The Impact of Ammonia Fuel on Marine Engine Lubrication: An Artificial Lubricant Ageing Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Engine Oil
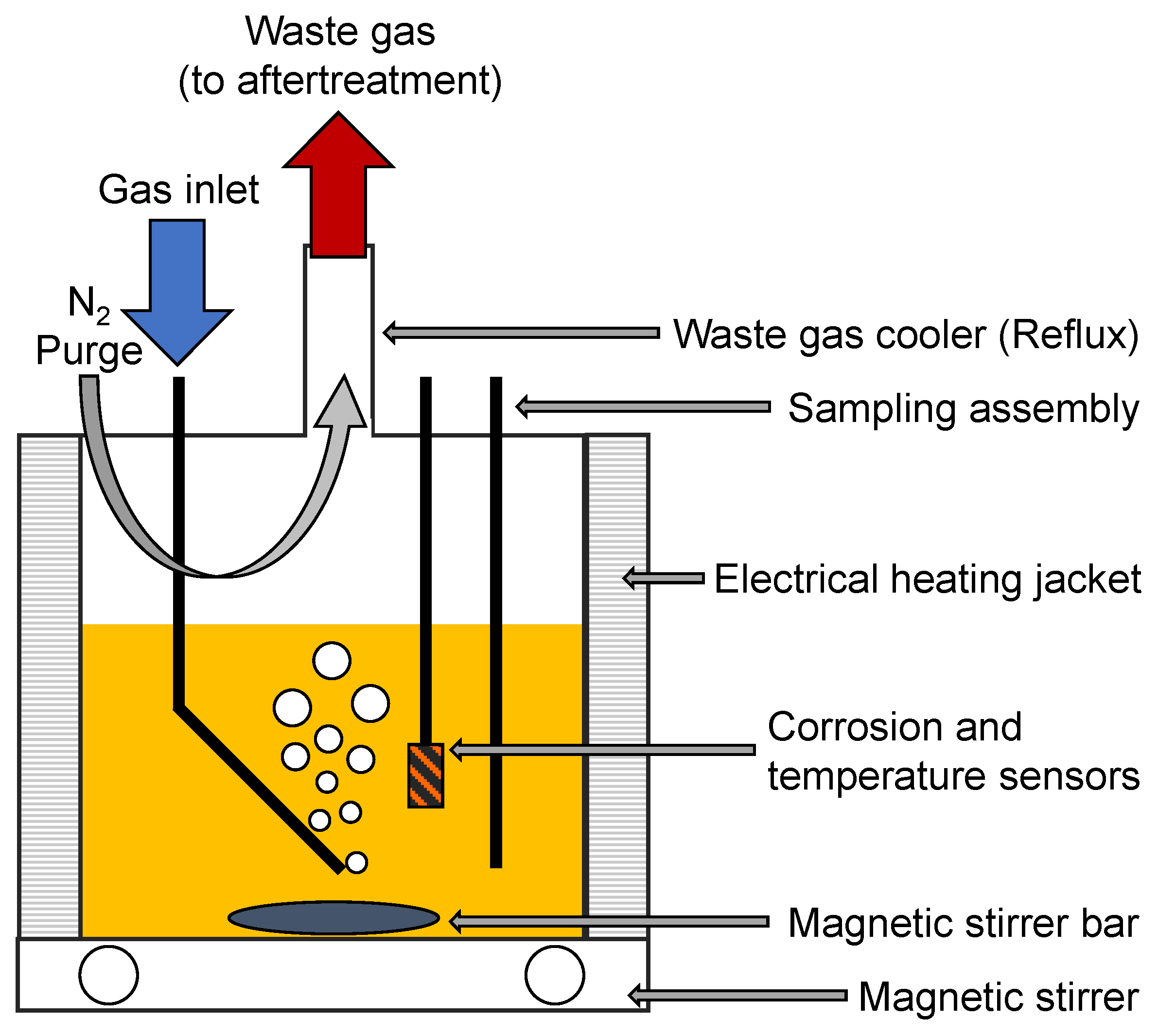
2.2. Artificial Alteration
- Synthetic air
- Synthetic air + 1000 ppm NH3
- Synthetic air + 21.7 vol% NH3
- Synthetic air + 1000 ppm NO2
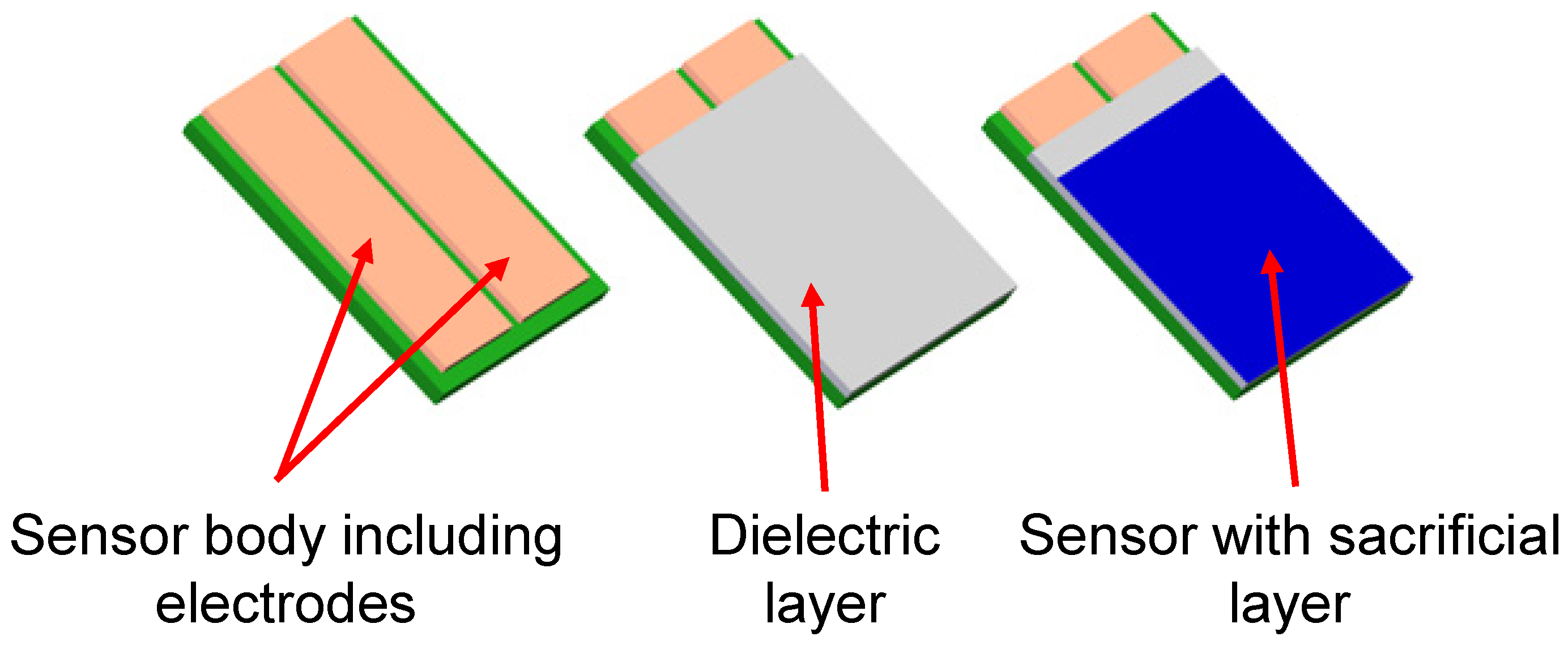
2.3. Online Corrosion Monitoring
2.4. Chemical Oil Analysis
- Fourier transform infrared spectroscopy (FT-IR) using a Tensor 27 FT-IR spectrometer (Bruker, Ettlingen, Germany) to determine the accumulation of relevant degradation products, namely
- ◦
- ◦
- nitration based on the peak height at 1630 cm−1 according to DIN 51453 [26] and
- ◦
- aminification based on the maximal peak height between 3086 cm−1 and 3586 cm−1 (details see Section 3.1).
- Water content by indirect Karl Fischer titration using an Oven Sample Processor 774 and a KF Coulometer 756 (Metrohm, Herisau, Switzerland) corresponding to DIN 51,777 [31] of the final samples.
2.5. Lubricant Performance Tests
- static deposit control performance using the micro coking test (MCT) according to GFC-LU-27-A-13 [32],
- dynamic deposit control performance by thermo-oxidation engine oil simulation test (TEOST MHT®) corresponding to ASTM D7097 [33] as well as
- extreme pressure properties (EP) according to ASTM D7421 [34] using an Optimol® SRV® 5 tribometer (Optimol Instruments Prüftechnik, Munich, Germany).
