Synthesis of Multi-butylnaphthalene Base Oils Catalyzed by Trifluoromethanesulfonic Acid and Its Lubricating Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Products Analysis
2.3. Physicochemical Properties Test
2.4. Tribological Tests
3. Results and Discussions
3.1. The Alkylation of Naphthalene and n-butene
3.2. Effect of Reaction Conditions
3.2.1. Catalyst Dosage
3.2.2. Reaction Temperature
3.2.3. Flow Rate of n-butene
3.2.4. Reaction Time
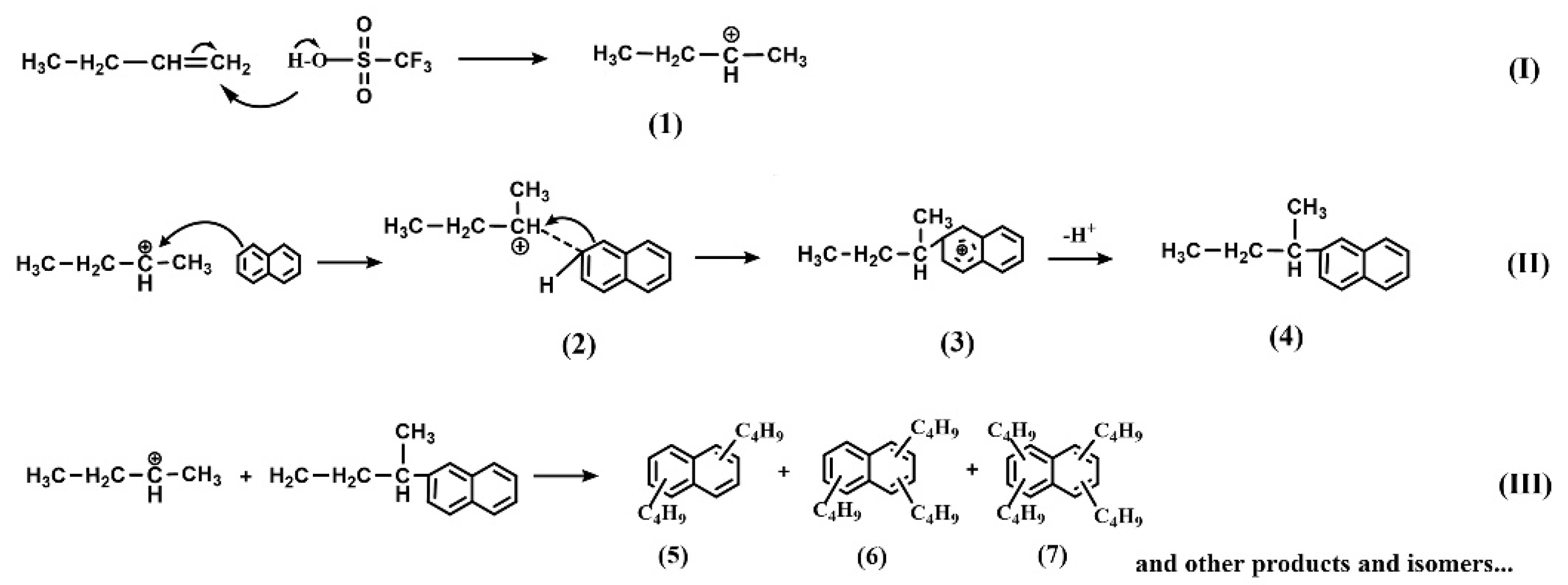
3.3. Catalytic Mechanism
3.4. Physical and Chemical Properties of Multi-butylnaphthalenes
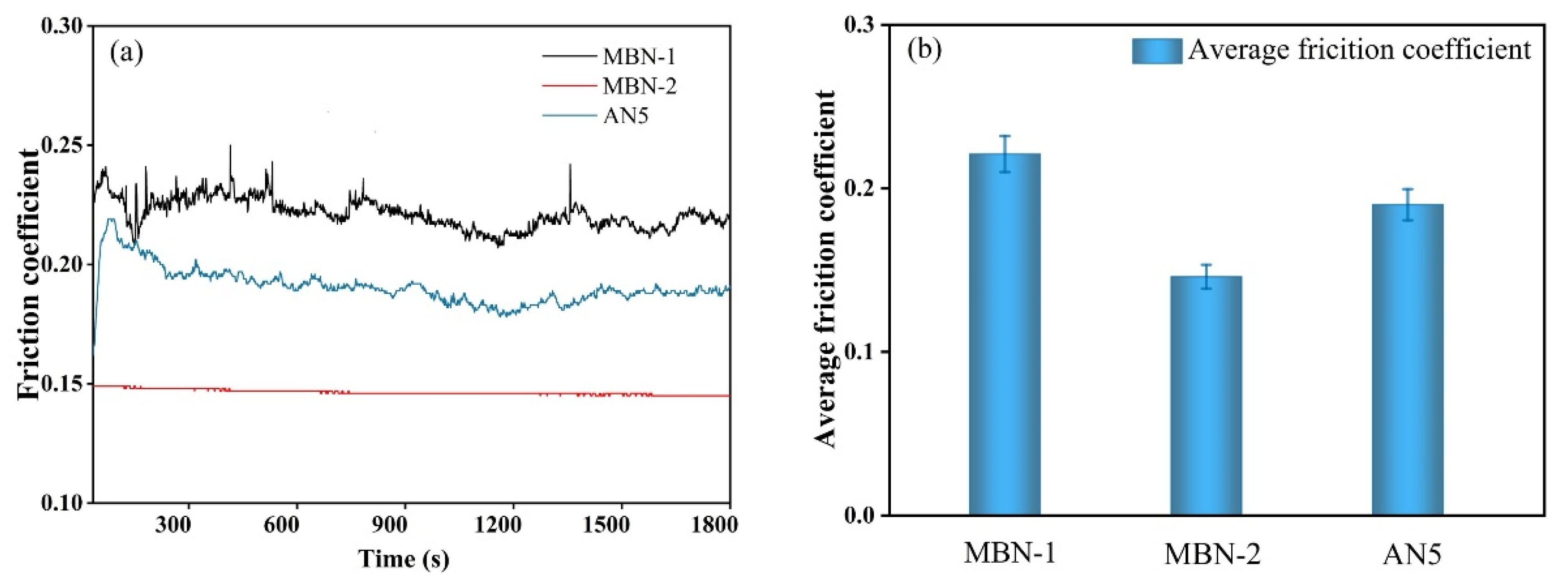
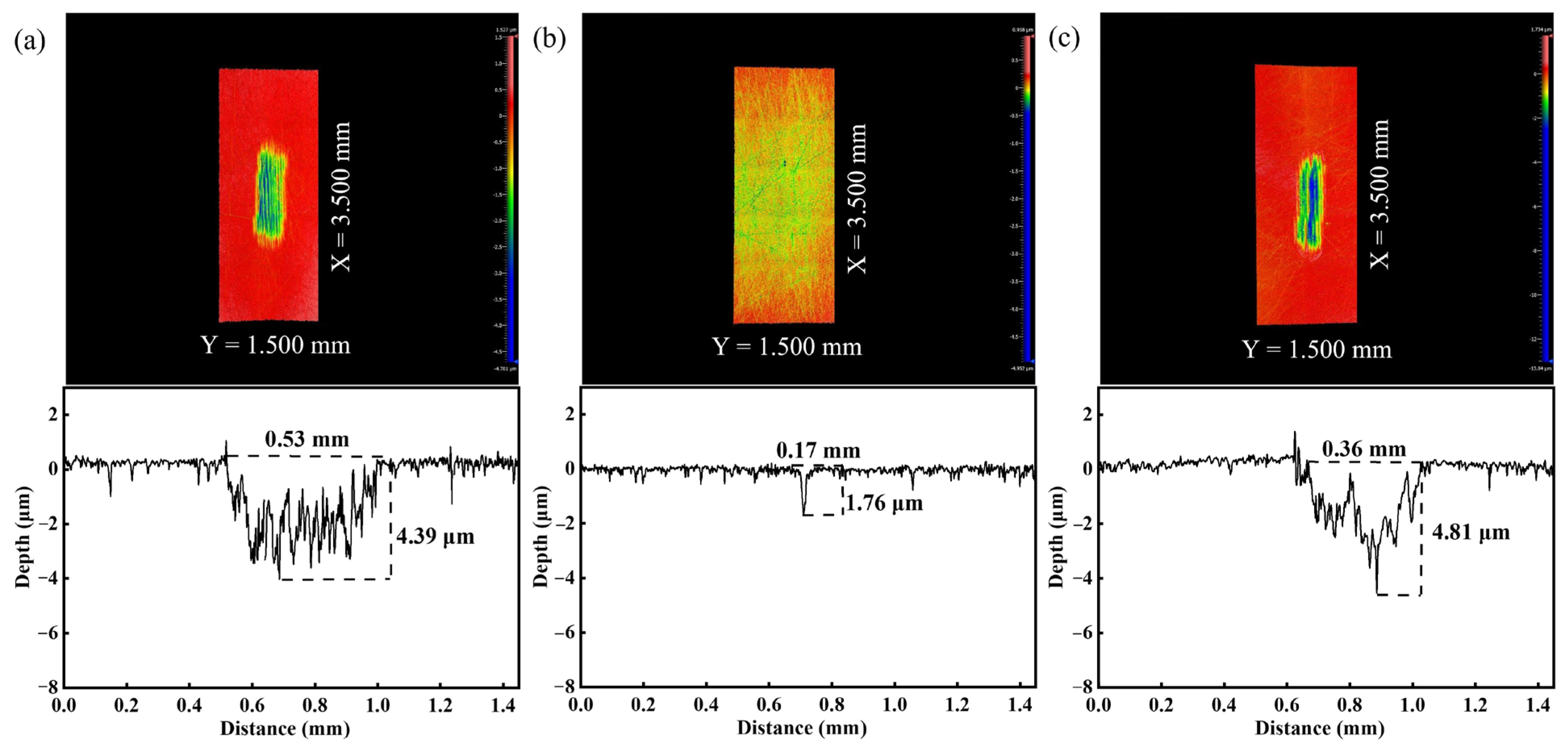
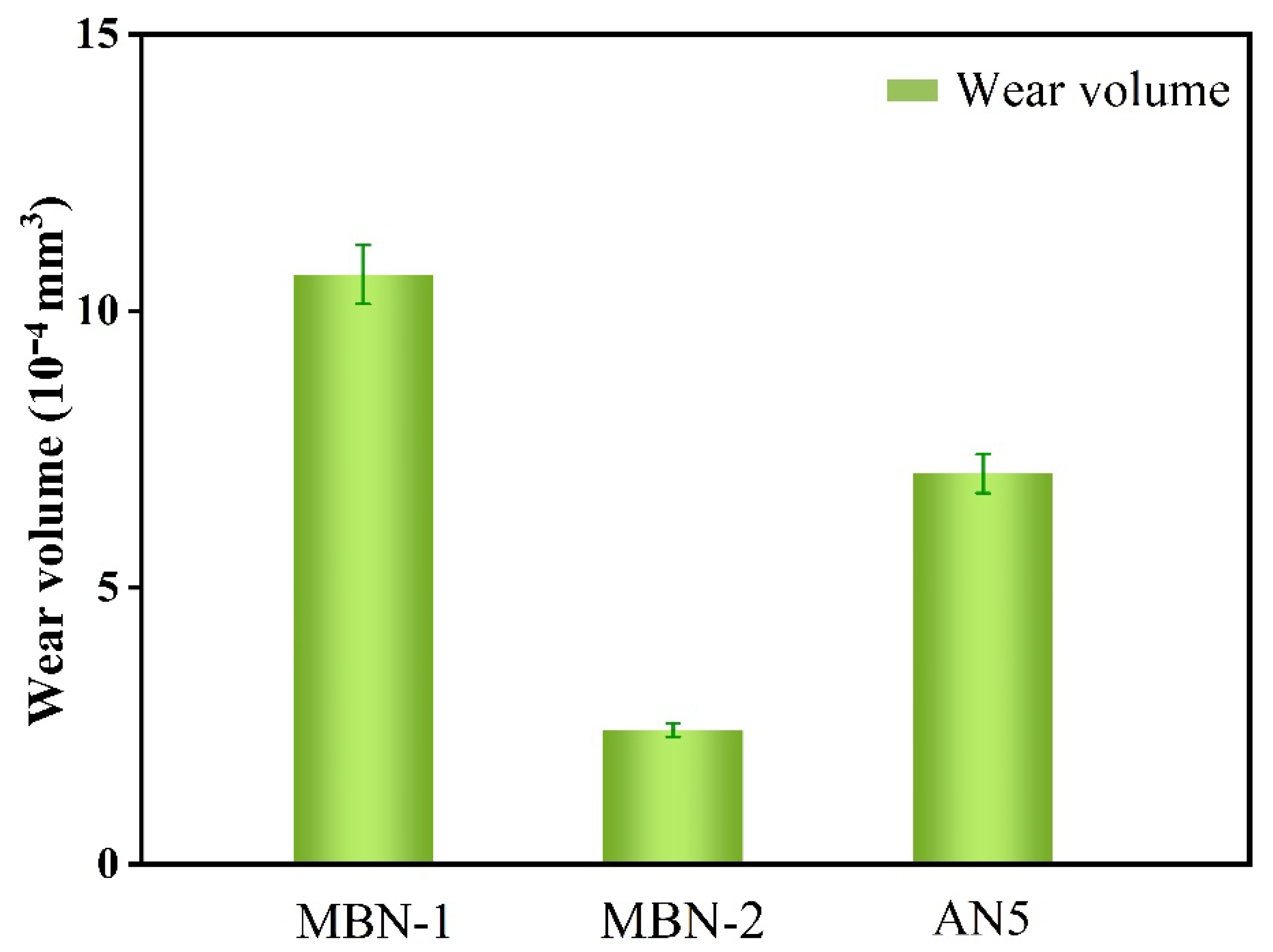
3.5. Tribological Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| TfOH | trifluoromethanesulfonic acid |
| GC | gas chromatograph |
| GC-MS | gas chromatography-mass spectrometry |
| Mono- | mono-butylnaphthalene |
| Di- | di-butylnaphthalenes |
| Tri- | tri-butylnaphthalenes |
| Tetra- | tetra-butylnaphthalenes |
| Penta- | penta-butylnaphthalenes |
| Hexa- | hexa-butylnaphthalenes |
| MBN-1 | 90.3% mono-/di-butylnaphthalenes |
| MBN-2 | 98.2% tri/tetra/penta/hexa-butylnaphthalenes |
References
- Kang, H.; Jung, Y.; Kwon, J. Changes in ecotoxicity of naphthalene and alkylated naphthalenes during photodegradation in water. Chemosphere 2019, 222, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Bruyneel, B.; Kamelia, L.; Wesseling, S.; Rietjens, I.; Boogaard, P. In vitro metabolism of naphthalene and its alkylated congeners by human and rat liver microsomes via alkyl side chain or aromatic oxidation. Chem. -Biol. Interact. 