Influence of Dry-Film Lubricants on Bond Strength and Corrosion Behaviour of 6xxx Aluminium Alloy Adhesive Joints for the Automotive Industry
Abstract
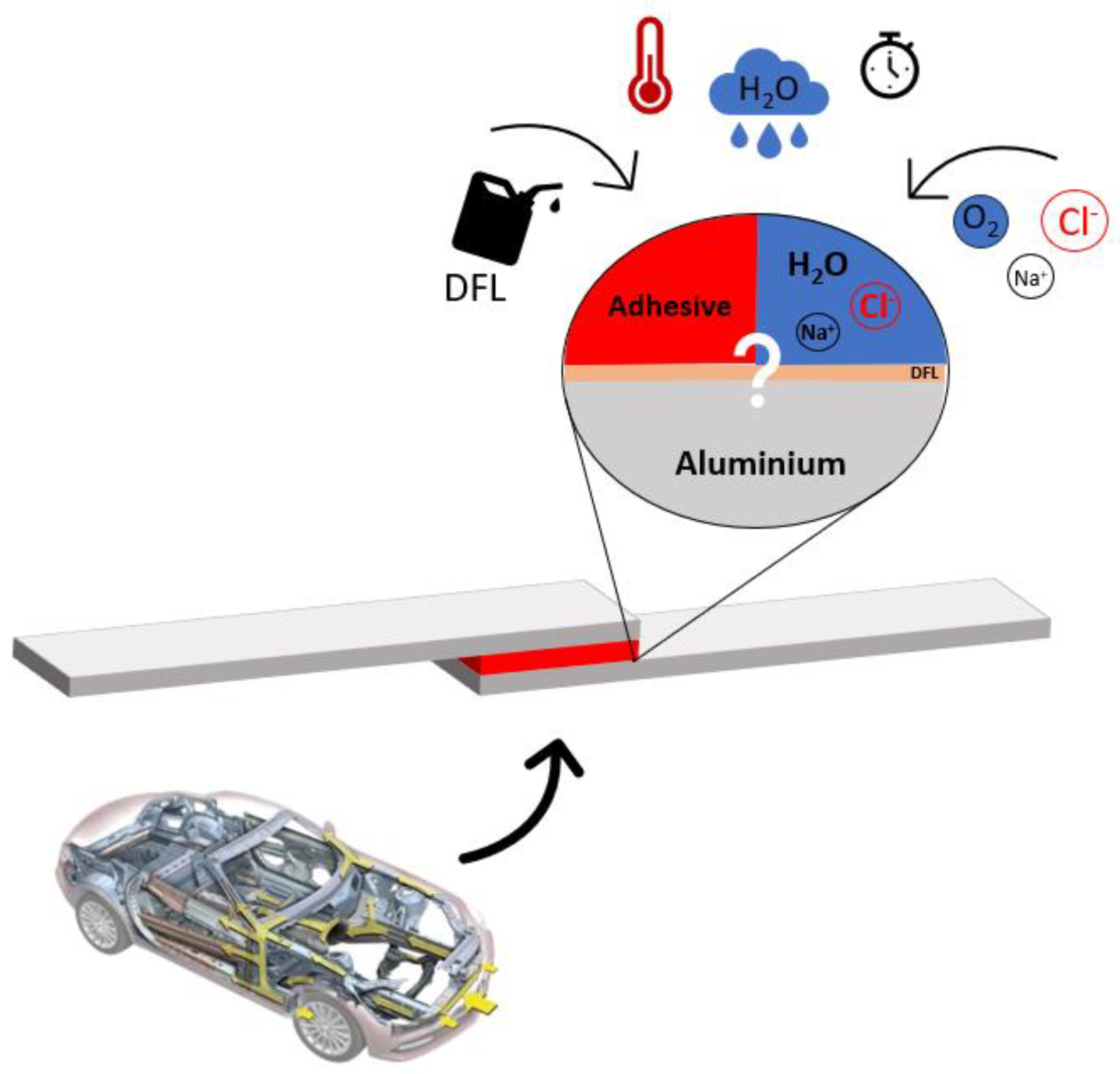
1. Introduction
2. Materials and Methods
2.1. Materials
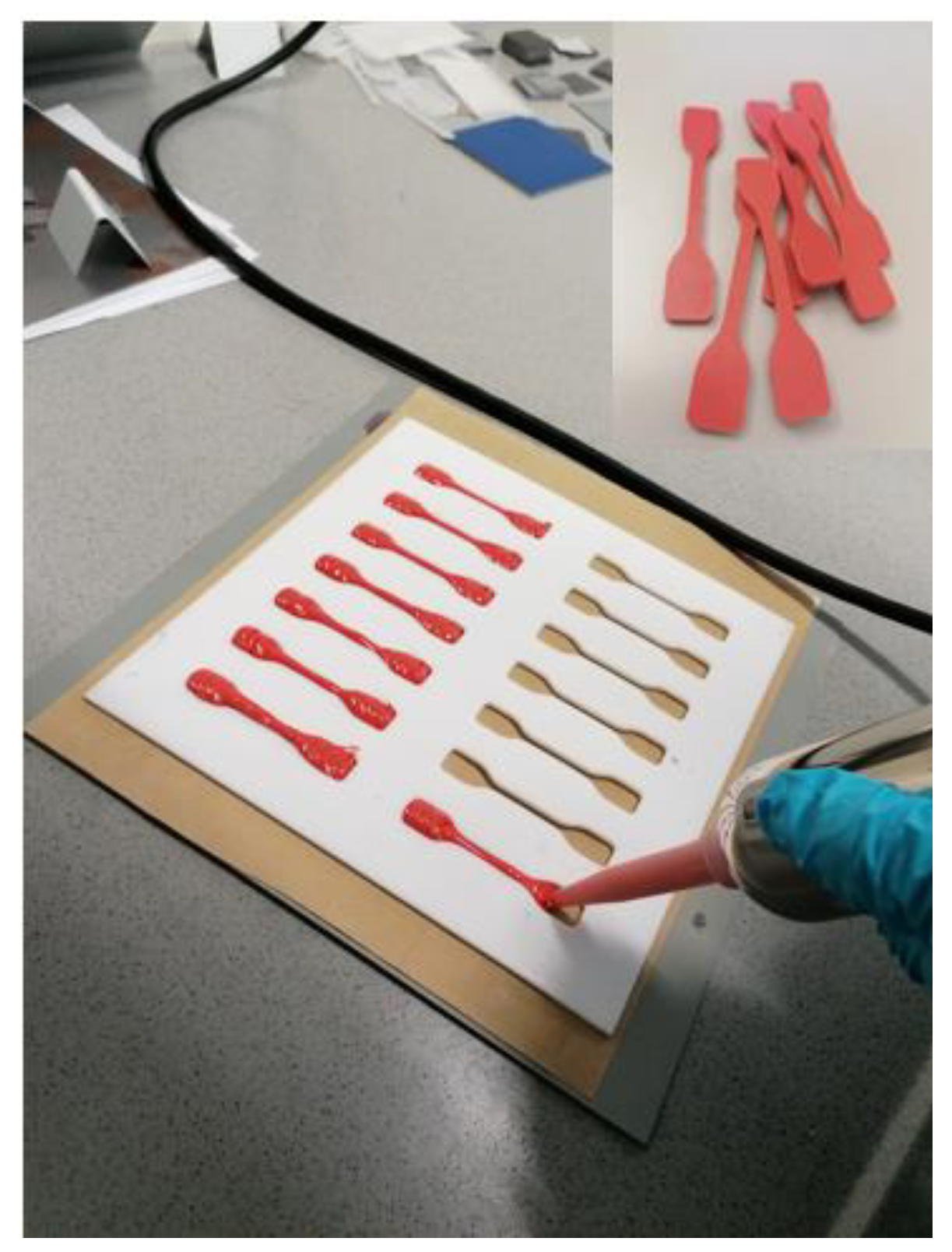
2.2. Sample Preparation
2.3. Testing and Characterisation
2.3.1. Corrosion Test
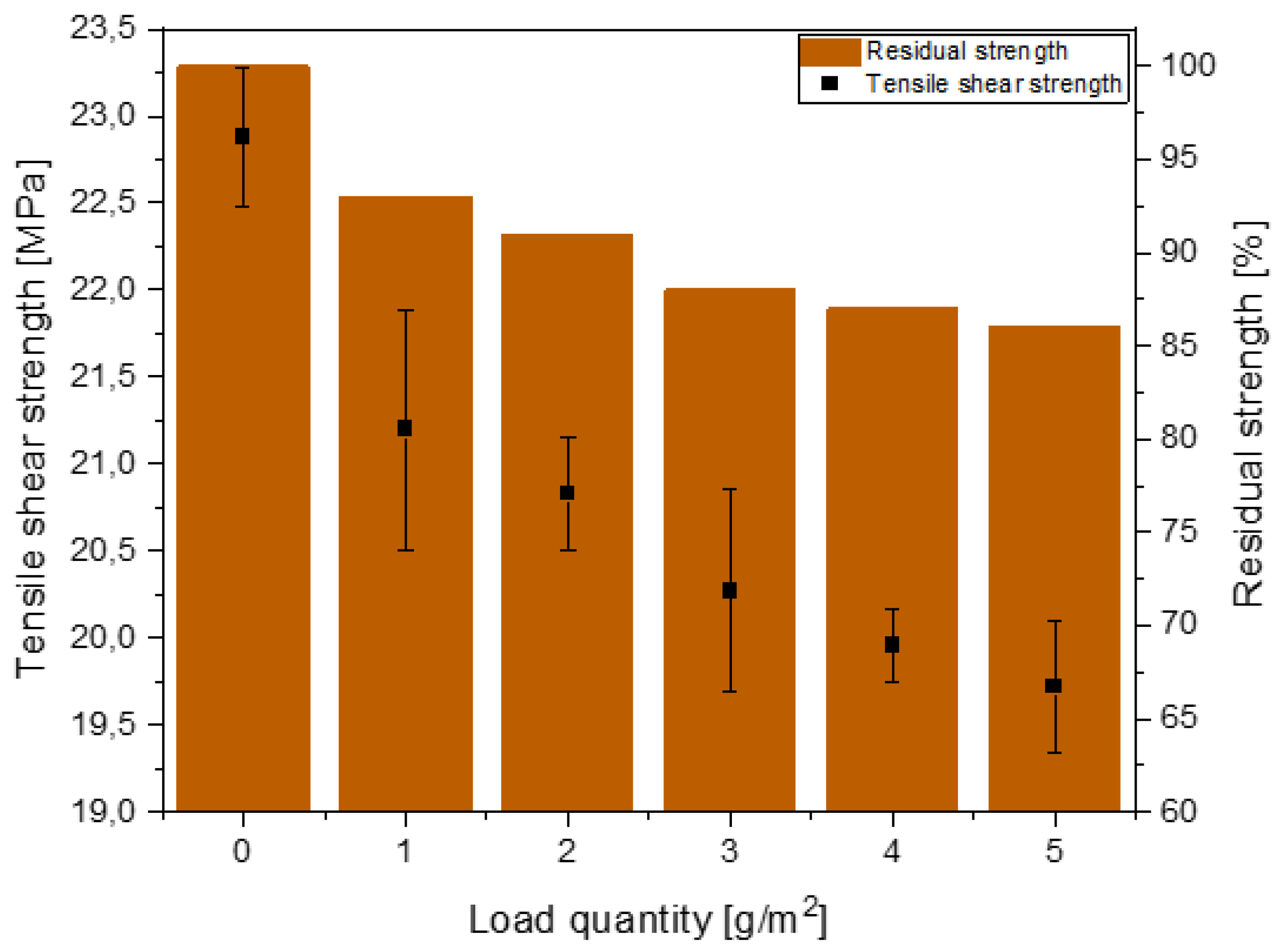
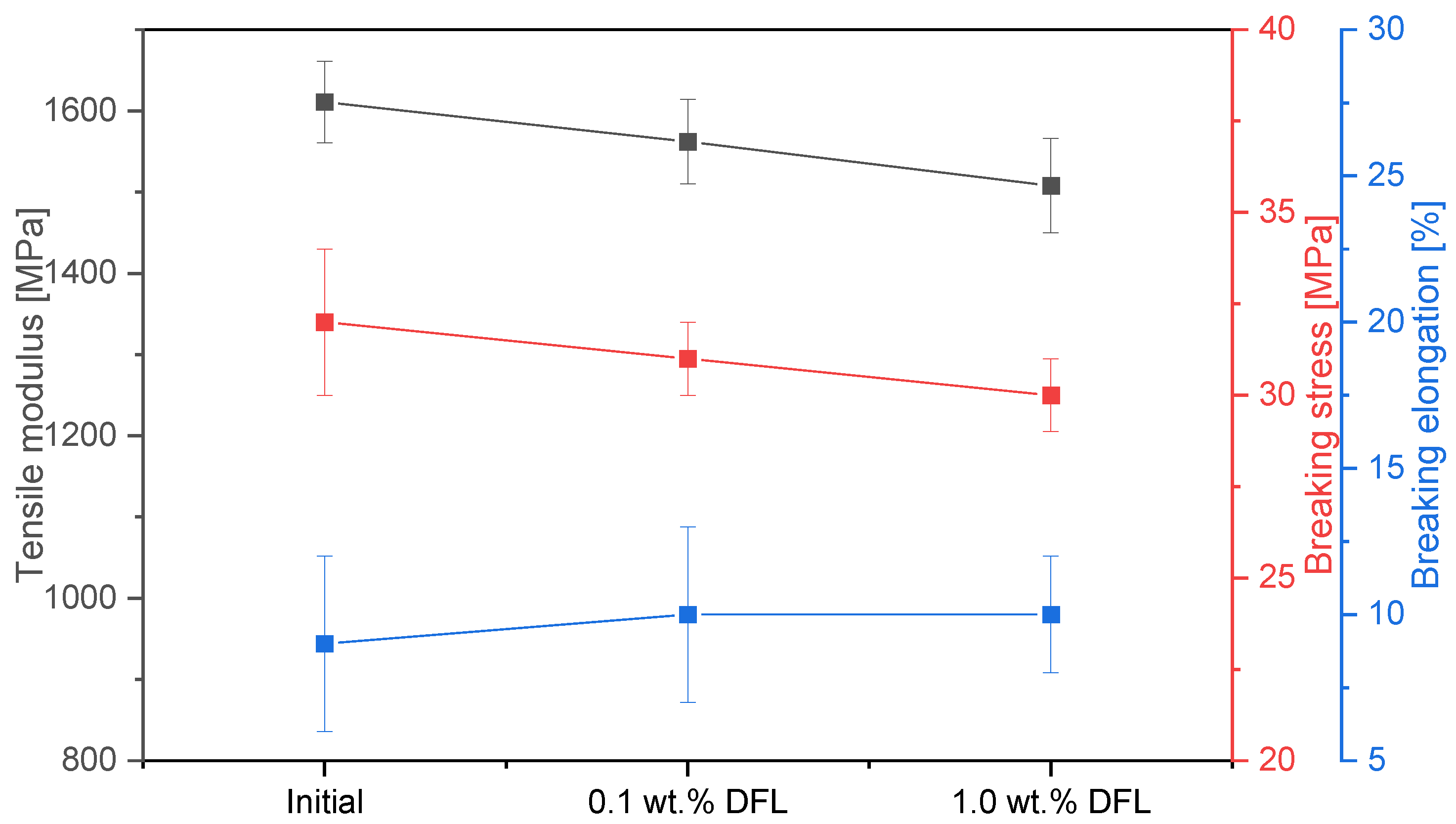
2.3.2. Tensile and Single-Lap Shear Test
2.3.3. Dynamic Mechanical Analysis (DMA)
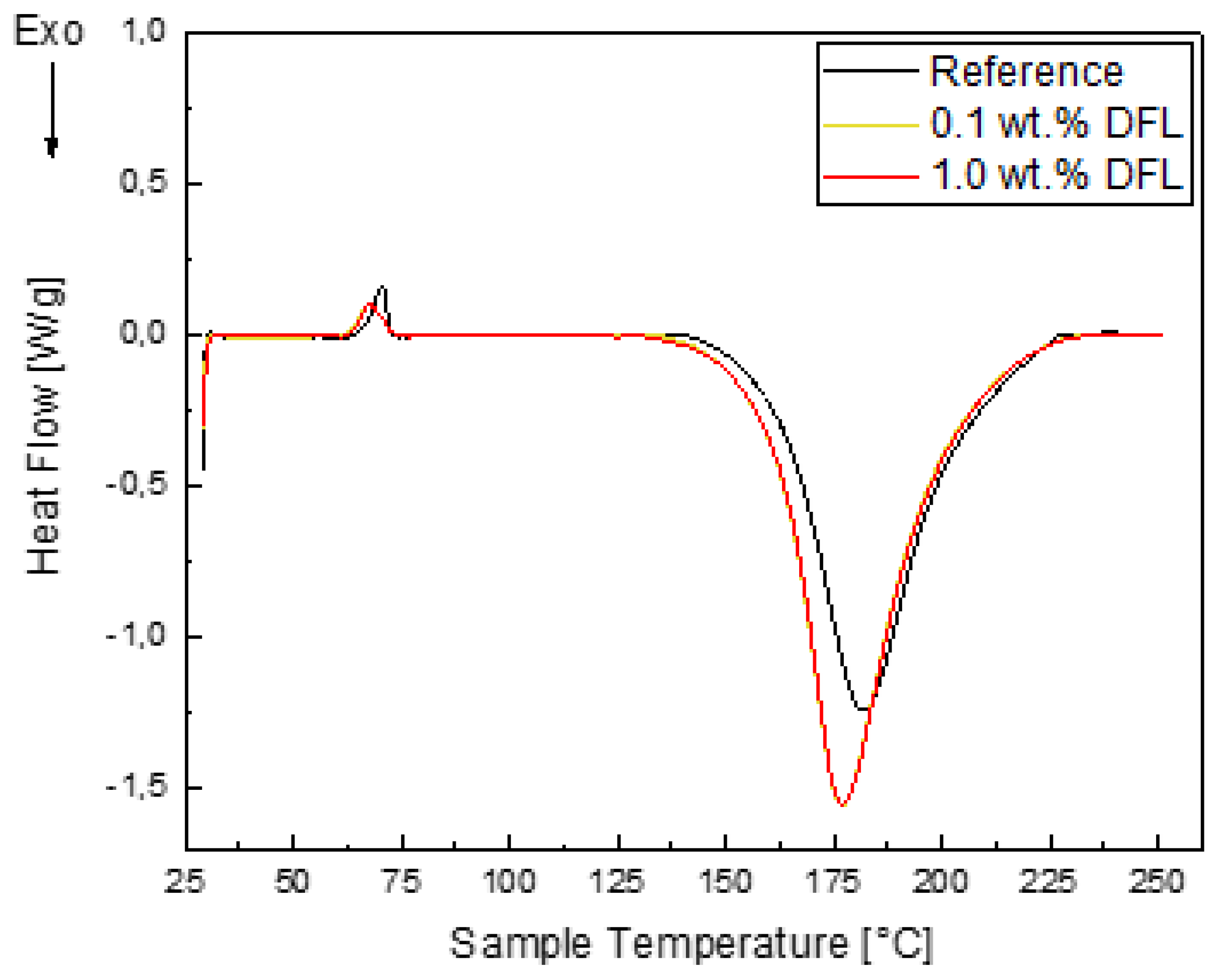
2.3.4. Differential Scanning Calorimetry (DSC)
2.3.5. Scanning Electron Microscopy (SEM)
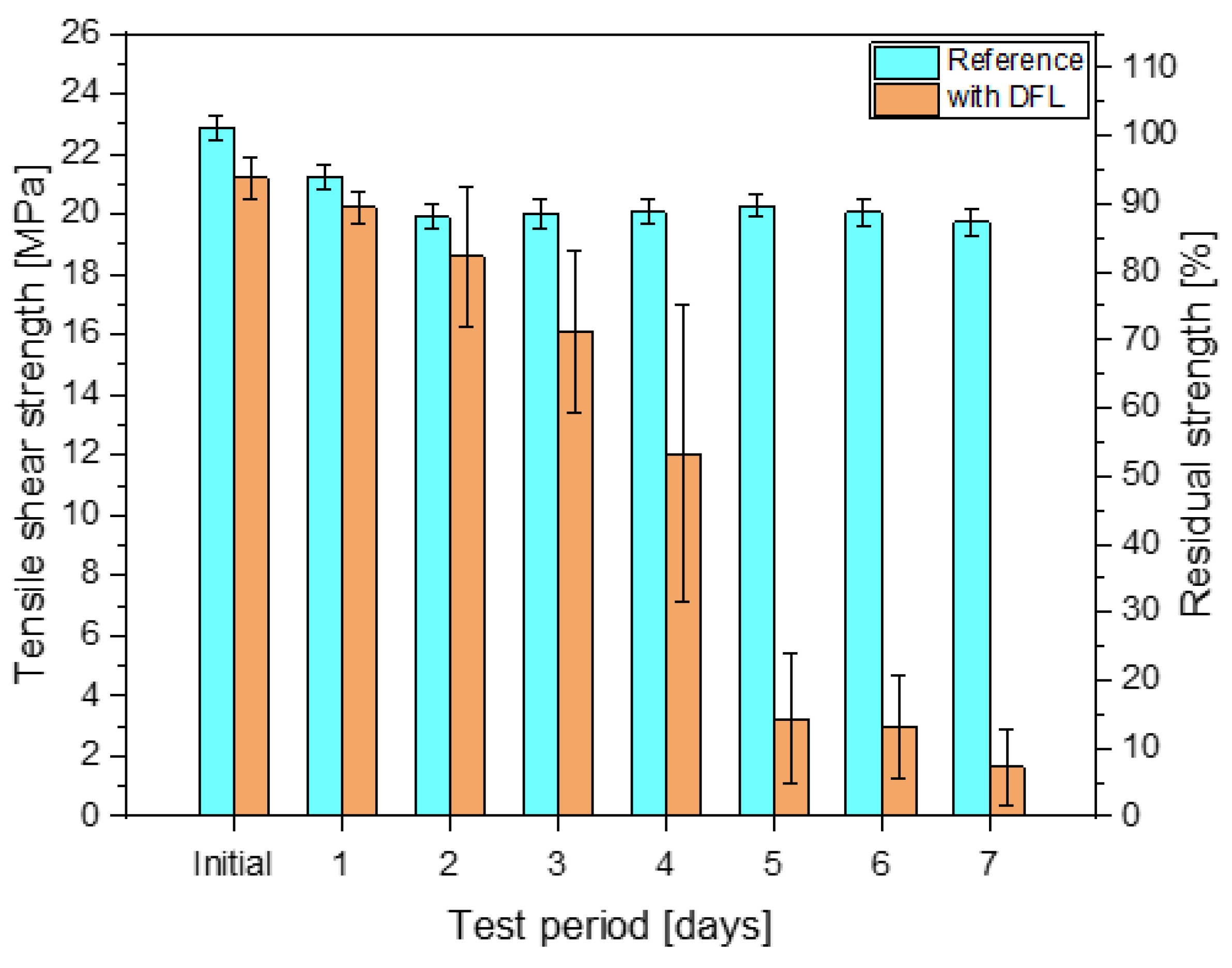
3. Results
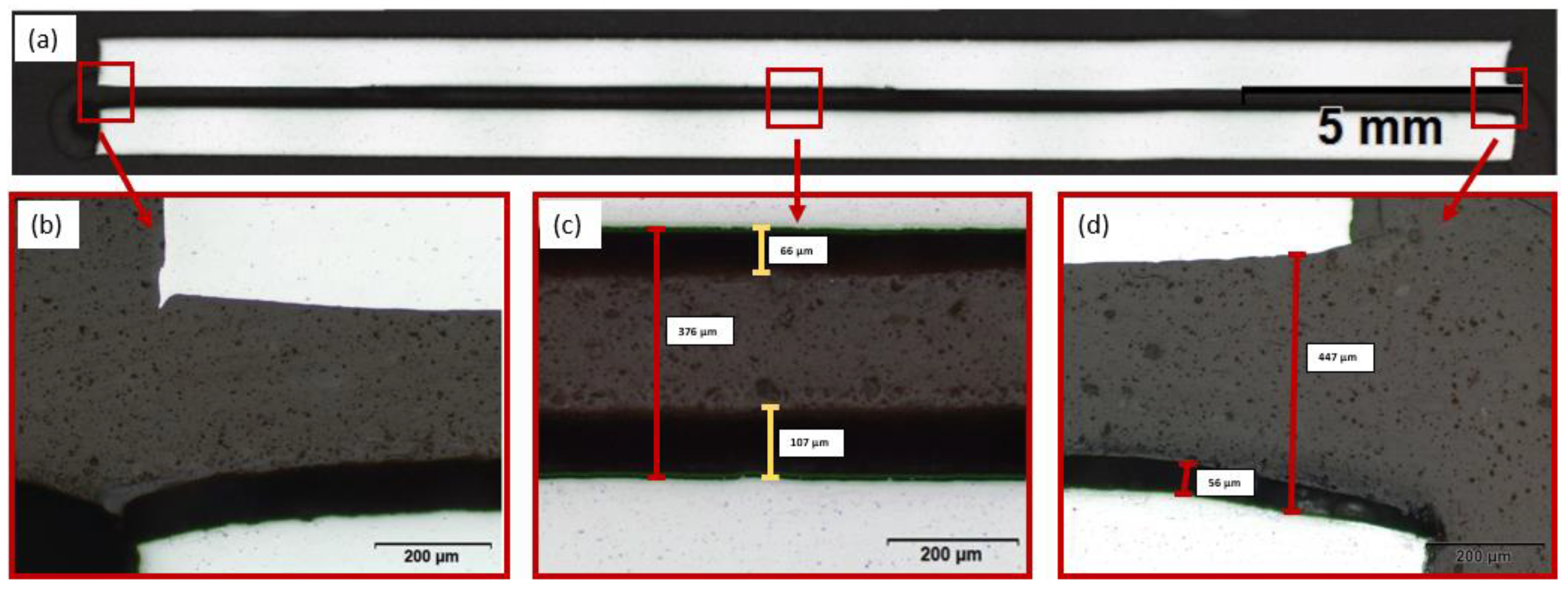
3.1. Displacement of DFL
3.2. Swelling of the Adhesive
3.3. Change in the Polymer Matrix
3.4. Corrosion Mechanism
4. Discussion and Conclusions
- In comparison with a nonlubricated reference, the DFL promoted a reinforced corrosive attack during a standardised immersion test procedure;
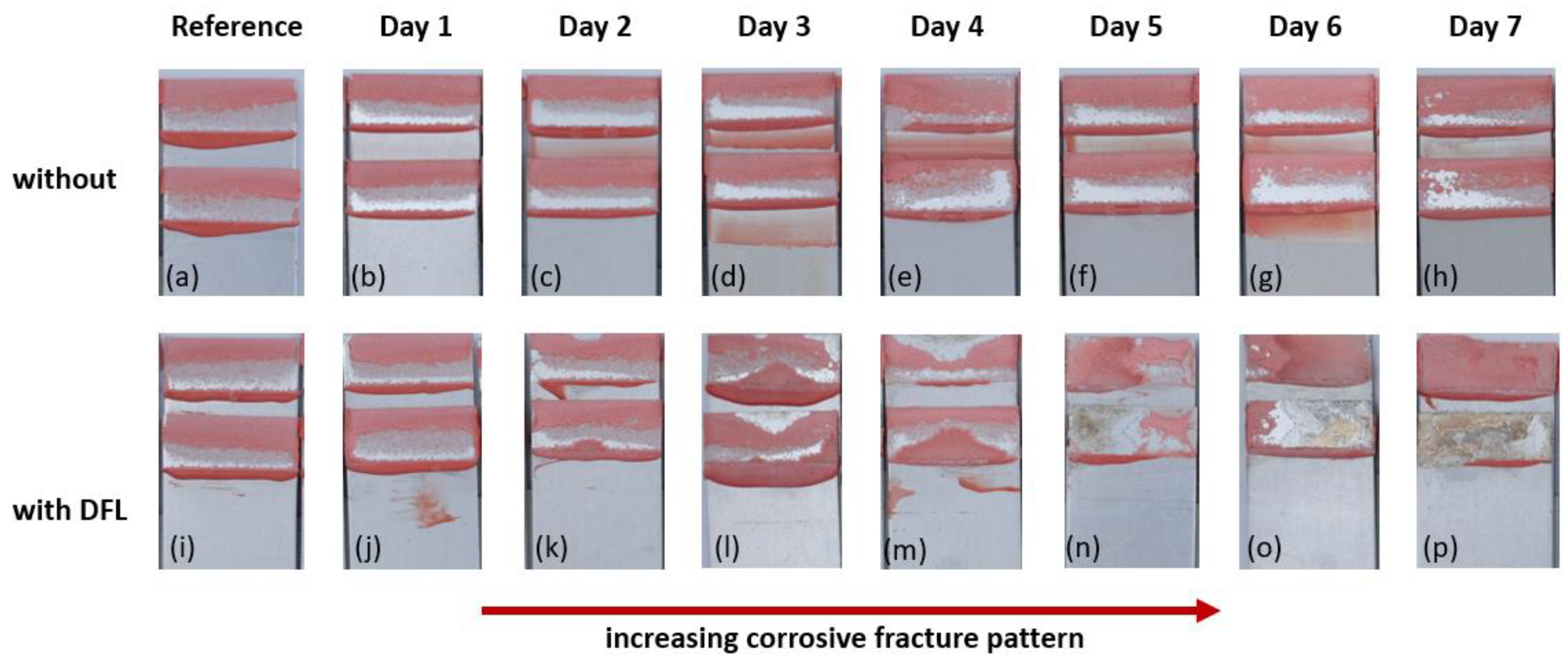
- The fracture pattern analysis indicated bondline corrosion already after 24 h of testing. A cross-section of a tested joint showed corrosive delamination of the adhesive, which led to loss of the mechanical strength of the joint;
- The load quantity of the dry lubricant already influenced the tensile shear strengths in the initial state. Microscopic characterisation showed a displacement out of the joint, which indicates an incomplete absorption of the DFL by the adhesive;
