Effect of Y on Microstructure and Properties of Al0.8FeCrCoNiCu0.5 High Entropy Alloy Coating on 5083 Aluminum by Laser Cladding
Abstract
1. Introduction
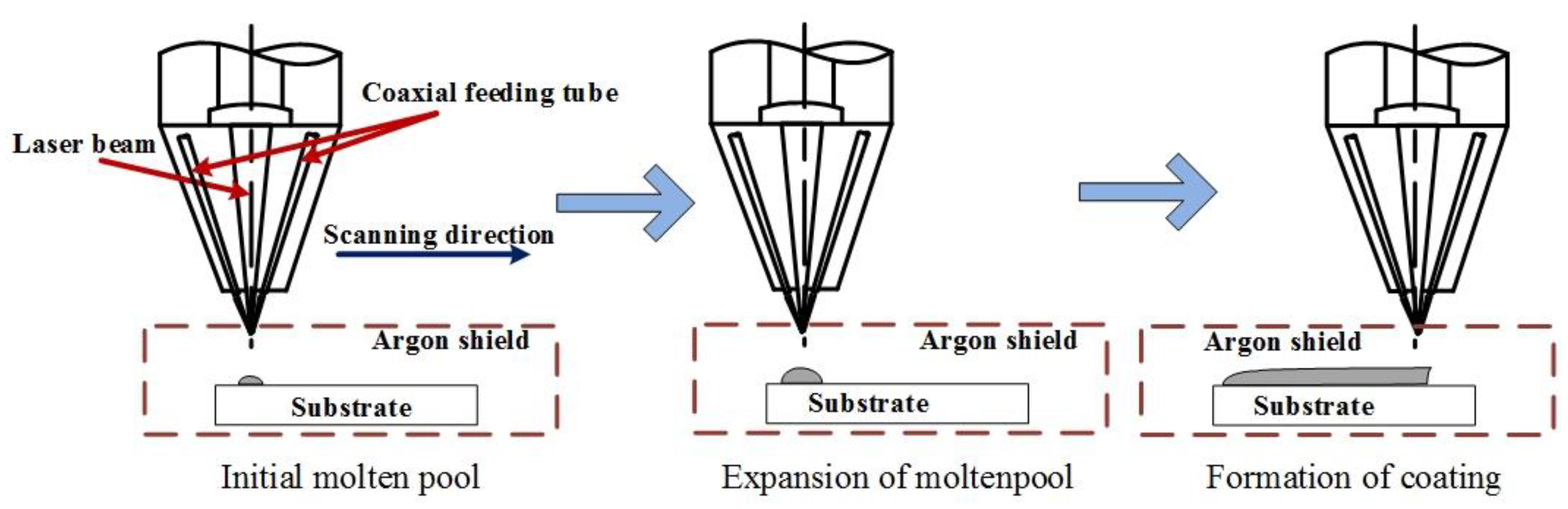
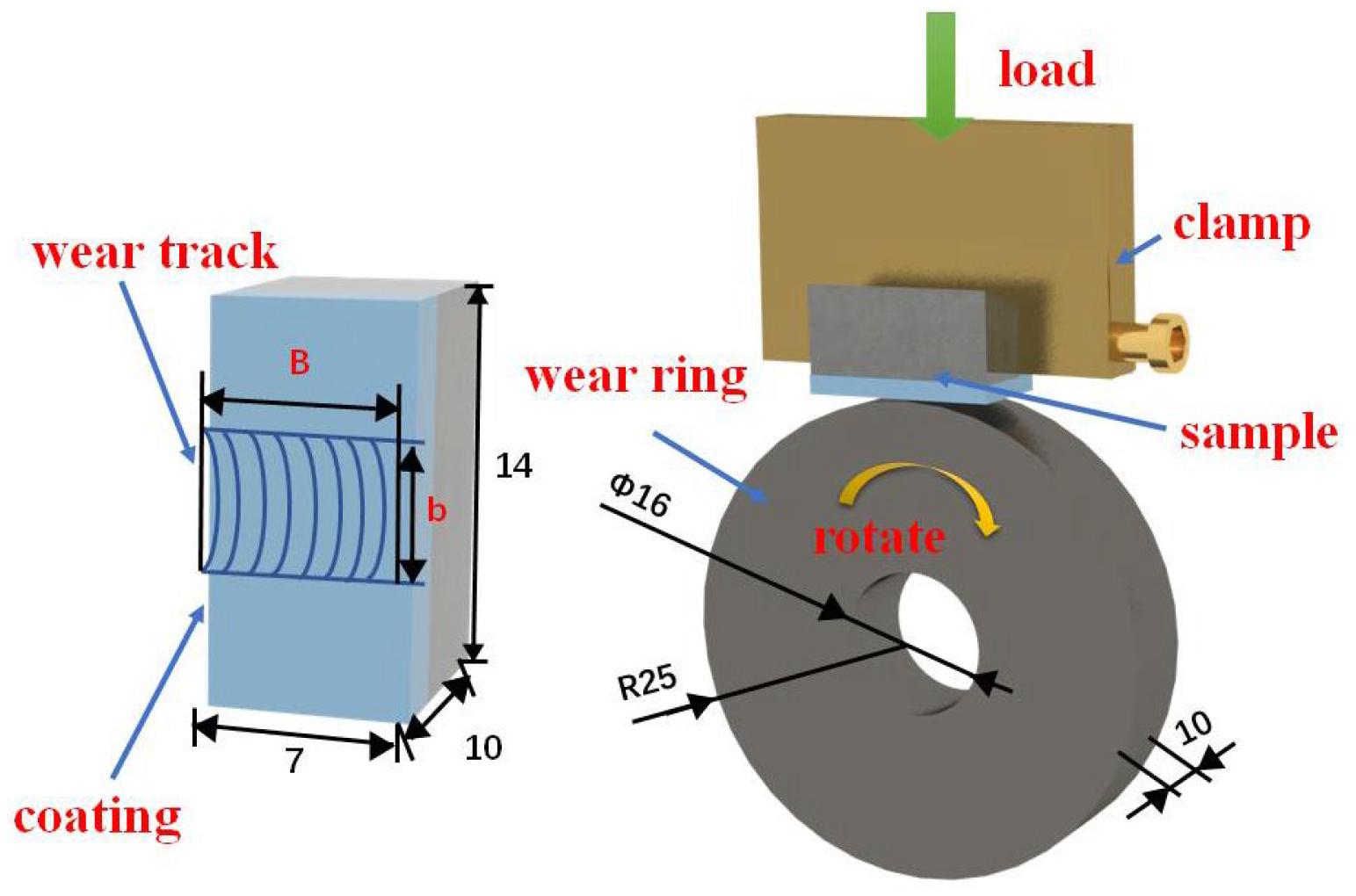
2. Materials and Methods
3. Results and Discussion
3.1. XRD Analysis
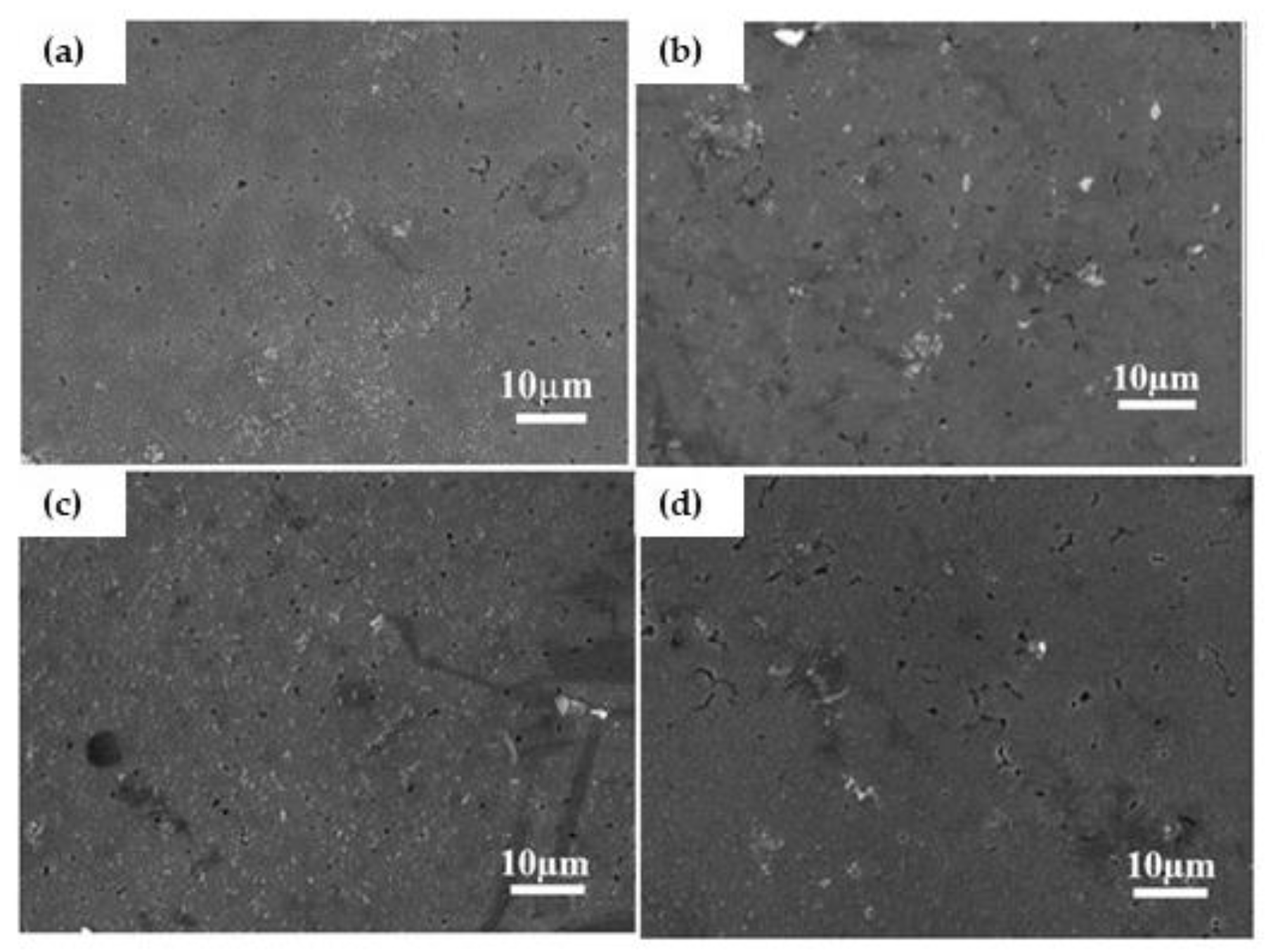
3.2. Microstructure
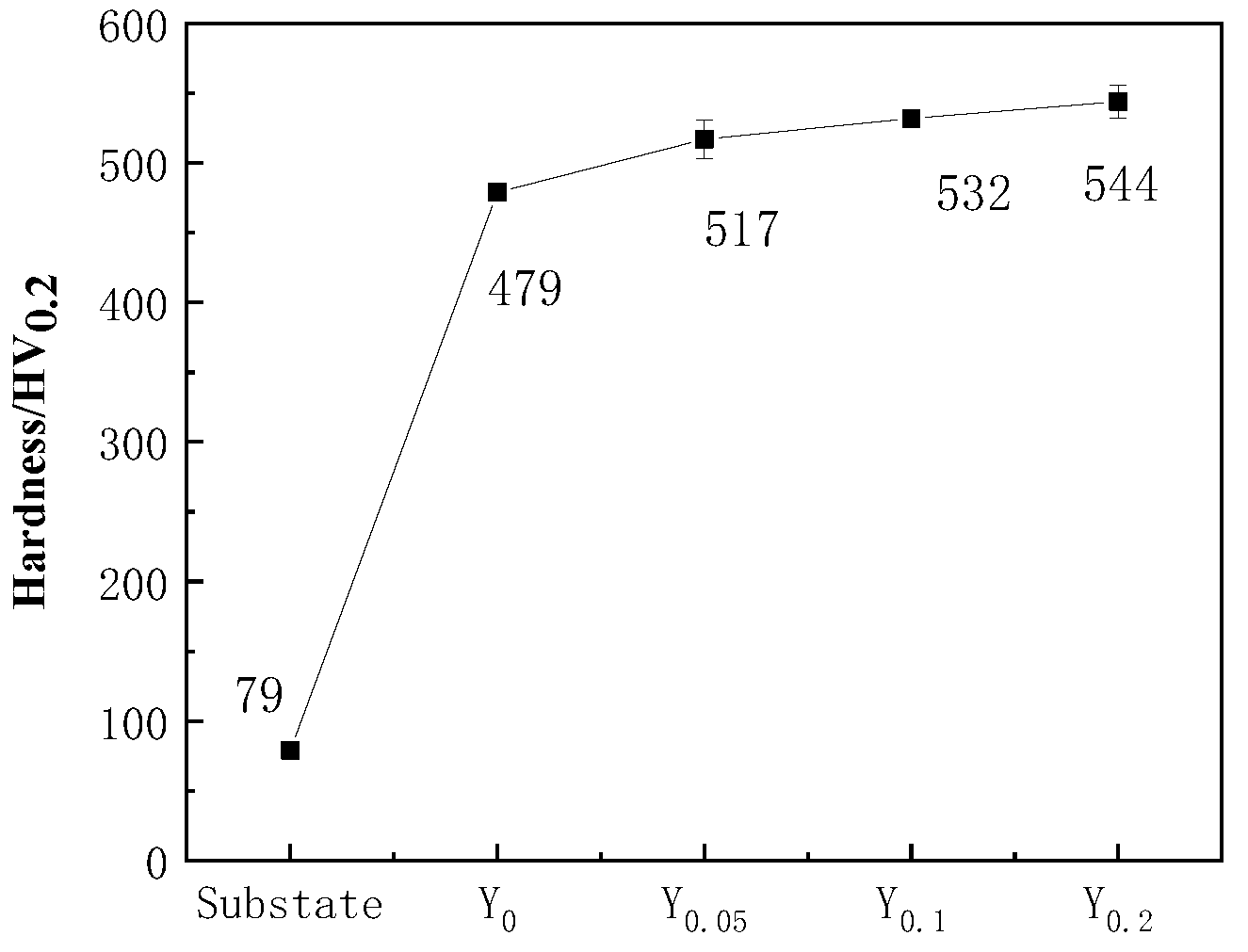
3.3. Hardness
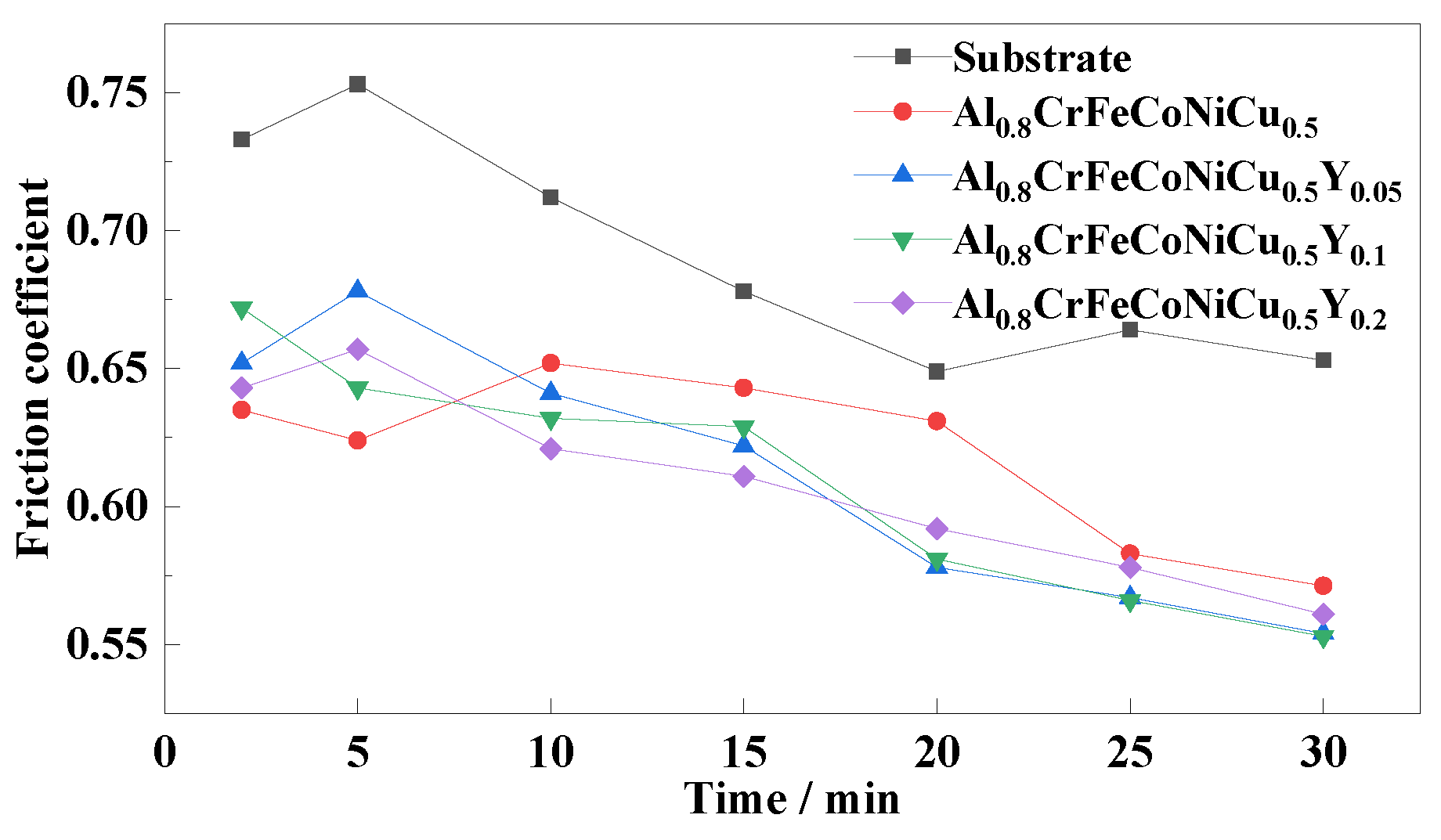
3.4. Friction and Wear Properties
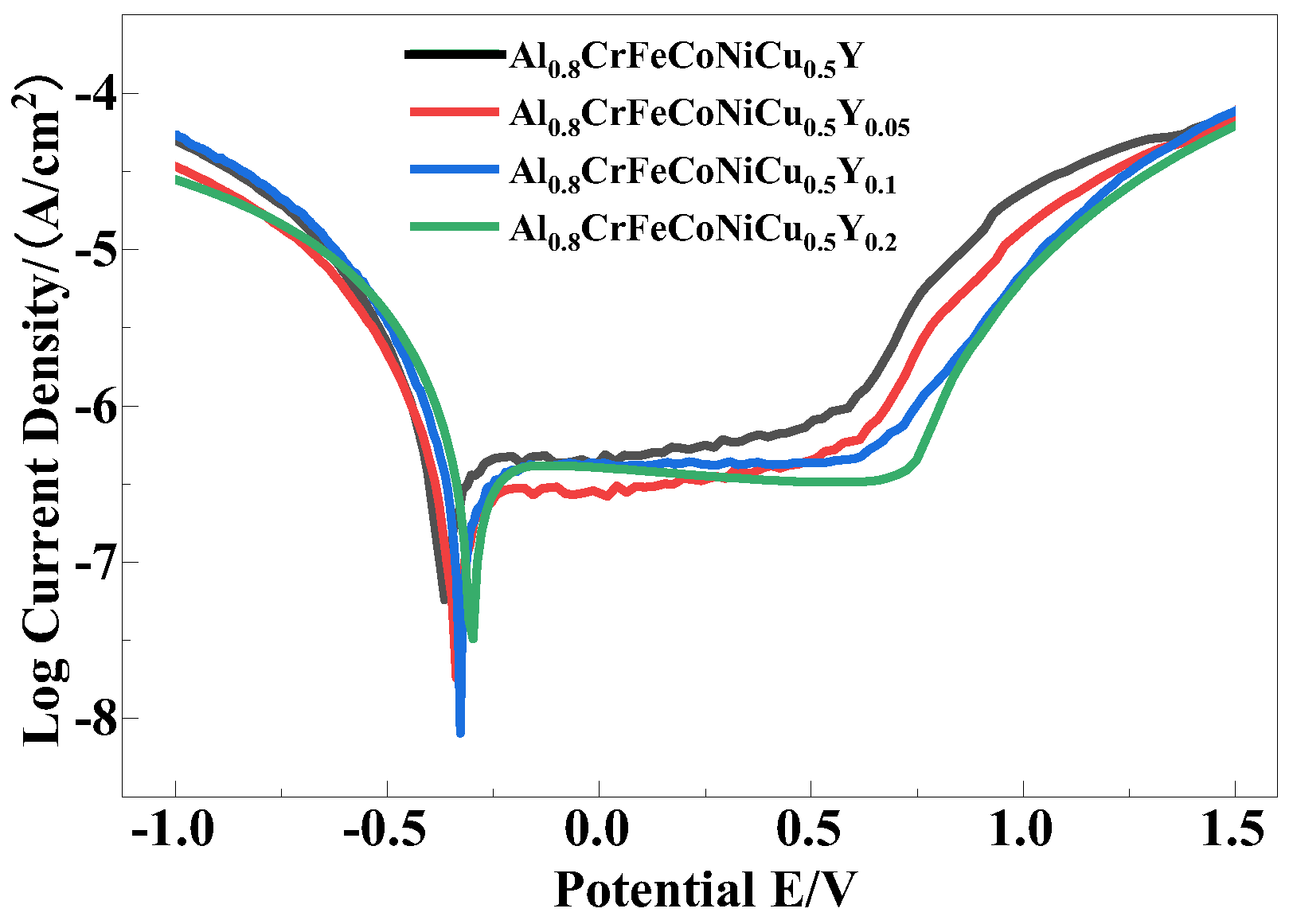
3.5. Corrosion Resistance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Yao, X.; Peng, K.; Chen, X.; Jiang, F.; Wang, K.; Wang, Q. Microstructure and mechanical properties of dual wire-arc additive manufactured Al-Co-Cr-Fe-Ni high entropy alloy. Mater. Lett. 2022, 326, 132928. [Google Scholar] [CrossRef]
- Shi, F.K.; Zhang, Q.K.; Xu, C.; Hu, F.Q.; Yang, L.J.; Zheng, B.Z.; Song, Z.L. In-situ synthesis of NiCoCrMnFe high entropy alloy coating by laser cladding. Opt. Laser Technol. 2022, 151, 108020. [Google Scholar] [CrossRef]
- Ivanov, I.V.; Emurlaev, K.I.; Kuper, K.E.; Akkuzin, S.A.; Bataev, I.A. Deconvolution-based peak profile analysis methods for characterization of CoCrFeMnNi high-entropy alloy. Heliyon 2022, 8, e10541. [Google Scholar] [CrossRef]