2020, 315, 108905. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Kuai, L.; Shi, L.; Meng, X.; Liu, N. Catalytic performance and industrial test of HY zeolite for alkylation of naphthalene and α-tetradecene. New J. Chem. 2022, 46, 2290–2299. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, Q.; Zhang, H.; Wang, R.; Zheng, L. Overview of research and application of alkyl naphthalene base oil. Proc. China–SAE Congr. 2021, 3, 62–65. [Google Scholar]
- Liang, Y.; Jiao, G. Study on the preparation of long chain alkylated naphthalenes. Pet. Process. Petrochem. 2009, 40, 22–25. [Google Scholar]
- Ivanova, I.; Kuznetsov, A.; Knyazeva, E.; Fajula, F. Design of hierarchically structured catalysts by mordenites recrystallization: Application in naphthalene alkylation. Catal. Today 2011, 168, 133–139. [Google Scholar] [CrossRef]
- Hourani, M.; Hessell, T.; Abramshe, R.; Liang, J. Alkylated naphthalenes as high-performance synthetic lubricating fluids. Tribol. Trans. 2007, 50, 82–87. [Google Scholar] [CrossRef]
- Cui, J.; Tang, Q.; Chen, C.; Xu, H.; Liu, L.; Dong, J. High-viscosity polyalkylphenanthrene oils: Synthesis and evaluation of lubricating properties. Lubr. Sci. 2022, 34, 527–536. [Google Scholar] [CrossRef]
- Cui, J.; Tang, Q.; Chen, C.; Xu, H.; Liu, L.; Dong, J. Synthesis of alkylated acenaphthene with high viscosity and an investigation of their lubrication property. CIESC J. 2022, 73, 3659–3668. [Google Scholar]
