Abstract
Graphene carbon materials show good tribological properties due to their unique layered structures. In this work, the tribological properties of graphene (GN) and fluorinated graphene (FGN) were studied in two kinds of synthetic hydrocarbon base stocks at different working conditions. Firstly, the structures of GN and FGN were characterized comparatively using FT-IR, Raman, XRD, and TGA. Secondly, the tribological properties of GN and FGN as the lubrication additives both in PAO6 and CTL6 were studied on a four-ball tester. Finally, the surfaces of friction counterparts, before and after tribological tests, were analyzed to disclose the lubrication mechanism using UV, micro-Raman, and EDS. The results show that GN and FGN can be stably dispersed in the selected synthetic hydrocarbon base stocks with 1 wt.% T161 as the dispersant, and the optimal addition of graphene additive is 100 ppm, which shows better friction reducing and anti-wear properties. GN and FGN also show better tribological performance at a higher load (not less than 392 N), and their compatibility with PAO6 is better. The worn surface analysis shows that the graphene additive participates in the lubrication film formation during friction by frictional chemical reaction with friction counterparts, which could improve the stability and tribological performance, resulting in an increased application temperature of synthetic hydrocarbon base stock by at least 10 °C.
1. Introduction
Global industrial synthetic and semisynthetic use continues to grow, driven by modern equipment, advanced technology, and government regulations. Synthetics are now required in many applications due to severe operating conditions or corrosive environments. Synthetic lubricants—formulated products consisting of synthetic base stocks plus additives—have physical and chemical properties that are generally superior to those of conventional mineral oil-based lubricants, including good thermal and oxidative stability [1], low-temperature fluidity [2], low volatility, high viscosity indexes, and fire resistance. As a result, synlubes are the lubricants of choice in applications with especially demanding performance requirements [3]. According to his Markit, the global market for synthetic base oils was 1.3 million metric tons in 2020, and polyalphaolefins (PAOs) dominated the market with a share of 47% (volume), followed by esters (28%), and polyalkylene glycols (PAG, 13%). Therefore, the compatibility of synthetic base stocks, especially synthetic hydrocarbons and additives, has become the focus of researchers in recent years [4,5].
In the past few decades, various new two-dimensional materials, including MXenes [6,7], MoS2 [8,9], WS2 [10], as well as graphene [11], have become a hot topic in the field of friction [12]. Among them, graphene has been widely considered by researchers due to its low surface energy and unique tribological properties [13,14,15] caused by easy-sliding multi-layer structures [16]. Omrani et al. [17] modified graphene using oleic acid and found that 0.02 wt.% oleic acid-modified graphene could be well-dispersed in PAO9 and improve the friction-reducing and anti-wear properties effectively. Yin et al. [18] prepared a boronized functional layer on the surface of bearing steel through an electrochemical boronization process, which improved the adsorption of GO on the bearing steel. By adding 1 wt.% GO nanosheets, an ultra-low friction state with a coefficient of friction (COF) of 0.03 was achieved. Ismail et al. [19] achieved a high oil-soluble graphene oxide that was modified through the copper-catalyzed cycloaddition, which reacted with an alkyne, and applied the prepared modified graphene oxide as an additive; the results showed that the modified graphene oxide could significantly improve the friction reducing and anti-wear performance of the base oil when the addition was 0.01 wt.%. At present, the dispersion stability of nanoparticle additives as liquid additives is particularly important. Shi et al. [20] used molecular dynamics (MD) simulation and experiments to study the dispersion behavior and mechanism of the dispersant polyisobutylene succinimide polyamine (PIB) concentration on soot aggregates under different pressures and temperatures and established static, limiting, and shear models.
Fluorinated graphene (FGN) is a derivative of graphene wherein its hydrogen atoms are partially or completely replaced by fluorine atoms [21]. Therefore, FGN not only inherits the sheet structure of graphene but also introduces fluorine atoms with a larger radius, which can weaken the interactions between interlayers and enhance the lubricating performance due to an easier shear capability [11]. Researchers found that FGN exhibited better tribological performance compared to pristine graphene at the macroscale [22]. To achieve better tribological properties, Fan et al. [23] prepared fluorinated graphene containing different F concentrations through direct fluorination with F2, which was added to liquid paraffin oil as an additive. The results show that fluorination with a C/F ratio of 1.0 could reduce friction and wear by 50.4% and 90.9%, respectively. They also [24] prepared fluorinated graphene oxide with a relatively low F content under mild temperature conditions, which could lead to a 47% and 31% lower wear rate compared to that with the lubrication of pure water and GO water suspension when used as the additive, respectively. With the assistance of microwave heating, Ma et al. [25] fulfiled the substitution of FGN with NaOH at a quite mild reaction conditions to give a high fluorine content HOFG with excellent water dispersibility. The study on the tribological behaviors of HOFGs showed that the extreme-pressure and wear-resistance properties of pure water could be improved to over 240% and up to 30%, respectively.
However, studies on the tribological properties of graphene additives at present mostly focus on a single oil or water system, and the performance of graphene additives in different base stocks with the same viscosity grade has not been explored. Meanwhile, the conditions of tribological tests are mostly carried out at room temperature, and the adaptability of graphene additives in different working conditions is not studied in detail. Therefore, two synthetic hydrocarbon base stocks, that is, PAO6 and CTL6, with the same viscosity grade were selected, and the high molecular weight polysuccinimide T161 was used as a dispersant to study the tribological performance of GN and FGN under different test conditions. The study of graphene additives in various synthetic hydrocarbon base stocks under different tribological conditions is helpful in establishing the compatibility law of graphene additives and synthetic base stocks and disclosing its lubrication mechanism in synthetic hydrocarbon base stocks, which will provide theoretical guidance for the subsequent formulation of research based on graphene additives.
