Mechanical and Tribological Properties of Ni-B and Ni-B-W Coatings Prepared by Electroless Plating
Abstract
:1. Introduction
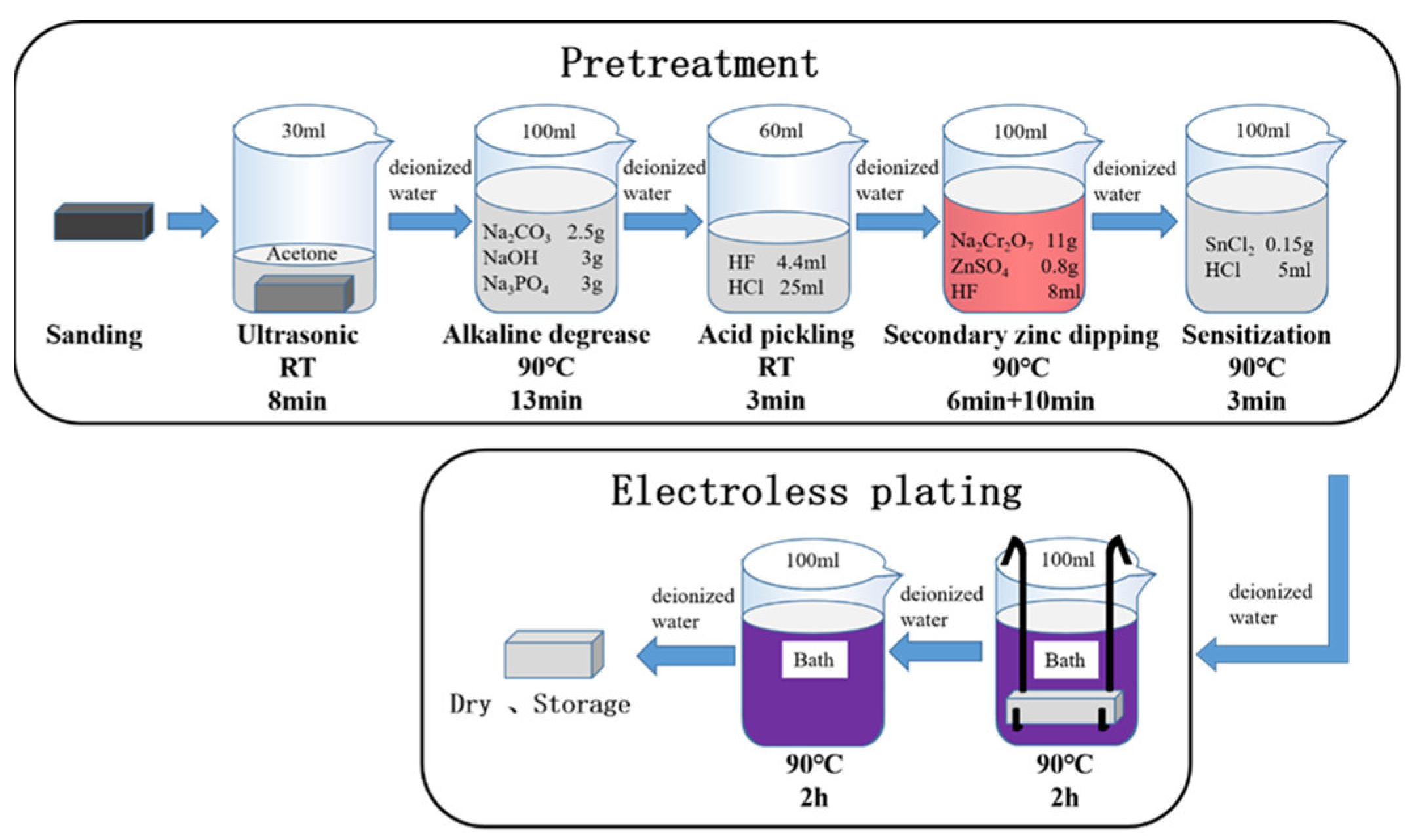
2. Materials and Methods
3. Results and Discussion
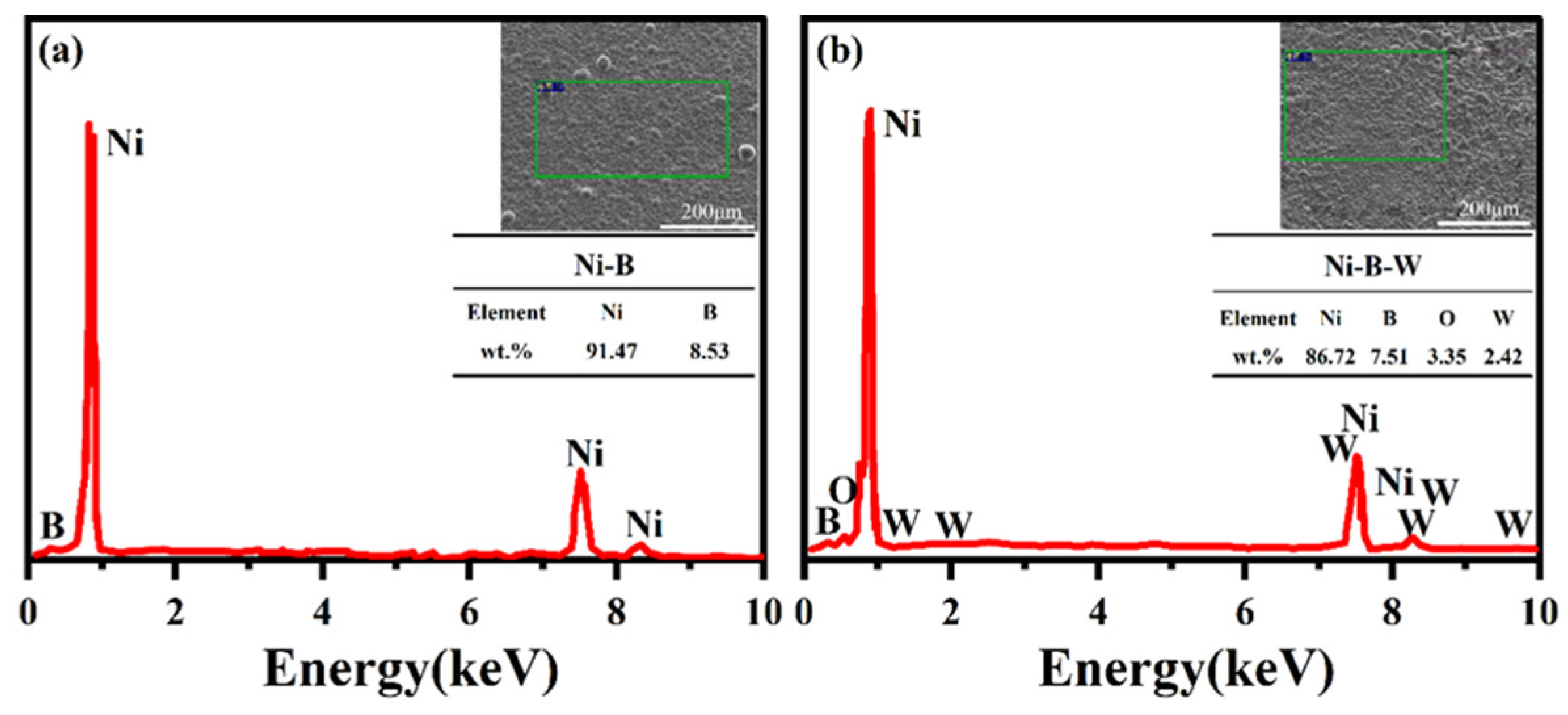
3.1. Compositions of Ni-B and Ni-B-W Coatings
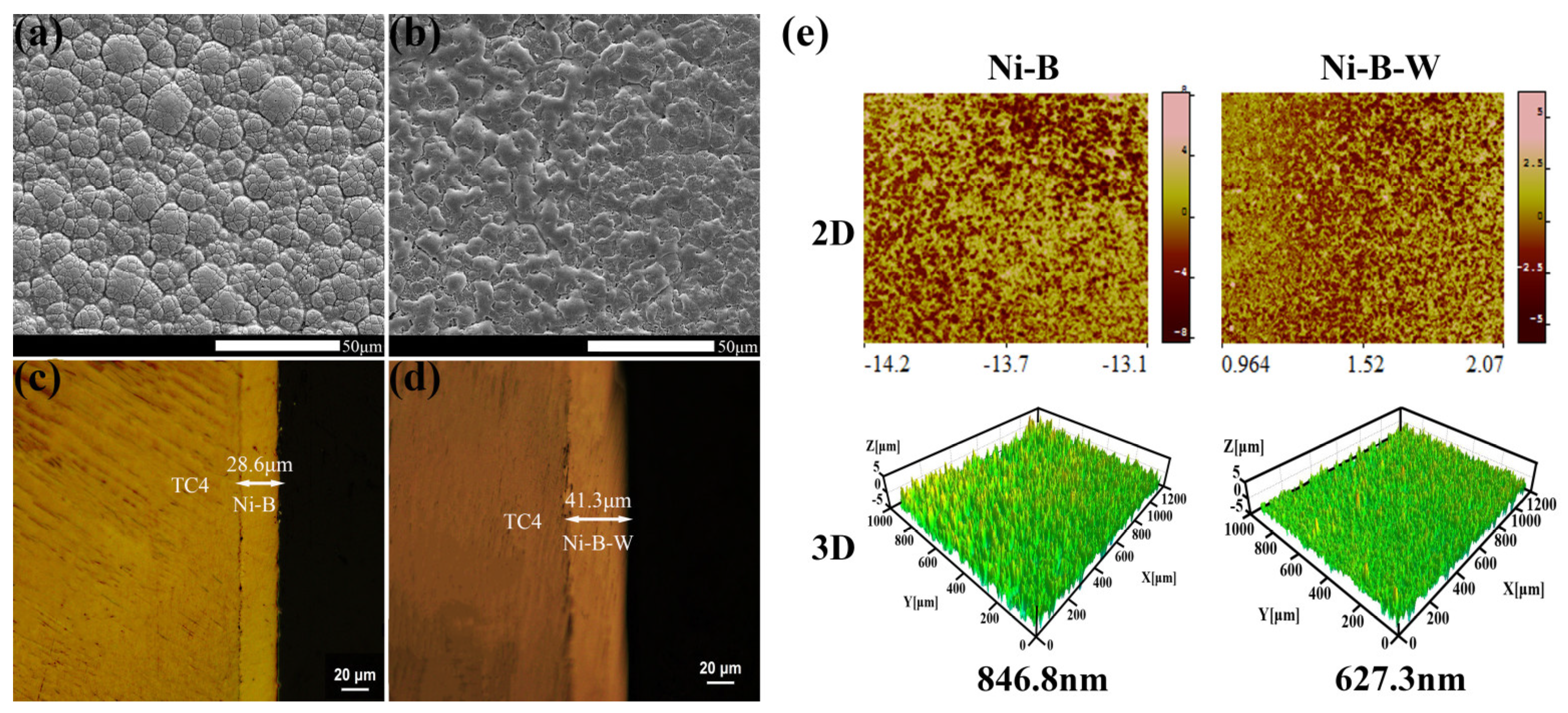
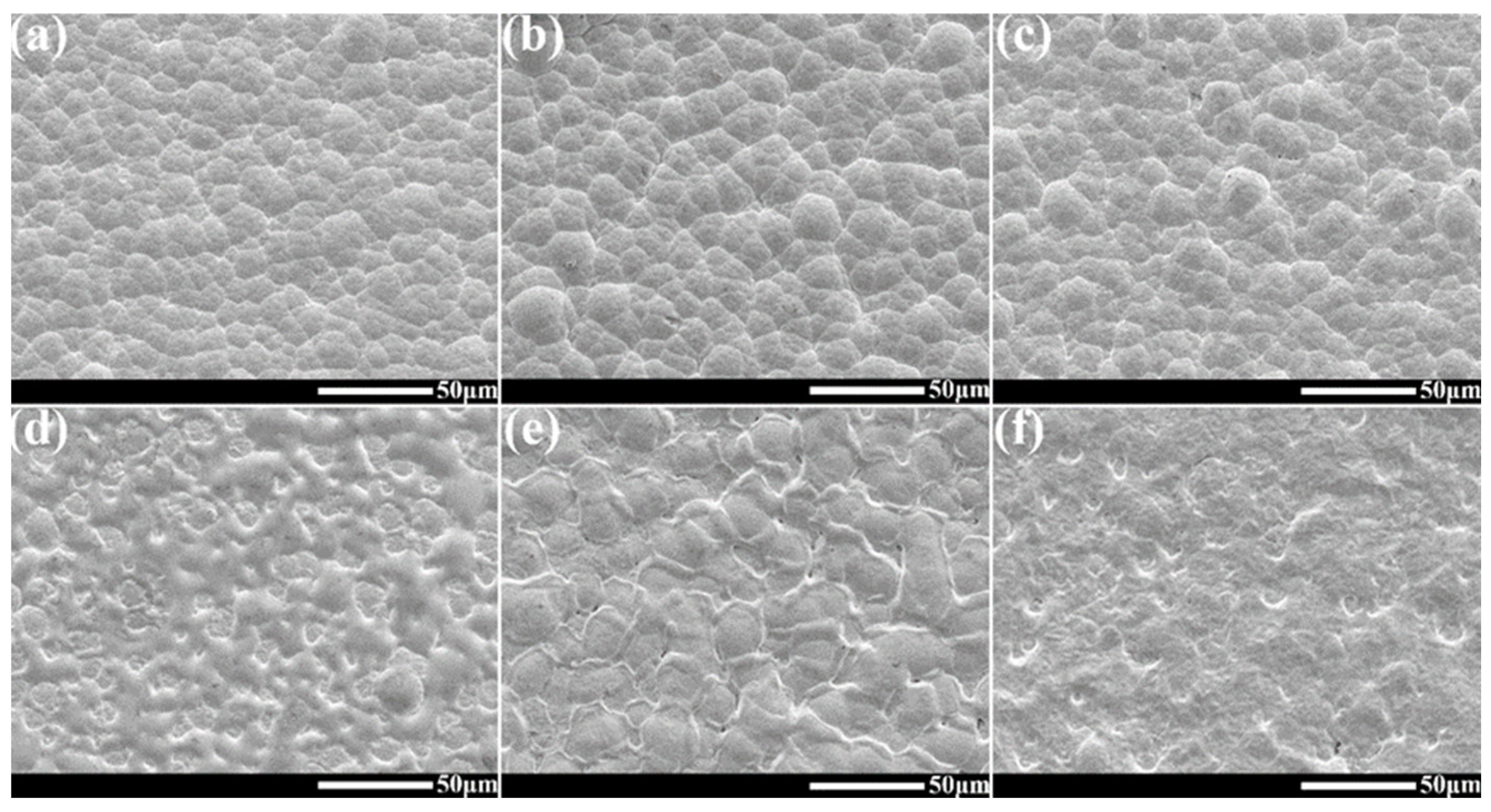
3.2. Morphologies of Ni-B and Ni-B-W Coatings
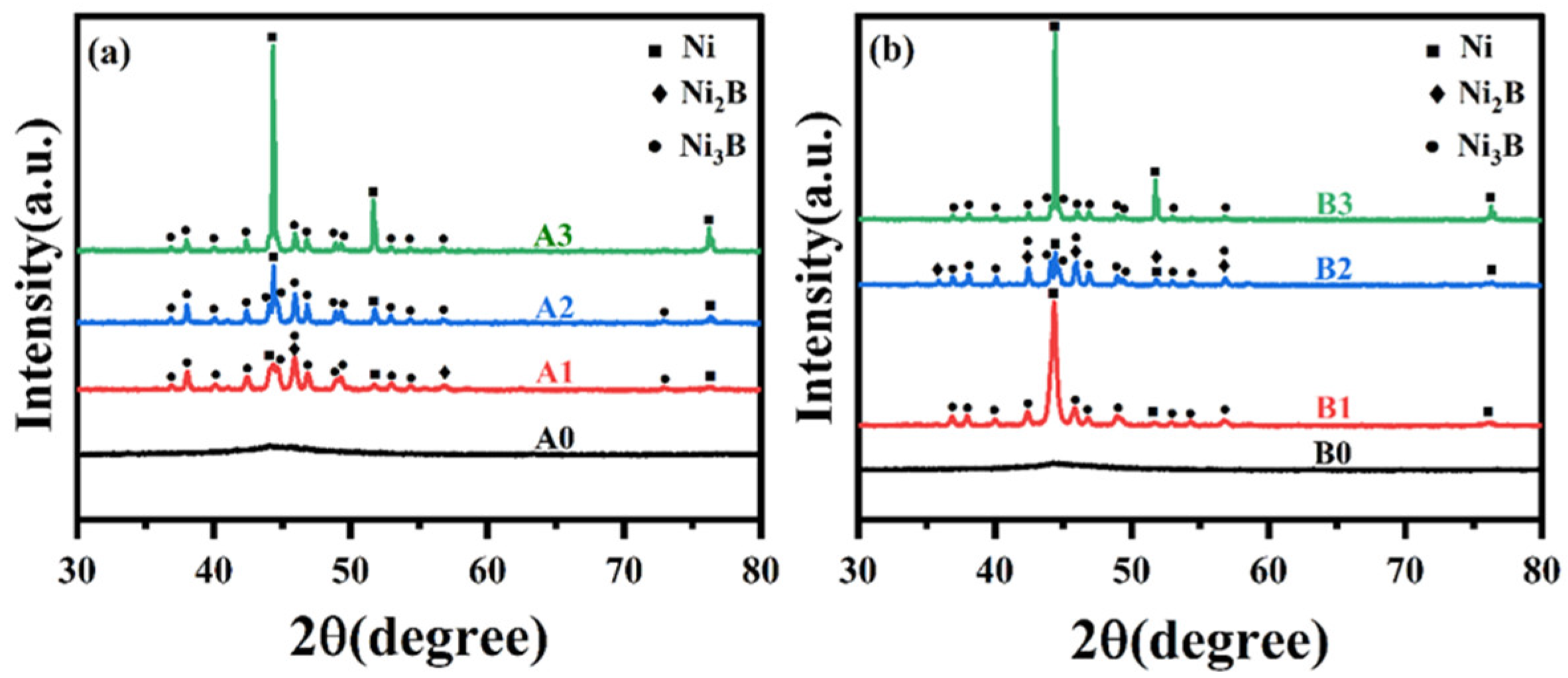
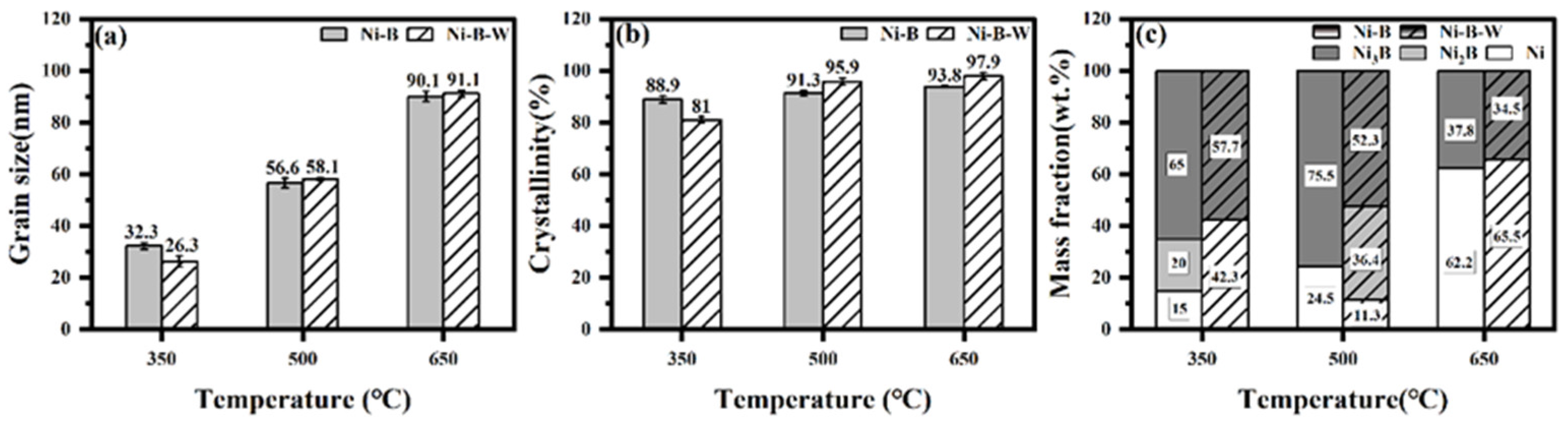
3.3. Microstructure of Ni-B and Ni-B-W Coatings
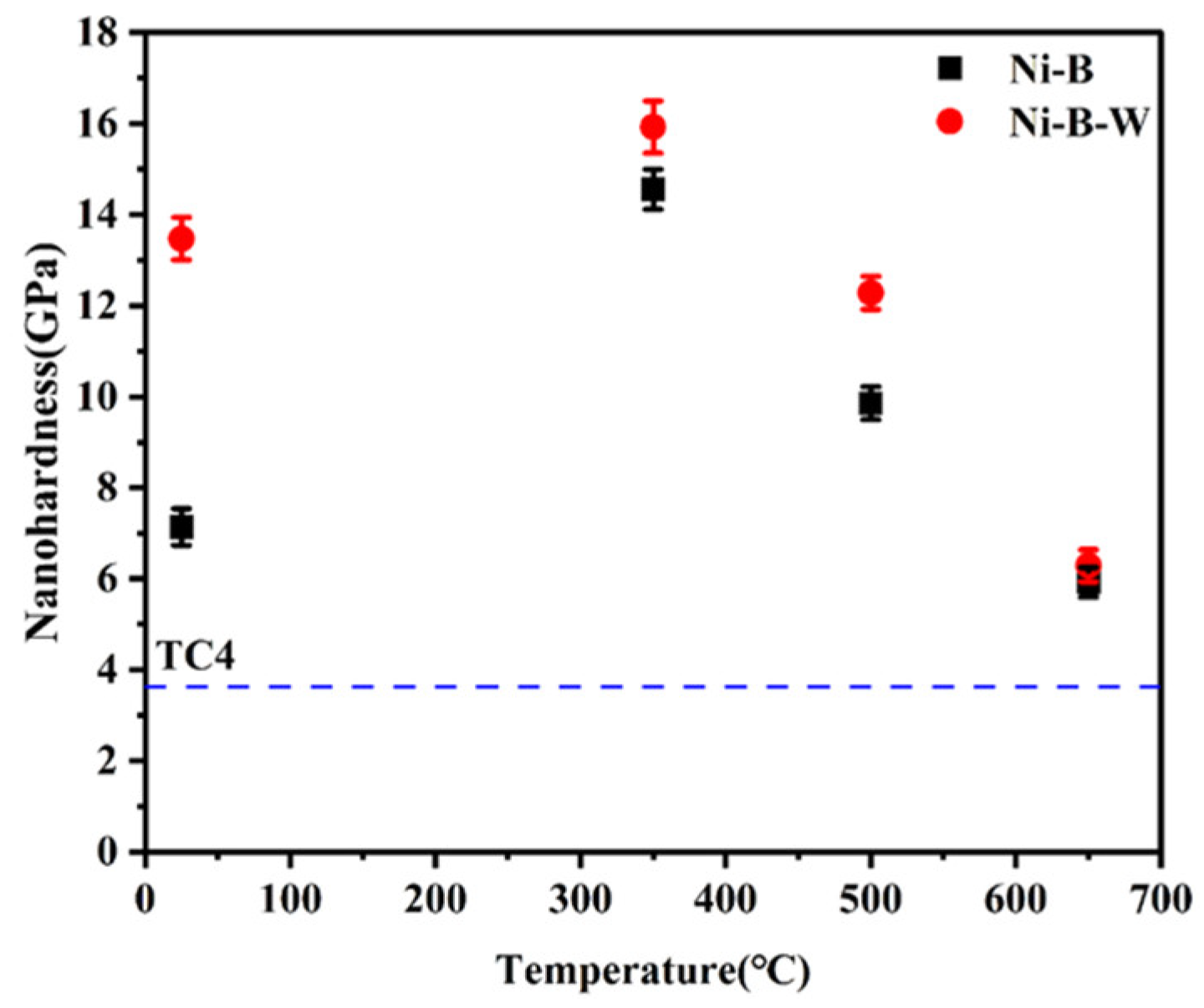
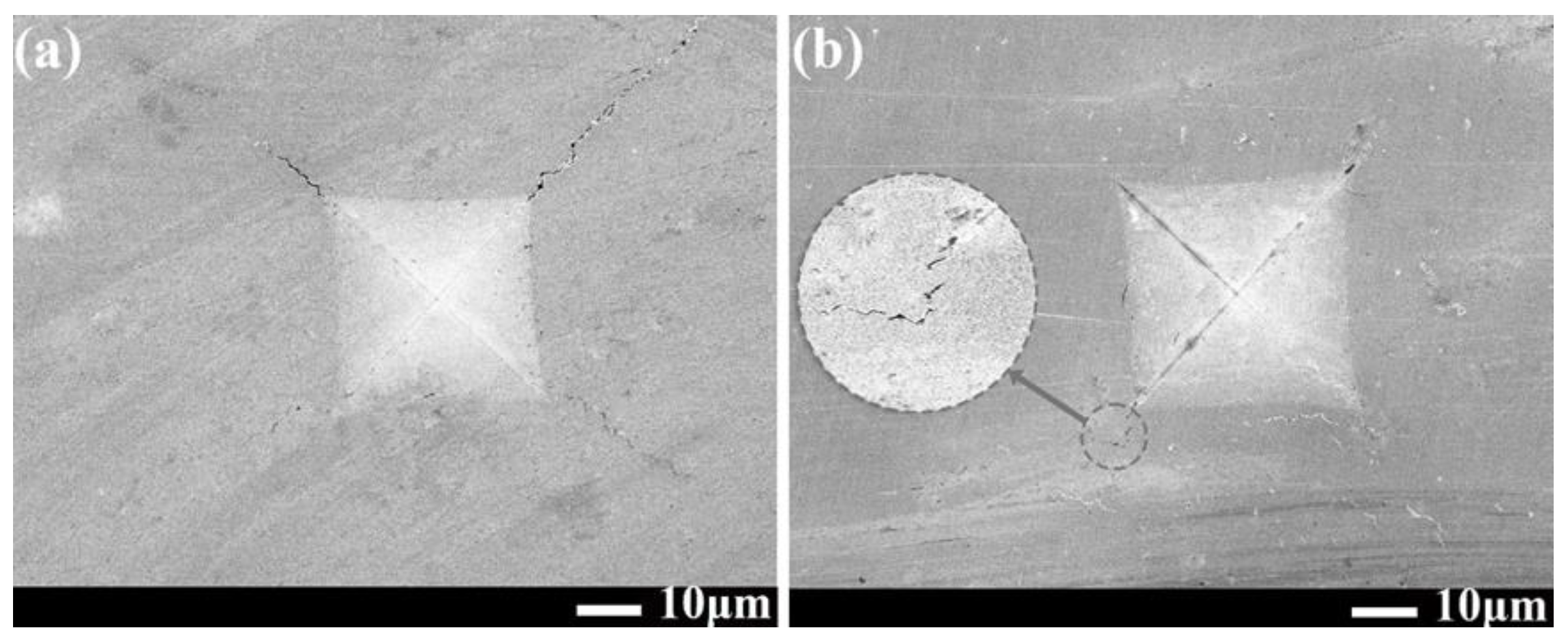
3.4. Mechanical Properties of Ni-B and Ni-B-W Coatings
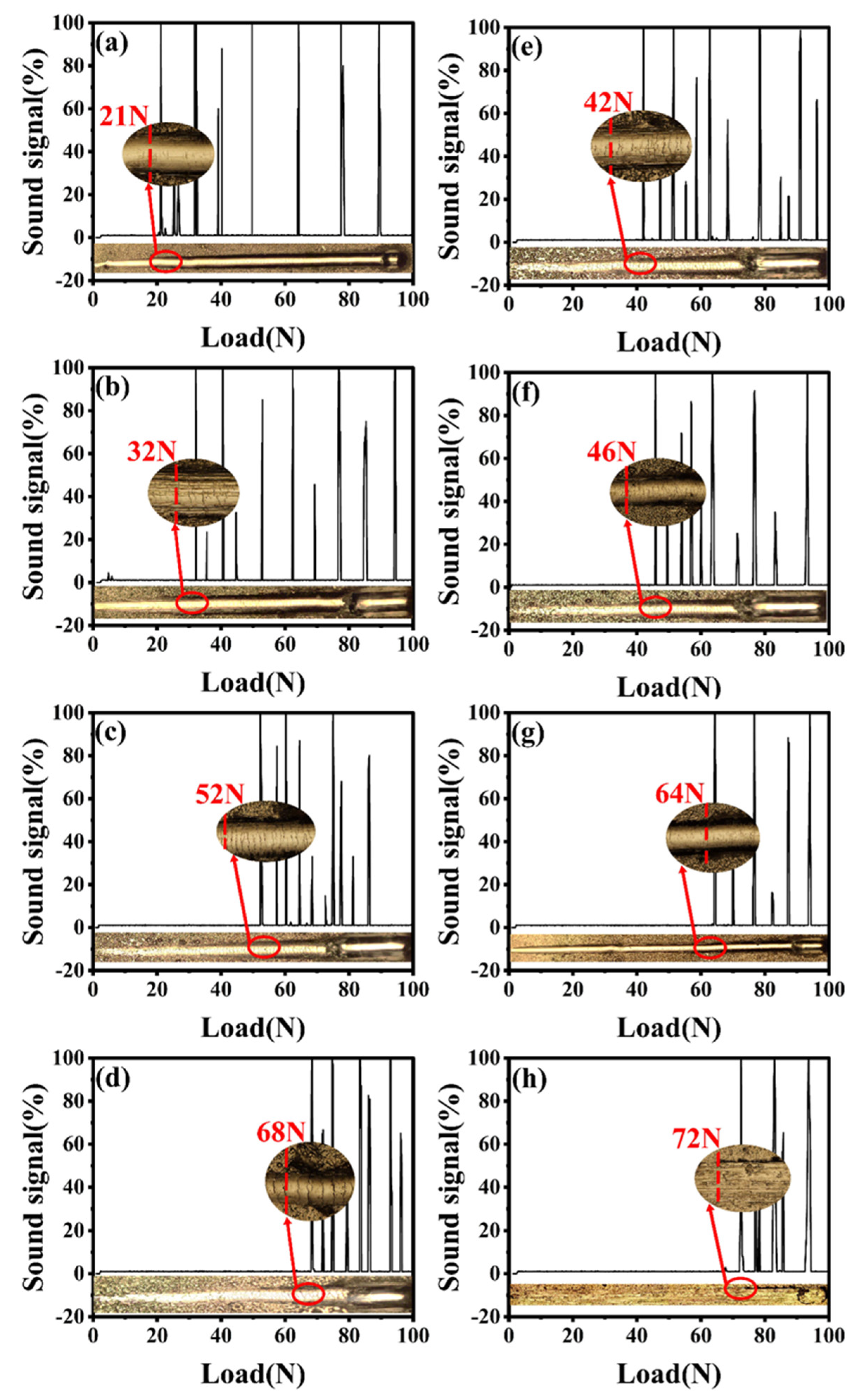
3.5. Tribological Properties of Ni−B and N−B−W Coatings
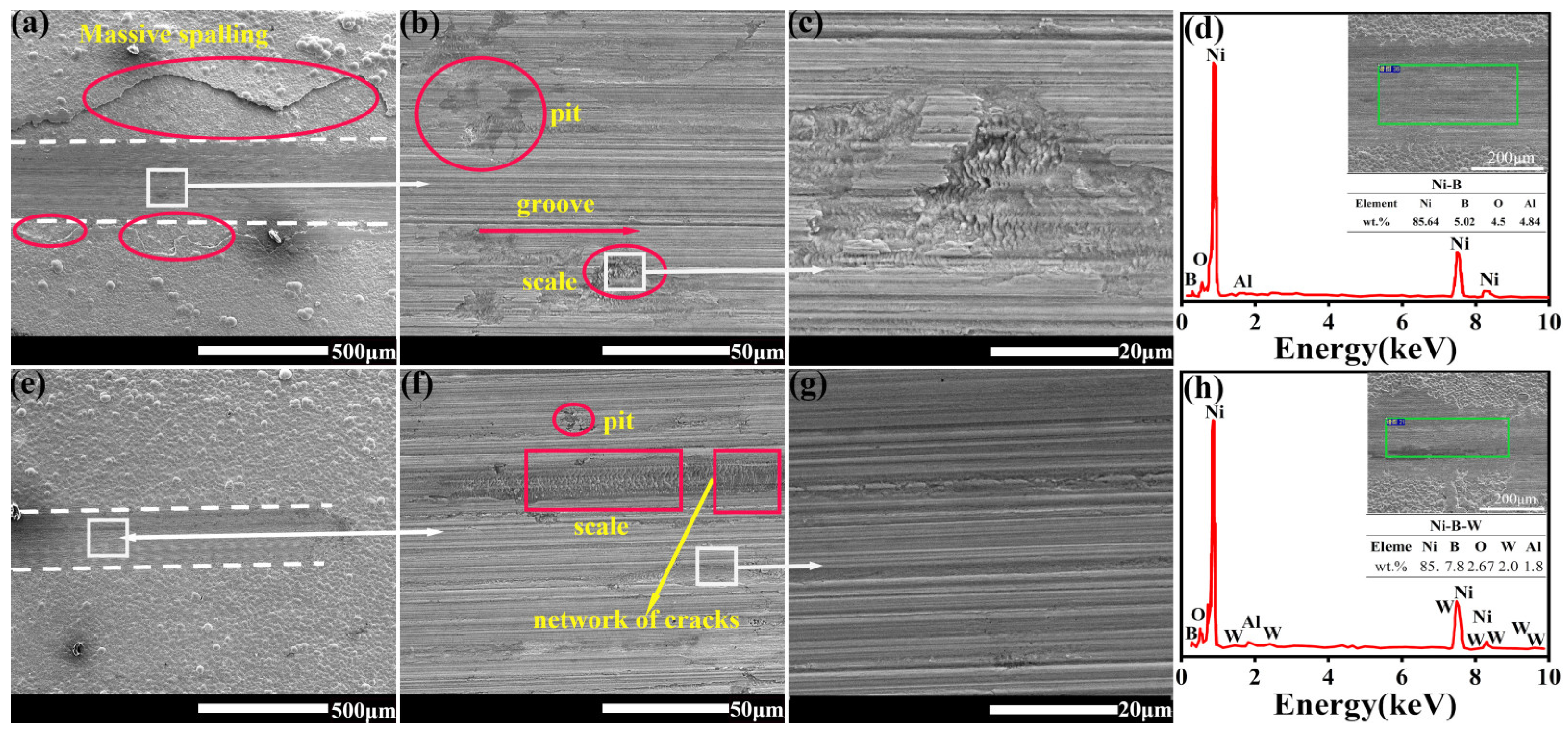
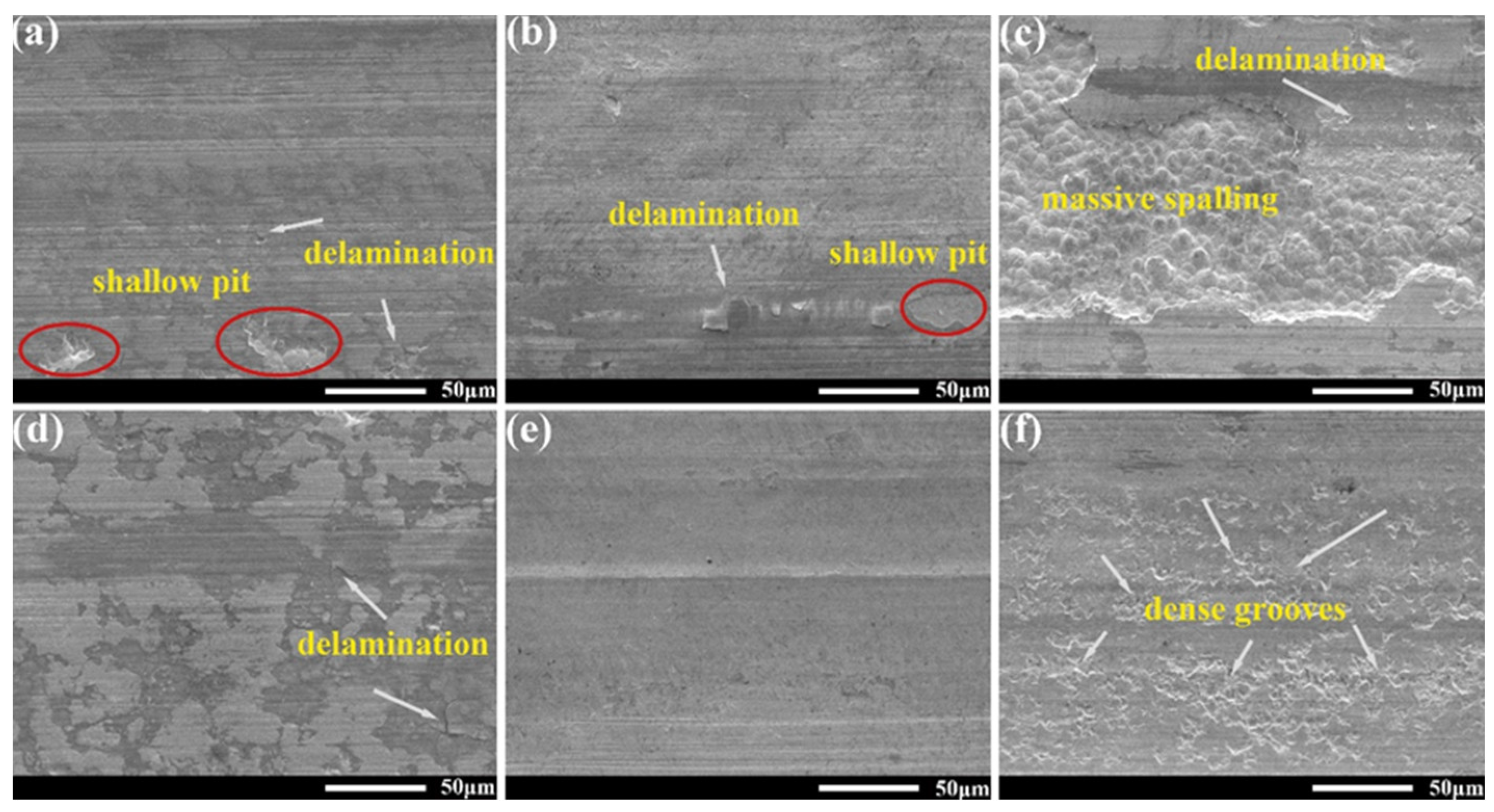
3.6. Wear Mechanism of Ni-B and Ni- B-W Coatings
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boyer, R.R. An overview on the use of titanium in the aerospace industry. Mater. Sci. Eng. A 1996, 213, 103–114. [Google Scholar] [CrossRef]
- Zhao, Q.; Sun, Q.; Xin, S.; Chen, Y.; Wu, C.; Wang, H.; Xu, J.; Wan, M.; Zeng, W.; Zhao, Y. High-strength titanium alloys for aerospace engineering applications: A review on melting-forging process. Mater. Sci. Eng. A 2022, 845, 143260. [Google Scholar] [CrossRef]
- Behera, A.; Sahoo, A.K.; Mohapatra, S.S. 14-Nickel-titanium smart hybrid materials for automotive industry. In Nickel-Titanium Smart Hybrid Materials; Elsevier: Amsterdam, The Netherlands, 2022; pp. 271–295. [Google Scholar]
- Cai, Z.; Chen, J.; Zhang, Z.; Li, K.; Yang, X.; Xie, G. Microstructure regulation of titanium-oxygen alloy with high strength and excellent ductility for biomedical applications. Intermetallics 2022, 148, 107648. [Google Scholar] [CrossRef]
