1. Introduction
The application of tribological components in space mechanisms is manifold [
1]. A distinction is made between one-shot devices (required to operate only once, e.g., deployment devices) and devices that operate continuously or periodically (e.g., solar-array-drive mechanisms and de-spin mechanisms). Lubricants are applied to reduce friction and wear to ensure such components operate efficiently and achieve longevity. The success of spacecraft missions is tied to the reliability of these mechanisms. Since it is often not practical or even impossible to implement redundancy in aerospace components, failure in lubrication is deemed a potential single point of failure to the whole system. The use of liquid lubricants (and greases based on them) in space applications is generally restricted to those with exceptionally low volatility [
2]. These include, for example, silicones, mineral oils, and different synthetic lubricants, such as polyalphaolefins (PAO), polyesters (POE), silahydrocarbons (SiHC), multiply alkylated cyclopentanes (MAC), and perfluoropolyethers (PFPE). Lubricant selection depends on the application, considering regimes of operation (boundary, mixed, elastohydrodynamic, and hydrodynamic) and environmental factors [
3,
4].
In demanding conditions and harsh environments, perfluoropolyether (PFPE) lubricants are frequently used due to their distinguishing characteristics, such as excellent lubricity, high thermal stability, chemical inertness, low vapor pressure, and nonflammability [
5]. There are different commercially available perfluoropolyether lubricants, referred to as Fomblin
® Z, Fomblin
® Y, Krytox™, and Demnum
®. They are produced by the polymerization of perfluorinated monomers via different synthesis routes. Fomblin
® Z and Demnum
® consist of linear molecules, whereas Fomblin
® Y and Krytox™ are composed of branched molecules with pendant trifluoromethyl groups (-CF
3) [
6].
The representative structural formulae of these different classes of PFPE lubricants are listed in
Table 1 [
7]. The physical properties of the respective lubricant depend on the structure of the polymeric chain [
8]. For example, a difference in thermal stability is shown for the four PFPE types [
9]. PFPE-Z has the lowest thermal stability as a result of the high ratio of difluoro formyl (−CF
2−O−) segments in the polymer chain. PFPE-K has the highest stability due to the shielding of every ether linkage by the adjacent carbon with a pendant trifluoromethyl group. Furthermore, the end groups of the lubricants also affect the thermal stability [
10,
11,
12].
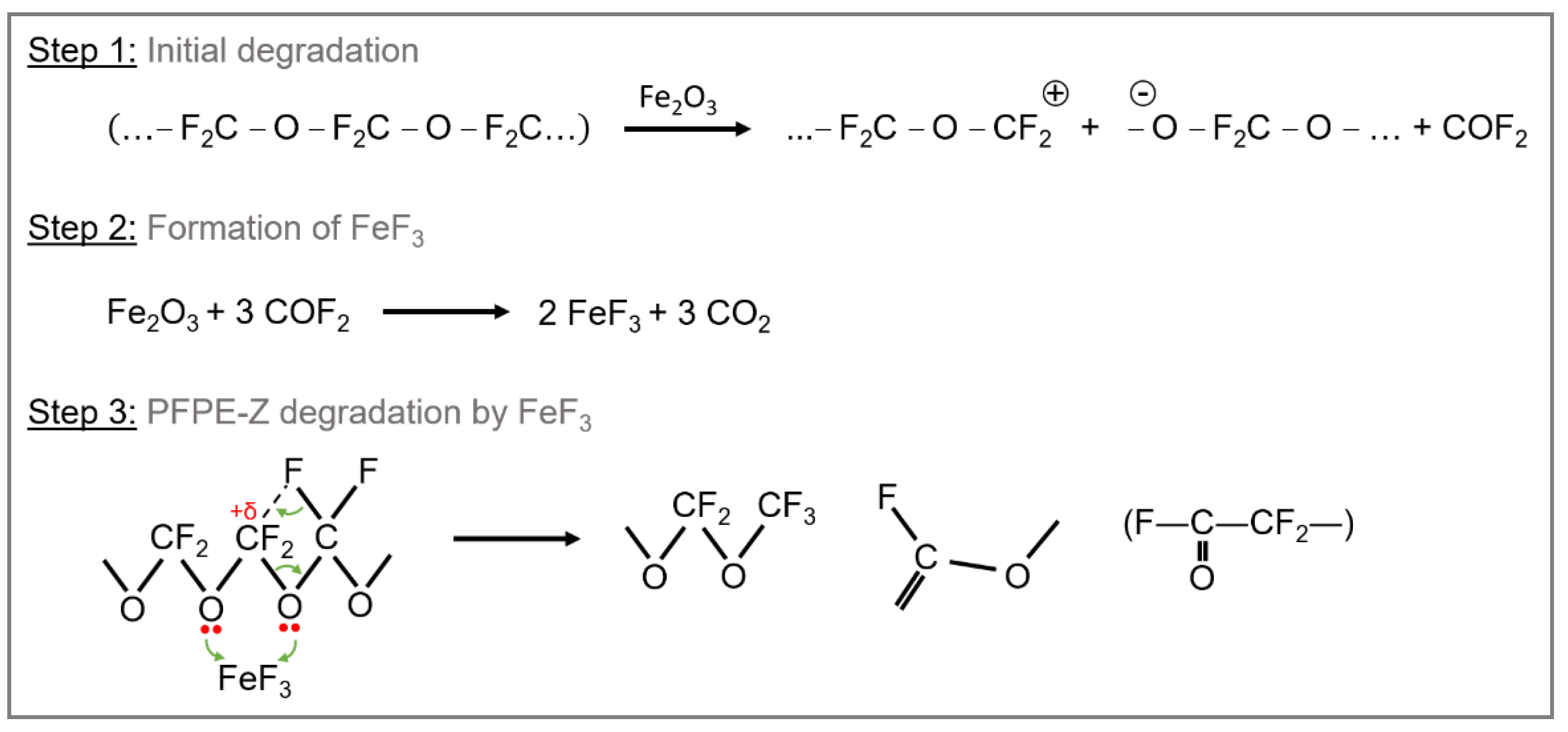
Different degradation mechanisms of PFPEs are discussed in the literature. Due to the application in tribological systems, the emphasis lies on degradation under sliding contacts. In addition to aerospace, another area of application includes recording disks and metal evaporated tapes for computer data storage, which motivates parts of the research on PFPE degradation. Established degradation mechanisms include mechanical degradation by a shearing process, electron-mediated degradation, thermal decomposition, and catalytic degradation [
13,
14,
15].