2. Materials and Methods
2.1. Materials
Graphene (GN) and fluorinated graphene (FGN) were purchased from Hubei Zhuoxi Fluorochemical Co., Ltd. (Yingcheng, China), and high molecular weight poly-(isobutylene succinimide) (T161, ashless dispersant) was purchased from Wuxi South Petroleum Additives Co., Ltd. (Wuxi, China). PAO6 (Durasyn 166, Ineos, London, UK), as well as CTL6 (Coal to oil, Lu’an Chemical Group, Changzhi, China), were commercially obtained and used as the synthetic hydrocarbon base stock.
2.2. Preparation of Oils
In general, 0~5 wt.% of the dispersant T161 was first added to the selected synthetic hydrocarbon base stock (PAO6 or CTL6) and stirred at 60 °C for 1 h to give the corresponding base oil. Further, the graphene additive (GN or FGN) was subsequently added to the prepared base oil with an addition of 0~200 ppm in mass percentage, and the mixture was ultrasonically dispersed for 10 min in a 20 °C water bath.
2.3. Characterization
The contents of C and H in GN and FGN were determined by an automatic element analyzer—Vario Micro Cube (Elementar Analysensysteme GmbH, Langenselbold, Germany)—according to the general rules of analytical methods for the element analyzer JY/T 017-2020. Additionally, the F content in FGN was determined by the oxygen bottle combustion-chemical titration method using a 25 mL Titrette Automatic Liquid Microtitrator (BRAND GMBH + CO KG, Wertheim, Germany) and a micro-analysis balance with a minimum fraction value of 1 ug, according to the general rules in the Chinese Pharmacopoeia (2020 edition)—oxygen bottle combustion method 0703. The detailed steps are as follows: FGN was accurately weighed and wrapped into the filter paper, which was decomposed using the oxygen bottle method, and 10 mL of deionized water was applied as the absorption solution.
The micro-morphologies of GN and FGN were tested using scanning electron microscopy with an accelerating voltage of 5~10 kV (SEM, JEOL JSM-6390LV). The specific surface area was achieved by the Brunauer-Emmett-Teller (BET) method, and the measurement was performed according to the physical adsorption of N2 via a fully automatic surface area and porosity analyzer (ASAP 2020 HD88, Micromeritics, Norcross, GA, USA).
Fourier transform infrared spectroscopy (FT-IR, Thermo Fisher Nicolet iN 10) was recorded by scanning from 500 to 4000 cm−1 in the Attenuated Total Reflectance (ATR) configuration. Raman spectroscopy (Thermo DXR) was detected with an excitation wavelength of 532 nm. X-ray diffraction (XRD, PANalytical XPert PRO) was carried out with Cu Kα radiation (λ = 0.154 nm), and the diffraction data were recorded as 2θ from 5~60° at a speed of 4° min−1. Thermo gravimetric analysis (TGA, TA Q500) was performed under an N2 atmosphere with a flow rate of 60 mL/min and a heating rate of 10 °C/min from 25 to 950 °C. Ultraviolet-visible spectroscopy (UV-Vis, Shimadzu UV-2700) was applied to analyze the chemical structure and composition changes of oils between, before, and after tribological tests. An energy dispersive spectrometer (EDS, OXFORD x-act) was used to analyze the components of the tribofilm after tribological tests.
2.4. Tribological Tests
The tribological behaviors of GN and FGN in the prepared oils were evaluated on a Tenkey MS-10A four-ball tester (Xiamen TenKey Automation Co., Ltd., Xiamen, China). The tribological tests were operated at 1200 rpm under different loads ranging from 96 to 490 N, the test duration was 30 min at varied temperatures (54~100 °C), the balls used were made of GCr15 bearing steel with a diameter of 12.7 mm, and the upper limit of COF was set to be 0.4. All tribological tests were performed at least three times to ensure repeatability.
3. Results and Discussion
3.1. Characterization of Graphene Additives
The content analyses of C, H, and F in GN and FGN were carried out by elemental analysis, as shown in Figure 1, and the O content in GN was obtained by subtraction of the C and H content from 100%. For GN, the element content of C, O, and H was 94.13%, 5.46%, and 0.41%, respectively, and the atomic number ratio of C, O, and H was estimated to be 19.13:0.83:1, indicating that GN contains a small amount of oxygen, which is likely to have been reduced by graphene oxide. The element content of C, F, and H was 36.29%, 63.36%, and 0.35% for FGN, and the atomic number ratio of C, F, and H was estimated to be 8.64:9.53:1; that is, the F/C ratio in FGN was as high as 1.1:1, indicating that FGN has a high degree of fluorination.
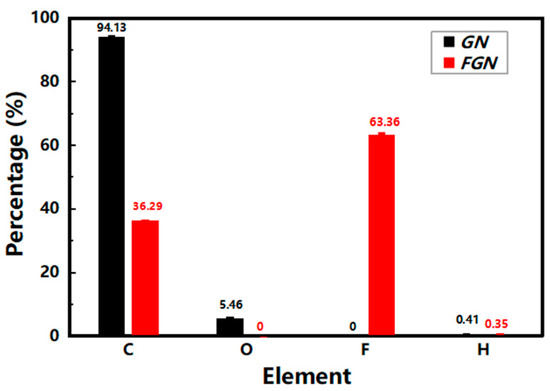
Figure 1.