- Chirico, C.; Romero, A.V.; Gordo, E.; Tsipas, S.A. Improvement of wear resistance of low-cost powder metallurgy β-titanium alloys for biomedical applications. Surf. Coat. Technol. 2022, 434, 128207. [Google Scholar] [CrossRef]
- Cheng, J.; Li, F.; Zhu, S.; Yu, Y.; Qiao, Z.; Yang, J. Electrochemical corrosion and tribological evaluation of TiAl alloy for marine application. Tribol. Int. 2017, 115, 483–492. [Google Scholar] [CrossRef]
- Zhao, Y.; Fan, Z.; Tan, Q.; Yin, Y.; Lu, M.; Huang, H. Interfacial and tribological properties of laser deposited TiOxNy/Ti composite coating on Ti alloy. Tribol. Int. 2021, 155, 106758. [Google Scholar] [CrossRef]
- Yuan, S.; Lin, N.; Zou, J.; Lin, X.; Liu, Z.; Yu, Y.; Wang, Z.; Zeng, Q.; Chen, W.; Tian, L.; et al. In-situ fabrication of gradient titanium oxide ceramic coating on laser surface textured Ti6Al4V alloy with improved mechanical property and wear performance. Vacuum 2020, 176, 109327. [Google Scholar] [CrossRef]
- Liu, X.; Meng, X.; Liu, H.; Shi, G.; Wu, S.; Sun, C.; Wang, M.; Qi, L. Development and characterization of laser clad high temperature self-lubricating wear resistant composite coatings on Ti-6Al-4V alloy. Mater. Des. 2014, 55, 404–409. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, K.; Sun, Z.; Xin, R. Simultaneous improvement of strength and elongation of laser melting deposited Ti-6Al-4V titanium alloy through three-stage heat treatment. J. Mater. Process. Technol. 2022, 306, 117607. [Google Scholar] [CrossRef]
- Feng, J.; Wang, J.; Yang, K.; Rong, J. Microstructure and performance of YTaO4 coating deposited by atmospheric plasma spraying on TC4 titanium alloy surface. Surf. Coat. Technol. 2022, 431, 128004. [Google Scholar] [CrossRef]
- Li, Y.L.; Wu, Y.Y.; Wang, W.Q.; Lei, M.; Li, X.W. Microstructure and mechanical properties of the Ni-B-Ti composite coating on TA2 prepared by pre-plating and laser remelting. Surf. Coat. Technol. 2021, 405, 126567. [Google Scholar] [CrossRef]