- Reunova, K.A.; Astafurova, E.G.; Melnikov, E.V.; Astafurov, S.V.; Panchenko, M.Y.; Moskvina, V.A. Effect of solid-solution treatment on the microstructure and mechanical properties of high-nitrogen FeMnCrNiCo high entropy alloys. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1014, 012047. [Google Scholar] [CrossRef]
- Dewangan, S.K.; Mangish, A.; Kumar, S.; Sharma, A.; Ahn, B.; Kumar, V. A review on High-Temperature Applicability: A milestone for high entropy alloys. Eng. Sci. Technol. Int. J. 2022, 35, 101211. [Google Scholar] [CrossRef]
- Karimzadeh, M.; Malekan, M.; Mirzadeh, H.; Li, L.; Saini, N. Effects of titanium addition on the microstructure and mechanical properties of quaternary CoCrFeNi high entropy alloy. Mater. Sci. Eng. A 2022, 856, 143971. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, T.; Li, G. Research on Wear and Corrosion Resistance of Ni60-WC Coating Fabricated by Laser on the Preheated Copper Alloy. Coatings 2022, 12, 1537. [Google Scholar] [CrossRef]
- Sha, M.; Jia, C.; Qiao, J.; Feng, W.; Ai, X.; Jing, Y.A.; Shen, M.; Li, S. Microstructure and Properties of High-Entropy AlxCoCrFe2.7MoNi Alloy Coatings Prepared by Laser Cladding. Met.–Open Access Metall. J. 2019, 9, 1243. [Google Scholar] [CrossRef]