- Displacement favours the diffusion of corrosive media. This diffusion leads to a swelling of the adhesive and forms cavities in the vicinity of inorganic fillers inside the polymer network. These voids additionally favour the diffusion of immersion solution, which promote bondline corrosion at the aluminium–adhesive interface;
- At the interface, the dry lubricant is directly in contact with the adhesive. The interaction between both the DFL and the adhesive leads to a change in the mechanical and thermal properties of the adhesive. Tensile tests and a DMA revealed a lubricant-induced plasticisation with a decrease in the glass transition temperature;
- The dry lubricant also affects the curing behaviour, which was observed with DSC analysis.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- European Aluminium Association. EAA Aluminium Automotive Manual—Joining; The Aluminium Automotive Manual, European Aluminium Association: Ljubljana, Slovenia, 2015; pp. 1–5. [Google Scholar]
- Alfano, M.; Morano, C.; Moroni, F.; Musiari, F.; Spennacchio, G.D.; Di Lonardo, D. Fracture toughness of structural adhesives for the automotive industry. Procedia Struct. Int. 2018, 8, 561–565. [Google Scholar] [CrossRef]
- Meschut, G.; Janzen, V.; Olfermann, T. Innovative and highly productive joining technologies for multi-material lightweight car body structures. J. Mater. Eng. Perform. 2014, 23, 1515–1523. [Google Scholar] [CrossRef]
- Barnes, T.; Pashby, I. Joining techniques for aluminium spaceframes used in automobiles. J. Mater. Process. Technol. 2002, 99, 72–79. [Google Scholar] [CrossRef]