The elemental analysis of GN and FGN.
The micro-morphologies of GN and FGN were conducted by SEM, and the results are displayed in Figure 2. Both GN and FGN showed coiled-layered structures; however, the layer spacing in GN was smaller than that in FGN, which can be seen in the circled portion in Figure 2a,c. At the same time, the lamellar folds in FGN were weaker than that in GN (see Figure 2b,d); this may be because the introduction of fluorine weakened the π-π stacking between graphene layers.
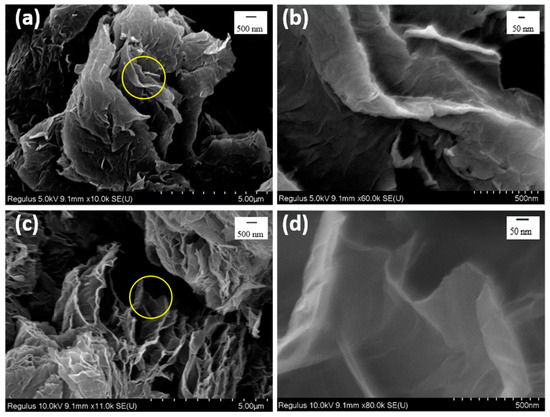
Figure 2.
SEM images: (a) 5 μm and (b) 500 nm of GN; (c) 5 μm and (d) 500 nm of FGN.
The specific surface area (SSA) and pore-size characterization of GN and FGN were further performed by N2 adsorption-desorption experiments, and the results are shown in Figure 3. Both GN and FGN are type IV isotherms according to the classification of the International Union of Pure and Applied Chemistry (IUPAC) (Figure 3a), and the H3 type hysteresis loops were obvious at high relative pressure, indicating the existence of mesoporous pores [26]. Meanwhile, the BET SSA of GN was higher than that of FGN, which was 526.1 m2/g and 313.2 m2/g, respectively, indicating that GN has more pores than FGN. The pore size in GN was bigger than that in FGN through the pore-size distribution calculated by DFT in Figure 3b. The fluorination process could cause the collapse of the porous structure, and the introduction of the F atom could significantly increase the weight per unit volume, so the formation of a C-F bond could result in the reduction of SSA and pore size [27].
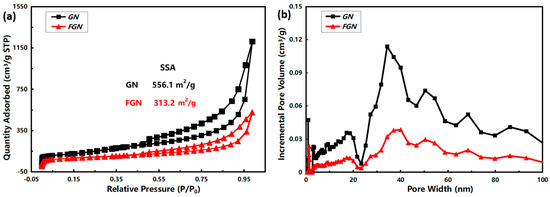
Figure 3.
N2 adsorption-desorption analysis of GN and FGN: (a) isotherm curves; (b) pore size distribution curves.
The FT-IR spectra of GN and FGN are shown in Figure 4a. The strong absorption peaks at 1213 and 1319 cm−1 in FGN are assigned to the stretching vibrations of covalent C-F bonds on the nanosheet and at the edge of the nanosheet for high F/C ratio, respectively [28,29], which is consistent with the result of the elemental analysis in Figure 1. The wide band at 3400 cm−1 in GN and FGN can be assigned to the O-H stretching vibration due to moisture, and the hydrogen bonding between F and H2O causes the O-H stretching vibration to be more significant in FGN [30]. Raman is widely used to characterize carbon materials, where the D peak at ~1340 cm−1 represents a defect of the carbon skeleton caused by Csp3, and the G peak at ~1580 cm−1 represents the carbon skeleton structure of Csp2, corresponding to the stretching vibration of C=C [31]. The calculated ID/IG can refer to the content ratio of Csp3/Csp2, which could estimate the degree of the defects and distinguish the disordered or ordered structure carbons [32]. The Raman results of GN and FGN are shown in Figure 4b. The ID/IG of FGN (1.25) is significantly higher than that of GN (1.01), indicating that the defects are increased after fluoridation in FGN. In addition, the G peak of FGN is downshifted, indicating that the defect type for FGN is mainly a point defect [33]. The layered structures of GN and FGN were further investigated by XRD, as shown in Figure 4c. FGN shows an obvious broad peak at 2θ = 12.3°, which is attributed to the diffraction peak of the crystal surface of the hexagonal crystal system (001) with high fluoride content, indicating that the regularity of FGN is high. The broad diffraction peak associated with the (001) reflection also indicates a high exfoliation degree for FGN. Based on Bragg’s law, the reduced value of 2θ indicates a larger interplanar distance for FGN [34]; the interplanar distance is calculated to be 0.72 nm, which is two times that of GN (0.35 nm). FGN has two more diffraction peaks located at 2θ = 26.2° and 42.7°, which originated from the (002) and (100) crystalline planes of graphite. The peak at 26.2° corresponds to the (002) reflection of graphite, indicating the existence of Csp3 due to the formation of C-F bonds in the FGN interlayers. The peak at 42.7° is attributed to the (100) reflection and is associated with the disorder induced by the fluorination in fully fluorinated graphene [35]. These data clearly illustrate that fluorination would give rise to a large interplanar distance due to the electrostatic repulsive force originating from the fluorine atom [36], which means an easier shear capability and the enhanced lubricant performance of FGN [11].