- Li, L.; Zhao, X.; Chen, C.; Xu, H.; Liu, L.; Dong, J. Highly selective synthesis of polyalkylated naphthalenes catalyzed by ionic liquids and their tribological properties as lubricant base oil. ChemistrySelect 2019, 4, 5284–5290. [Google Scholar] [CrossRef]
- Lucas, N.; Bordoloi, A.; Amrute, A.; Kasinathan, P.; Vinu, A.; Bohringer, W.; Fletcher, J.; Halligudi, S. A comparative study on liquid phase alkylation of 2-methylnaphthalene with long chain olefins using different solid acid catalysts. Appl. Catal. A Gen. 2009, 352, 74–80. [Google Scholar] [CrossRef]
- Sugi, Y.; Joseph, S.; Ramadass, K.; Indirathankam, S.; Premkumar, S.; Dasireddy, V.; Yang, J.; Muhtaseb, A.; Liu, Q.; Kubota, Y.; et al. The isopropylation of naphthalene over a beta zeolite with BEA topology. The selectivity of the products. Mol. Catal. 2021, 505, 111521. [Google Scholar] [CrossRef]
- Sugi, Y.; Joseph, S.; Ramadass, K.; Indirathankam, S.; Premkumar, S.; Dasireddy, V.; Yang, J.; Muhtaseb, A.; Liu, Q.; Kubota, Y.; et al. The isopropylation of naphthalene over USY Zeolite with FAU topology. The selectivities of the products. Bull. Chem. Soc. Jpn. 2021, 94, 606–615. [Google Scholar] [CrossRef]
- Lachter, E.; Gil, R.; Tabak, D. Alkylation of toluene with aliphatic alcohols and 1-octene catalyzed by cation-exchange resins. React. Funct. Polym. 2000, 44, 1–7. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, G.; Wu, G.; Hu, R. The Alkylation of isobutane and 2-butene in rotating packed bed reactor: Using ionic liquid and solid acid as catalysts. Ind. Eng. Chem. Res. 2020, 59, 14767–14775. [Google Scholar] [CrossRef]
