Impact of Water Content on the Superlubricity of Ethylene Glycol Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
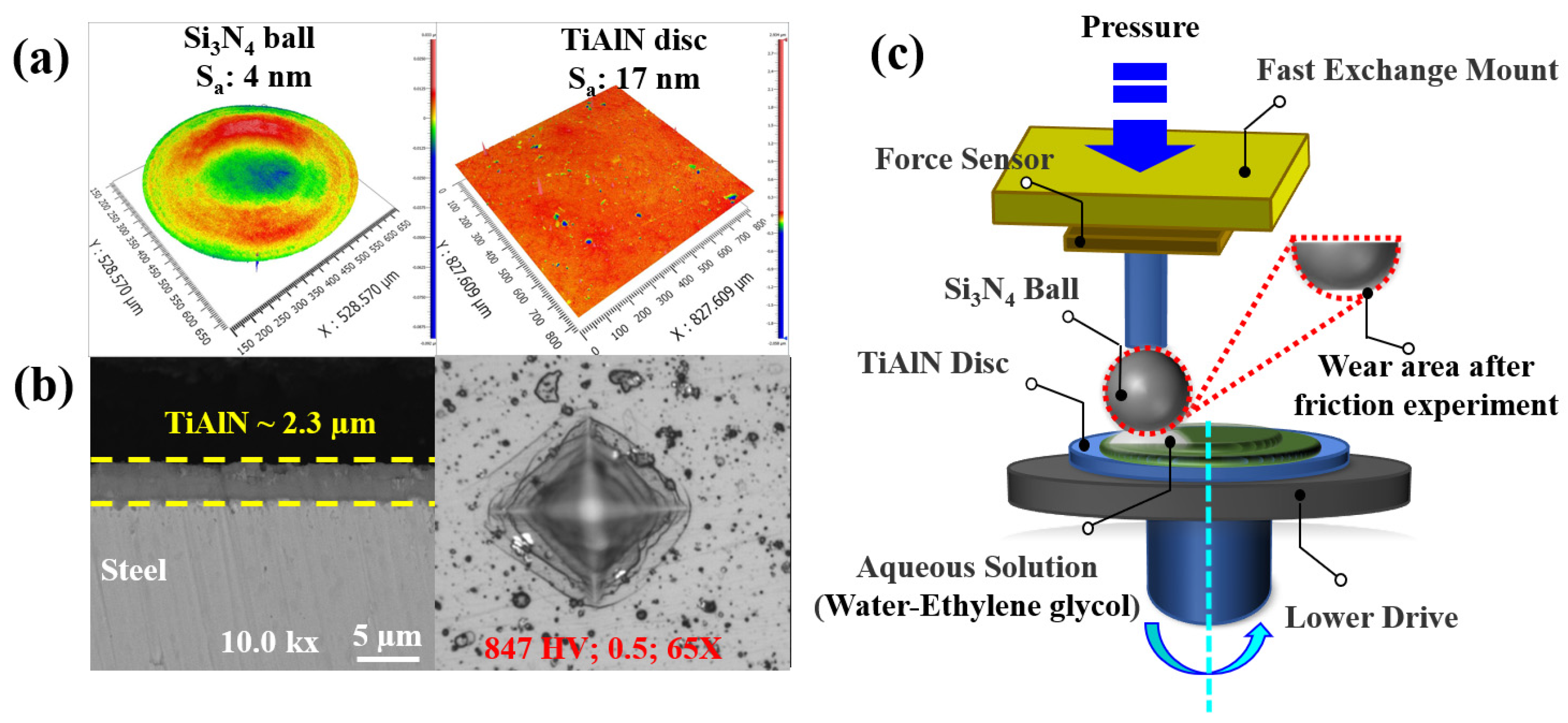
2.2. Experimental Methods
3. Results and Discussion
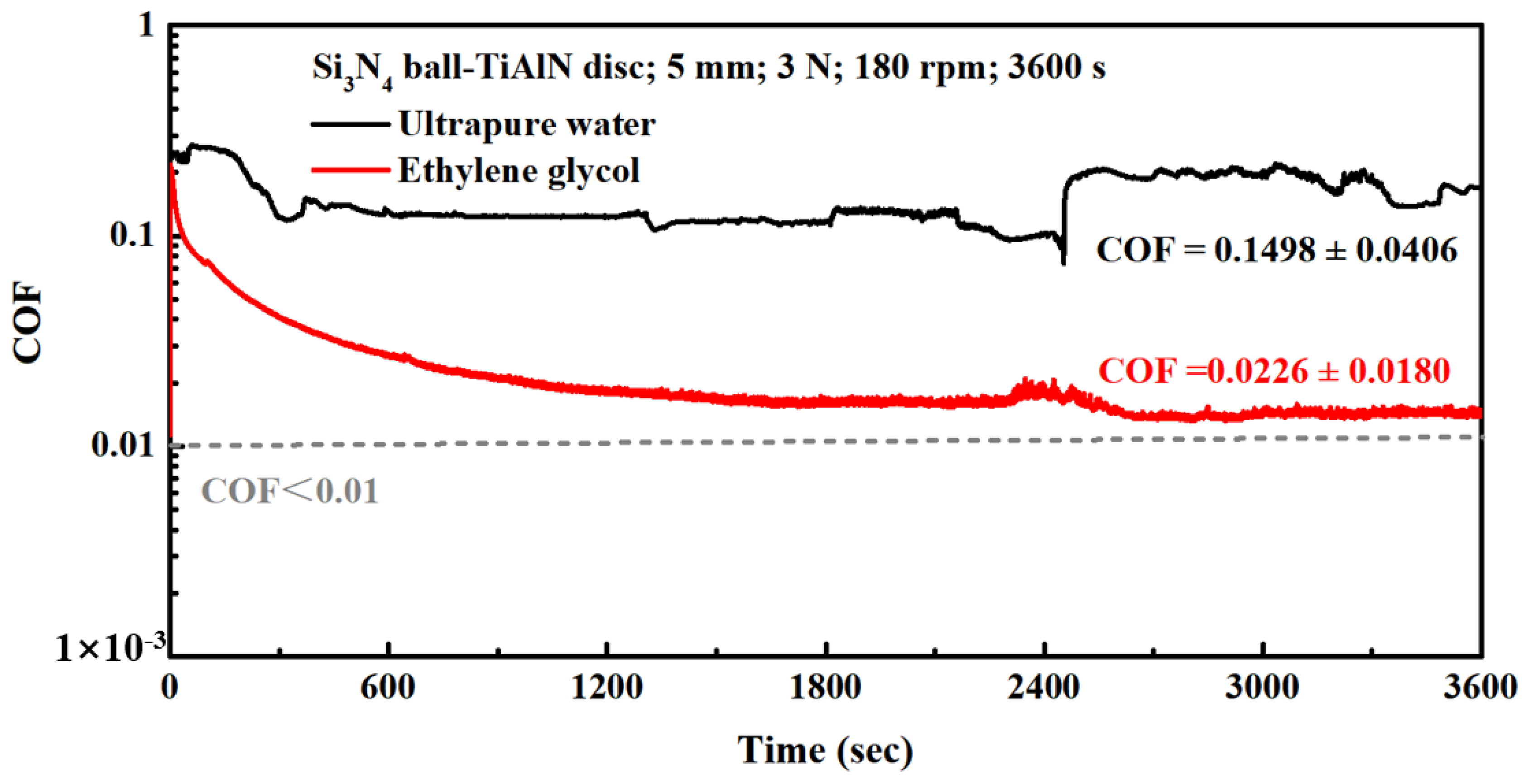
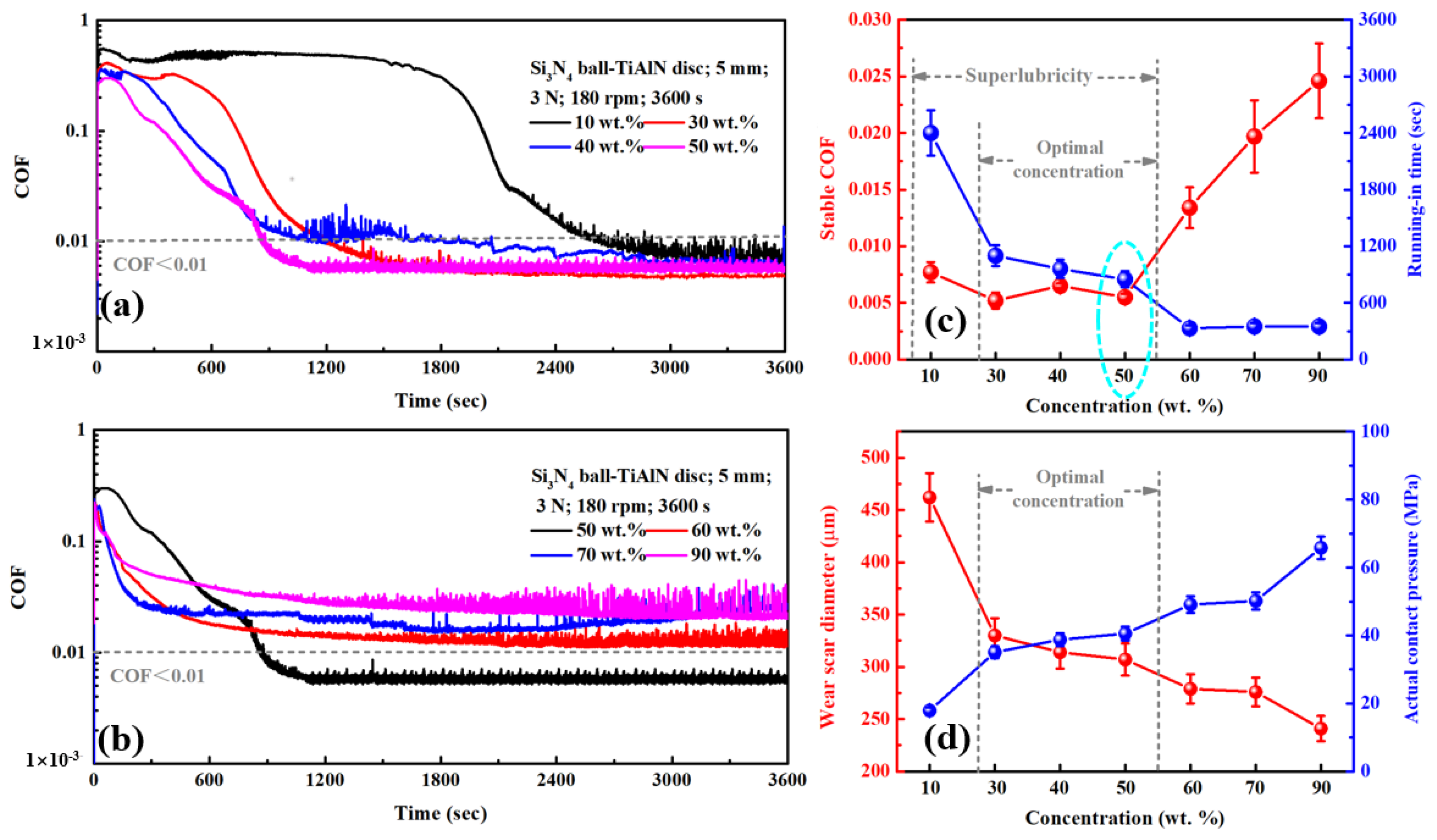
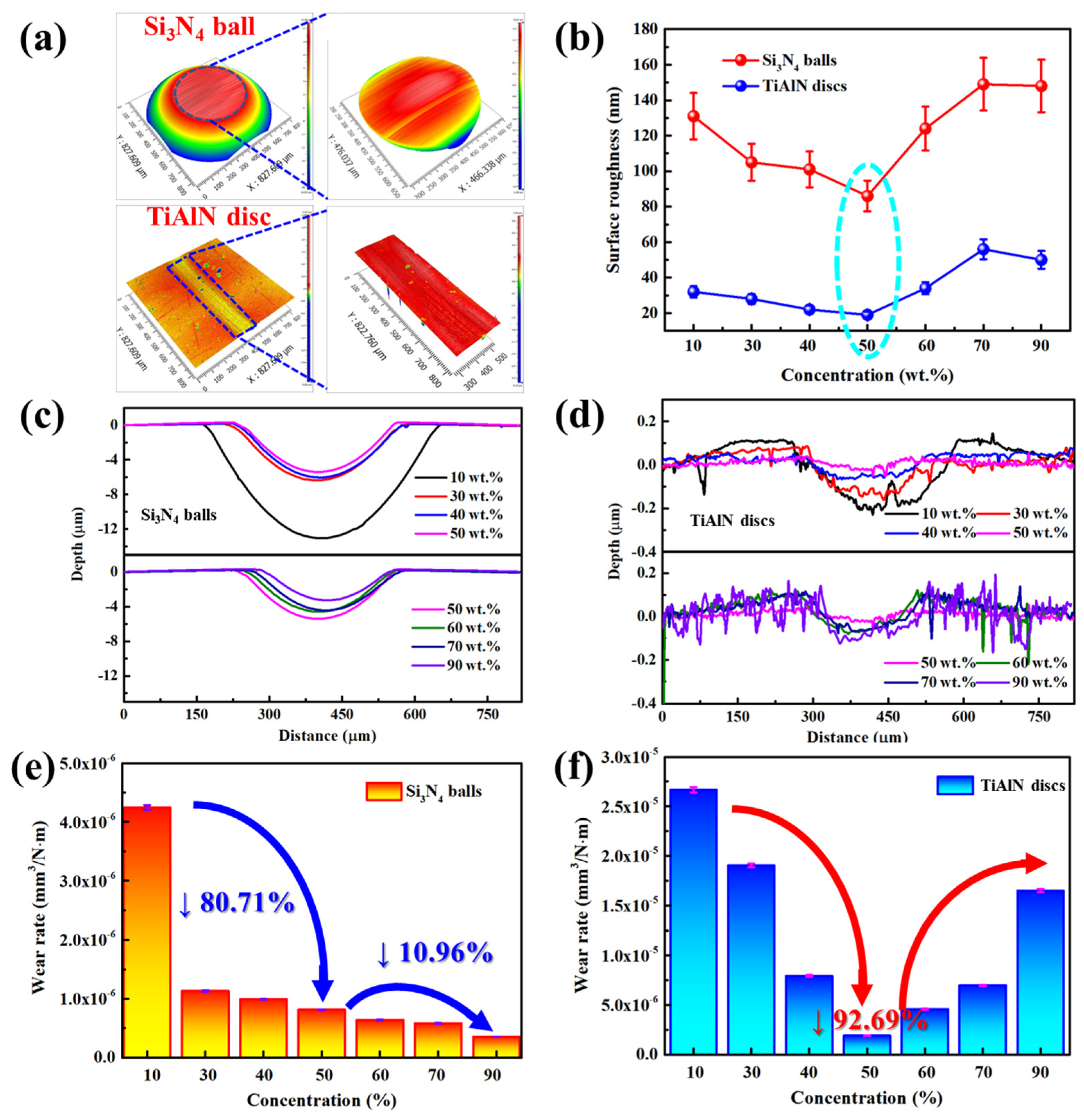
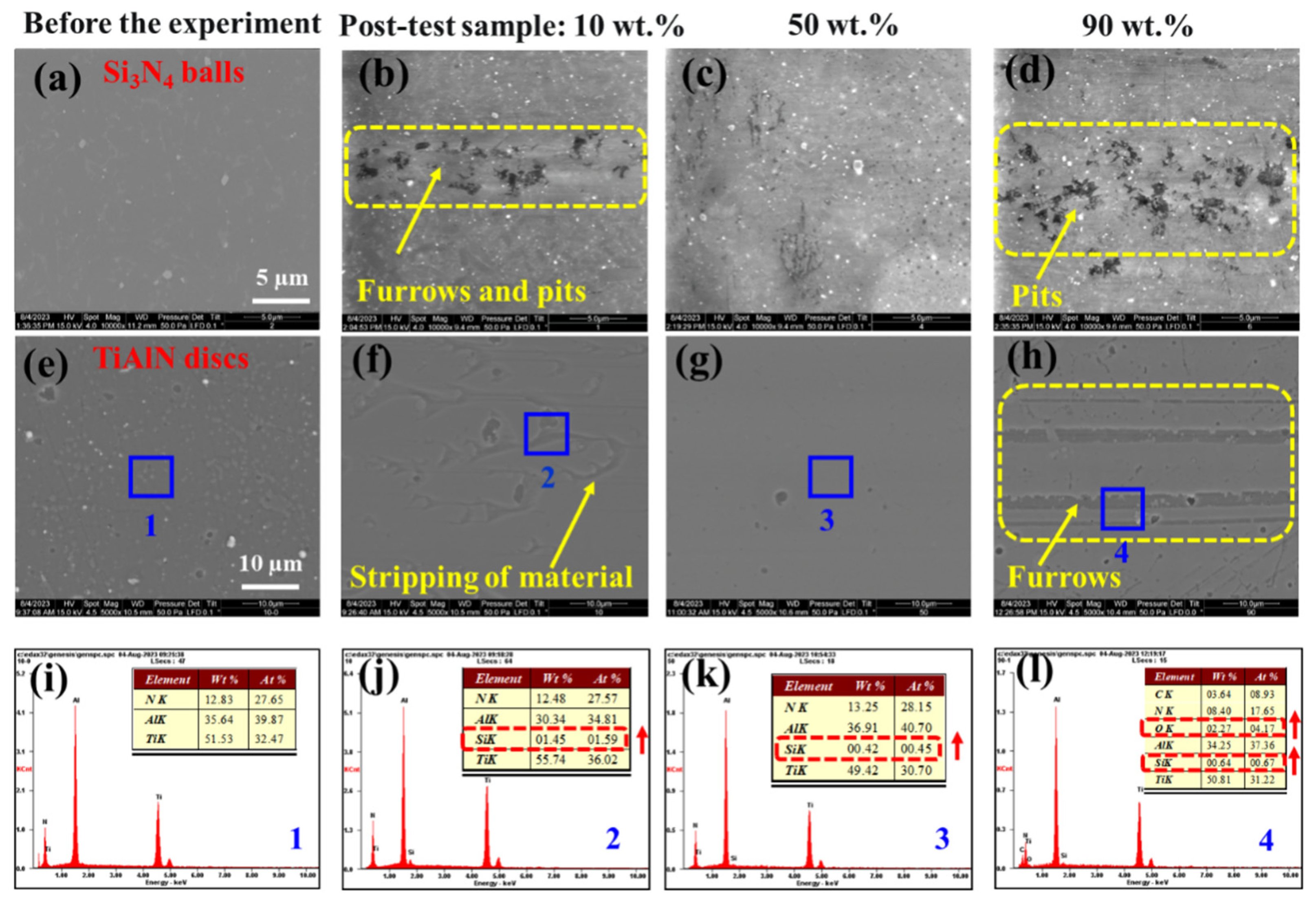
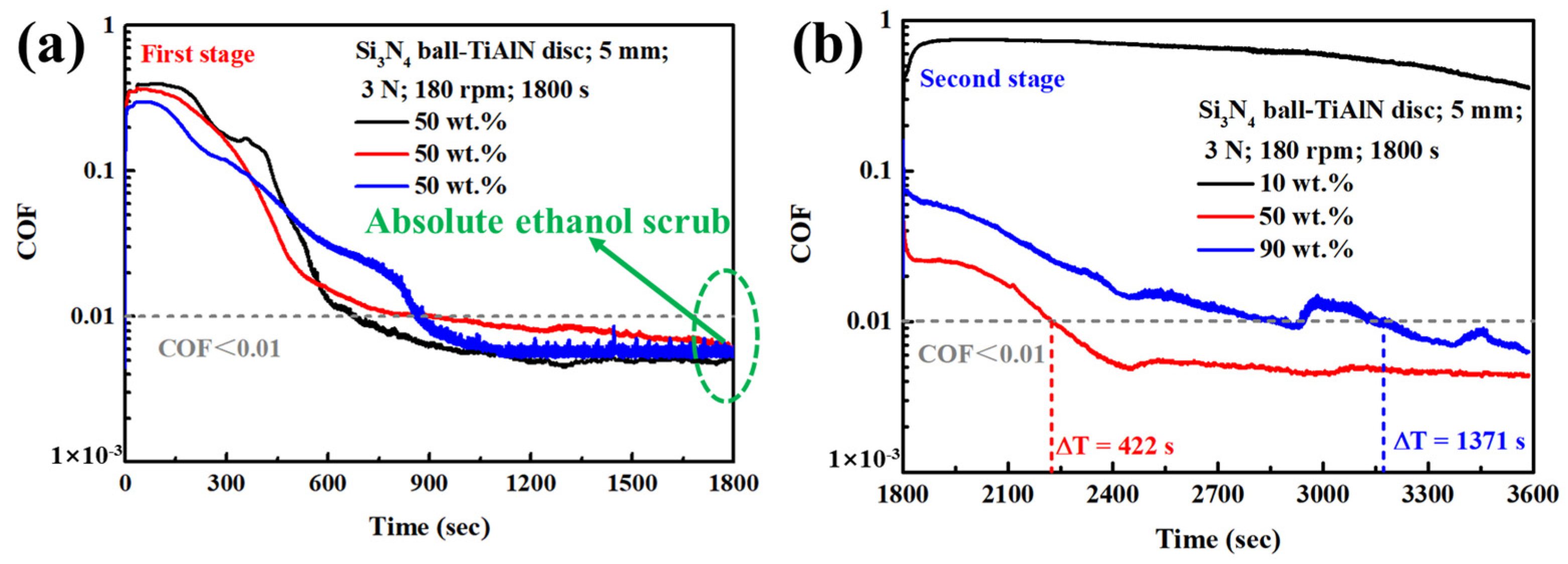
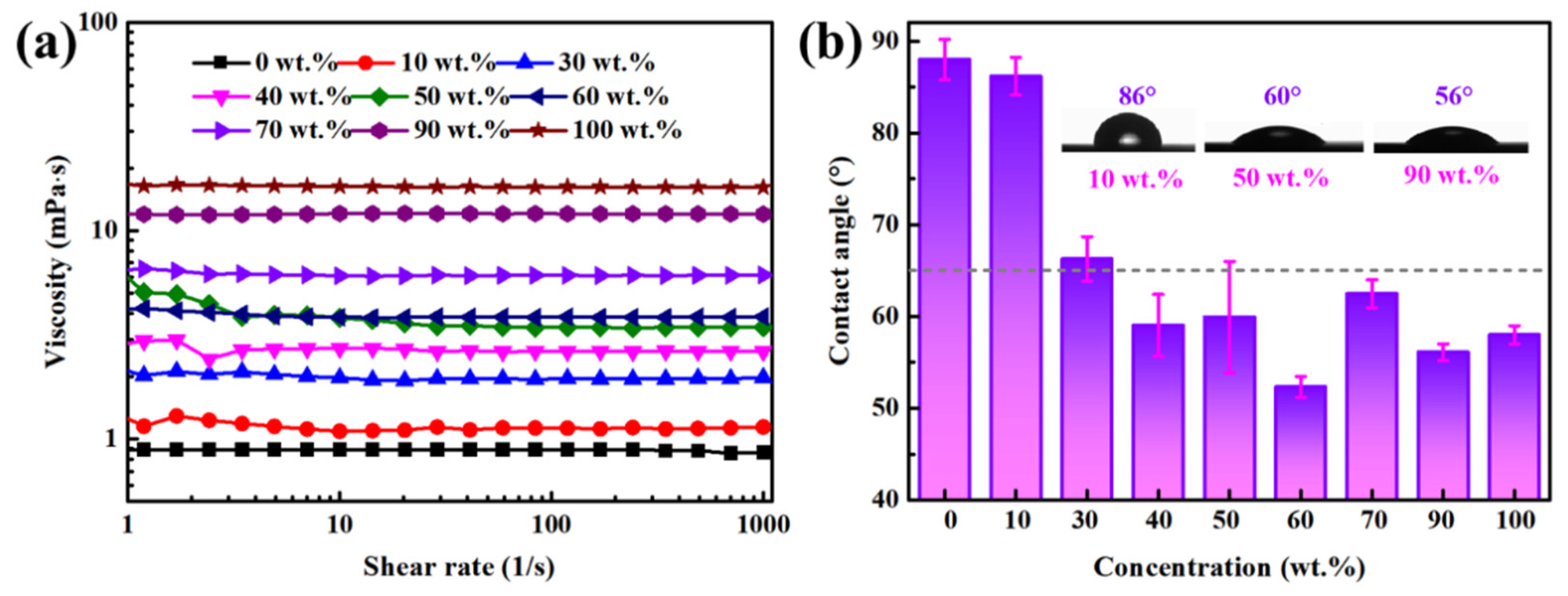
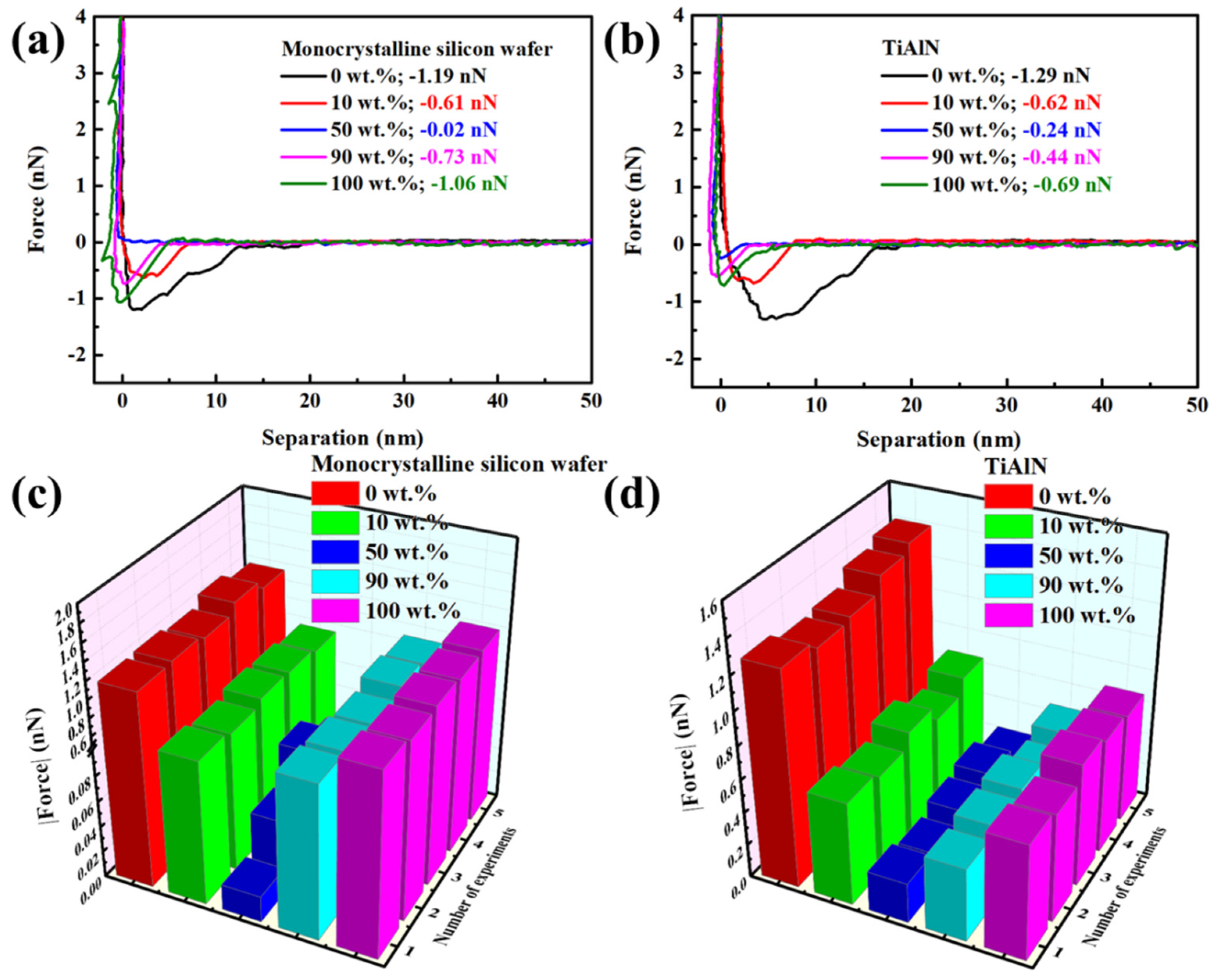
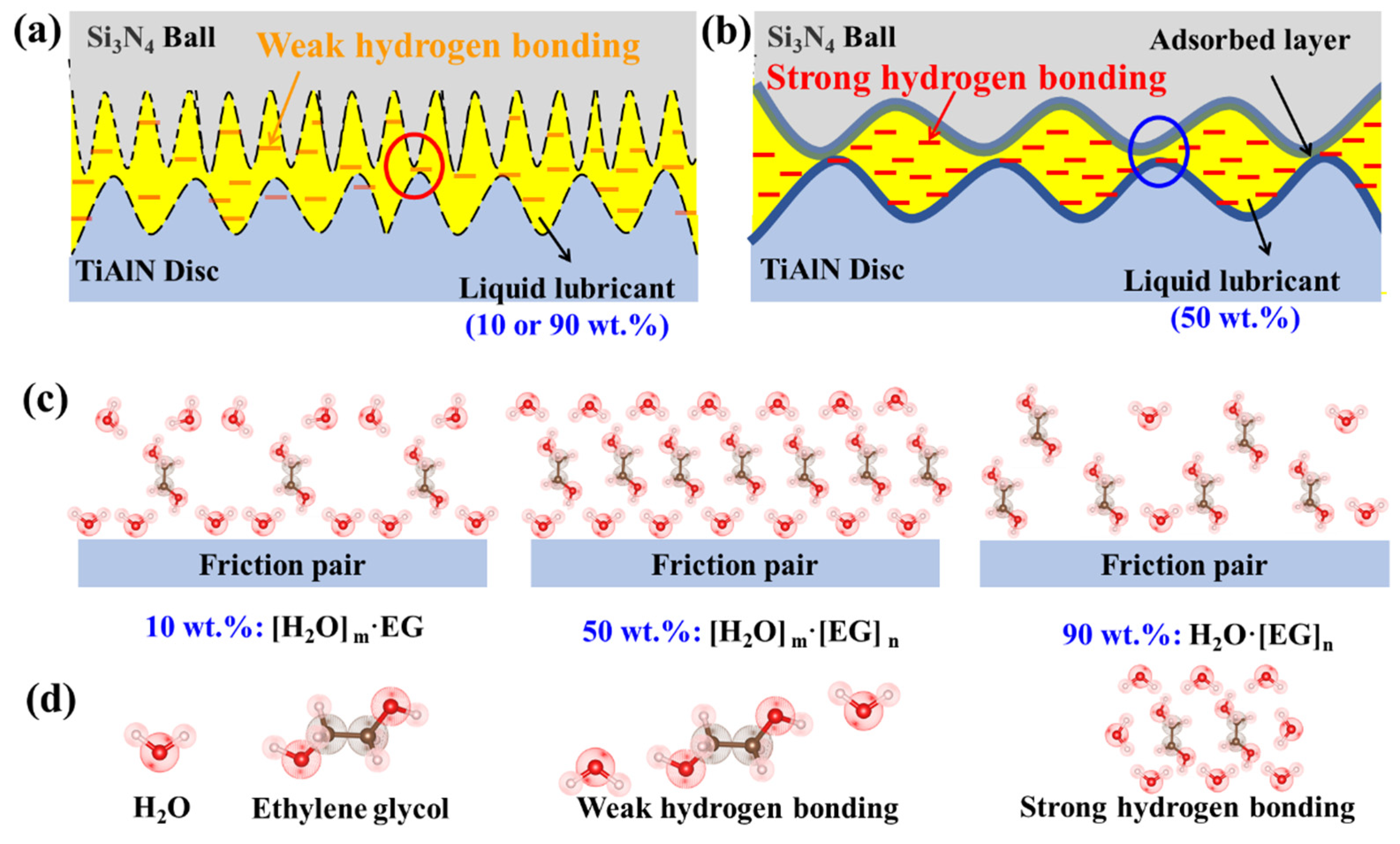
3.1. Results
3.2. Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Stellman, J.M. Encyclopaedia of Occupational Health and Safety; International Labour Organization: Geneva, Switzerland, 1998. [Google Scholar]
- Goto, T.; Yin, S.; Sato, T. Morphological control of zinc oxide and application to cosmetics. Int. J. Nanotechnol. 2013, 10, 48–56. [Google Scholar] [CrossRef]
- Seiler, J.; Hackmann, J.; Lanzerath, F.; Bardow, A. Refrigeration below zero C: Adsorption chillers using water with ethylene glycol as antifreeze. Int. J. Refrig. 2017, 77, 39–47. [Google Scholar] [CrossRef]
- Nguyen, T.; Zhao, M.; Geng, S.; Ivey, D. Ethylene Glycol as an Antifreeze Additive and Corrosion Inhibitor for Aqueous Zinc-Ion Batteries. Batter. Supercaps. 2022, 5, e202100420. [Google Scholar] [CrossRef]