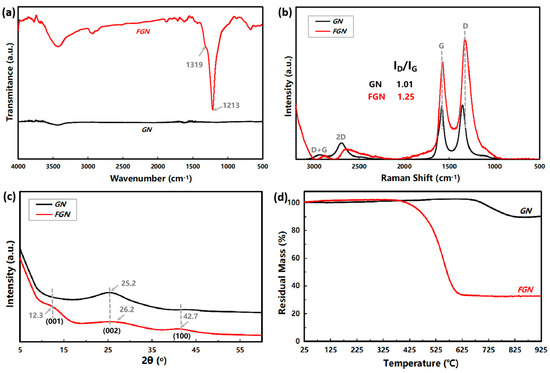
Figure 4.
Characterizations of GN and FGN: (a) FT-IR; (b) Raman; (c) XRD; and (d) TGA.
The thermal stabilities of GN and FGN are displayed using TGA, as shown in Figure 4d and Table 1. The initial weight loss temperature (Ti) and the maximum weight loss temperature (Tmax) of GN were 547 °C and 721 °C, respectively, which is much higher than that of FGN (Ti and Tmax was 347 °C and 551 °C, respectively). Meanwhile, the weight loss at Tmax of GN and FGN were 10.5% and 68.5%, respectively. For FGN, it appears that a sharp weight loss from 350 to 600 °C was caused by the defluorination process [37]; therefore, GN’s thermal stability is much better.

Table 1.
TGA analysis of GN and FGN.
3.2. Stability Monitoring of the Prepared Oils
According to the study reported by Kong et al. [4], high molecular weight poly-(isobutylene succinimide) could improve the dispersibility of graphene in PAO4 effectively. T161 is one high molecular weight poly-(isobutylene succinimide), and the images of the GN or FGN dispersion oils with or without T161 are shown in Figure 5. The settlement of GN could be observed in the dispersion oils, both in PAO6 and CTL6 without T161, after 1 day, and the settlement was almost complete after 7 days (in Figure 5a,e). Meanwhile, the dispersion oils with 1 wt.% T161, shown in Figure 5c,g, kept good dispersion after 7 days; that is, T161 can effectively improve the dispersion stability of GN in the synthetic hydrocarbon base stocks. For FGN, whether the oil was with or without T161, no significant FGN settlement was observed over time (Figure 5b,d,f,h). Combined with the characterization results of Raman and XRD, the introduction of F atoms in FGN could increase its interlayer spacing, making it easier to be exfoliated and stably dispersed in oils. However, lacking recognition of the gray-white FGN in dispersion oils may also cause settlement that was not observed.

Figure 5.
Images of dispersion oils with 100 ppm of graphene additives over time: (a) GN, (b) FGN, (c) GN-1%T161, and (d) FGN-1%T161 in PAO6; (e) GN, (f) FGN, (g) GN-1%T161, and (h) FGN-1%T161 in CTL6.
3.3. Tribological Performance
3.3.1. Tribological Performance of Graphene Additives at Different Additions
In order to select a suitable addition of graphene additive, GN dispersion oils at various concentrations of 0 ppm, 50 ppm, 100 ppm, 150 ppm, and 200 ppm in mass percentage were prepared separately using the base oil PAO6 containing 1 wt.% T161. The tribological behaviors of the prepared oils were compared with the base stock of PAO6, and the results are shown in Figure 6. When the test temperature was 85 °C, and the load was 392 N, PAO6 and 1%T161-PAO6 appeared as spikes in friction profiles (Figure 6b), and the wear scars on the steel balls were irregular (Figure 6d). The spikes disappeared when adding different concentrations of GN, which would provide effective protection for friction counterparts. When the GN concentration was 100 ppm, the friction profile was more stable, and the average COF (ave. COF) and wear scar diameter (ave. WSD) were smaller (Figure 6c,d); that is, lubrication performance was optimal at this time. Therefore, all subsequent additions of graphene additives in this study used 100 ppm.
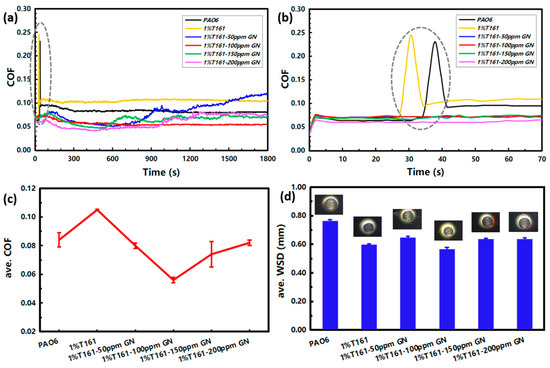
Figure 6.
Tribological behaviors of dispersion oils with different additions of GN in PAO6: (a) the full (0~1800 s) and (b) locally amplified (0~70 s) friction profiles; (c) ave. COF; (d) ave. WSD and wear scars. All tests were run at 85 °C with a load of 392 N.