- Liu, H.; Gao, W.; Liu, J.; Du, X.; Li, X.; Yang, H. Microstructure and Properties of CoCrFeNiTi High-Entropy Alloy Coating Fabricated by Laser Cladding. J. Mater. Eng. Perform. 2020, 29, 7170–7178. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, C.L.; Zhang, C.H.; Guan, M.; Tan, J.Z. Laser surface alloying of FeCoCrAlNi high-entropy alloy on 304 stainless steel to enhance corrosion and cavitation erosion resistance. Opt. Laser Technol. 2016, 84, 23–31. [Google Scholar] [CrossRef]
- Na, Y.S.; Lim, K.R.; Chang, H.J.; Kim, J. Effect of Trace Additions of Ti on the Microstructure of AlCoCrFeNi-Based High Entropy Alloy. Sci. Adv. Mater. 2016, 8, 1984–1988. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, G.J.; Dai, P.Q. Evolution of the microstructure and properties of laser-clad FeCrNiCoBx high-entropy alloy coatings. Mater. Sci. Technol. 2016, 32, 1666–1672. [Google Scholar] [CrossRef]
- Ma, S.G.; Zhang, Y. Effect of Nb addition on the microstructure and properties of AlCoCrFeNi high-entropy alloy. Mater. Sci. Eng. A 2012, 532, 480–486. [Google Scholar] [CrossRef]
- He, Y.Z.; Zhang, J.L.; Zhang, H.; Song, G.S. Effects of Different Levels of Boron on Microstructure and Hardness of CoCrFeNiAlxCu0.7Si0.1By High-Entropy Alloy Coatings by Laser Cladding. Coatings 2017, 7, 7. [Google Scholar] [CrossRef]