- Zhang, L.C.; Chen, L.Y.; Wang, L.Q. Surface modification of titanium and titanium alloys: Technologies, developments, and future interests. Adv. Eng. Mater. 2020, 22, 1901258. [Google Scholar] [CrossRef]
- Koshuro, V.; Fomina, M.; Zakharevich, A.; Fomin, A. Superhard Ta-O-N coatings produced on titanium using induction physical vapor deposition. Ceram. Int. 2022, 48, 19467–19483. [Google Scholar] [CrossRef]
- Liu, R.; Yuan, S.; Lin, N.; Zeng, Q.; Wang, Z.; Wu, Y. Application of ultrasonic nanocrystal surface modification (UNSM) technique for surface strengthening of titanium and titanium alloys: A mini review. J. Mater. Res. Technol. 2021, 11, 351–377. [Google Scholar] [CrossRef]
- Zhang, B. Chapter 1—History-from the discovery of electroless plating to the present. In Amorphous and Nano Alloys Electroless Depositions; Elsevier: Oxford, UK, 2016; pp. 3–48. [Google Scholar]
- Gunji, T.; Umehashi, Y.; Tsunoi, H.; Yokoi, K.; Kawai, A.; Matsumoto, F. Preparation of chemical-resistant atomically ordered Sn-Ni alloy films by electroless plating. J. Alloy. Compd. 2021, 877, 160100. [Google Scholar] [CrossRef]
- Hamid, Z.A.; Hassan, H.B.; Attyia, A.M. Influence of deposition temperature and heat treatment on the performance of electroless Ni-B films. Surf. Coat. Technol. 2010, 205, 2348–2354. [Google Scholar] [CrossRef]
- Samanta, S.; Mondal, K.; Dutta, M.; Singh, S.B. Electroless NiP coatings over API X70 steel: Effect of composition on the H-permeation and corrosion resistance. Surf. Coat. Technol. 2021, 409, 126928. [Google Scholar] [CrossRef]
- Kaliaraj, G.S.; Vishwakarma, V.; Dawn, S.S.; Karthik, A.; Vigneshwaran, S.; Naidu, G.D. Reduction of sulphate reducing bacterial survival by Cu-Ni, Zn-Ni and Cu-Zn-Ni coatings using electroless plating technique for oil/diesel pipeline applications. Mater. Today Proc. 2021, 45, 6804–6806. [Google Scholar] [CrossRef]