3.3.2. Tribological Performance of Graphene Additives under Different Test Loads
The tribological performance of graphene additives with increasing loads is displayed in Figure 7, from which we can see that all the dispersion oils in PAO6, as well as CTL6, cannot fulfill the tribological tests at 490 N. This means that their COFs exceed the set upper limit of 0.4. Apart from 1%T161-100 ppm FGN in CTL6, the COF of the base stock, 1%T161, 1%T161-100 ppm GN, and 1%T161-100 ppm FGN, shows a trend of first reducing and then increasing with an increasing load (Figure 7a,b), which is in line with the Stribeck curve [38]; that is, the thickness of the oil film decreases with the increasing load, and the transition from mixed lubrication to boundary lubrication occurs. According to the wear mechanism reported by J. F. Archard in 1953 [39], the wear is proportional to the positive pressure, so the higher the load, the more serious the wear (see Figure 7c,d). In PAO6, T161 increased the friction, but it also stabilized the oil film against wear (Figure 7a,c). When the test load was higher, such as at 392 N, the addition of GN and FGN could effectively reduce friction and wear. However, when further increasing the load to 490 N, none of the samples could operate stably. In CTL6, GN and FGN showed good friction-reducing performance at low test loads (<392 N) and stabilized the oil film at high loads (490 N), fulfilling the friction test that base stock cannot (Figure 7b,d).
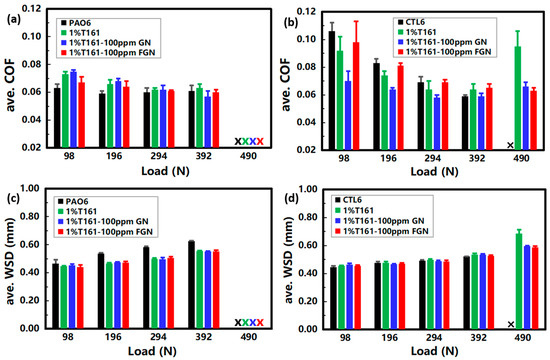
Figure 7.
Tribological performance of dispersion oils when the test load varied from 98~490 N: (a) ave. COF and (c) ave. WSD in PAO6; (b) ave. COF and (d) ave. WSD in CTL6. The tests were run at 54 °C, where “x” represents that the tribological test cannot be successfully completed.
3.3.3. Tribological Performance of Graphene Additives at Different Test Temperatures
When the test temperature rises, the viscosity of base stocks will decrease to different degrees, and the thickness and stability of the oil films are the key factors in determining lubrication performance. In PAO6, the addition of T161 at 75~80 °C could stabilize the oil film and reduce the COF and WSD (Figure 8a,c); however, when the temperature rose to 85 °C or higher, T161 also increased friction (see Figure 8a). Meanwhile, GN or FGN could stabilize the oil film in time, which could relieve friction and wear effectively. In CTL6, the application of T161 at 75~85 °C could stabilize the oil film (Figure 8b,d), but it increased friction at 90 °C or higher temperatures (Figure 8b). At this time, GN or FGN could play an important role in friction reduction and anti-wear, and FGN showed better tribological performance. Overall, GN or FGN can increase the application temperature of PAO6 and CTL6 by at least 10 °C at 1200 rpm with a load of 392 N.
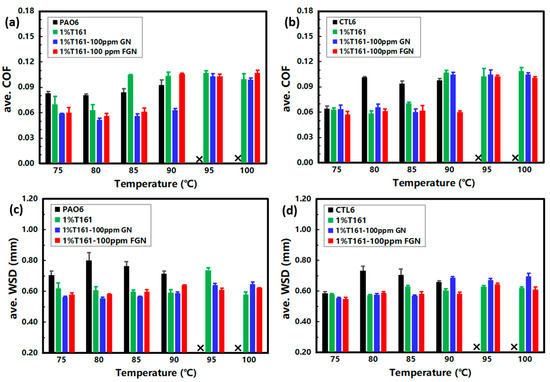
Figure 8.
Tribological performance of dispersion oils when the test temperature varied from 75~100 °C: (a) ave. COF and (c) ave. WSD in PAO6; (b) ave. COF and (d) ave. WSD in CTL6. The tests were run with a load of 392 N, where “x” represents that the tribological test cannot be successfully completed.
3.3.4. Tribological Performances of Graphene Additives in Different Base Stocks
When studying the influence of test load and temperature on the tribological performance of graphene additives, it was found that the performance in different base stocks was quite different, and the further friction-reducing and anti-wear properties in PAO6 and CTL6 were compared, as shown in Figure 9. From the figure, it can be seen that CTL6 shows a lower ave. COF and WSD compared to PAO6, meaning CTL6 has better friction-reducing and anti-wear properties. In PAO6, the ave. COF from low to high is 1%T161-100 ppm FGN~1%T161-100 ppm GN < 1%T161 < PAO6; namely, FGN and GN have a comparable friction-reducing property in PAO6. However, the order of ave. COF in CTL6 is 1%T161-100 ppm FGN < 1%T161-100 ppm GN~1%T161~CTL6; that is, the friction-reducing performance of FGN is better than that of GN in CTL6. In PAO6, ave. WSD from small to large is 1%T161-100 ppm GN~1%T161-100 ppm FGN < 1%T161 < PAO6; namely, both GN and FGN have comparable anti-wear performance in PAO6. However, the order of ave. WSD in CTL6 is 1%T161-100 ppm FGN~1%T161-100 ppm GN < 1%T161~CTL6; that is, the anti-wear performance of FGN and GN in CTL6 is also comparable. Overall, FGN shows a better tribological performance in both PAO6 and CTL6, combined with the XRD characterization in Section 3.1, which may be attributed to the easier shear capability after fluorination.