- Chen, H.; Wang, D.; Na, J.; Chen, X. Effect of sealing treatment on mechanical properties of CFRP-Aluminum alloy single lap joints. Int. J. Adhes. Adhes. 2022, 119, 103236. [Google Scholar] [CrossRef]
- Cavezza, F.; Boehm, M.; Terryn, H.; Hauffman, T. A Review on Adhesively Bonded Aluminium Joints in the Automotive Industry. Metals 2020, 10, 730. [Google Scholar] [CrossRef]
- Viana, G.M.S.O.; Costa, M.; Banea, M.D.; Da Silva, L.F.M. A review on the temperature and moisture degradation of adhesive joints. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2017, 231, 488–501. [Google Scholar] [CrossRef]
- Goede, M.; Stehlin, M.; Rafflenbeul, L.; Kopp, G.; Beeh, E. Super light car-lightweight construction thanks to a multi-material design and function integration. Eur. Transp. Res. Rev. 2009, 1, 5–10. [Google Scholar] [CrossRef]
- Mercier, D.; Rouchaud, J.-C.; Barthés-Labrousse, M.-G. Interaction of amines with native aluminium oxide layers in non-aqueous environment: Application to the understanding of the formation of epoxy-amine/metal Interphases. Appl. Surf. Sci. 2008, 254, 6495–6503. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, X. Moisture sorption-desorption-resorption characteristics and its effect on the mechanical behaviour of the epoxy system. Polymer 2005, 46, 11994–12003. [Google Scholar] [CrossRef]
- Lörinci, G.; Matuschek, G.; Fekete, J.; Gebefügi, I.; Kettrup, A. Investigation of thermal degradation of some adhesives used in the automobile industry by thermal analysis/mass spectrometry and GC-MS. Thermochim. Act. 1995, 263, 73–86. [Google Scholar] [CrossRef]