Several studies have demonstrated that the presence of metal surfaces (e.g., stainless-steel surfaces) has an adverse effect on the thermal stability of PFPEs. The loss of thermal stability is explained by an initial formation of a surface oxide under oxidative conditions, followed by the formation of a surface halide (e.g., FeF
3), which catalyzes the degradation [
16,
17,
18,
19]. However, the progression of degradation via this pathway is highly dependent on local contact conditions [
20]. Different metal oxides and ceramics have been reported to initiate this catalytic degradation mechanism in Z-type lubricants up to varying degrees depending on the strength of the Lewis acid [
21,
22,
23]. However, due to AISI 440C steel being the most common bearing material for space mechanisms [
20] and its use in previous studies, [
24,
25] FeF
3 was selected within this work to simulate the generated Lewis acid-catalyst in steel-on-steel sliding contacts (in accordance with the mechanism displayed in
Figure 1) [
16,
26,
27].
The action of repeated shearing under high contact stresses releases COF
2 from the lubricant, which in return, reacts with iron in the steel and forms iron fluoride (FeF
3). FeF
3 initiates the catalytic degradation of the PFPE lubricant and, as a consequence, results in the release of yet more COF
2. The subsequent chain reaction, often referred to as the autocatalytic effect (ACE) in the literature, leads to accelerated degradation of the lubricant [
20,
26]. The initial conversion of surface Fe
2O
3 to FeF
3 progresses slowly compared to the rapid degradation of the lubricant catalyzed by surface FeF
3. A significant difference between K-type PFPEs and Z-types PFPEs was reported. No degradation in K-lube was observed, compared to a significant vulnerability of Z-lube to the catalyzed degradation mechanism. The differences were attributed to the presence of acetal units (-O-CF
2-O-) in the polymeric chains of the Z-type polymers [
28]. An intramolecular disproportionation reaction dominates the degradation process. The acetal units are presumed to be highly susceptible to this reaction. Still, it can also occur at other ether linkages, given adequate activation energy or the presence of a sufficiently strong Lewis acid. It was found that the disproportionation reaction occurred in the main chain of Z-type lubricants, whereas the reaction was limited to chain ends in K-type lubricants [
29]. Earlier studies found that, in addition, the structure of the lubricant end groups had a significant effect on the thermal stability in the presence of a Lewis acid in Z-type lubricants [
11,
30].
Future space exploration is headed towards increasingly demanding and longer-lasting missions [
31,
32]. However, an increase in operating time and extended periods of storage (either on-ground or in-flight) have to be considered. Storage was initially viewed to be of negligible impact. Despite this, there are growing concerns over the effect of extended storage durations on subsequent lubricant performance. At present, little explicit data on the effect of spacecraft lubricants are available. Therefore, no industry-established models can be employed [
33]. Considering the impact of degradation on the lubricants, localized effects, for example, thermal degradation of small amounts of lubricant at asperities, are expected, instead of a negligible impact on the performance of the bulk lubricant. Yet, during long periods of storage, generated reactive species, e.g., due to catalytic degradation, should be considered as potentially harmful [
34]. In general, lifetime predictions of polymeric materials are often performed by the extrapolation of accelerated aging data. Failure times or degradation rates are hereby determined at elevated temperatures and subsequently extrapolated to ambient conditions. These extrapolations are commonly based on the very widely used Arrhenius relationship, where the assumption is that a chemical degradation processes reaction rate (k) or the degradation time (1/k) versus the inverse temperature (1/T) have a linear relationship, and extrapolations to lower temperatures are easy to realize. However, the accuracy of this approach in more complex thermal degradation processes (e.g., based on free radical reaction sequences) that have many individual reaction steps is often controversial. Despite these concerns, certain degradation processes can be well described using a simple linear Arrhenius relationship. It was shown that the key weakness is often the limited available experimental data and the necessity of extrapolation of several orders of magnitude to cover ambient aging conditions, which may take decades of aging. To expand the confidence in the extrapolation, more comprehensive data sets covering a large temperature range are required [
35,
36].
The presented work aims to gain further insight into the catalytic degradation mechanism and its impact on the properties of two types of PFPE lubricants that are commonly used in space applications. The aging of lubricants, in general, is accelerated at higher temperatures [
37]. A possible correlation between degradation behavior under accelerated aging and the behavior under long-term storage (e.g., on-ground storage in ambient conditions) is explored, and characterization methods that give insight into the degradation pathway and how it can be used to characterize the properties of the lubricants are investigated.
2. Materials and Methods
2.1. Materials
Fomblin® Z25 (Batch 31221SA) was purchased from MasCom Technologies GmbH (Bremen, Germany). Krytox™ 143AC (Batch K5236) was supplied by H. Costenoble GmbH & Co. KG (Eschborn, Germany). Iron(III) fluoride (FeF3) was obtained from Sigma-Aldrich. All materials were used without further treatment.
2.2. Gravimetric Analysis
A micro-balance (MC 210 S, Sartorius AG, Göttingen, Germany) was used to determine the sample mass during storage at increased temperatures. Five individual samples were prepared for each aging condition, and the mass of the sample vials, including the lubricant and catalyst, was determined after defined intervals with a duration starting from 12 h up to a cumulative duration of 2000 h. The samples were removed from the oven after each aging interval, and the weight was determined after cooling off for approximately 20 min. Reference samples of both lubricants were stored at room temperature. The mass loss was evaluated by calculating the amount of evaporated products based on the difference in mass.
2.3. Fourier-Transform Infrared (FTIR) Spectroscopy
FTIR measurements were carried out on the FTIR spectrometer Spectrum Two (Perkin Elmer, Waltham, MA, USA) in attenuated total reflectance (ATR) mode. Approximately 10 μL of the liquid samples was applied on the ATR crystal. Four scans with a resolution of 4 cm−1 in the range between 4000 cm−1 and 650 cm−1 were accumulated, and peak locations and absorption peak areas were calculated with the software ”Spectrum” by Perkin Elmer.