3. Results
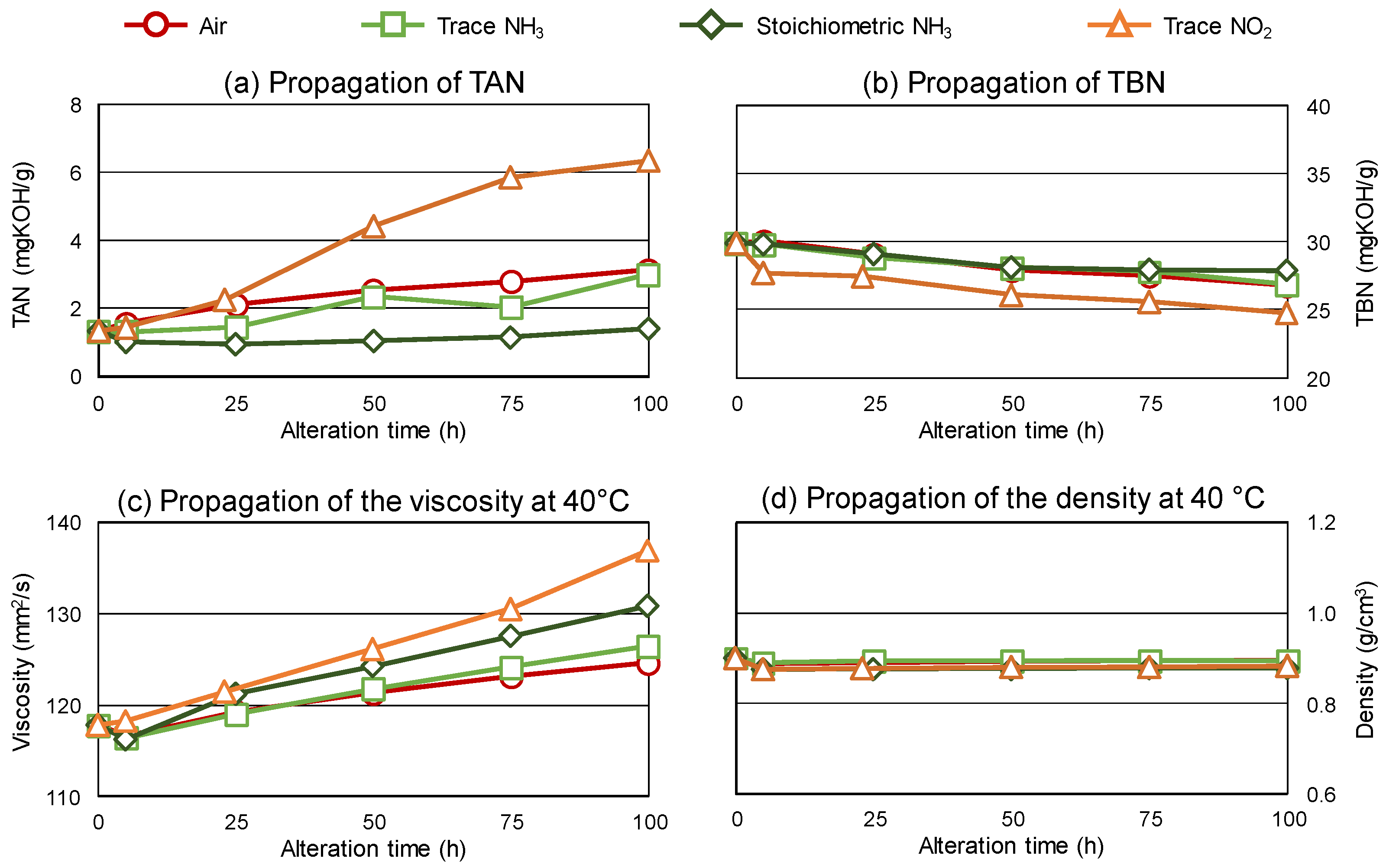
3.1. Propagation of Alterations
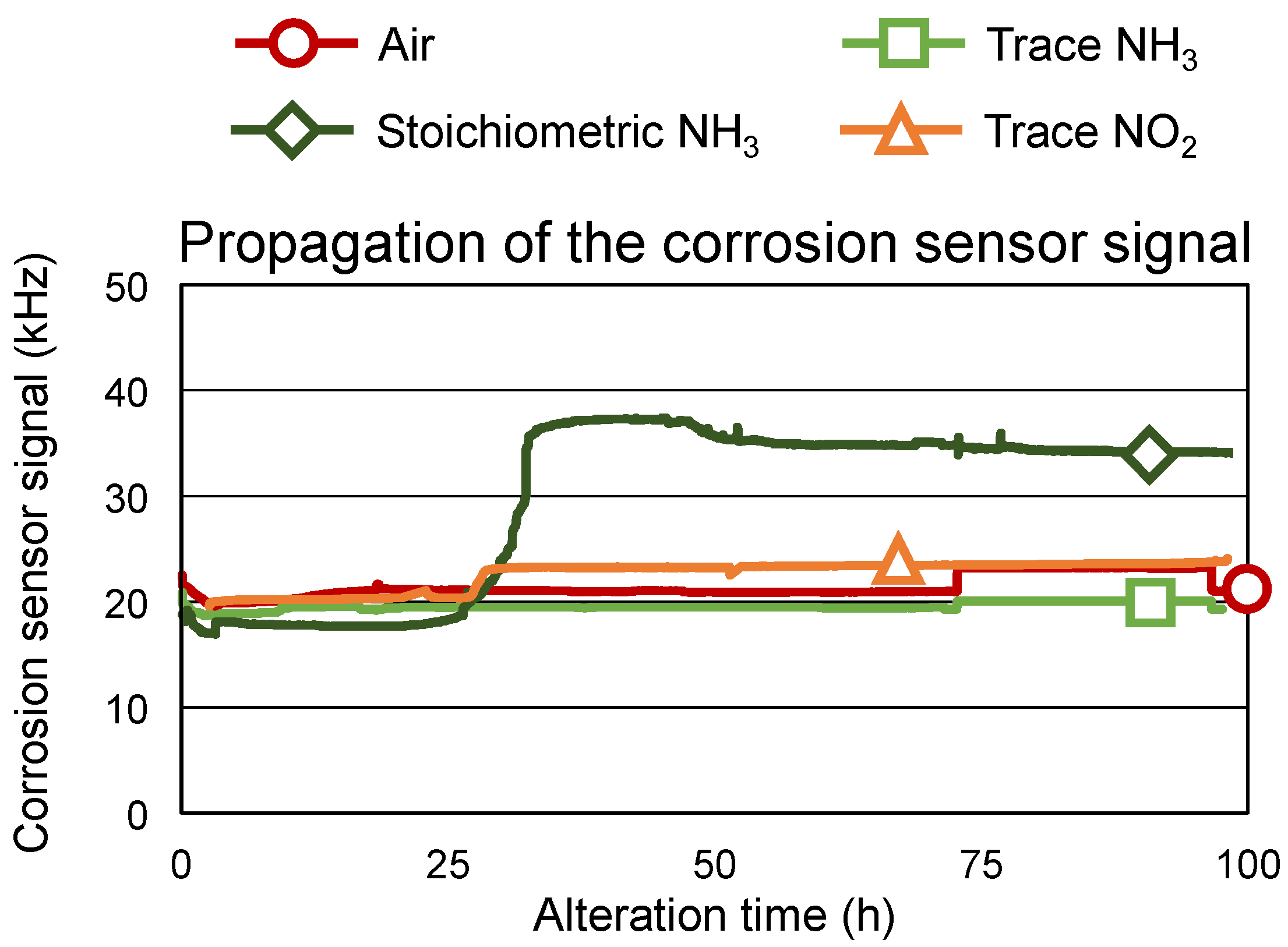
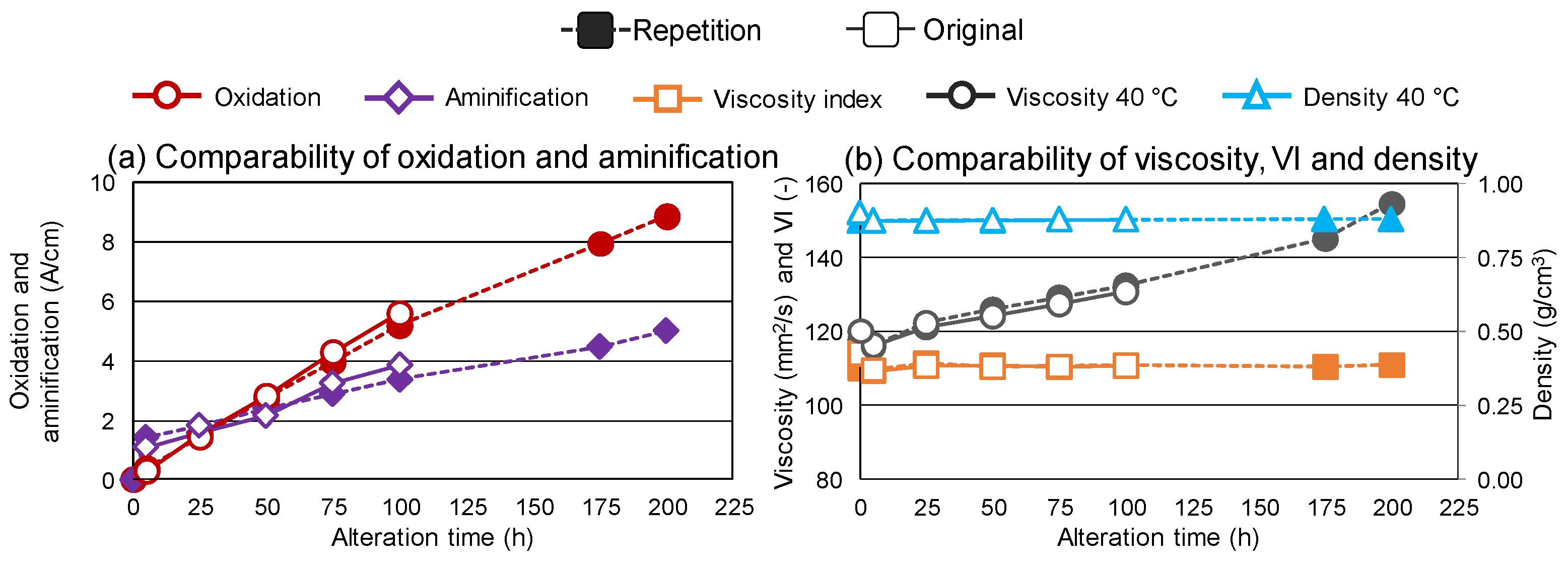
3.2. Repeatability and Extended Alteration Time
3.3. Relationship of Degradation Processes and Overall Trends of Oil Degradation
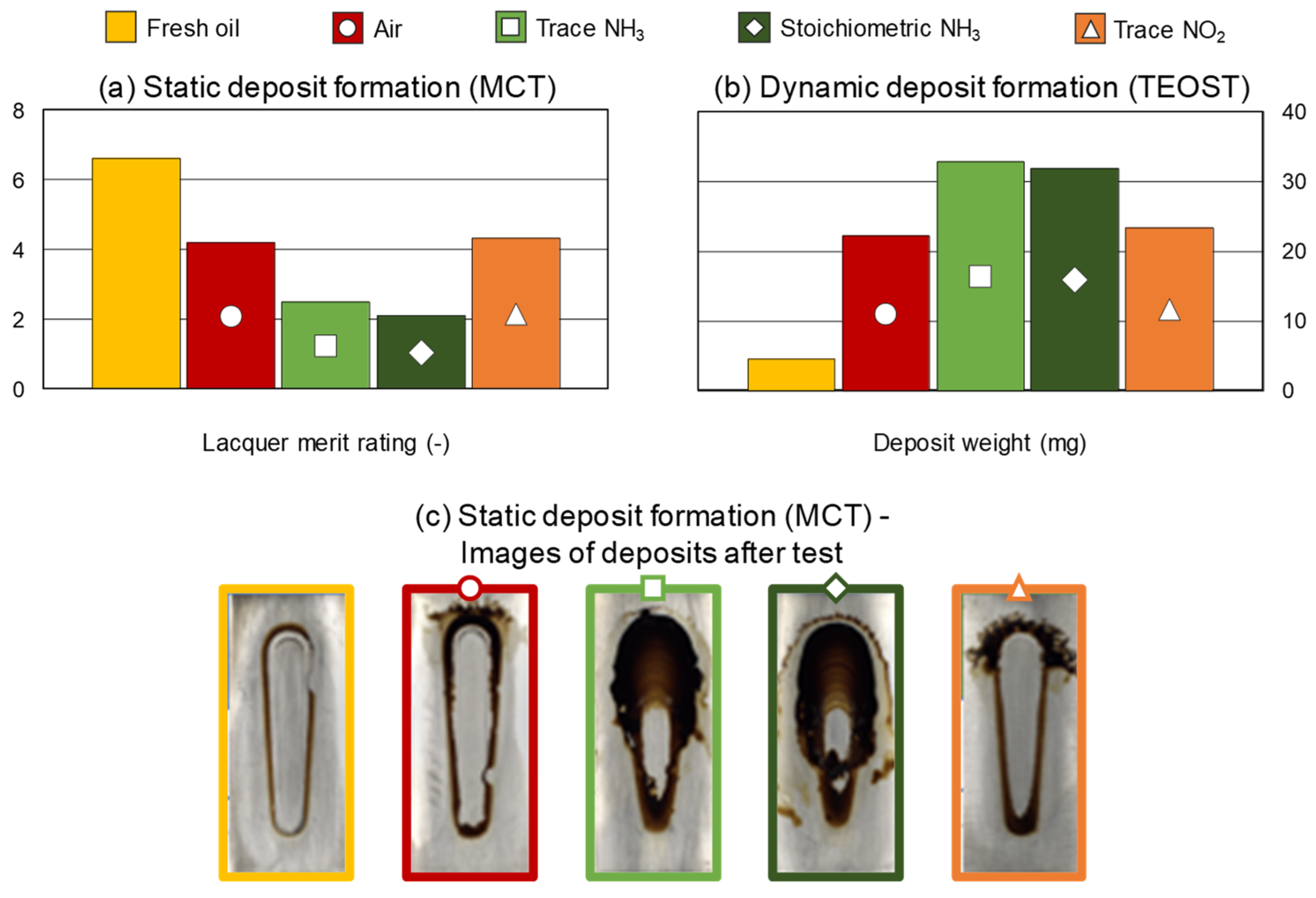
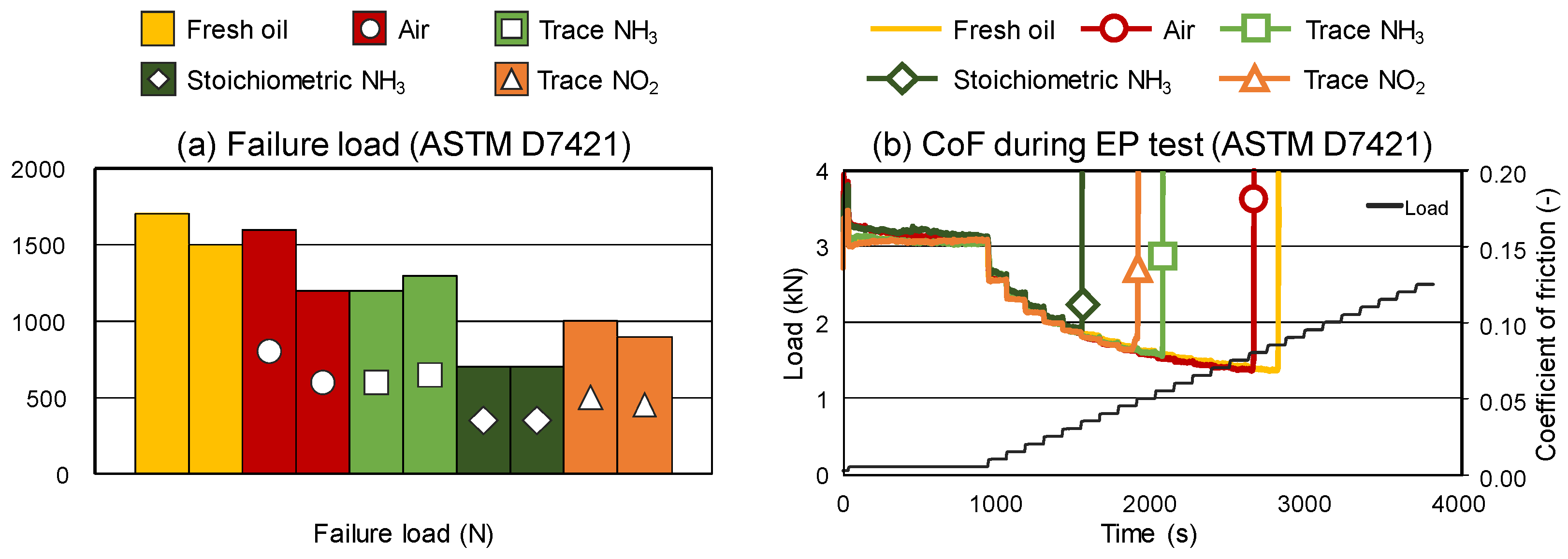
3.4. Performance Results
4. Discussion
5. Conclusions
- Oxidation was low when utilizing stoichiometric NH3 as a reaction gas mixture compared to air.
- The presence of aminic degradation products was indicated in the oils altered with trace and stoichiometric NH3 reaction gas.