- Ocaña, R.; Arenas, J.; Alía, C.; Narbón, J. Evaluation of degradation of structural adhesive joints in functional automotive applications. Procedia Eng. 2015, 132, 716–723. [Google Scholar] [CrossRef][Green Version]
- Hirsch, J. Recent development in aluminium for automotive applications. Trans. Nonferrous Met. Soc. China 2014, 24, 1995–2002. [Google Scholar] [CrossRef]
- Gruber, R.; Singewald, T.D.; Bruckner, T.M.; Hader-Kregl, L.; Hafner, M.; Groiss, H.; Duchoslav, J.; Stifter, D. Investigation of oxide thickness on technical aluminium alloys-a comparison of characterization methods. Metal 2023, 13, 1322. [Google Scholar] [CrossRef]
- Meiler, M.; Jaschke, H. Lubrication of aluminium sheet metal within the automotive industry. Adv. Mater. Res. 2005, 6–8, 551–558. [Google Scholar] [CrossRef]
- Wu, D.; Ma, L.; Liu, B.; Zhang, D.; Minhas, B.; Qian, H.; Terryn, H.A.; Mol, J.M. Long-term deterioration of lubricant-infused nanoporous anodic aluminium oxide surface immersed in NaCl solution. J. Mater. Sci. Tech. 2021, 64, 57–65. [Google Scholar] [CrossRef]
- Blanck, S.; Loehlé, S.; Steinmann, S.N.; Michel, C. Adhesion of lubricant on aluminium through adsorption of additive head-groups on γ-alumina: A DFT study. Tribol. Int. 2020, 145, 106140. [Google Scholar] [CrossRef]
- Zeller+Gmelin Mulidraw Drylube E1. Available online: https://www.zeller-gmelin.de/zgSite/en/Lubricants/Forming/Sheet-Metal-Forming/Multidraw-Drylube-E-1/p/22200?s=93A303F60CD8C34B29EFE69CA7585F7EFE92BF58 (accessed on 25 July 2023).
- Richtlinie DVS 3302: Kleben im Karosseriebau: Bewertung von Bruchbildern. 2018. Available online: https://www.dvs-regelwerk.de/regelwerke/richtlinie-dvs-3302-09-2018 (accessed on 31 July 2023).