2.4. Evolved Gas Analysis (EGA)
Combined thermal and infrared analysis (TG-IR), also referred to as evolved gas analysis (EGA), was carried out on an EGA 4000 instrument (Perkin Elmer, USA). Measurements were performed with approximately 5 mg of lubricant samples containing 1 wt.% FeF3 catalyst weighed into aluminum pans. The samples were heated from 40 °C to 600 °C with a heating rate of 10 K/min under N2-atmosphere. FTIR spectra were recorded at five-second intervals over the entire measurement duration and obtained in a spectral range between 4000 cm−1 and 650 cm−1 with a resolution of 4 cm−1.
2.5. Cone Plate Rheometry
Dynamic viscosity measurements of the lubricant samples were carried out on an MCR702 rheometer (Anton Paar, Graz, Austria) equipped with the CP50-0.5 cone-plate measurement setup (50 mm diameter, 0.5° angle). Approximately 325 μL of the sample was applied onto the rheometer plate, and shear rate sweep tests at shear rates ranging from 1 s−1 to 100 s−1 were conducted at 20 °C.
2.6. Tribological Testing
The friction behavior of the PFPE lubricants was investigated in a ball-on-disc configuration using a Universal-Micro-Tribometer UMT-2 (Bruker, Billerica, MA, USA) equipped with a DFH-10-G force sensor for the range of 1 N to 100 N. The ball-on-disc sliding tests were carried out using AISI 440C (1.4125) steel balls (HSI-Solutions GmbH, Wien, Austria) with a diameter of 6 mm on discs of the same material. Discs with a diameter of 81 mm, a thickness of 3.55 mm, and an average roughness (Ra) between 0.5 μm and 0.8 μm were used. Prior to the measurement, the discs were degreased with acetone, and for each measurement, 20 mg of lubricant was applied to the disc with a piston-operated pipette. A stencil was used during the application to ensure consistent positioning of the applied lubricant for each measurement. After the application of the lubricant, 5 revolutions with a pre-load of 1 N and a velocity of 10 rpm were performed in order to achieve an even distribution on the surface. After this initial procedure, the tests were conducted with a constant normal load of 25 N, a sliding speed of 0.15 m/s for 90 min, and an accumulated sliding distance of 810 m.
2.7. Accelerated Thermo-Catalytic Aging
Thermo-catalytic aging of all samples was performed in standard UT6060 convection ovens (Heraeus, Hanau, Germany) in ambient air. For the accelerated aging tests, 2.5 mL of liquid lubricant was weighed into 10 mL glass head-space vials, and for samples including the catalyst, 1 wt.% of FeF3 was added and homogeneously distributed within the liquid lubricant sample. All samples were sealed with aluminum lids without a septum in order to allow gas exchange and prevent overpressure.
3. Results
The influence of accelerated thermo-catalytic aging on the lubricants’ properties was investigated for two different types of liquid perfluoropolyether (PFPE) lubricants, Z-type Fomblin
® Z25 and K-type Krytox™ 143AC. To investigate the aging mechanism and degradation processes and the related changes to the physico-chemical properties of the lubricants, accelerated aging was performed at three temperature levels (180 °C, 200 °C, and 220 °C) in ambient air for multiple aging durations up to a maximum of 2000 h. Samples of both lubricants, with and without the addition of FeF
3 as a catalyst, were kept in individual glass vials and enclosed in convection ovens under an ambient atmosphere for the respective aging duration, and the different analyses were conducted subsequently after reaching the target aging duration of each sample. Typical on-ground storage conditions included ISO8 or ISO5 cleanroom conditions primarily to avoid particulate contamination. Furthermore, nitrogen purging was sometimes employed as an additional protective measure [
33,
34]. PFPE oils were found to be less stable in environments that contained oxygen. However, Sianesi et al. [
22] concluded that the decomposition temperature for PFPEs in air and inert atmosphere are close in proximity to each other.
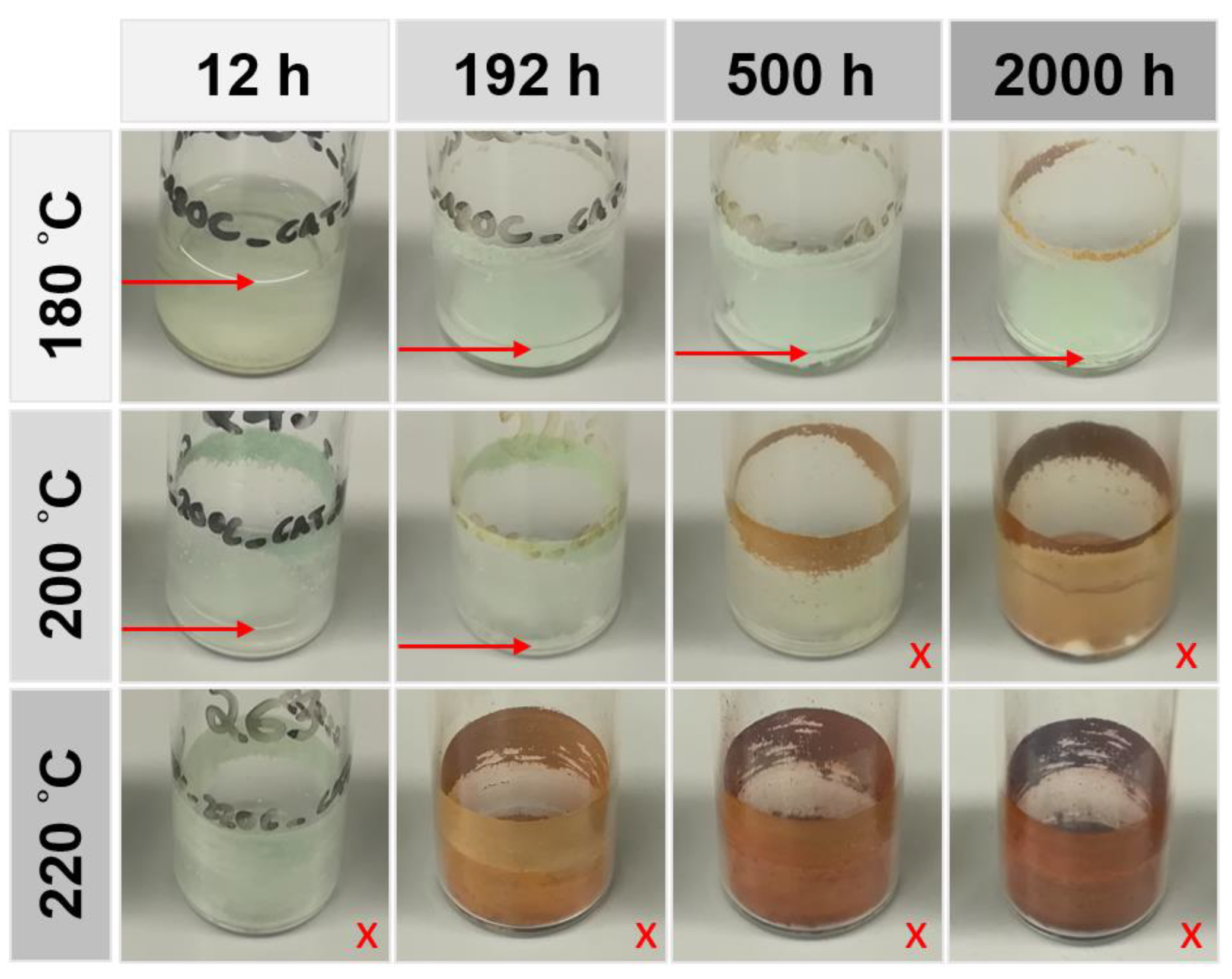
Samples that were aged for long periods were regularly manually mixed to reduce the impact of diffusion effects or large differences in the relative surface area of catalytic species, e.g., by agglomeration. Selected images of the aged samples are shown in
Figure 2.
