Lubricating Oil Consumption Measurement on Large Gas Engines
Abstract
:1. Introduction
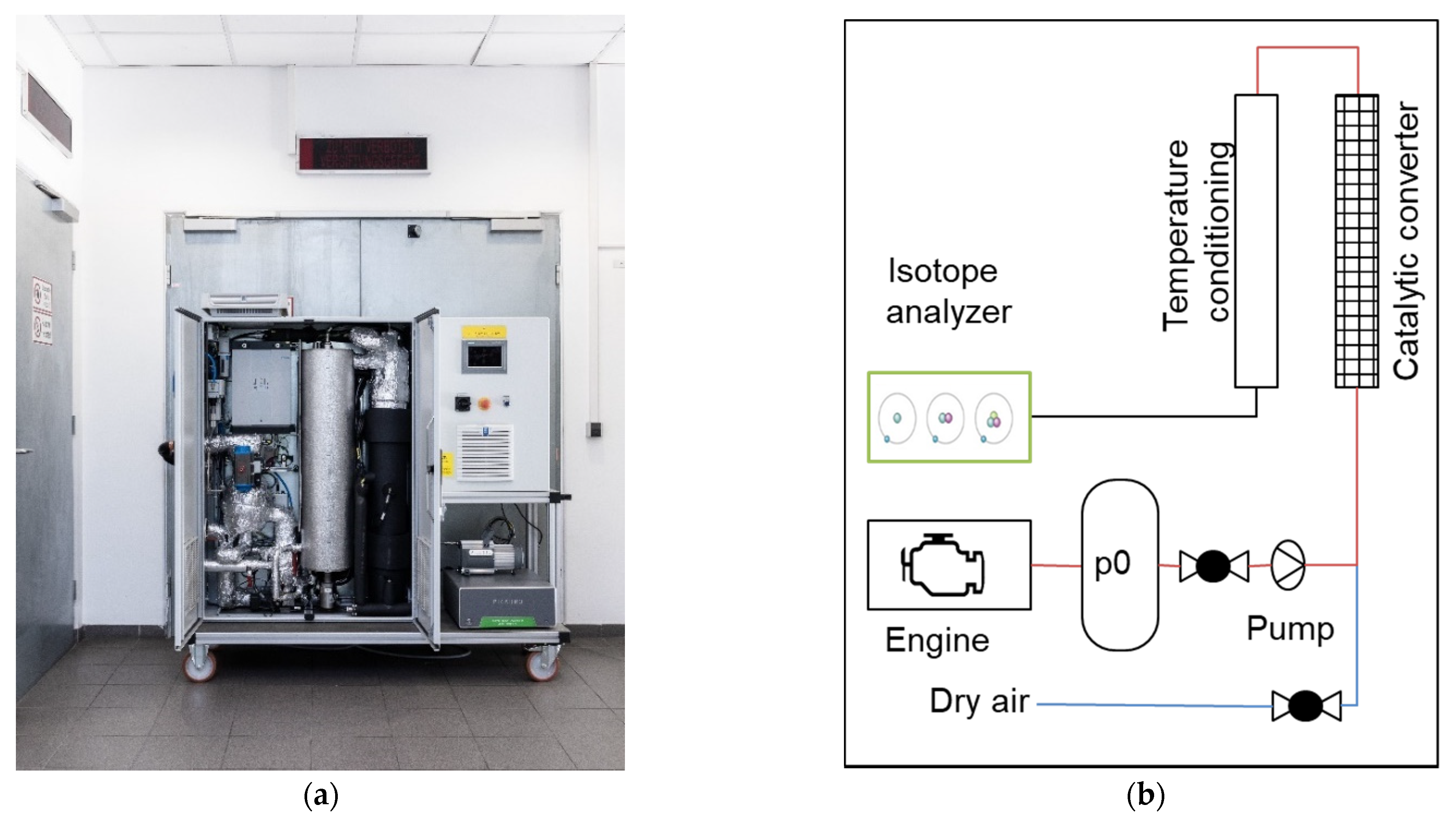