- Abrahami, S.T.; Hauffman, T.; de Kok, J.M.; Terryn, H.; Mol, J.M. Adhesive bonding and corrosion performance investigated as a function of aluminum oxide chemistry and adhesives. Corrosion 2017, 73, 903–914. [Google Scholar] [CrossRef]
- Nijemeisland, M.; Meteleva-Fischer, Y.V.; Garcia, S.J. Identifying interfacial failure mode in aerospace adhesive bonds by broadband dielectric spectroscopy. Int. J. Adhes. Adhes. 2022, 118, 103246. [Google Scholar] [CrossRef]
- LeBozec, N.; Thierry, D. Influence of test parameters in an automotive cyclic test on the corrosion and mechanical performance of joined materials. Mater. Corros. 2015, 66, 1051–1059. [Google Scholar] [CrossRef]
- Singewalda, T.D.; Brucknera, T.M.; Grubera, R.; Schimo-Aichhorna, G.; Hader-Kregla, L.; Poellerb, S.; Muellerb, M.; Kernc, C.; Luckenederc, G.; Stellnbergerc, K.-H.; et al. Water-uptake in hollow glass microspheres and their influence on cathodic and anodic delamination along the polymer/metal-Interface. Corros. Sci. 2022, 196, 110045. [Google Scholar] [CrossRef]
- Bruckner, T.M.; Singewald, T.D.; Gruber, R.; Hader-Kregl, L.; Klotz, M.; Müller, M.; Luckeneder, G.; Rosner, M.; Kern, C.; Hafner, M.; et al. Water absorption and leaching of a 1K structural model epoxy adhesive for the automotive industry. Polym. Test. 2023, 117, 107870. [Google Scholar] [CrossRef]
- Loh, W.; Crocombe, A.; Wahab, M.A.; Ashcroft, I. Modelling anomalous moisture uptake, swelling and thermal characteristics of a rubber toughened epoxy adhesive. Int. J. Adhes. Adhes. 2005, 25, 1–12. [Google Scholar] [CrossRef]