No visible changes occurred in samples of Fomblin® Z25 and Krytox™ 143AC where no FeF3 was added, independent of aging temperature and duration. A distinctive change in color from light green to a brownish hue was observed in the FeF3 catalyst with rising aging temperatures and durations. The samples of Fomblin® Z25, which contained 1 wt.% of the catalyst, exhibited rapid mass loss. In contrast, no visible changes were detected in samples of Krytox™ 143AC, which contained the catalyst at all tested temperature levels and aging durations. This behavior is explored in detail within the following chapters.
3.1. Gravimetric Analysis
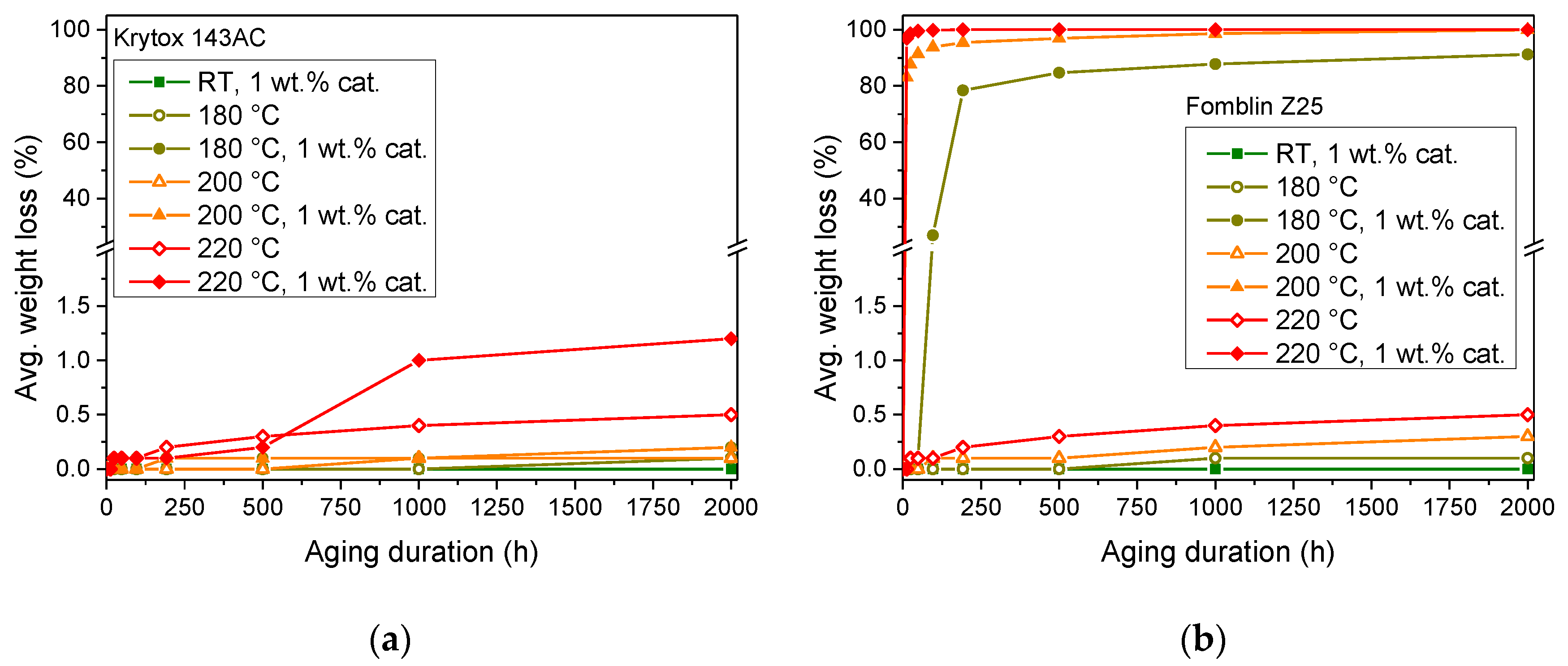
Gravimetric analysis was conducted to monitor the changes in sample mass during accelerated aging and detect the possible formation of volatile decomposition products. The measured averaged weight loss of samples of the Z-type and the K-type lubricant are depicted in
Figure 3. These measurements aim to indicate the degree of degradation over time.