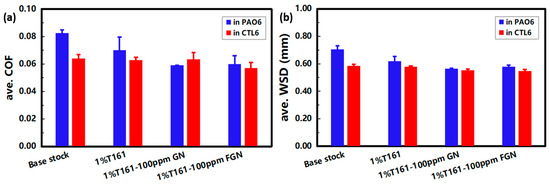
Figure 9.
Comparison of (a) friction reducing and (b) anti-wear performance of graphene additives in PAO6 and CTL6. The tests were run at 75 °C with a load of 392 N.
3.3.5. The Lubrication Mechanism
To better understand the lubricating mechanism of the graphene additive in synthetic base stock, UV-Vis, micro-Raman, and EDS were applied to analyze the friction counterparts of 1%T161-100 ppm GN in PAO6 before and after the tribological test, which was run at 85 °C with a load of 392 N, and the results are displayed in Figure 10 and Figure 11.
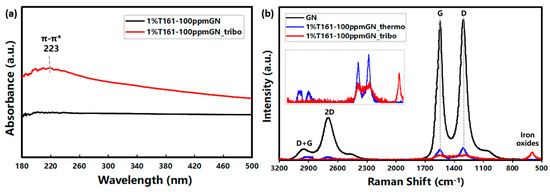
Figure 10.
(a) UV-Vis spectra and (b) micro-Raman analysis of the friction counterparts.
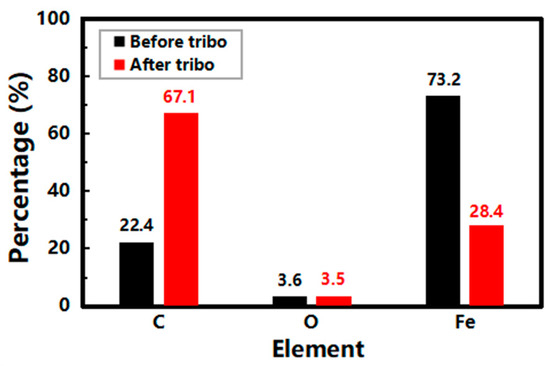
Figure 11.
EDS analysis of the friction counterpart before and after the tribological tests.
Compared to the UV spectra before and after the tribological test in Figure 10a, there is a wide peak at 223 nm that can be assigned to the absorption of the π-π* transition on the wear scar, which is probably caused by the π-π* transition of C=C in the GN skeleton, indicating that the tribofilm contains the GN skeleton. The lubricating film during friction may be an adsorption film formed by simple physical adsorption or a reaction film generated by the frictional chemical reaction. In order to distinguish the composition of the lubricating film, a steel ball was soaked the oil and tested in an 85 °C oven for 0.5 h to form the thermal adsorption film. The results are shown in Figure 10b; 1%T161-100 ppmGN_thermo and 1%T161-100 ppmGN_tribo represent the thermal adsorption and the tribofilms after the tribological test, respectively. The weak Raman signal intensity of both thermal adsorption and tribofilms compared to GN is due to the small addition of GN (only 100 ppm). The Raman response of the thermal adsorption film is nearly the same as GN; that is, the D peak at ~1340 cm−1, G peak at ~1580 cm−1, 2D peak at ~2720 cm−1, and D + G peak at ~2940 cm−1. However, besides the D peak, G peak, and D + G peak, the peak that appeared at ~600 cm−1 can be assigned to iron oxides, indicating that the lubrication film during friction is formed by the frictional chemical reaction between GN and friction counterparts, which is different from the thermal adsorption film.
In Figure 11, the surface of the friction counterpart before tribological tests is composed of Fe (73.2%) and C (22.4%). However, the content of Fe and C became 28.4% and 67.1% after tribological tests, respectively. The C content increased more than three times, and the Fe content decreased by nearly 90%, while the O content was essentially unchanged. This means that the tribofilm is mainly composed of C. Combining the analysis of the UV and micro-Raman results, GN is involved in the formation of tribofilms, which improves the tribological performance of the base stock.
4. Conclusions
The study of graphene additives in various synthetic hydrocarbon base stocks at different tribological conditions is helpful in establishing compatibility law. In this work, the tribological properties of GN and FGN were studied in PAO6 and CTL6 under different working conditions. The results show that GN and FGN can be stably dispersed in the selected synthetic hydrocarbon base stocks with 1 wt.% T161 as the dispersant, and the optimal addition of the graphene additive is 100 ppm, which shows better friction-reducing and anti-wear properties. GN and FGN show better tribological performance at a higher load (not less than 392 N), and their compatibility with PAO6 is better. The worn surface analysis discloses that the graphene additive participates in the formation of lubrication film during friction by a frictional chemical reaction with friction counterparts, which could improve the stability and tribological performance, resulting in an increased application temperature of synthetic hydrocarbon base stock by at least 10 °C. These results provide theoretical guidance for the formulation of research based on graphene additives.
Author Contributions
Conceptualization, H.Y., X.S. and Y.T.; methodology, H.Y. and X.S.; validation, H.Y.; formal analysis, H.Y. and G.D.; investigation, G.D.; data curation, H.Y. and G.D.; writing—original draft preparation, H.Y. and G.D.; writing—review and editing, H.Y. and G.D.; supervision, H.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ling Chuang Research Project of China National Nuclear Corporation that was granted by CAS Key Laboratory of Nuclear Materials and Safety Assessment of the Chinese Academy of Sciences.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could influence the work reported in this paper.