- Singh, T.; Jain, M.; Ganguli, D.; Ravi, K. Evaluation of water glycol hydraulic fluids: A tribological approach. J. Synth. Lubric. 2006, 23, 177–184. [Google Scholar] [CrossRef]
- Wang, J.; Xu, C.; Wang, J.; Li, C.; Zhao, G.; Wang, X. Tribological properties of three S-Alkyl-N, N-dicarboxymethyl dithiocarbamates as additives in water–glycol hydraulic fluid. Tribol. Trans. 2013, 56, 374–384. [Google Scholar] [CrossRef]
- Peyghambarzadeh, S.; Hashemabadi, S.; Hoseini, S.; Jamnani, M. Experimental study of heat transfer enhancement using water/ethylene glycol based nanofluids as a new coolant for car radiators. Int. Commun. Heat Mass Transfer 2011, 38, 1283–1290. [Google Scholar] [CrossRef]
- Azmi, W.; Hamid, K.; Usri, N.; Mamat, R.; Sharma, K. Heat transfer augmentation of ethylene glycol: Water nanofluids and applications—A review. Int. Commun. Heat Mass Transfer 2016, 75, 13–23. [Google Scholar] [CrossRef]
- Soltani, F.; Hajian, M.; Toghraie, D.; Gheisari, A.; Sina, N.; Alizadeh, A. Applying Artificial Neural Networks (ANNs) for prediction of the thermal characteristics of engine oil–based nanofluids containing tungsten oxide-MWCNTs. Case Stud. Therm. Eng. 2021, 26, 101122. [Google Scholar] [CrossRef]