2. Materials and Methods
The Measurement Device
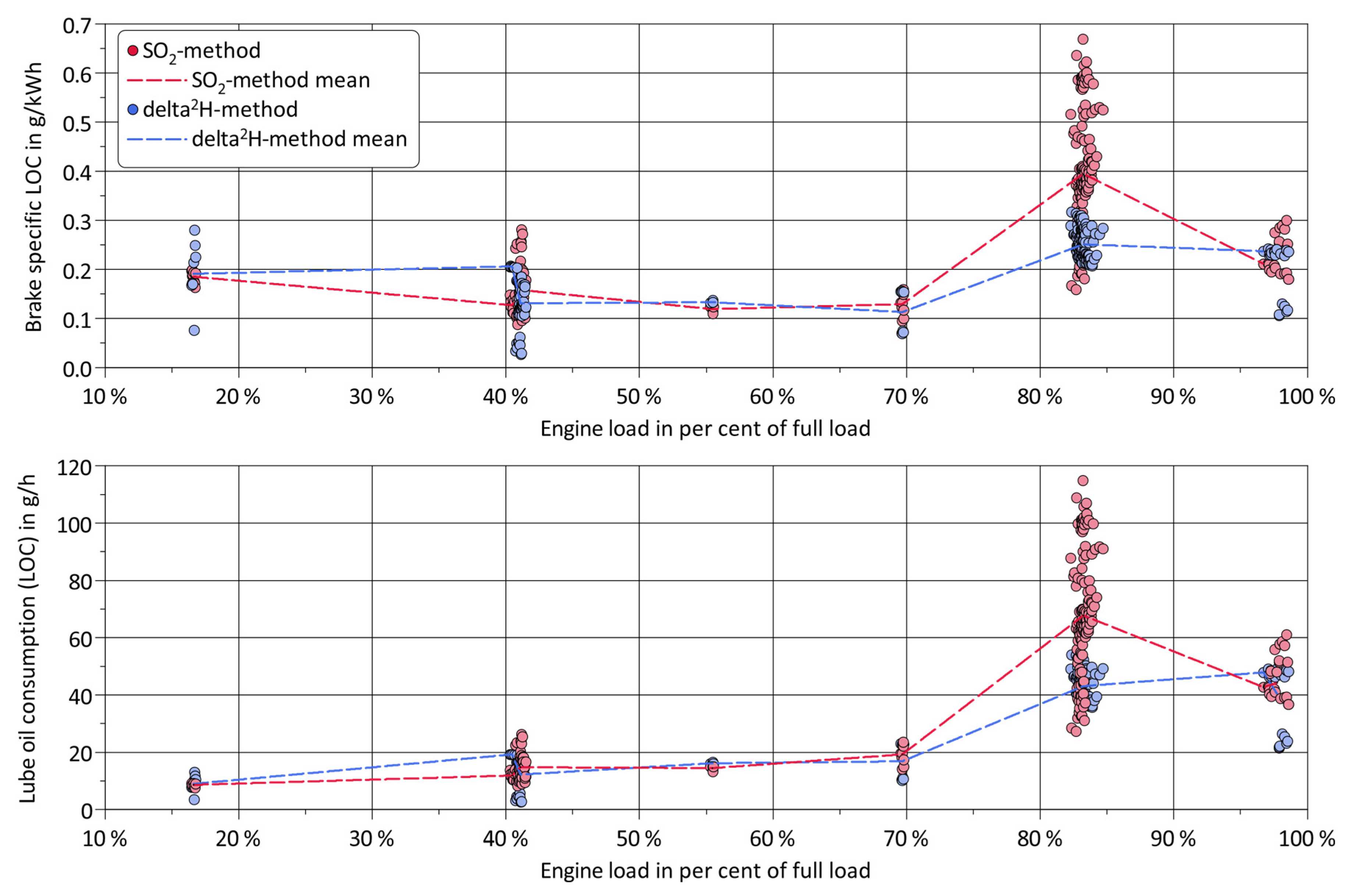