- An increase in kinematic viscosity can be attributed to the aminic species.
- Corrosiveness against copper was shown when stoichiometric NH3 was used as a reaction gas.
- Both trace and stoichiometric NH3 concentration impacted the deposit control performance severely compared to air or NO2.
- Lubricants altered with stoichiometric NH3 reached the lowest failure load (EP performance) amongst the samples.
- Furthermore, the repeatability of the novel methodology was demonstrated, including the possibility of achieving more pronounced oil degradation by extending the alteration time.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFR | air-to-fuel ratio |
| ∆H0 | standard enthalpy |
| EP | extreme pressure |
| FT-IR | Fourier-transform infrared spectroscopy |
| GHG | greenhouse gas |
| ICE | internal combustion engine |
| IDLH | immediate danger to life or health |
| MCT | micro-coking test |
| NH3 | ammonia |
| NO2 | nitrogen dioxide |
| NOX | nitrogen oxides |
| OEM | original equipment manufacturer |
| PCB | printed circuit board |
| PEL | permissible exposure limits |
| SCR | selective catalytic reduction |
| TAN | total acid number |
| TBN | total base number |
| TEOST | thermo-oxidation engine oil simulation test |
| VI | viscosity index |
| ZDDP | zinc-dialkyldithiophosphate |
References
- DVN Group. IMO GHG Strategy. Available online: https://www.dnv.com/maritime/hub/decarbonize-shipping/key-drivers/regulations/imo-regulations/ghg-vision.html (accessed on 8 March 2023).
- European Maritime Safety Agency. Update on Potential of Biofuels in Shipping. Available online: https://www.emsa.europa.eu/newsroom/latest-news/item/4834-update-on-potential-of-biofuels-for-shipping.html (accessed on 8 March 2023).