- Aminov, R.; Mazitova, A.; Khusnutdinov, R. Benzene alkylation with cycloolefins under the action of [Et3NH] + [Al2Cl7]– ionic liquid. Russ. J. Gen. Chem. 2019, 89, 2171–2177. [Google Scholar] [CrossRef]
- Chen, C.; Tang, Q.; Xu, H.; Liu, L.; Tang, M.; Li, X.; Dong, J. Alkylation of naphthalene with n-butene catalyzed by liquid coordination complexes and its lubricating properties. Chin. J. Chem. Eng. 2021, 39, 306–313. [Google Scholar] [CrossRef]
- Davis, J.; Ruether, T.; Dorman, S. Boronium based ionic liquids: Salts of boron centered cations as promising salts for electrochemical applications. ECS Trans. 2013, 50, 293–299. [Google Scholar] [CrossRef]
- Kore, R.; Mishra, M.; Gonela, U.; Rogers, R. Chloroaluminate liquid clathrates: Is it the cations or the anions that drive the solubility of aromatics? Ind. Eng. Chem. Res. 2020, 59, 18419–18424. [Google Scholar] [CrossRef]
- Ipatieff, V.; Komarewsky, V.; Pines, H. Phosphoric acid as a catalyst for the destructive alkylation of hydrocarbons. J. Am. Chem. Soc. 2002, 58, 918–919. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Z.; Qiao, W.; Wang, G.; Lübo, C. An efficient method for the alkylation of α-methylnaphthalene with various alkylating agents using methanesulfonic acid as novel catalysts and solvents. Catal. Lett. 2005, 102, 219–222. [Google Scholar] [CrossRef]