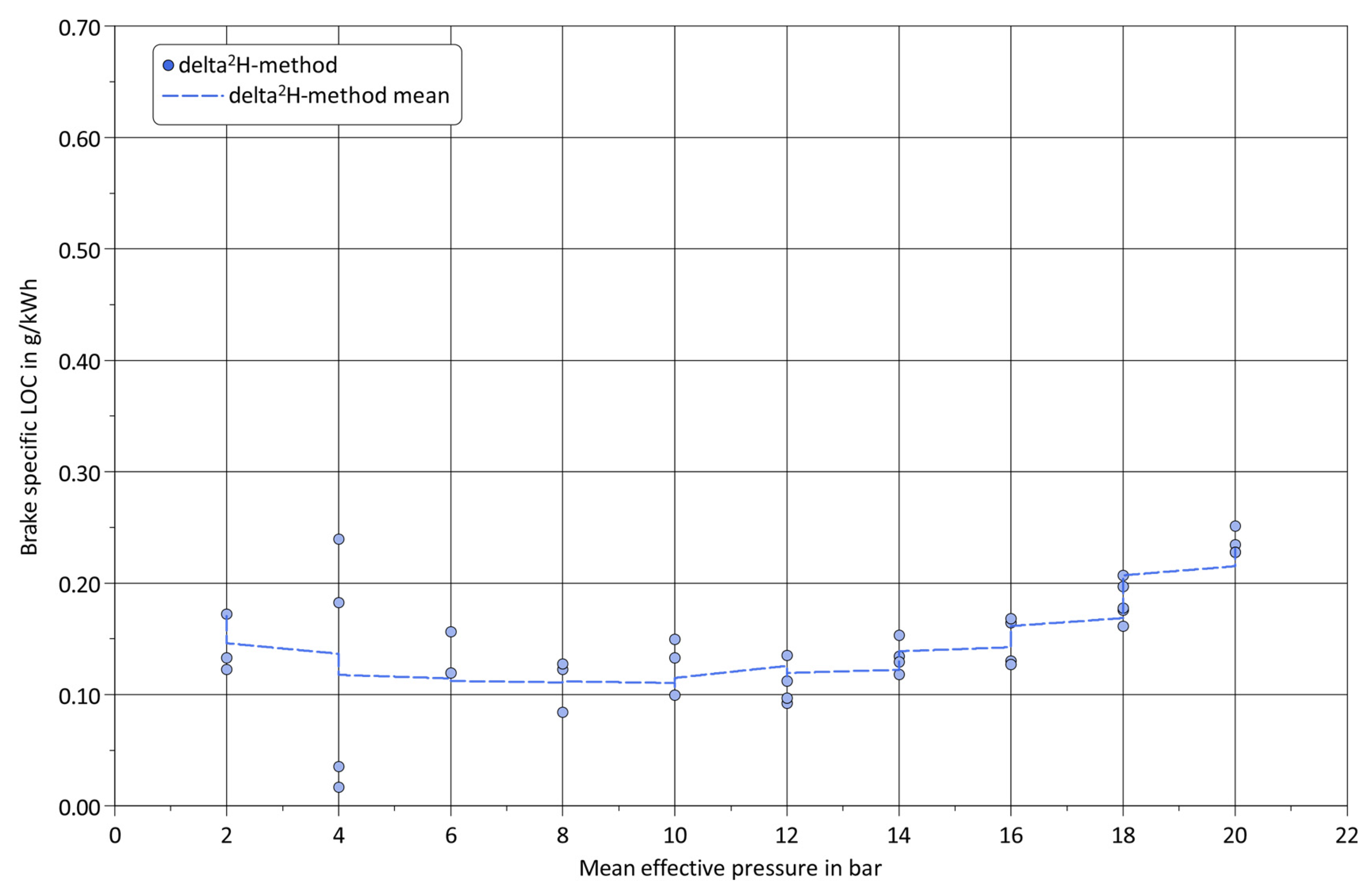
3. Results and Discussion