- Tai, R.C.L.; Szklarska-Smialowska, Z. Effect of fillers on the degradation of automotive epoxy adhesives in aqueous solutions: Part I Absorption of water by different fillers-incorporated automotive epoxy adhesives. J. Mater. Sci. 1993, 28, 6199–6204. [Google Scholar] [CrossRef]
- Al-Harthi, M.; Kahraman, R.; Yilbas, B.; Sunar, M.; Aleem, B.J.A. Influence of water immersion on the single-lap shear strength of aluminum joints bonded with aluminum-powder-filled epoxy adhesive. J. Adhes. Sci. Tech. 2004, 18, 1699–1710. [Google Scholar] [CrossRef]
- Han, X.; Jin, Y.; Zhang, W.; Hou, W.; Yu, Y. Characterisation of moisture diffusion and strength degradation in an epoxy-based structural adhesive considering a post-curing process. J. Adhes. Sci. Tech. 2018, 32, 1643–1657. [Google Scholar] [CrossRef]
- Sugiman, S.; Salman, S.; Maryudi, M. Effects of volume fraction on water uptake and tensile properties of epoxy filled with inorganic fillers having different reactivity to water. Mater. T. Commun. 2020, 24, 101360. [Google Scholar] [CrossRef]
- Bruckner, T.; Singewald, T.; Gruber, R.; Hader-Kregl, L.; Müller, M.; Kern, C.; Hafner, M.; Paulik, C. Influence of hollow glass microspheres on 1K epoxy structural adhesive for the automotive industry. Int. J. Adhes. Adhes. 2023, 124, 103396. [Google Scholar] [CrossRef]
- Stuart, B. The application of Raman spectroscopy to the tribology of polymers. Tribol. Int. 1998, 31, 687–693. [Google Scholar] [CrossRef]
- Doyle, G.; Pethrick, R.A. Environmental effects on the ageing of epoxy adhesive joints. Int. J. Adhes. Adhes. 2009, 29, 77–90. [Google Scholar] [CrossRef]