- Saito, S.; Saito, S.; Ohwada, T.; Shudo, K. The Hammett acidity function H0 of trifluoromethanesulfonic acid-trifluoroacetic acid and related acid systems. A versatile nonaqueous acid system. Chem. Pharm. Bull. 2008, 39, 2718–2720. [Google Scholar] [CrossRef]
- Song, S.; Liu, Q.; Lu, S. Alkylation of naphthalene catalyzed by trifluoromethanesulfonic acid. Pet. Process. Petrochem. 2018, 49, 71–74. [Google Scholar]
- Wang, Y.; You, X.; Song, C.; Wang, X.; Duan, H.; Zhang, Q. A Production Method of Alkyl Naphthalene. CN114507110A, 11 January 2022. [Google Scholar]
- Shen, P.; Zhang, S. Comprehensive utilization of C4 resource as by-product of methanol to olefin process. Contemp. Chem. Ind. 2012, 41, 1333–1336. [Google Scholar]
- Anonymous. Yangzi Petrochemical utilizes the pyrolysis C4 resources to expand butene-1 and butadiene production capacity. China Pet. Process. Petrochem. Technol. 2007, 1, 55. [Google Scholar]
- Jia, X.; Yang, Z.; Han, C. Domestic 1-butene production capacity and technology. Chem. Enterp. Manag. 2021, 31, 150–151. [Google Scholar]
- Zhou, S. Comprehensive utilization of C4/C5 friction. Ethyl. Ind. 2022, 34, 5–8. [Google Scholar]
- Zhao, G.; Qi, P.; Guo, L.; He, Y. Separation technology of 1-butene and development of downstream products. Petrochem. Technol. 2008, 37, 114–116. [Google Scholar]