- Ma, L.; Chen, Y.; Renner, P.; Parkinson, D.; Fang, A.; Liang, H. Synthesis and Morphological Characterization of Electroless-Deposited Ni-P Coatings on Diamond Abrasives. Lubricants 2021, 9, 20. [Google Scholar] [CrossRef]
- Pal, S.; Jayaram, V. Effect of microstructure on the hardness and dry sliding behavior of electroless Ni–B coating. Materialia 2018, 4, 47–64. [Google Scholar] [CrossRef]
- Vitry, V.; Hastir, J.; Mégret, A.; Yazdani, S.; Yunacti, M.; Bonin, L. Recent advances in electroless nickel-boron coatings. Surf. Coat. Technol. 2022, 429, 127937. [Google Scholar] [CrossRef]
- Yildiz, R.A.; Göksenli, A.; Yüksel, B.H.; Muhaffel, F.; Aydeniz, A. Effect of annealing temperature on the corrosion resistance of electroless produced Ni-B-W coatings. Adv. Mater. Res. 2013, 2249, 651. [Google Scholar] [CrossRef]
- Algul, H.; Uysal, M.; Alp, A. A comparative study on morphological, mechanical and tribological properties of electroless NiP, NiB and NiBP coatings. Appl. Surf. Sci. Adv. 2021, 4, 100089. [Google Scholar] [CrossRef]
- Ranganatha, S.; Venkatesha, T.V.; Vathsala, K. Development of electroless Ni-Zn-P/nano-TiO2 composite coatings and their properties. Appl. Surf. Sci. 2010, 256, 7377–7383. [Google Scholar] [CrossRef]
- Valova, E.; Armyanov, S.; Hristova, G.; Vassilev, T.; Steenhaut, O.; Dille, J.; Hubin, A.; Vandendael, I. Electroless deposited Ni-Ce-P coatings. Surf. Coat. Technol. 2016, 304, 468–475. [Google Scholar] [CrossRef]
- Zhu, L.; Luo, L.; Luo, J.; Wu, Y.; Li, J. Effect of electroless plating Ni-Cu-P layer on brazability of cemented carbide to steel. Surf. Coat. Technol. 2012, 206, 2521–2524. [Google Scholar] [CrossRef]
- Zuxiao, Y.U.; Jian, C.; Shixiong, H.A.O.; Xiaoli, T. Study of process for electoless plating of Ni-Co-P alloy coating. J. Mater. Prot. 2007, 40, 22–24. [Google Scholar]