- Chateauminois, A.; Chabert, B.; Soulier, J.P.; Vincent, L. Dynamic mechanical analysis of epoxy composites plasticized by water: Artifact and reality. Polym. Compos. 1995, 16, 288–296. [Google Scholar] [CrossRef]
- Carbas, R.J.C.; Marques, E.A.S.; da Silva, L.F.M.; Lopes, A.M. Effect of cure temperature on the glass transition temperature and mechanical properties of epoxy adhesives. Adhesion 2014, 90, 104–119. [Google Scholar] [CrossRef]
- Zhou, J.; Lucas, J.P. Hygrothermal effects of epoxy resin. Part II: Variations of glass transition temperature. Polymer 1999, 40, 5513–5522. [Google Scholar] [CrossRef]
- Puentes, J.; Restrepo-Zapata, N.C.; Chaloupka, A.; Duddleston, L.J.L.; Rudolph, N.; Osswald, T.A. Quasi-isothermal DSC testing of epoxy adhesives using initial fast heating rates. Appl. Poly. 2017, 134, 45425. [Google Scholar] [CrossRef]
- Maggiore, S.; Pedemonte, M.; Bazurro, A.; Stagnaro, P.; Utzeri, R.; Luciano, G. Characterization of the effect on an epoxy adhesive in hybrid FSW-bonding aluminium-steel joints for naval application. Int. J. Adhes. Adhes. 2020, 103, 102702. [Google Scholar] [CrossRef]
- Chen, Y.; Li, M.; Yang, X.; Wei, K. Durability and mechanical behavior of CFRP/Al structural joints in accelerated cyclic corrosion environments. Int. J. Adhes. Adhes. 2020, 102, 102695. [Google Scholar] [CrossRef]




| Parameter | Value |
|---|---|
| Appearance/colour | solid/brown |
| Density at 15 °C | 870 kg/m3 |
| Viscosity at 100 °C Flashpoint | 11 mm2/s >220 °C |
| Corrosion Test | Parameters | ||||
|---|---|---|---|---|---|