The gravimetric analysis of Krytox™ 143AC generally shows a low mass loss compared to the results of Fomblin® Z25. The first signs of an impact of the catalyst on the K-type lubricant may be visible after 1000 h at 220 °C (1.0% mass loss). However, this is not ascertainable within the limited duration of the gravimetric analysis. Since no rapid evaporation occurs, the autocatalytic regime was not reached. Samples of Krytox™ 143AC, which contain 1 wt.% of FeF3, reached a maximum average mass loss of 1.2 % after 2000 h at 220 °C. For samples where no catalyst was added, 0.5 % mass loss was measured after 2000 h at 220 °C.
Fomblin® Z25 with 1 wt.% of FeF3 exhibited rapid mass loss, with 96.9% lost after only 12 h at 220 °C. After longer aging durations at this temperature level, the mass loss further increased and reached 100% after 192 h. Samples of Fomblin® Z25 aged at 200 °C show a mass loss of 83.1% after 12 h, with an increasing mass loss and 100% evaporation of the sample after the full aging duration of 2000 h. At 180 °C, an induction period with only a minor mass loss of 0.1% occurred, followed by a clearly noticeable mass loss after 96 h. However, only two of the five samples exhibited a noticeable mass loss of 69.8% and 65.1%, respectively. At the longer aging durations, all five samples aged at 180 °C in the presence of the catalyst demonstrated pronounced evaporation. The mass loss reached levels between 71.6% and 82.7% after a duration of 2000 h. Samples of the Z-type PFPE, which did not contain the catalyst, exhibited comparatively low mass loss of 0.5% at 220 °C, 0.3% at 200 °C, and 0.1% at 180 °C after 2000 h.
3.2. Fourier Transform Infrared (FTIR) Spectroscopy
FTIR spectra were obtained for both types of investigated lubricants to identify non-volatile degradation products and changes to the chemical structure of the lubricants during accelerated aging. A comparison between the spectra of the pristine lubricant and the spectra obtained after aging at increased temperatures for several aging durations is shown in
Figure 4.
The most prominent signals for the obtained FTIR spectra of Krytox™ 143AC appear in the range of 1360 cm
−1 and 930 cm
−1. The signals at 1303 cm
−1, 1226 cm
−1, and 1179 cm
−1 correspond to C-F stretching vibrations of CF
3 and CF
2 groups, and the peak at 1116 cm
−1 is related to stretching vibrations of the C-O bonds. The sharp signal at 980 cm
−1 is associated with stretching vibrations of the pendant CF
3 groups. For the K-type lubricant, no significant changes occur in the FTIR spectra after thermo-catalytic aging at the different aging temperatures and aging durations up to 2000 h. The FTIR spectra of Fomblin
® Z25 show the most prominent signals in a spectral range between 1370 cm
−1 and 925 cm
−1, which is attributed to combined signals of C-F stretching and C-O-C stretching modes (CF
2 stretching vibrations at 1184 cm
−1, CF
2 deformation vibrations at 684 cm
−1, coupled stretching vibrations of C-O-C and C-F bonds at 1043 cm
−1, and a signal involving C-O-C deformation at 814 cm
−1) [
5,
38,
39].
In lubricant samples of Fomblin
® Z25, where partial degradation and mass loss occurred due to the addition of FeF
3 as a catalyst, changes in the FTIR spectra could be detected. A new emerging sharp signal at 1780 cm
−1 assigned to C=O stretching vibrations, a broad weak signal between 3400 cm
−1 and 2700 cm
−1, assigned to O-H stretching vibrations can be observed, and an apparent shift of the C-O band at 1043 cm
−1 to higher wavenumbers is noticed in the FTIR spectra. These changes are explained by the formation of acyl fluoride due to the catalytic decomposition of the Z-type PFPE lubricant at the acetal unit (-O-CF
2-O-), with subsequent hydrolysis to carboxylic acids and formation of hydrogen fluoride. Hydrolysis and carboxylic acid formation are explained by the presence of residual moisture within the samples [
40]. The mentioned changes to the spectra were only observed in spectra that exhibit partial degradation, starting from samples after accelerated aging for 48 h at 180 °C (no changes were detected after 12 h and 24 h) and 12 h at 200 °C. Due to the rapid mass loss of Fomblin
® Z25 samples containing FeF
3, the number of samples available for characterization was limited to an aging duration of 2000 h at 180 °C and 192 h at 200 °C. Furthermore, the increase in characteristic signals for C=O and O-H moieties is most pronounced for the sample after 48 h of aging duration and decreases again after 96 h and 192 h, respectively. Additionally, a stronger shift of the C-O band to higher wavenumbers is initially seen. Samples aged for more than 500 h do not show characteristic signals for C=O and O-H groups. However, the shift of the C-O peak is still present.
3.3. Evolved Gas Analysis (EGA)
Evolved gas analysis (EGA) was performed to investigate the volatile species generated under catalytic decomposition of the PFPE lubricants. This measurement technique combines thermogravimetric analysis (TGA) with FTIR spectroscopy. Thus, conclusions on the evolved gaseous species under a defined heating profile can be drawn. Measurements were conducted for both types of PFPE lubricants, with added 1 wt.% of FeF3 in order to corroborate the proposed decomposition mechanism and identify the gaseous decomposition products.
The evolution of the sample weight over time is depicted in
Figure 5a (Fomblin
® Z25) and
Figure 5c (Krytox™ 143AC). The mass loss of Fomblin
® Z25 has an approximate onset temperature of 200 °C and proceeds rapidly, reaching the zero-level at approximately 250 °C. In comparison, Krytox™ 143AC displays a slower instating mass loss at a significantly higher temperature between 250 °C and 300 °C, and the zero-value is only reached above 400 °C.
The obtained FTIR spectra of the volatile gaseous species detected during the thermo-catalytic decomposition of Fomblin
® Z25 and Krytox™ 143AC are shown in
Figure 5b,d, respectively. At the measurements’ beginning and before the decompositions’ onset (spectra taken at 100 °C), no signals other than a peak at approximately 2360 cm
−1 are detected. This peak is assigned to atmospheric influences and, therefore, not further considered. Exemplary spectra obtained during both materials’ main decomposition phase are displayed. Both spectra were obtained at 50% weight loss, at 227 °C for the Z-type and 370 °C for the K-type lubricant.