- Rahmani, S.; Omrani, A.; Hosseini, S.R. Impact of barium in improving corrosion resistance and properties of electroless Ni-Ba-B alloy deposits. Met. Mater. Int. 2020, 26, 979–988. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Barman, T.K.; Sahoo, P. Wear and friction characteristics of electroless Ni-B-W coatings at different operating temperatures. Mater. Res. Express 2018, 5, 026526. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Barman, T.K.; Sahoo, P. Corrosion resistance of electroless Ni-B-W-Mo coatings using electrochemical impedance spectroscopy. Port. Electrochim. Acta 2019, 37, 193–203. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, T.; Zhang, Y.; Shao, Y.; Meng, G.; Wang, F. Friction, wear and corrosion resistance of multi-layer electroless Ni-W-P coating on AZ91D magnesium alloy. China Surf. Eng. 2019, 32, 53–62. [Google Scholar]
- Palaniappa, M.; Seshadri, S.K. Friction and wear behavior of electroless Ni-P and Ni-W-P alloy coatings. Wear 2008, 265, 735–740. [Google Scholar] [CrossRef]
- Bulbul, F. The effects of deposition parameters on surface morphology and crystallographic orientation of electroless Ni-B coatings. Met. Mater. Int. 2011, 17, 67–75. [Google Scholar] [CrossRef]
- Celik, I.; Karakan, M.; Bulbul, F. Investigation of structural and tribological properties of electroless Ni-B coated pure titanium. Proc. Inst. Mech. Eng. Part J-J. Eng. Tribol. 2016, 230, 57–63. [Google Scholar] [CrossRef]
- Cheng, X.; Rao, Q. A study of microstructure of electroless nickel-boron alloy coatings. Electroplat. Pollut. Control 2012, 32, 19–21. [Google Scholar]
- Das, S.K.; Sahoo, P. Influence of process parameters on microhardness of electroless Ni-B coatings. Adv. Mech. Eng. 2012, 4, 703168. [Google Scholar] [CrossRef]
- Pan, J.; Chen, R.; Wu, C. Research on micro-structure of electroless Ni-B coatings. In Proceedings of the 2nd International Conference on Materials and Products Manufacturing Technology, Guangzhou, China, 22–23 September 2012; pp. 1641–1645. [Google Scholar]