References
- Pournorouz, Z.; Mostafavi, A.; Pinto, A.; Bokka, A.; Jeon, J.; Shin, D. Enhanced thermophysical properties via PAO superstructure. Nanoscale Res. Lett. 2017, 12, 29. [Google Scholar] [CrossRef] [PubMed]
- Nifant’ev, I.; Bagrov, V.; Vinogradov, A.; Vinogradov, A.; Ilyin, S.; Sevostyanova, N.; Batashev, S.; Ivchenko, P. Methylenealkane-Based Low-Viscosity Ester Oils: Synthesis and Outlook. Lubricants 2020, 8, 50. [Google Scholar] [CrossRef]
- Dong, S.Q.; Mi, P.K.; Xu, S.; Zhang, J.; Zhao, R.D. Preparation and Characterization of Single-Component Poly-α-olefin Oil Base Stocks. Energy Fuels 2019, 33, 9796–9804. [Google Scholar] [CrossRef]
- Kong, S.; Wang, J.; Hu, W.; Li, J. Effects of Thickness and Particle Size on Tribological Properties of Graphene as Lubricant Additive. Tribol. Lett. 2020, 68, 112. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, C.; Dong, R.; Shi, Y.; Wang, Y.; Bai, Y.; Zhang, J.; Cai, M.; Zhou, F.; Novel, N. P-containing oil-soluble ionic liquids with excellent tribological and anticorrosion performance. Tribol. Int. 2018, 132, 118–129. [Google Scholar] [CrossRef]
- Pogorielov, M.; Smyrnova, K.; Kyrylenko, S.; Gogotsi, O.; Zahorodna, V.; Pogrebnjak, A. MXenes—A new class of two-dimensional materials: Structure, properties and potential applications. Nanomaterials 2021, 11, 3412. [Google Scholar] [CrossRef]
- Rakhadilov, B.K.; Maksakova, O.V.; Buitkenov, D.B.; Kylyshkanov, M.K.; Pogrebnjak, A.D.; Antypenko, V.P.; Konoplianchenko, Y.V. Structural-phase and tribo-corrosion properties of composite Ti3SiC2/TiC MAX-phase coatings: An experimental approach to strengthening by thermal annealing. Appl. Phys. A 2022, 128, 145. [Google Scholar] [CrossRef]
- Liu, T.; Qin, J.; Wang, J.; Li, J. On the Tribological Properties of RGO–MoS2 Composites Surface Modified by Oleic Acid. Tribol. Lett. 2022, 70, 14. [Google Scholar] [CrossRef]
- Xu, Z.; Lou, W.; Wu, X.; Wang, X.; Hao, J. Investigating the tribological behavior of PEGylated MoS2 nanocomposites as additives in polyalkylene glycol at elevated temperature. RSC Adv. 2017, 7, 53346–53354. [Google Scholar] [CrossRef]
- Ratoi, M.; Niste, V.B.; Zekonyte, J. WS2 nanoparticles-potential replacement for ZDDP and friction modifier additives. RSC Adv. 2014, 4, 21238–21245. [Google Scholar] [CrossRef]
- Berman, D.; Erdemir, A.; Sumant, A.V. Graphene: A new emerging lubricant. Mater. Today 2014, 17, 31–42. [Google Scholar] [CrossRef]
- Omrani, E.; Menezes, P.L.; Rohatgi, P.K. Effect of Micro-and Nano-Sized Carbonous Solid Lubricants as Oil Additives in Nanofluid on Tribological Properties. Lubricants 2019, 7, 25. [Google Scholar] [CrossRef]
- Pape, F.; Poll, G. Investigations on Graphene Platelets as Dry Lubricant and as Grease Additive for Sliding Contacts and Rolling Bearing Application. Lubricants 2019, 8, 3. [Google Scholar] [CrossRef]
- Wu, Q.; Li, H.; Wu, L.; Bo, Z.; Wang, C.; Cheng, L.; Wang, C.; Peng, C.; Li, C.; Hu, X.; et al. Synergistic Lubrication and Antioxidation Efficacies of Graphene Oxide and Fullerenol as Biological Lubricant Additives for Artificial Joints. Lubricants 2022, 11, 11. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, S.; Shi, Q.; Ge, X.; Wang, W. Graphene-family lubricant additives: Recent developments and future perspectives. Lubricants 2022, 10, 215. [Google Scholar] [CrossRef]
- Kim, K.-S.; Lee, H.-J.; Lee, C.; Lee, S.-K.; Jang, H.; Ahn, J.-H.; Kim, J.-H.; Lee, H.-J. Chemical Vapor Deposition-Grown Graphene: The Thinnest Solid Lubricant. ACS Nano 2011, 5, 5107–5114. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, M.; Zhu, H.; Yu, T.; Wang, K.; Wei, J.; Ji, F.; Li, X.; Li, Z.; Zhang, P.; et al. Tribological properties of oleic acid-modified graphene as lubricant oil additives. J. Phys. D Appl. Phys. 2011, 44, 225303. [Google Scholar] [CrossRef]
- Yin, S.; Wu, H.; Yi, X.; Huang, Z.; Ye, C.; Li, P.; Zhang, Y.; Shi, J.; Hua, K.; Wang, H. Enhanced graphene oxide adhesion on steel surface through boronizing functionalization treatment: Toward the robust ultralow friction. Carbon 2023, 206, 201–210. [Google Scholar] [CrossRef]
- Ismail, N.A.; Bagheri, S. Highly oil-dispersed functionalized reduced graphene oxide nanosheets as lube oil friction modifier. Mater. Sci. Eng. B 2017, 222, 34–42. [Google Scholar] [CrossRef]