- Cai, Y.; Zhu, L.; Cui, Y.; Shan, M.; Han, J. Fracture and wear mechanisms of FeMnCrNiCo + x(TiC) composite high-entropy alloy cladding layers. Appl. Surf. Sci. 2020, 543, 148794. [Google Scholar] [CrossRef]
- Wang, C.; Gao, Y.; Wang, R.; Wei, D.; Cai, M.; Fu, Y. Microstructure of laser-clad Ni60 cladding layers added with different amounts of rare-earth oxides on 6063 Al alloys. J. Alloys Compd. 2018, 740, 1099–1107. [Google Scholar] [CrossRef]
- Wang, C.; Gao, Y.; Zeng, Z.; Fu, Y. Effect of rare-earth on friction and wear properties of laser cladding Ni-based coatings on 6063Al. J. Alloys Compd. 2017, 727, 278–285. [Google Scholar] [CrossRef]
- Das, A.K. Effect of rare earth oxide additive in coating deposited by laser cladding: A review. Mater. Today Proc. 2022, 52, 1558–1564. [Google Scholar] [CrossRef]
- Liang, C.J.; Wang, C.L.; Zhang, K.X.; Liang, M.L.; Xie, Y.G.; Liu, W.J.; Yang, J.J.; Zhou, S.F. Nucleation and strengthening mechanism of laser cladding aluminum alloy by Ni-Cr-B-Si alloy powder based on rare earth control. J. Mater. Process. Technol. 2021, 294, 117145. [Google Scholar] [CrossRef]
- Hu, Z.; Zhan, Y.; Zhang, G.; She, J.; Li, C. Effect of rare earth Y addition on the microstructure and mechanical properties of high entropy AlCoCrCuNiTi alloys. Mater. Des. 2010, 31, 1599–1602. [Google Scholar] [CrossRef]