- Matta, C.; Joly-Pottuz, L.; Bouchet, M.; Martin, J.; Kano, M.; Zhang, Q.; Goddard, W. Superlubricity and tribochemistry of polyhydric alcohols. Phys. Rev. B 2008, 78, 85436. [Google Scholar] [CrossRef]
- Jia, W.; Tian, J.; Bai, P.; Li, S.; Zeng, H.; Zhang, W.; Tian, Y. A novel comb-typed poly (oligo (ethylene glycol) methylether acrylate) as an excellent aqueous lubricant. J. Colloid Interface Sci. 2019, 539, 342–350. [Google Scholar] [CrossRef]
- Liu, W.; Wang, H.; Liu, Y.; Zhang, C.; Luo, J. Controllable superlubricity system of polyalkylene glycol aqueous solutions under various applied conditions. Macromol. Mater. Eng. 2020, 305, 2000141. [Google Scholar] [CrossRef]
- Du, C.; Yu, T.; Wu, Z.; Zhang, L.; Shen, R.; Li, X.; Feng, M.; Feng, Y.; Wang, D. Achieving macroscale superlubricity with ultra-short running-in period by using polyethylene glycol-tannic acid complex green lubricant. Friction 2023, 11, 748–762. [Google Scholar] [CrossRef]
- Liu, W.; Wang, H.; Liu, Y. Adjustable superlubricity system using polyalkylene glycol with various acid aqueous solutions. Friction 2023, 11, 1138–1149. [Google Scholar] [CrossRef]
- Han, T.; Yi, S.; Zhang, C.; Li, J.; Chen, X.; Luo, J.; Banquy, X. Superlubrication obtained with mixtures of hydrated ions and polyethylene glycol solutions in the mixed and hydrodynamic lubrication regimes. J. Colloid Interface Sci. 2020, 579, 479–488. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, X.; Yu, H.; Chen, H.; Feng, D.; Qian, D. Insight into macroscale superlubricity of polyol aqueous solution induced by protic ionic liquid. Friction 2022, 10, 2000–2017. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, P.; Ma, K.; Han, F.; Chen, G.; Wei, X. Hydrogen bonding interactions between ethylene glycol and water: Density, excess molar volume, and spectral study. Sci. China Ser. B-Chem. 2008, 51, 420–426. [Google Scholar] [CrossRef]
- Ninni, L.; Camargo, M.; Meirelles, A. Water activity in poly (ethylene glycol) aqueous solutions. Thermochim. Acta 1999, 328, 169–176. [Google Scholar] [CrossRef]
- Gmbh, R. Automotive Handbook; Society of Automotive Engineers: Germany, Bosch, 2004. [Google Scholar]
- Baudot, A.; Odagescu, V. Thermal properties of ethylene glycol aqueous solutions. Cryobiology 2004, 48, 283–294. [Google Scholar] [CrossRef] [PubMed]
- De, O.; Freitas, L. Molecular dynamics simulation of liquid ethylene glycol and its aqueous solution. J. Mol. Struct.-Theochem. 2005, 728, 179–187. [Google Scholar]
- Wang, Y.; Zhao, N.; Fang, B.; Li, H.; Bi, X.; Wang, H. Effect of different solvent ratio (ethylene glycol/water) on the preparation of Pt/C catalyst and its activity toward oxygen reduction reaction. RSC Adv. 2015, 5, 56570–56577. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Opalinska, A.; Chudoba, T.; Gierlotka, S.; Mukhovskyi, R.; Pietrzykowska, R.; Sobczak, K.; Lojkowski, W. Effect of water content in ethylene glycol solvent on the size of ZnO nanoparticles prepared using microwave solvothermal synthesis. J. Nanomater. 2016, 2016, 2789871. [Google Scholar] [CrossRef]
- Azmi, W.; Usri, N.; Mamat, R.; Sharma, K.; Noor, M. Force convection heat transfer of Al2O3 nanofluids for different based ratio of water: Ethylene glycol mixture. Appl. Therm. Eng. 2017, 112, 707–719. [Google Scholar] [CrossRef]
- Yan, S.; Lin, B.; Zhang, X.; Wang, A.; Zhou, X. Investigation of the running-in process of silicon nitride sliding in aqueous solutions of ethylene glycol. Tribol. Int. 2015, 90, 386–392. [Google Scholar] [CrossRef]
- Weng, L.; Chen, C.; Zuo, J.; Li, W. Molecular dynamics study of effects of temperature and concentration on hydrogen-bond abilities of ethylene glycol and glycerol: Implications for cryopreservation. J. Phys. Chem. A 2011, 115, 4729–4737. [Google Scholar] [CrossRef]
- Krishnan, K.; Krishnan, R. Raman and Infrared Spectra of Ethylene Glycol. In Proceedings of the Indian Academy of Sciences-Section A; Springer: New Delhi, India, 1966; Volume 64, pp. 111–122. [Google Scholar]