The spectra obtained from volatile decomposition products of Krytox™ 143AC show similar signals related to C-F and C-O-C stretching vibrations in the range from 1400 cm
−1 to 1050 cm
−1. Additionally, a peak related to CF
3 groups, characteristic for K-type PFPE lubricants, is detected at 908 cm
−1. The spectra of the volatile species generated from the decomposition of Fomblin
® Z25 show characteristic signals in the range of 1980 cm
−1 to 1870 cm
−1, which are attributed to C=O stretching of the acyl fluoride species due to cleavage of the perfluoropolyether molecules. No indication of hydrolysis of the degradation products is observed, since the measurements were performed in dry N
2 atmosphere. Signals between 1300 cm
−1 to 1100 cm
−1 are assigned to stretching vibrations of C-F and ether bonds from the volatile species [
40]. These results are in line with previously reported results where the decomposition products of PFPE fluids contain COF
2, CF
3COF, perfluoro olefins, and volatile acid fluorides in inert atmospheres. In contrast, additional hydrogen fluoride may occur in moist atmospheres [
6].
3.4. Cone Plate Rheometry
A cone plate rheometer setup was used to perform dynamic viscosity measurements of the pristine and aged lubricants in order to investigate a potential impact on the dynamic viscosity during accelerated aging due to changes in molecular structure (e.g., chain scission).
Figure 6a depicts the initial characterization of both lubricants through shear rate sweep tests, where a mean value was calculated from eleven measurement points between 1 s
−1 and 100 s
−1 with three repetitions per sample. In comparison,
Figure 6b represents mean dynamic viscosity values of the aged samples over the aging duration.
Dynamic viscosity measurements of Krytox™ 143AC show no clear impact of the accelerated aging regime, independent of the addition of FeF3 to the lubricant samples. All obtained viscosity values lie in the range of the pristine sample (1645 mPa·s). Some scattering of the measured values is observed; this is most likely related to the measurement method (e.g., dependence on the accuracy of the sample quantity or gap trimming). As no significant changes at short aging durations were expected, samples were only measured upwards of 192 h to reduce the measurement workload to a reasonable level.
Due to the cleavage and subsequent evaporation of Fomblin® Z25 during thermo-catalytic aging, insufficient sample volume for viscosity measurements at longer aging durations was available. Nonetheless, before complete evaporation occurred, samples of the Z-type lubricant exhibited a clear decrease in the dynamic viscosity after accelerated aging in the presence of FeF3. A significant reduction in the average dynamic viscosity was observed in samples that exhibited pronounced mass loss in gravimetric analysis. However, if no significant mass loss was detected, no