- Chiong, M.C.; Kang, H.S.; Shaharuddin, N.M.R.; Mat, S.; Quen, L.K.; Ten, K.H.; Ong, M.C. Challenges and opportunities of marine propulsion with alternative fuels. Renew Sustain. Energy Rev. 2021, 149, 111397. [Google Scholar] [CrossRef]
- Al-Aboosi, F.Y.; El-Halwagi, M.M.; Moore, M.; Nielsen, R.B. Renewable ammonia as an alternative fuel for the shipping industry. Curr. Opin. Chem. Eng. 2021, 31, 100670. [Google Scholar] [CrossRef]
- Dimitriou, P.; Javaid, R. A review of ammonia as a compression ignition engine fuel. Int. J. Hydrogen Energy 2020, 45, 7098–7118. [Google Scholar] [CrossRef]
- Chehade, G.; Dincer, I. Progress in green ammonia production as potential carbon-free fuel. Fuel 2021, 299, 120845. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Van Herle, J.; Maréchal, F.; Desideri, U. Techno-economic comparison of green ammonia production processes. Appl. Energy 2020, 259, 114135. [Google Scholar] [CrossRef]
- Sánchez, A.; Martín, M. Optimal renewable production of ammonia from water and air. J. Clean. Prod. 2018, 178, 325–342. [Google Scholar] [CrossRef]
- Zamfirescu, C.; Dincer, I. Using ammonia as a sustainable fuel. J. Power Sources 2008, 185, 459–465. [Google Scholar] [CrossRef]
- Chai, W.S.; Bao, Y.; Jin, P.; Tang, G.; Zhou, L. A review on ammonia, ammonia-hydrogen and ammonia-methane fuels. Renew Sustain. Energy Rev. 2021, 147, 111254. [Google Scholar] [CrossRef]
- Berwal, P.; Kumar, S.; Khandelwal, B. A comprehensive review on synthesis, chemical kinetics, and practical application of ammonia as future fuel for combustion. J. Energy Inst. 2023, 99, 273–298. [Google Scholar] [CrossRef]
- Kobayashi, H.; Hayakawa, A.; Kunkuma, K.D.; Somarathne, A.; Okafor, E.C. Science and technology of ammonia combustion. Proc. Combust. Inst. 2019, 37, 109–133. [Google Scholar] [CrossRef]
- Zhou, X.; Li, T.; Wang, N.; Wang, X.; Chen, R.; Li, S. Pilot diesel-ignited ammonia dual fuel low-speed marine engines: A comparative analysis of ammonia premixed and high-pressure spray combustion modes with CFD simulation. Renew Sustain. Energy Rev. 2023, 173, 113108. [Google Scholar] [CrossRef]
- Ezzat, M.F.; Dincer, I. Comparative assessments of two integrated systems with/without fuel cells utilizing liquefied ammonia as a fuel for vehicular applications. Int. J. Hydrogen Energy 2018, 43, 4597–4608. [Google Scholar] [CrossRef]
- Gill, S.S.; Chatha, G.S.; Tsolakis, A.; Golunski, S.E.; York, A.P.E. Assessing the effects of partially decarbonising a diesel engine by co-fuelling with dissociated ammonia. Int. J. Hydrogen Energy 2012, 37, 6074–6083. [Google Scholar] [CrossRef]
- Agocs, A.; Besser, C.; Brenner, J.; Budnyk, S.; Frauscher, M.; Dörr, N. Engine Oils in the Field: A Comprehensive Tribological Assessment of Engine Oil Degradation in a Passenger Car. Tribol. Lett. 2022, 70, 28. [Google Scholar] [CrossRef]
- Dörr, N.; Agocs, A.; Besser, C.; Ristic, A.; Frauscher, M. Engine Oils in the Field: A Comprehensive Chemical Assessment of Engine Oil Degradation in a Passenger Car. Tribol. Lett. 2019, 67, 68. [Google Scholar] [CrossRef]
- Agocs, A.; Nagy, A.L.; Tabakov, Z.; Perger, J.; Rohde-Brandenburger, J.; Schandl, M.; Besser, C.; Dörr, N. Comprehensive assessment of oil degradation patterns in petrol and diesel engines observed in a field test with passenger cars—Conventional oil analysis and fuel dilution. Tribol. Int. 2021, 161, 107079. [Google Scholar] [CrossRef]
- Agocs, A.; Budnyk, S.; Frauscher, M.; Ronai, B.; Besser, C.; Dörr, N. Comparing oil condition in diesel and gasoline engines. Ind. Lubr. Tribol. 2020, 72, 1033–1039. [Google Scholar] [CrossRef]
- CEC, L. 48-A00: Oxidation Stability of Lubricating Oils Used in Automotive Transmissions by Artificial Ageing; Coordinating European Council for the Development of Performance Tests for Fuels, Lubricants and Other Fluids: Brussels, Belgium, 2014. [Google Scholar]
- Besser, C.; Schneidhofer, C.; Dörr, N.; Novotny-Farkas, F.; Allmaier, G. Investigation of long-term engine oil performance using lab-based artificial ageing illustrated by the impact of ethanol as fuel component. Tribol. Int. 2012, 46, 174–182. [Google Scholar] [CrossRef]
- Besser, C.; Agocs, A.; Ronai, B.; Ristic, A.; Repka, M.; Jankes, E.; McAleese, C.; Dörr, N. Generation of engine oils with defined degree of degradation by means of a large scale artificial alteration method. Tribol. Int. 2019, 132, 39–49. [Google Scholar] [CrossRef]
- Besser, C.; Agocs, A.; Ristic, A.; Frauscher, M. Implementation of Nitration Processes in Artificial Ageing for Closer-to-Reality Simulation of Engine Oil Degradation. Lubricants 2022, 10, 298. [Google Scholar] [CrossRef]
- Agocs, A.; Budnyk, S.; Besser, C.; Ristic, A.; Frauscher, M.; Ronai, B.; Dörr, N. Production of Used Engine Oils with Defined Degree of Degradation in a Large-scale Device: Correlation of Artificially Altered Oils with Field Samples. ACTA Tech. Jaurinensis 2020, 13, 131–150. [Google Scholar] [CrossRef]
- Schneidhofer, C.; Sen, S.; Dörr, N. Determination of the Impact of Biogas on the Engine Oil Condition Using a Sensor Based on Corrosiveness. In Biofuel Production—Recent Developments and Prospects; Bernardes, D.S., Ed.; InTech: London, UK, 2011; pp. 577–596. [Google Scholar]
- DIN 51453:2004-10; Testing of Lubricants—Determination of Oxidation and Nitration of Used Motor Oils—Infrared Spectrometric Method. Deutsches Institut für Normung: Berlin, Germany, 2004. [CrossRef]
- ASTM D664-18e2; Standard Test Method for Acid Number of Petroleum Products by Potentiometric Titration. ASTM International: West Conshohocken, PA, USA, 2018. [CrossRef]
- DIN ISO 3771:1985–04; Petroleum Products; Total Base Number; Perchloric Acid Potentiometric Titration Method. Deutsches Institut für Normung: Berlin, Germany, 1985.