- Qian, W.; Chen, H.; Feng, C.; Zhu, L.; Wei, H.; Han, S.; Li, G.; Lin, H.; Jiang, J. Microstructure and properties of the Ni-B and Ni-B-Ce ultrasonic-assisted electroless coatings. Surf. Rev. Lett. 2018, 25, 1950006. [Google Scholar] [CrossRef]
- Rao, Q.; Wang, H.; Fan, X.; Zhou, Y. Morphology and formation mechanism of electroless Ni-B alloy coating. J. Shanghai Jiaotong Univ. 2003, 37, 1965–1968. [Google Scholar]
- Vitry, V.; Bonin, L. Increase of boron content in electroless nickel-boron coating by modification of plating conditions. Surf. Coat. Technol. 2017, 311, 164–171. [Google Scholar] [CrossRef]
- Lopez, J.R.; Mendez, P.F.; Perez-Bueno, J.J.; Trejo, G.; Stremsdoerfer, G.; Meas, Y. The effect of boron content, crystal structure, crystal size on the hardness and the corrosion resistance of electrodeposited Ni-B coatings. Int. J. Electrochem. Sci. 2016, 11, 4231–4244. [Google Scholar] [CrossRef]
- Oraon, B.; Majumdar, G.; Ghosh, B. Improving hardness of electroless Ni-B coatings using optimized deposition conditions and annealing. Mater. Des. 2008, 29, 1412–1418. [Google Scholar] [CrossRef]
- Krishnaveni, K.; Sankara Narayanan, T.S.N.; Seshadri, S.K. Electroless Ni-B coatings: Preparation and evaluation of hardness and wear resistance. Surf. Coat. Technol. 2005, 190, 115–121. [Google Scholar] [CrossRef]
- Aydeniz, A.I.; Goksenli, A.; Dil, G.; Muhaffel, F.; Calli, C.; Yuksel, B. Electroless Ni-B-W coatings for improving hardness, wear and corrosion resistance. Mater. Tehnol. 2013, 47, 803–806. [Google Scholar]
- Mukhopadhyay, A.; Barman, T.K.; Sahoo, P. Co-deposition of W and Mo in electroless Ni-B coating and its effect on the surface morphology, structure, and tribological behavior. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2021, 235, 149–161. [Google Scholar] [CrossRef]