- Zhang, L.J.; Zhang, M.D.; Zhou, Z.; Fan, J.T.; Cui, P.; Yu, P.F.; Jing, Q.; Ma, M.Z.; Liaw, P.K.; Li, G.; et al. Effects of rare-earth element, Y.; additions on the microstructure and mechanical properties of CoCrFeNi high entropy alloy. Mater. Sci. Eng. A 2018, 725, 437–446. [Google Scholar] [CrossRef]
- Lu, S.F.; Ma, L.; Wang, J.; Du, Y.S.; Li, L.; Zhao, J.T.; Rao, G.H. Effect of configuration entropy on magnetocaloric effect of rare earth high-entropy alloy. J. Alloys Compd. 2021, 874, 159918. [Google Scholar] [CrossRef]
- Guo, K.; Gou, G.Q.; Lv, H.; Shan, M.L. Jointing of CFRP/5083 Aluminum Alloy by Induction Brazing: Processing, Connecting Mechanism, and Fatigue Performance. Coatings 2022, 12, 1559. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, Z.H.; Yue, X.J.; Wang, P.H.; Zhai, Y.C. Water jet impact damage mechanism and dynamic penetration energy absorption of 2A12 aluminum alloy. Vacuum 2022, 206, 111532. [Google Scholar] [CrossRef]
- Lv, B.J.; Wang, S.; Xu, T.W.; Guo, F. Effects of minor Nd and Er additions on the precipitation evolution and dynamic recrystallization behavior of Mg-6.0Zn-0.5Mn alloy. J. Magnes. Alloy. 2021, 9, 840–852. [Google Scholar] [CrossRef]
- Li, Y.; Shi, Y.; Olugbade, E. Microstructure, mechanical, and corrosion resistance properties of Al0.8CrFeCoNiCux high-entropy alloy coatings on aluminum by laser cladding. Mater. Res. Express 2020, 7, 026504. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Wang, Z.T.; Yu, H.; Ning, Y.Q. Microstructural origin and control mechanism of the mixed grain structure in Ni-based superalloys. J. Alloys Compd. 2022, 900, 163515. [Google Scholar] [CrossRef]
- Cui, C.; Wu, M.; Miao, X.; Zhao, Z.; Gong, Y. Microstructure and corrosion behavior of CeO2/FeCoNiCrMo high-entropy alloy coating prepared by laser cladding. J. Alloys Compd. 2022, 890, 161826. [Google Scholar] [CrossRef]
- Arif, Z.U.; Khalid, M.Y.; ur Rehman, E.; Ullah, S.; Atif, M.; Tariq, A. A review on laser cladding of high-entropy alloys, their recent trends and potential applications. J. Manuf. Process. 2021, 68, 225–273. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, J.L.; Gao, Y.R.; Liu, Z.H.; Mai, Q.Q. Effect of heat treatment process on the micro machinability of 7075 aluminum alloy. Vacuum 2023, 207, 111574. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, Z.; Liu, J.; Yu, J.; Mai, Q.; Yue, X. Effect of aging plus cryogenic treatment on the machinability of 7075 aluminum alloy. Vacuum 2023, 208, 111692. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Li, G.; Tang, L. Friction and wear behavior of Ni-based alloy coatings with different amount of WC–TiC ceramic particles. J. Mater. Sci. 2023, 58, 1116–1126. [Google Scholar] [CrossRef]