- Berenblyum, A.; Katsman, E.; Hommeltoft, S. Mathematical description of isobutane alkylation with butenes in the presence of trifluoromethanesulfonic acid. Ind. Eng. Chem. Res. 2004, 43, 6988–6993. [Google Scholar] [CrossRef]
- Wang, H.; Ma, S.; Zhou, Z.; Li, M.; Wang, H. Alkylation of isobutane with butene catalyzed by deep eutectic ionic liquids. Fuel 2020, 269, 117419. [Google Scholar] [CrossRef]
- Jin, S.; Yu, H.; Zhao, C.; Yang, X.; Liu, X. Study on 1-hexene oligomerication catalyzed by trifluoromethanesulfonic acid. Ind. Catal. 2012, 20, 41–43. [Google Scholar]
- Wang, Y.; Yang, S.; Bian, G.; Song, L. A novel three-step tandem reaction for efficient syntheses of bulky anthracenyl esters from 2-benzylbenzoic acids. Synthesis 2017, 49, 1884–1888. [Google Scholar]
- Liu, N.; Pu, X.; Wang, X.; Shi, L. Study of alkylation on a Lewis and Brønsted acid hybrid catalyst and its industrial test. J. Ind. Eng. Chem. 2014, 20, 2848–2857. [Google Scholar] [CrossRef]
- Li, C.; Qi, X.; Tang, X. Synthesis of 2-isopropyl naphthalene catalyzed by Et3NHCl-AlCl3 ionic liquids. China Pet. Process. Petrochem. Technol. 2014, 16, 60–65. [Google Scholar]
- Chen, S.; Wu, T.; Zhao, C. Conversion of lipid into high-viscosity branched bio-lubricant base oil. Green Chem. 2020, 22, 7348–7354. [Google Scholar] [CrossRef]
- Zhmud, B. Beyond the aniline point: Critical solution point for the oil/aniline system as a measure of oil solubility. Fuel 2007, 86, 2545–2550. [Google Scholar] [CrossRef]