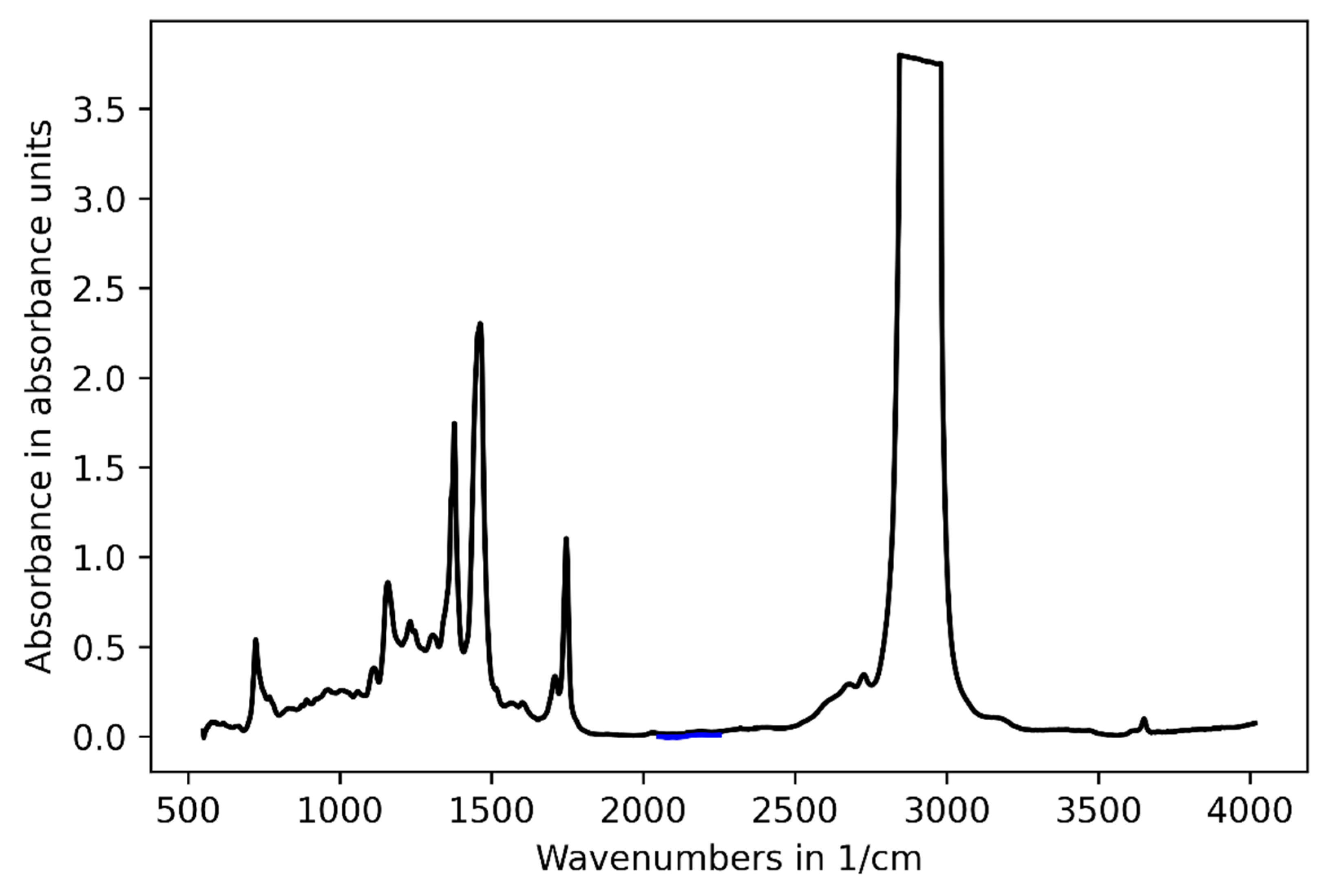