- Chou, Y.L.; Yeh, J.W.; Shih, H.C. The effect of molybdenum on the corrosion behaviour of the high-entropy alloys Co1.5CrFeNi1.5Ti0.5Mox in aqueous environments. Corros. Sci. 2010, 52, 2571–2581. [Google Scholar] [CrossRef]
- Li, Y.; Shi, Y. Phase assemblage and properties of laser cladded TixCrFeCoNiCu high-entropy alloy coating on aluminum. Mater. Res. Express 2020, 7, 036519. [Google Scholar] [CrossRef]



| Alloy | Abbreviation | Al | Cr | Fe | Co | Ni | Cu | Y |
|---|---|---|---|---|---|---|---|---|
| Al0.8FeCrCoNiCu0.5 | Y0 | 15.84 | 19.80 | 19.80 | 19.80 | 19.80 | 4.95 | 0 |
| Al0.8FeCrCoNiCu0.5Y0.05 | Y0.05 | 14.95 | 18.69 | 18.69 | 18.69 | 18.69 | 9.34 | 0.93 |
| Al0.8FeCrCoNiCu0.5Y0.1 | Y0.1 | 14.81 | 18.51 | 18.51 | 18.51 | 18.51 | 9.25 | 1.85 |
| Al0.8FeCrCoNiCu0.5Y0.2 | Y0.2 | 14.54 | 18.18 | 18.18 | 18.18 | 18.18 | 9.09 | 3.63 |
| Alloy | Length (mm) | Width (mm) | Wear Coefficient (mm3/Nm) |
|---|---|---|---|
| Substrate | 7.00 | 14.00 | 3.50 × 10−4 |
| Al0.8FeCrCoNiCu0.5 | 6.72 | 4.82 | 5.45 × 10−6 |
| Al0.8FeCrCoNiCu0.5Y0.05 | 6.72 | 3.49 | 3.95 × 10−6 |
| Al0.8FeCrCoNiCu0.5Y0.1 | 6.68 | 3.52 | 4.04 × 10−6 |
| Al0.8FeCrCoNiCu0.5Y0.2 | 7.00 | 4.32 | 8.65 × 10−6 |
| Solution | Samples | Ecorr (V) | Icorr (A/cm2) |
|---|---|---|---|
| Substrate [35] | −1.32 | 4.12 × 10−5 | |
| 3.5% NaCl | Al0.8FeCrCoNiCu0.5 | −0.361 | 1.67 × 10−7 |