- Lu, R.; Tani, H.; Koganezawa, S.; Hata, M. Alkylated polyphenyl ethers as high-performance synthetic lubricants. Lubricants 2022, 10, 275. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, D.; Shi, L.; Yu, M.; Saikat, G. Effect of molecular structure on dispersion of carbon nanotubes by natural organic matter surrogates. China Environ. Sci. 2018, 38, 3429–3436. [Google Scholar]
- Jiang, D.; Hu, L.; Feng, D. Crown-type ionic liquids as lubricants for steel-on-steel system. Tribol. Lett. 2011, 41, 417–424. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, C.; Dong, R.; Shi, Y.; Wang, Y.; Bai, Y.; Zhang, J.; Cai, M.; Zhou, F.; Liu, W. Physicochemical and tribological properties of gemini-type halogen-free dicationic ionic liquids. Friction 2021, 9, 344–355. [Google Scholar] [CrossRef]
- Yao, M.; Fan, M.; Liang, Y.; Zhou, F.; Xia, Y. Imidazolium hexafluorophosphate ionic liquids as high temperature lubricants for steel-steel contacts. Wear 2010, 268, 67–71. [Google Scholar] [CrossRef]
- Jin, C.; Ye, C.; Phillips, B.; Zabinski, J.; Liu, X.; Liu, W.; Shreeve, J. Polyethylene glycol functionalized dicationic ionic liquids with alkyl or polyfluoroalkyl substituents as high temperature lubricants. J. Mater. Chem. 2006, 16, 1529–1535. [Google Scholar] [CrossRef]
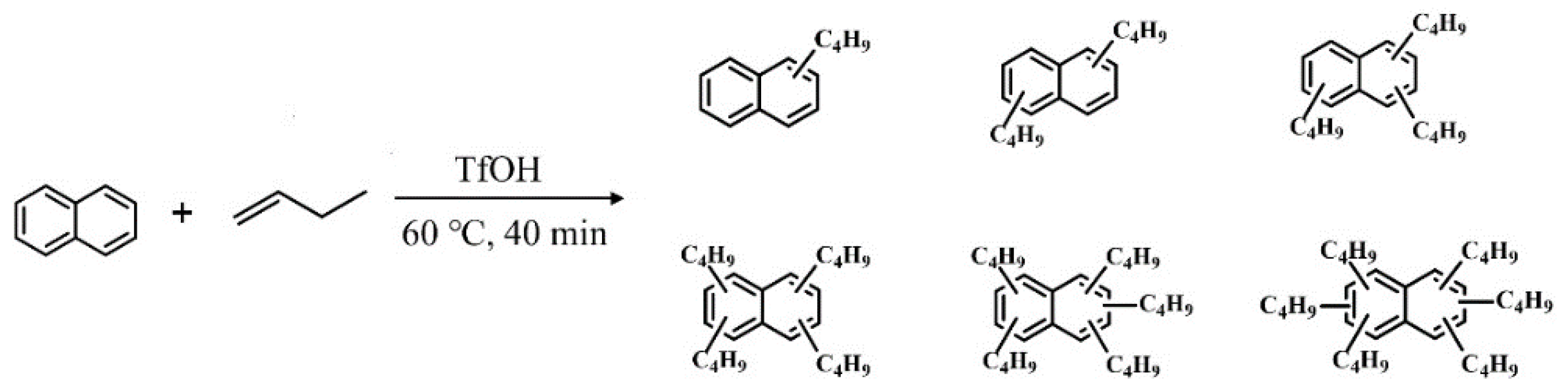

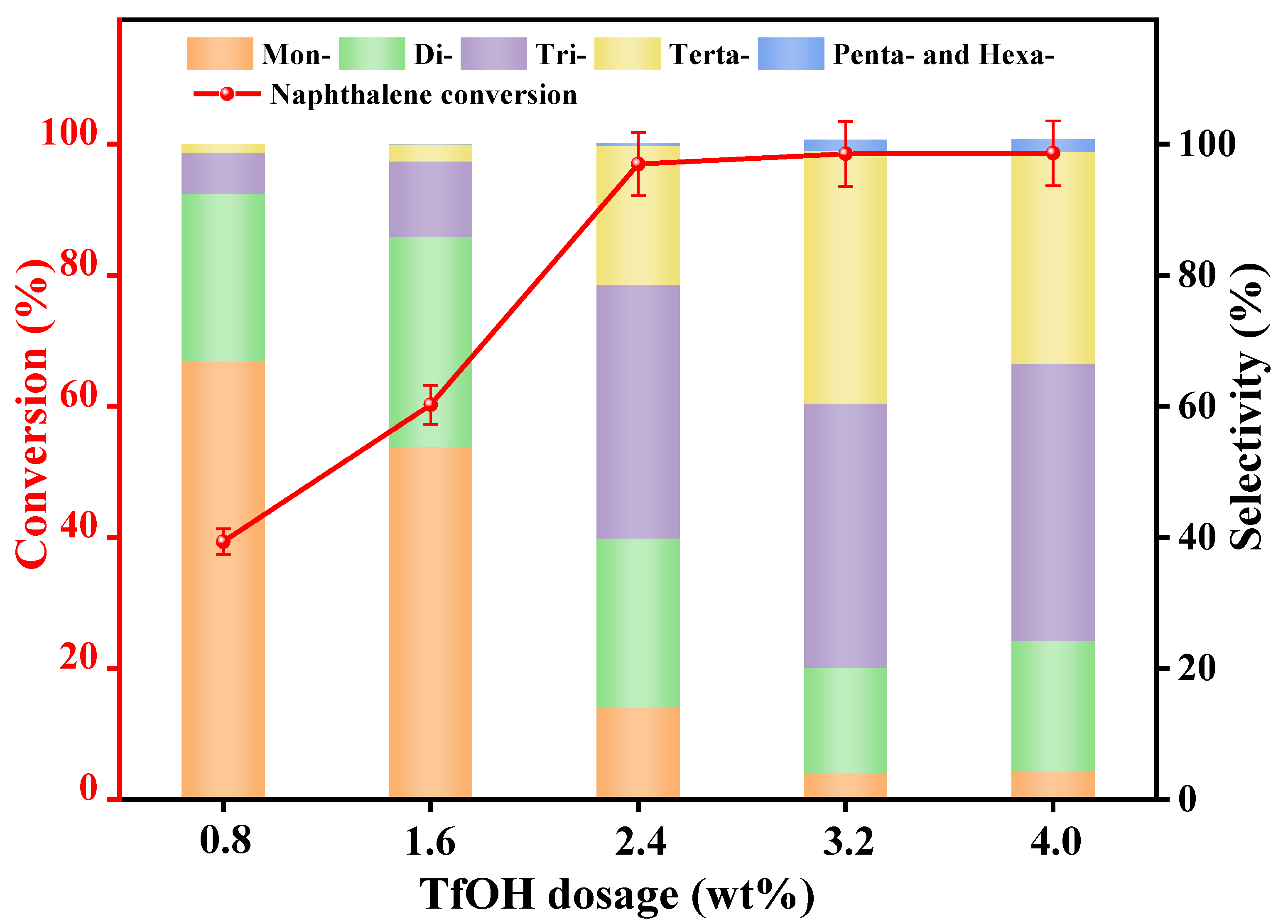
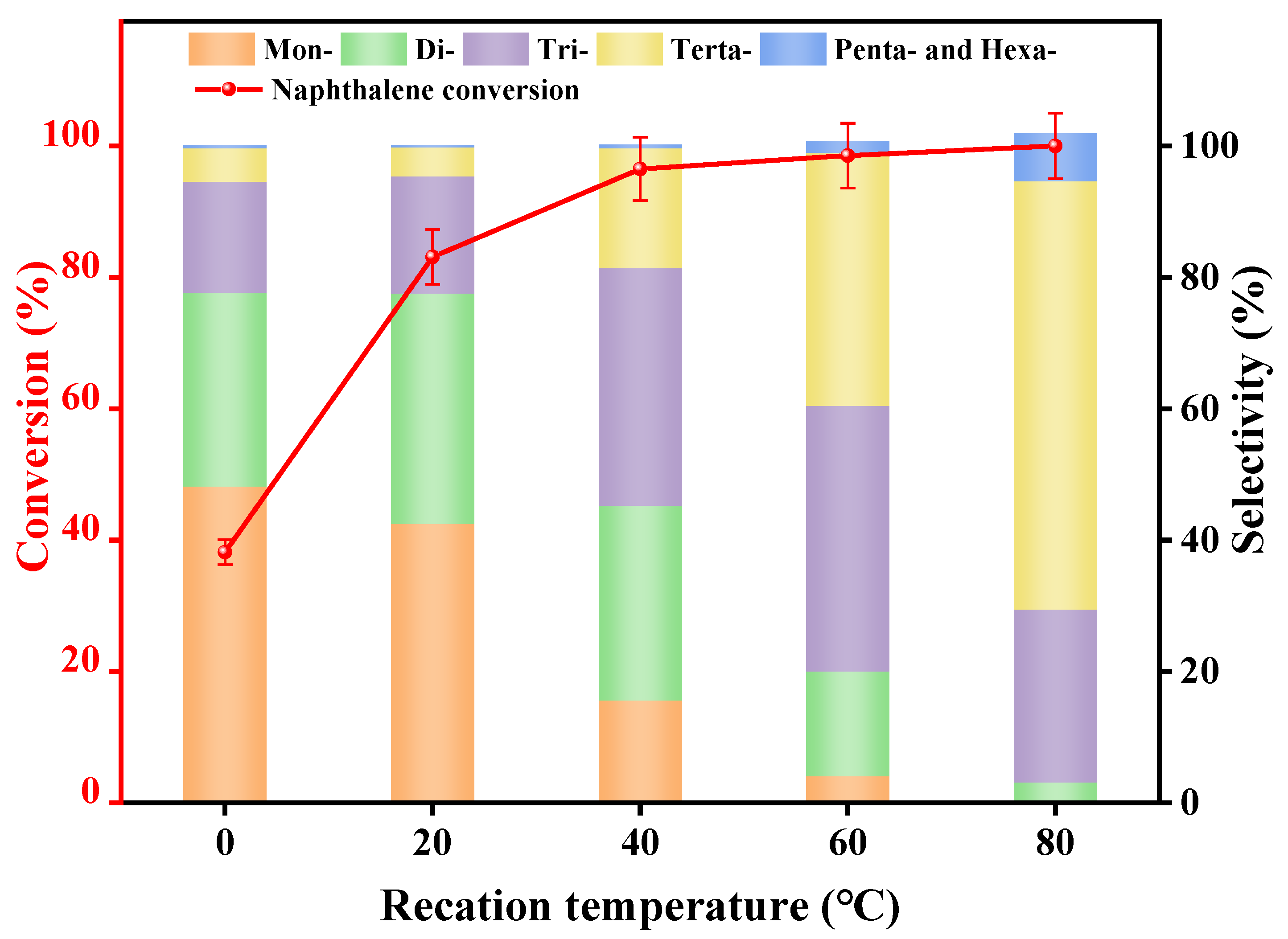
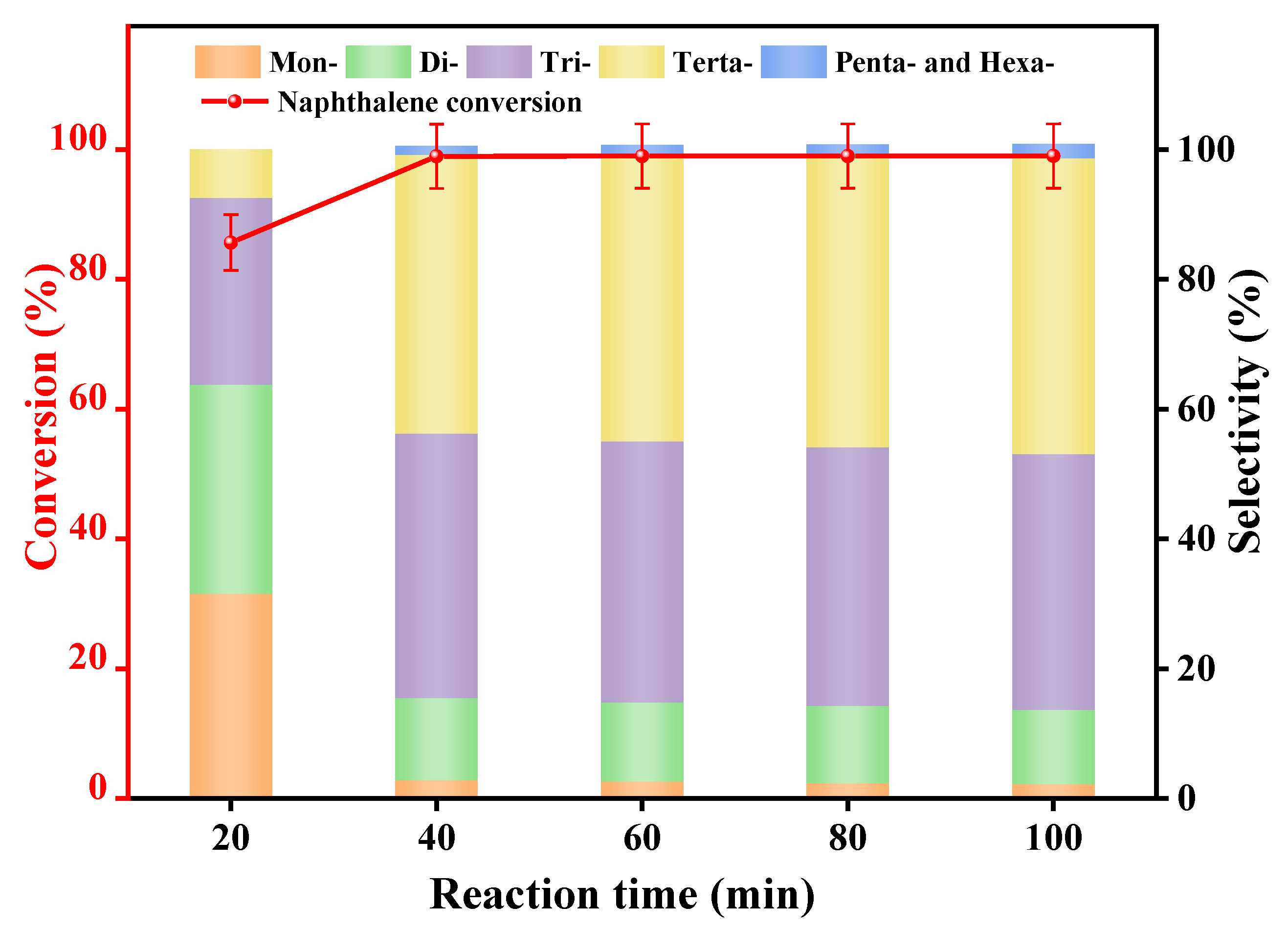

| Synthetic Oils | Proportion of Various Alkylated Products (%) | ||||
|---|---|---|---|---|---|
| mono- | di- | tri- | tetra- | penta- and hexa- | |