- Liu, H.; Viejo, F.; Guo, R.X.; Glenday, S.; Liu, Z. Microstructure and corrosion performance of laser-annealed electroless Ni-W-P coatings. Surf. Coat. Technol. 2010, 204, 1549–1555. [Google Scholar] [CrossRef]
- Drovosekov, A.B.; Ivanov, M.V.; Krutskikh, V.M.; Lubnin, E.N.; Polukarov, Y.M. Chemically deposited Ni-W-B coatings: Composition, structure, and properties. Prot. Met. 2005, 41, 55–62. [Google Scholar] [CrossRef]
- Biswas, P.; Kalyan Das, S.; Sahoo, P. Duplex electroless Ni-P/Ni-P-W coatings: Effect of heat treatment on tribological and corrosion performance. Mater. Today Proc. 2022, 66, 2237–2244. [Google Scholar] [CrossRef]
- Estupinan, F.A.; Moreno, C.M.; Olaya, J.J.; Ardila, L.C. Wear Resistance of TiAlCrSiN Coatings Deposited by Means of the Co-Sputtering Technique. Lubricants 2021, 9, 64. [Google Scholar] [CrossRef]
- Li, Z.; Farhat, Z.; Jarjoura, G.; Fayyad, E.; Abdullah, A.; Hassan, M. Synthesis and characterization of scratch-resistant Ni-P-Ti-based composite coating. Tribol. Trans. 2019, 62, 880–896. [Google Scholar] [CrossRef]
- Balaraju, J.N.; Priyadarshi, A.; Kumar, V.; Manikandanath, N.T.; Kumar, P.P.; Ravisankar, B. Hardness and wear behaviour of electroless Ni-B coatings. Mater. Sci. Technol. 2016, 32, 1654–1665. [Google Scholar] [CrossRef]


| Element | Ti | Al | V | Fe | C | N | H | O |
|---|---|---|---|---|---|---|---|---|
| Wt.% | 89.82 | 6.12 | 3.97 | 0.05 | 0.01 | 0.01 | <0.1 | 0.01 |
| Coatings | Bath Composition (g/L) | |||||
|---|---|---|---|---|---|---|
| NiCl2·6H2O | NaOH | C2H8N2 | NaBH4 | Pb(NO3)2 | Na2WO4·2H2O | |
| Ni-B | 24.00 | 39.00 | 59.00 | 0.60 | 0.01 | - |
| Ni-B-W | 24.00 | 39.00 | 59.00 | 0.60 | 0.01 | 20.00 |
| Sample No. | Deposition Conditions | Annealing Temperature (℃) | ||
|---|---|---|---|---|
| PH | Temperature (℃) | Time (h) | ||
| A0 | 13 ± 0.5 | 90 ± 1 | 2 + 2 | - |
| A1 | 13 ± 0.5 | 90 ± 1 | 2 + 2 | 350 |
| A2 | 13 ± 0.5 | 90 ± 1 | 2 + 2 | 500 |