| Al0.8FeCrCoNiCu0.5Y0.05 | −0.336 | 8.70 × 10−8 | |
| Al0.8FeCrCoNiCu0.5Y0.1 | −0.328 | 6.33 × 10−8 | |
| Al0.8FeCrCoNiCu0.5Y0.2 | −0.298 | 1.54 × 10−7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Shi, Y.; Wang, H.; Zhou, B.; Li, D.; Lin, H.; Wang, J. Effect of Y on Microstructure and Properties of Al0.8FeCrCoNiCu0.5 High Entropy Alloy Coating on 5083 Aluminum by Laser Cladding. Lubricants 2023, 11, 50. https://doi.org/10.3390/lubricants11020050
Li Y, Shi Y, Wang H, Zhou B, Li D, Lin H, Wang J. Effect of Y on Microstructure and Properties of Al0.8FeCrCoNiCu0.5 High Entropy Alloy Coating on 5083 Aluminum by Laser Cladding. Lubricants. 2023; 11(2):50. https://doi.org/10.3390/lubricants11020050
Chicago/Turabian StyleLi, Yanzhou, Yan Shi, Hongxin Wang, Binjun Zhou, Defa Li, Hua Lin, and Junqi Wang. 2023. "Effect of Y on Microstructure and Properties of Al0.8FeCrCoNiCu0.5 High Entropy Alloy Coating on 5083 Aluminum by Laser Cladding" Lubricants 11, no. 2: 50. https://doi.org/10.3390/lubricants11020050
APA StyleLi, Y., Shi, Y., Wang, H., Zhou, B., Li, D., Lin, H., & Wang, J. (2023). Effect of Y on Microstructure and Properties of Al0.8FeCrCoNiCu0.5 High Entropy Alloy Coating on 5083 Aluminum by Laser Cladding. Lubricants, 11(2), 50. https://doi.org/10.3390/lubricants11020050