strong change in viscosity arose either. For samples aged at 180 °C, the average dynamic viscosity dropped to 51 mPa∙s at the 48 h measurement. The measured values were 83 mPa∙s and 110 mPa∙s after 96 h and 192 h of aging, respectively. At the aging temperature of 200 °C, only the 12 h sample contained enough volume to carry out a viscosity measurement, which resulted in an average dynamic viscosity of 53 mPa∙s. As observed in gravimetry, at 220 °C an immediate degradation occurred and the Z-type lubricant could not be further analyzed. The dynamic viscosities of the samples of Fomblin® Z25, which did not contain the catalyst, are all in the same order of magnitude of the values measured for the unaged material (462 mPa·s).
3.5. Tribological Testing
The tribological performance of the aged lubricants was evaluated using tribological testing in a ball-on-disc (BoD) setup, which provides a unidirectional sliding point-loaded contact and can be used to simulate boundary-lubricated contacts [
41]. The selected test parameter and contact conditions (Hertzian stress) were based on previous experimental work [
42,
43]. Exemplary curves of the evolution of the coefficient of friction (COF) of the two investigated PFPE lubricants are depicted in
Figure 7. Tribological testing was conducted with the pristine samples, as well as aged samples with and without the addition of 1 wt.% FeF
3.
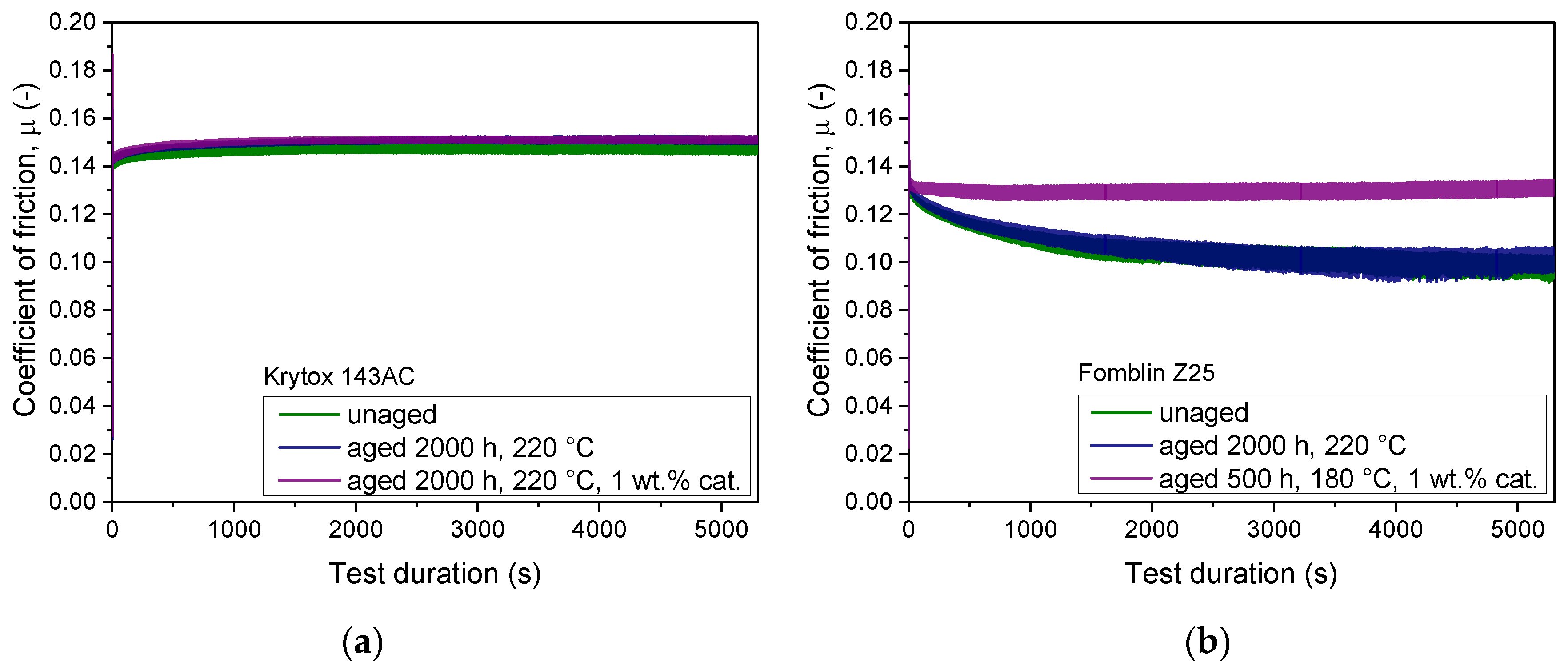
Comparison of the evolution of coefficient of friction shows a smooth running-in behavior of both types of PFPE lubricants. The COF value of the K-type lubricant (
Figure 7a) increases during running-in until it approaches a constant level after approximately 1500 s. In contrast, the running-in phase of the Z-type lubricant (
Figure 7b) lasts considerably longer and within this phase the COF steadily declines. The mean coefficient of friction of Krytox™ 143AC after running-in is around 0.15, whereas Fomblin
® Z25 reaches a significantly lower mean COF of approximately 0.10. Ball-on-disc tribological tests of Krytox™ 143AC show a very consistent evolution of the coefficient of friction, independent of aging temperature, aging duration, or the addition of 1 wt.% of catalyst in all investigated accelerated aging conditions. Samples of Fomblin
® Z25, which were aged without the addition of the catalyst, demonstrate no distinct changes in the coefficient of friction curves compared to the pristine material. However, thermo-catalytic aging of Fomblin
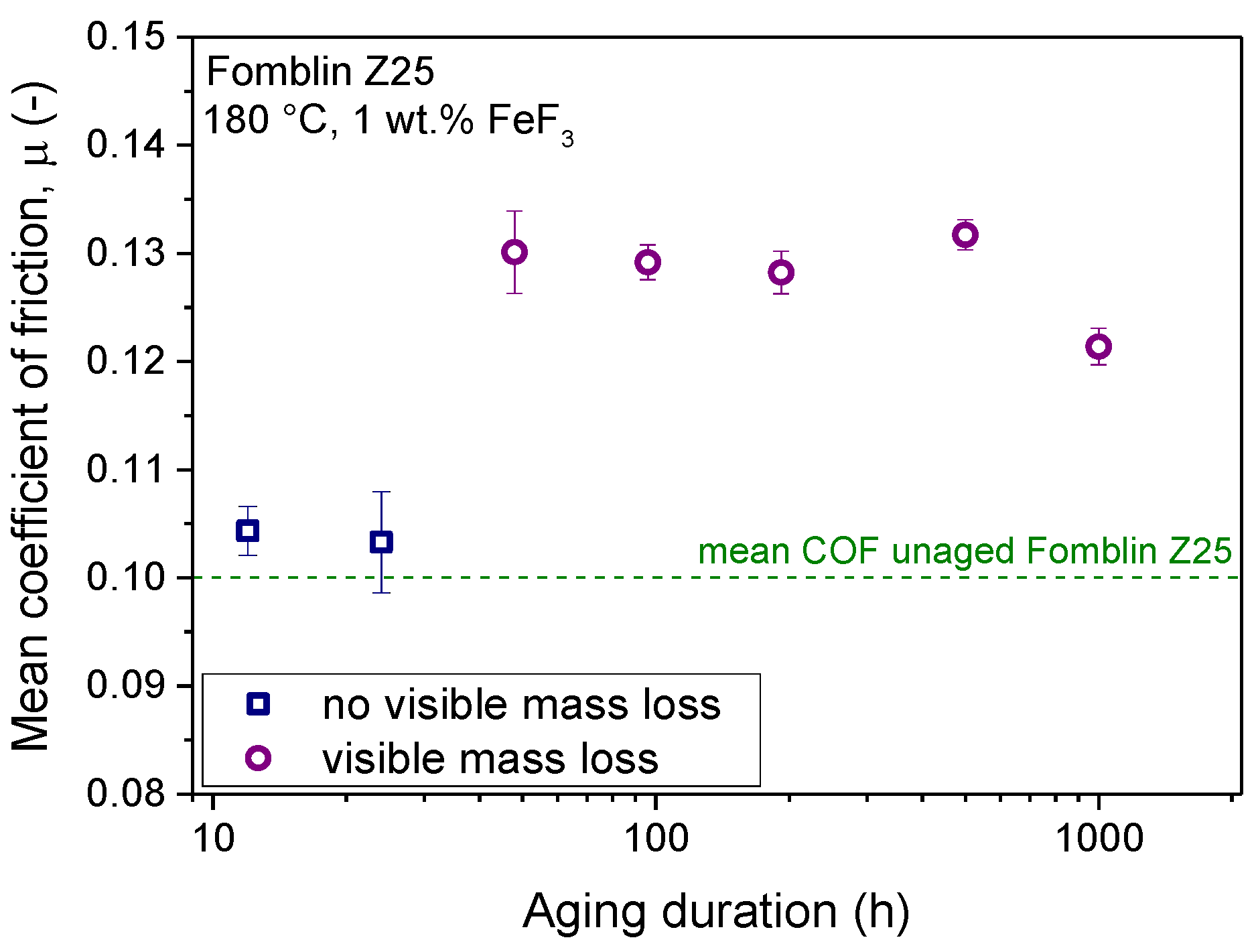
® Z25 led to an increase in the mean coefficient of friction in samples where visible mass loss occurred. This increase led to a mean coefficient of friction around 0.13, although no clear trend between the magnitude of the increase in COF and the aging duration can be established. These elevated mean COF values of the aged Z-type lubricant are compared in
Figure 8.