- ASTM D7042–21; Standard Test Method for Dynamic Viscosity and Density of Liquids by Stabinger Viscometer (and the Calculation of Kinematic Viscosity). ASTM International: West Conshohocken, PA, USA, 2021. [CrossRef]
- ASTM D2270–10; Standard Practice for Calculating Viscosity Index from Kinematic Viscosity at 40 °C and 100 °C. ASTM International: West Conshohocken, PA, USA, 2016. [CrossRef]
- DIN 51777:2020–04; Petroleum Products—Determination of Water Content Using Titration According to Karl Fischer. Deutsches Institut für Normung: Berlin, Germany, 2020. [CrossRef]
- GFC-LU-27-A-13; Microcoking Test for Automotive Lubricants. Carburants, Lubrifiants & Fluides pour le Transport: Paris, France, 2018.
- ASTM D7097–19; Standard Test Method for Determination of Moderately High Temperature Piston Deposits by Thermo-Oxidation Engine Oil Simulation Test—TEOST MHT. ASTM International: West Conshohocken, PA, USA, 2020. [CrossRef]
- ASTM D7421–19; Standard Test Method for Determining Extreme Pressure Properties of Lubricating Oils Using High-Frequency, Linear-Oscillation (SRV) Test Machine. ASTM International: West Conshohocken, PA, USA, 2020. [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2000; pp. 10815–10837. [Google Scholar]


| Marine Engine Oil SAE-40 | |
|---|---|
| Kinematic viscosity 40 °C | 120 mm2/s |
| Kinematic viscosity 100 °C | 14 mm2/s |
| Viscosity index (VI) | 110 |
| Total base number (TBN) | 30 mg KOH/g |
| Total acid number (TAN) | 1.5 mg KOH/g |
| Contact | Punctual (Ball/Disc) | ||
|---|---|---|---|
| Test specimens | Ball | Disc | |
| Dimensions | Ø10 mm | Ø24 × 7.9 mm | |
| Material | steel 100Cr6 | steel 100Cr6 | |
| Hardness | 60 ± 2 HRC | 60 ± 2 HRC | |
| Final surface quality | Ra = 0.055 ± 0.003 µm | 0.035 µm ≤ Ra ≤ 0.065 µm | |
| Movement | Oscillating | ||
| Frequency | 50 Hz | ||
| Stroke | 2 mm | ||
| Load | 100–2500 N | ||
| Time | Max 64 min | ||
| Temperature | 80 °C | ||
| Test Protocol | Force | Contact Pressure | Temperature | Time | |
|---|---|---|---|---|---|
| Step 1 | Running-in | 100 N | 2200 MPa | 80 °C | 15 min |
| Step 2 | Test | +100 N/load stage | Up to 6400 MPa | 80 °C | 2 min/load stage |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agocs, A.; Rappo, M.; Obrecht, N.; Schneidhofer, C.; Frauscher, M.; Besser, C. The Impact of Ammonia Fuel on Marine Engine Lubrication: An Artificial Lubricant Ageing Approach. Lubricants 2023, 11, 165. https://doi.org/10.3390/lubricants11040165
Agocs A, Rappo M, Obrecht N, Schneidhofer C, Frauscher M, Besser C. The Impact of Ammonia Fuel on Marine Engine Lubrication: An Artificial Lubricant Ageing Approach. Lubricants. 2023; 11(4):165. https://doi.org/10.3390/lubricants11040165
Chicago/Turabian StyleAgocs, Adam, Maria Rappo, Nicolas Obrecht, Christoph Schneidhofer, Marcella Frauscher, and Charlotte Besser. 2023. "The Impact of Ammonia Fuel on Marine Engine Lubrication: An Artificial Lubricant Ageing Approach" Lubricants 11, no. 4: 165. https://doi.org/10.3390/lubricants11040165
APA StyleAgocs, A., Rappo, M., Obrecht, N., Schneidhofer, C., Frauscher, M., & Besser, C. (2023). The Impact of Ammonia Fuel on Marine Engine Lubrication: An Artificial Lubricant Ageing Approach. Lubricants, 11(4), 165. https://doi.org/10.3390/lubricants11040165