- Wang, Y.; Li, F.; Fang, W.; Sun, C.; Men, Z. Study of hydrogen bonding interactions in ethylene glycol-water binary solutions by Raman spectroscopy. Spectrochim. Acta A 2021, 260, 119916. [Google Scholar] [CrossRef] [PubMed]
- Nalam, P.; Clasohm, J.; Mashaghi, A.; Spencer, N. Macrotribological Studies of Poly(L-lysine)-graft-Poly(ethylene glycol) in Aqueous Glycerol Mixtures. Tribol. Lett. 2009, 37, 541–552. [Google Scholar] [CrossRef]
- Wen, X.; Fang, J.; Bai, P.; Li, Y.; Meng, Y.; Ma, L.; Tian, Y. Significantly Affected Lubrication Behavior of Silicone Oil Lubricated Si3N4/Glass Contact after Cleaning with Different Solvents. Langmuir 2022, 39, 155–167. [Google Scholar] [CrossRef]
- Wen, X.; Bai, P.; Li, Y.; Cao, H.; Li, S.; Wang, B.; Fang, J.; Meng, Y.; Ma, T.; Tian, Y. Effects of Abrasive Particles on Liquid Superlubricity and Mechanisms for Their Removal. Langmuir 2021, 37, 3628–3636. [Google Scholar] [CrossRef]
- Han, T.; Zhang, C.; Chen, X.; Li, J.; Wang, W.; Luo, J. The Contribution of Tribo-Induced Silica Layer to Macroscale Superlubricity of Hydrated Ions. J. Phys. Chem. C 2019, 123, 20270–20277. [Google Scholar] [CrossRef]
- Wen, X.; Bai, P.; Meng, Y.; Ma, L.; Tian, Y. High-Temperature Superlubricity Realized with Chlorinated-Phenyl and Methyl-Terminated Silicone Oil and Hydrogen-Ion Running-in. Langmuir 2022, 38, 10043–10051. [Google Scholar] [CrossRef]