| A3 | 13 ± 0.5 | 90 ± 1 | 2 + 2 | 650 |
| B0 | 13 ± 0.5 | 90 ± 1 | 2 + 2 | - |
| B1 | 13 ± 0.5 | 90 ± 1 | 2 + 2 | 350 |
| B2 | 13 ± 0.5 | 90 ± 1 | 2 + 2 | 500 |
| B3 | 13 ± 0.5 | 90 ± 1 | 2 + 2 | 650 |
| Coating | Wt.% | |||
|---|---|---|---|---|
| Ni | B | W | O | |
| Ni-B | 91.47 | 8.53 | - | - |
| Ni-B-W | 86.72 | 7.51 | 2.42 | 3.35 |
| Sample | Nanohardness H (GPa) | Modulus of Elasticity E (Gpa) | Load P (N) | Average Crack Length c (×10−5m) | Fracture Toughness Kc (Mpa·m0.5) |
|---|---|---|---|---|---|
| A0 | 7.1 | 106.2 | 10 | 3.7 | 2.8 |
| A1 | 14.6 | 109.8 | 10 | 2.8 | 2.9 |
| A2 | 9.9 | 113.5 | 10 | 2.3 | 5.0 |
| A3 | 5.9 | 110.1 | 10 | 2.2 | 6.9 |
| B0 | 13.5 | 72.1 | 10 | 2.5 | 3.1 |
| B1 | 15.9 | 82.6 | 10 | 2.3 | 3.3 |
| B2 | 12.3 | 80.3 | 10 | 2.2 | 3.9 |
| B3 | 6.3 | 78.7 | 10 | 2.1 | 6.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, F.; Hu, H.; Yu, J.; Lai, J.; He, H.; Zhang, Y.; Qi, H.; Wang, D. Mechanical and Tribological Properties of Ni-B and Ni-B-W Coatings Prepared by Electroless Plating. Lubricants 2023, 11, 42. https://doi.org/10.3390/lubricants11020042
Zhao F, Hu H, Yu J, Lai J, He H, Zhang Y, Qi H, Wang D. Mechanical and Tribological Properties of Ni-B and Ni-B-W Coatings Prepared by Electroless Plating. Lubricants. 2023; 11(2):42. https://doi.org/10.3390/lubricants11020042
Chicago/Turabian StyleZhao, Fan, Hong Hu, Jiaxin Yu, Jianping Lai, Hongtu He, Yafeng Zhang, Huimin Qi, and Dongwei Wang. 2023. "Mechanical and Tribological Properties of Ni-B and Ni-B-W Coatings Prepared by Electroless Plating" Lubricants 11, no. 2: 42. https://doi.org/10.3390/lubricants11020042
APA StyleZhao, F., Hu, H., Yu, J., Lai, J., He, H., Zhang, Y., Qi, H., & Wang, D. (2023). Mechanical and Tribological Properties of Ni-B and Ni-B-W Coatings Prepared by Electroless Plating. Lubricants, 11(2), 42. https://doi.org/10.3390/lubricants11020042