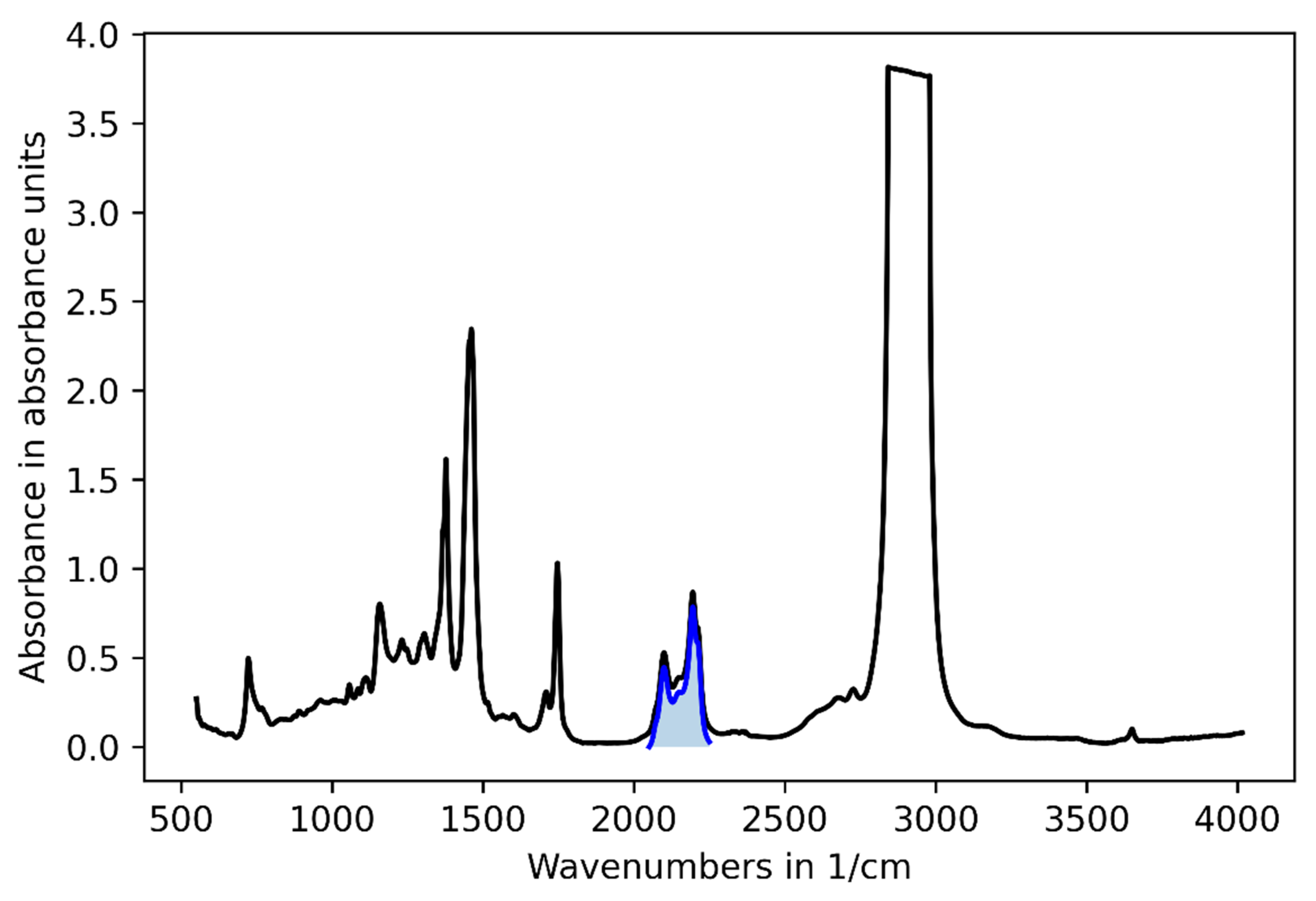
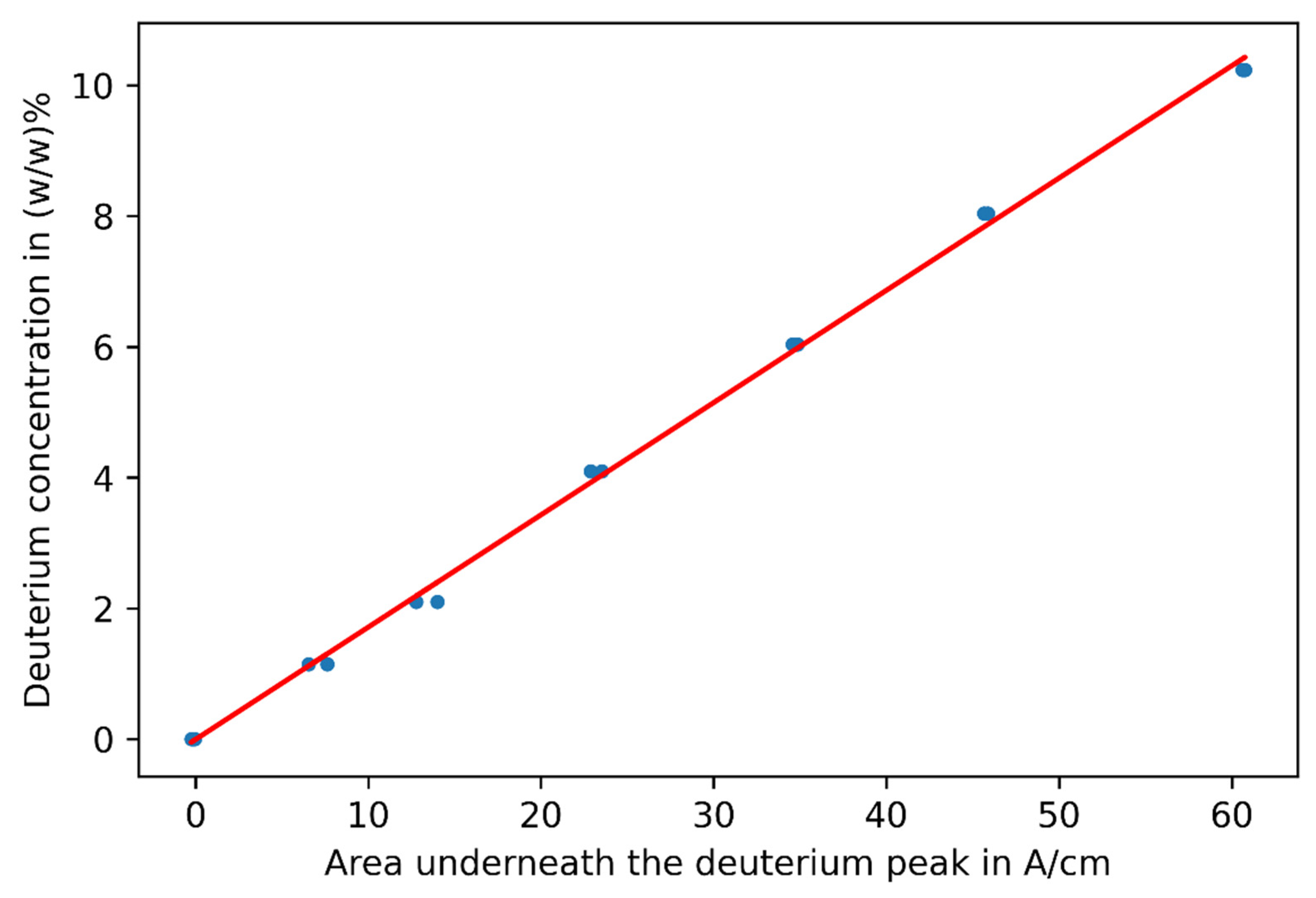