4. Discussion
In the presented work, accelerated aging tests of two different types of PFPE lubricants were performed. Fomblin® Z25, a linear Z-type lubricant, and Krytox™ 143AC, a branched K-type lubricant with pendant trifluoromethyl groups (-CF3), were selected. A catalyst, 1 wt.% of iron(III) fluoride (FeF3), was added to induce degradation in the PFPE lubricants via a thermo-catalytic degradation mechanism. This comparably high amount of catalytic species was intentionally selected to accelerate the degradation and reduce the impact of catalyst amount on the degradation rate. Thermo-oxidative aging was performed for selected aging durations up to 2000 h at 180 °C, 200 °C, and 220 °C in convection ovens in ambient air. Initial analysis and further analysis during accelerated aging at regular intervals were performed to monitor changes in the lubricants’ physico-chemical properties. The proposed degradation mechanism of the lubricant by a Lewis acid-induced catalytic process describes the acetal unit (−O−CF2−O−) as a starting point for the decomposition. Due to the lack of acetal units in branched K-type PFPE lubricants, such as Krytox™ 143AC, these lubricants are expected to show higher stability in this thermo-catalytic degradation process.
To be able to monitor the changes in sample mass and detect the possible formation of volatile decomposition products during accelerated aging and compare it to reference samples kept in ambient conditions, gravimetric analysis was conducted. Pristine Fomblin® Z25 and Krytox™ 143AC behaved in a comparable manner, with minor mass loss of a maximum of 0.5% after 2000 h at 220 °C, which is attributed to evaporation losses. In contrast, the addition of 1 wt.% of FeF3 led to rapid degradation of Fomblin® Z25 (96.9% mass loss after 12 h at 220 °C), whereas the mass loss of Krytox™ 143AC did not exceed 1.2%, even after 2000 h of aging at 220 °C.
Previous studies support the rapid decomposition of Z-type PFPEs due to chain scission, which occurs randomly throughout the polymer chains. This was also established here during gravimetric analysis. In contrast, during the degradation of K-type PFPEs, the remaining fluid’s molecular weight (chain length) decreased more monotonically because of the stepwise loss of monomer units at the chain ends [
29]. The measured 1.2% mass loss of Krytox™ 143AC after 2000 h of aging at 220 °C might be an indicator of instating degradation of the lubricant. However, as no additional longer aging period was investigated, this alone is not sufficient to conclude whether this mass loss is related to thermo-catalytic degradation. Differences between both types of lubricants also were recognizable during FTIR spectroscopy, where non-volatile degradation products and changes to the chemical structure of the lubricants due to degradation were investigated. During the accelerated aging of Fomblin
® Z25 samples, which contain 1 wt.% of FeF
3, additional emerging signals were assigned to C=O stretching vibrations and O-H stretching vibrations. In addition, an apparent shift of the C-O band was noticed. A plausible explanation for these findings would be a chain scission due to catalytic degradation and the formation of carboxylic acid species due to the presence of residual moisture. No visible changes occurred to the spectra of Krytox™ 143AC samples independent of aging temperature, duration, or the addition of the catalyst. Dynamic viscosity measurements furthermore supported those results. Fomblin
® Z25 demonstrated a decrease in the dynamic viscosity of partially degraded samples compared to the lubricant without any catalyst addition or lubricant samples with the catalyst where no visible mass loss had yet occurred. The observed behavior of an initially substantial reduction in viscosity, followed by complete evaporation, further underlines that in Z-type lubricants, chain scission reactions are expected both in mid-sections of the molecule and at end sections. Krytox™ 143AC demonstrated no distinguishable impact of the FeF
3 catalyst on the dynamic viscosity after accelerated aging. Evolved gas analysis (EGA) exhibited a significant gap between the onset temperatures of degradation of both types of lubricants. The catalytic decomposition of the Z-type PFPE started at approximately 200 °C, whereas the K-type PFPE had an onset temperature higher than 250 °C. Furthermore, the decomposition of Fomblin
® Z25 occurred more rapidly compared to Krytox™ 143AC. The recorded spectra obtained from volatile decomposition products exhibited similar signals related to C-F and C-O-C stretching vibrations. Krytox™ 143AC demonstrated a peak related to CF
3 groups, which is characteristic of K-type PFPE lubricants. Additionally, signals related to C=O stretching of the acyl fluoride species due to cleavage of the Z-type PFPE were detected in Fomblin
® Z25. No indication of hydrolysis of the degradation products was observed since the experiments were conducted under a dry N
2 atmosphere. Ball-on-disc sliding tests were carried out on AISI 440C (1.4125) steel discs with AISI 440C (1.4125) steel balls as a counterpart. In the tribological tests, an increase in the mean coefficient of friction was observed for samples of Fomblin
® Z25, which partially degraded due to the presence of FeF
3. However, other than this increase, no clear trend of the evolution of the coefficient of friction and the aging duration could be established. Aged samples of Fomblin
® Z25 where no catalyst was added did not show any change in the evolution of friction compared to the pristine material. This preservation of lubricating properties also applies to all aged samples of Krytox™ 143AC with and without the addition of FeF
3.
The performed accelerated aging experiments and selected characterization techniques are suitable for detecting changes caused by thermo-catalytic degradation in perfluoropolyether lubricants. However, concerning the extrapolation of results obtained at high aging temperatures to ambient conditions, caution should be exercised because errors in lifetime estimations can result from deviations in the degradation mechanism. Aging experiments at lower temperatures may not lead to identifiable degradation in a reasonable timeframe for experiments due to the high stability of the PFPE lubricants.
Accelerated aging data where correlations between different lubricant properties that change during aging, for example, a correlation between viscosity and the mass loss, can be utilized for structural equation modeling (SEM). However, the rapid catalyst-induced evaporation of Fomblin® Z25 resulted in a low number of data points, which prevented a meaningful evaluation of the thermo-catalytic degradation. Samples of Fomblin® Z25 where no FeF3 was added and all samples of Krytox™ did not show any significant changes during accelerated aging. Therefore, no long-term data could be derived. In future aging studies of PFPE lubricants, the overall aging duration should be extended to allow for more moderate aging temperatures, thus enabling a partial degradation without complete evaporation to obtain more data to study the degradation mechanism further. In addition, a detailed consideration of the degradation profile and occurring mechanism should be performed for each investigated material to mitigate the challenges of finding suitable aging conditions across different types of PFPE lubricants based on differences in their degradation mechanism.