, 8486–8493. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, H.; Cui, H.; Zhou, H.; Wan, H.; Chen, J. Preparation of Cu–Ni bimetallic nanoparticles surface-capped with dodecanethiol and their tribological properties as lubricant additive. Particuology 2017, 34, 89–96. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Wan, H.; Chen, J.; Zhou, H. Synthesis and Tribological Properties of Stearic Acid-Modified Anatase (TiO2) Nanoparticles. Tribol. Lett. 2010, 41, 409–416. [Google Scholar] [CrossRef]
- Xiong, X.; Kang, Y.; Yang, G.; Zhang, S.; Yu, L.; Zhang, P. Preparation and Evaluation of Tribological Properties of Cu Nanoparticles Surface Modified by Tetradecyl Hydroxamic Acid. Tribol. Lett. 2012, 46, 211–220. [Google Scholar] [CrossRef]
- Lahouij, I.; Vacher, B.; Martin, J.M.; Dassenoy, F. IF-MoS2 based lubricants: Influence of size, shape and crystal structure. Wear 2012, 296, 558–567. [Google Scholar] [CrossRef]
- Chang, J.; Wang, G.; Cheng, C.; Lin, W.; Hsu, J. Strategies for photoluminescence enhancement of AgInS2 quantum dots and their application as bioimaging probes. J. Mater. Chem. 2012, 22, 10609–10618. [Google Scholar] [CrossRef]
- Cai, T.; Liu, D.; Zhao, L.N.; Ye, M.T.; Liu, S.G. In situ tribochemical sulfurization of polyisobutylene-based molybdenum species for enhanced tribo-performance. Tribol. Int. 2019, 136, 556–569. [Google Scholar] [CrossRef]
- Wu, B.; Song, H.; Zhang, Q.; Hu, X. Controllable synthesis and friction reduction of ZnFe2O4@C microspheres with diverse core-shell architectures. Tribol. Int. 2021, 153, 106614–106630. [Google Scholar] [CrossRef]
- Han, X.L.; Li, Q.Y.; Hao, H.; Liu, C.Y.; Li, R.; Yu, F.; Lei, J.W.; Jiang, Q.Q.; Liu, Y.; Hu, J.C. Facile one-step synthesis of quaternary AgInZnS quantum dots and their applications for causing bioeffects and detecting Cu2+. RSC Adv. 2020, 10, 9172–9181. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Jiang, C.; Zhao, Q.; Wang, X.; Lou, W. Tribo-Dependent Photoluminescent Behavior of Oleylamine-Modified AgInS2 and AgInS2-ZnS Nanoparticles as Lubricant Additives. Lubricants 2023, 11, 280. https://doi.org/10.3390/lubricants11070280
Sun Y, Jiang C, Zhao Q, Wang X, Lou W. Tribo-Dependent Photoluminescent Behavior of Oleylamine-Modified AgInS2 and AgInS2-ZnS Nanoparticles as Lubricant Additives. Lubricants. 2023; 11(7):280. https://doi.org/10.3390/lubricants11070280
Chicago/Turabian StyleSun, Yiping, Cheng Jiang, Qin Zhao, Xiaobo Wang, and Wenjing Lou. 2023. "Tribo-Dependent Photoluminescent Behavior of Oleylamine-Modified AgInS2 and AgInS2-ZnS Nanoparticles as Lubricant Additives" Lubricants 11, no. 7: 280. https://doi.org/10.3390/lubricants11070280
APA StyleSun, Y., Jiang, C., Zhao, Q., Wang, X., & Lou, W. (2023). Tribo-Dependent Photoluminescent Behavior of Oleylamine-Modified AgInS2 and AgInS2-ZnS Nanoparticles as Lubricant Additives. Lubricants, 11(7), 280. https://doi.org/10.3390/lubricants11070280