| MBN-1 | 59.5 | 30.8 | 8.4 | 1.3 | 0 |
| MBN-2 | 0.4 | 1.4 | 36.5 | 57.8 | 3.9 |
| Properties | MBN-1 | MBN-2 | AN5 |
|---|---|---|---|
| KV40 (mm2·s−1) | 8.0 | 275.3 | 28.1 |
| KV100 (mm2·s−1) | 1.9 | 11.2 | 4.7 |
| Density (g·cm−3) | 0.9593 | 0.9230 | 0.9070 |
| Flash point (°C) | 140 | 173 | 222 |
| Pour point (°C) | −59 | −12.5 | −51 |
| Aniline point (°C) | 17.1 * | 18.6 | 32.0 |
| Oxidative onset temperature (°C) | 203.10 | 234.51 | 233.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Chen, C.; Tang, Q.; Liu, L.; Dong, J. Synthesis of Multi-butylnaphthalene Base Oils Catalyzed by Trifluoromethanesulfonic Acid and Its Lubricating Properties. Lubricants 2023, 11, 156. https://doi.org/10.3390/lubricants11040156
Li Y, Chen C, Tang Q, Liu L, Dong J. Synthesis of Multi-butylnaphthalene Base Oils Catalyzed by Trifluoromethanesulfonic Acid and Its Lubricating Properties. Lubricants. 2023; 11(4):156. https://doi.org/10.3390/lubricants11040156
Chicago/Turabian StyleLi, Yuanyuan, Chen Chen, Qiong Tang, Lei Liu, and Jinxiang Dong. 2023. "Synthesis of Multi-butylnaphthalene Base Oils Catalyzed by Trifluoromethanesulfonic Acid and Its Lubricating Properties" Lubricants 11, no. 4: 156. https://doi.org/10.3390/lubricants11040156
APA StyleLi, Y., Chen, C., Tang, Q., Liu, L., & Dong, J. (2023). Synthesis of Multi-butylnaphthalene Base Oils Catalyzed by Trifluoromethanesulfonic Acid and Its Lubricating Properties. Lubricants, 11(4), 156. https://doi.org/10.3390/lubricants11040156