- Dye, R. Ethylene glycols technology. Korean J. Chem. Eng. 2001, 18, 571–579. [Google Scholar] [CrossRef]
- Inhibited Ethylene Glycol-Based Heat Transfer Fluid. Available online: https://www.dow.com (accessed on 22 October 2023).
- Gupta, S. Viscosity of Water. In Viscometry for Liquids: Calibration of Viscometers; Springer International Publishing: Cham, Switzerland, 2014; pp. 197–226. [Google Scholar]
- Giguère, P. Bifurcated hydrogen bonds in water. J. Raman Spectrosc. 1984, 15, 354–359. [Google Scholar] [CrossRef]
- Du, Q.; Freysz, E.; Shen, Y. Vibrational spectra of water molecules at quartz/water interfaces. Phys. Rev. Lett. 1994, 72, 238. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Baskar, P.; Balamurugan, K.; Das, S.; Subramanian, V. On the perturbation of the H-bonding interaction in ethylene glycol clusters upon hydration. J. Phys. Chem. A 2012, 116, 4239–4247. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ozaki, Y.; Czarnecki, M. Molecular structure and hydrogen bonding in pure liquid ethylene glycol and ethylene glycol–water mixtures studied using NIR spectroscopy. Phys. Chem. Chem. Phys. 2013, 15, 18694–18701. [Google Scholar] [CrossRef] [PubMed]

| EG Concentration (wt.%) | Freezing Point (°C/°F) | Boiling Point (°C @ 101 kPa/°F @ 760 mmHG) | Degree Brix | Refractive Index 22 °C/72 °F |