| Immersion testing | Exposure time | Temperature | Media | Description | Device |
| 168 h | 70 °C | 2 L of 5 wt.% NaCl | fully immersed | closed chamber | |
| Immersion Time (Days) | Volume Change in DI Water (vol.%) | Volume Change in NaCl Solution(vol.%) |
|---|---|---|
| 7 | 17 | 12 |
| 14 | 18 | 14 |
| 21 | 19 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gruber, R.; Singewald, T.D.; Bruckner, T.M.; Hader-Kregl, L.; Hafner, M.; Stifter, D. Influence of Dry-Film Lubricants on Bond Strength and Corrosion Behaviour of 6xxx Aluminium Alloy Adhesive Joints for the Automotive Industry. Lubricants 2023, 11, 437. https://doi.org/10.3390/lubricants11100437
Gruber R, Singewald TD, Bruckner TM, Hader-Kregl L, Hafner M, Stifter D. Influence of Dry-Film Lubricants on Bond Strength and Corrosion Behaviour of 6xxx Aluminium Alloy Adhesive Joints for the Automotive Industry. Lubricants. 2023; 11(10):437. https://doi.org/10.3390/lubricants11100437
Chicago/Turabian StyleGruber, Ralph, Tanja Denise Singewald, Thomas Maximilian Bruckner, Laura Hader-Kregl, Martina Hafner, and David Stifter. 2023. "Influence of Dry-Film Lubricants on Bond Strength and Corrosion Behaviour of 6xxx Aluminium Alloy Adhesive Joints for the Automotive Industry" Lubricants 11, no. 10: 437. https://doi.org/10.3390/lubricants11100437
APA StyleGruber, R., Singewald, T. D., Bruckner, T. M., Hader-Kregl, L., Hafner, M., & Stifter, D. (2023). Influence of Dry-Film Lubricants on Bond Strength and Corrosion Behaviour of 6xxx Aluminium Alloy Adhesive Joints for the Automotive Industry. Lubricants, 11(10), 437. https://doi.org/10.3390/lubricants11100437