- Shi, J.; Yi, X.; Wang, J.; Jin, G.; Lu, Y.; Wu, H.; Fan, X. Carbonaceous soot dispersion characteristic and mechanism in lubricant with effect of dispersants by molecular dynamics simulation and experimental studies. Carbon 2022, 200, 253–263. [Google Scholar] [CrossRef]
- Nair, R.R.; Ren, W.; Jalil, R.; Riaz, I.; Kravets, V.G.; Britnell, L.; Blake, P.; Schedin, F.; Mayorov, A.S.; Yuan, S.; et al. Fluorographene: A two-dimensional counterpart of Teflon. Small 2010, 6, 2877–2884. [Google Scholar] [CrossRef]
- Matsumura, K.; Chiashi, S.; Maruyama, S.; Choi, J. Macroscale tribological properties of fluorinated graphene. Appl. Surf. Sci. 2018, 432, 190–195. [Google Scholar] [CrossRef]
- Fan, K.; Chen, X.; Wang, X.; Liu, X.; Liu, Y.; Lai, W.; Liu, X. Toward excellent tribological performance as oil-based lubricant additive: Particular tribological behavior of fluorinated graphene. ACS Appl. Mater. Interfaces 2018, 10, 28828–28838. [Google Scholar] [CrossRef]
- Fan, K.; Liu, J.; Wang, X.; Liu, Y.; Lai, W.; Gao, S.; Qin, J.; Liu, X. Towards enhanced tribological performance as water-based lubricant additive: Selective fluorination of graphene oxide at mild temperature. J. Colloid Interface Sci. 2018, 531, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Li, Z.; Jia, W.; Hou, K.; Wang, J.; Yang, S. Microwave-assisted synthesis of hydroxyl modified fluorinated graphene with high fluorine content and its high load-bearing capacity as water lubricant additive for ceramic/steel contact. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125931. [Google Scholar] [CrossRef]
- Meloni, D.; Monaci, R.; Solinas, V.; Auroux, A.; Dumitriu, E. Characterization of the active sites in mixed oxides derived from LDH precursors by physico-chemical and catalytic techniques. Appl. Catal. A Gen. 2008, 350, 86–95. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Xu, D.; Liu, Y.; Lai, W.; Liu, X. Activation effect of porous structure on fluorination of graphene based materials with large specific surface area at mild condition. Carbon 2017, 124, 288–295. [Google Scholar] [CrossRef]
- Boopathi, S.; Narayanan, T.N.; Kumar, S.S. Improved heterogeneous electron transfer kinetics of fluorinated graphene derivatives. Nanoscale 2014, 6, 10140–10146. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.-H.; Hao, G.-P.; Sun, Q. Can Carbon Spheres Be Created through the Stober Method? Angew. Chem.-Int. Ed. 2011, 50, 9023–9025. [Google Scholar] [CrossRef]
- Bon, S.B.; Valentini, L.; Verdejo, R.; Garcia Fierro, J.L.; Peponi, L.; Lopez-Manchado, M.A.; Kenny, J.M. Plasma Fluorination of Chemically Derived Graphene Sheets and Subsequent Modification with Butylamine. Chem. Mater. 2009, 21, 3433–3438. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman Spectrum of Graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Yang, H.; Li, J.; Zeng, X. Correlation between Molecular Structure and Interfacial Properties of Edge or Basal Plane Modified Graphene Oxide. ACS Appl. Nano Mater. 2019, 1, 2763–2773. [Google Scholar] [CrossRef]
- Fan, K.; Fu, J.; Liu, X.; Liu, Y.; Lai, W.; Liu, X.; Wang, X. Dependence of the fluorination intercalation of graphene toward high-quality fluorinated graphene formation. Chem. Sci. 2019, 10, 5546–5555. [Google Scholar] [CrossRef]
- Ci, X.; Zhao, W.; Luo, J.; Wu, Y.; Ge, T.; Xue, Q.; Gao, X.; Fang, Z. How the fluorographene replaced graphene as nanoadditive for improving tribological performances of GTL-8 based lubricant oil. Friction 2020, 9, 488–501. [Google Scholar] [CrossRef]
- Quan, Y.; Liu, Q.; Li, K.; Zhang, H.; Yang, Y.; Zhang, J. Simultaneous fluorination and purification of natural block coaly graphite into fluorinated graphene with tunable fluorination degree. Mater. Today Commun. 2022, 32, 104130. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, L.; Wu, X.; Hu, W. Experimental Sensing and Density Functional Theory Study of H2S and SOF2 Adsorption on Au-Modified Graphene. Adv. Sci. 2015, 2, 1500101. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Feng, Y.; Li, Y.; Qin, C.; Zhang, Q.; Feng, W. Solvothermally exfoliated fluorographene for high-performance lithium primary batteries. Nanoscale 2014, 6, 2634–2641. [Google Scholar] [CrossRef]
- Lu, X.; Khonsari, M.; Gelinck, E. The Stribeck curve: Experimental results and theoretical prediction. J. Tribol. 2006, 128, 789–794. [Google Scholar] [CrossRef]
- Archard, J.F. Contact and Rubbing of Flat Surfaces. J. Appl. Phys. 1953, 24, 981–988. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).