|---|---|---|---|---|
| 10 | −3.2/26.2 | 101.1/214 | 6.8 | 1.3428 |
| 30 | −14.1/6.7 | 104.4/220 | 19.2 | 1.3635 |
| 40 | −22.3/−8.1 | 105.6/222 | 25.3 | 1.3741 |
| 50 | −33.8/−28.9 | 107.2/225 | 31.2 | 1.3849 |
| 60 | −48.3/−54.9 | 110.0/230 | 36.6 | 1.3952 |
| 70 | -/- | 116.7/242 | 41.7 | 1.4055 |
| 90 | −29.8/−21.6 | 140.6/285 | 51.2 | 1.4255 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Gong, P.; Bai, P.; Wen, X.; Meng, Y.; Ding, J.; Tian, Y. Impact of Water Content on the Superlubricity of Ethylene Glycol Solutions. Lubricants 2023, 11, 466. https://doi.org/10.3390/lubricants11110466
Li L, Gong P, Bai P, Wen X, Meng Y, Ding J, Tian Y. Impact of Water Content on the Superlubricity of Ethylene Glycol Solutions. Lubricants. 2023; 11(11):466. https://doi.org/10.3390/lubricants11110466
Chicago/Turabian StyleLi, Lvzhou, Peng Gong, Pengpeng Bai, Xiangli Wen, Yonggang Meng, Jianning Ding, and Yu Tian. 2023. "Impact of Water Content on the Superlubricity of Ethylene Glycol Solutions" Lubricants 11, no. 11: 466. https://doi.org/10.3390/lubricants11110466
APA StyleLi, L., Gong, P., Bai, P., Wen, X., Meng, Y., Ding, J., & Tian, Y. (2023). Impact of Water Content on the Superlubricity of Ethylene Glycol Solutions. Lubricants, 11(11), 466. https://doi.org/10.3390/lubricants11110466