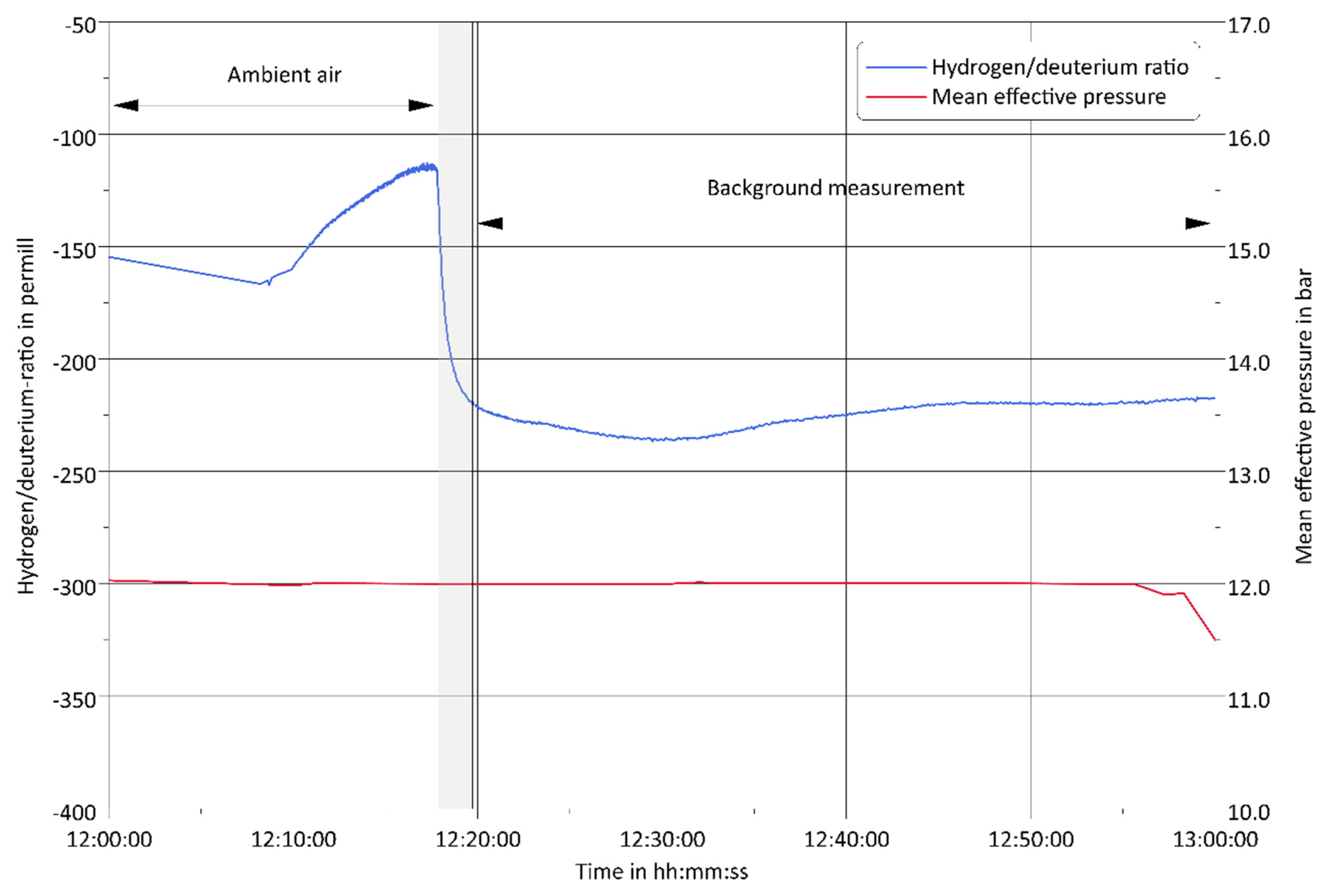
3.1. Challenge 1: Amount of Tracer
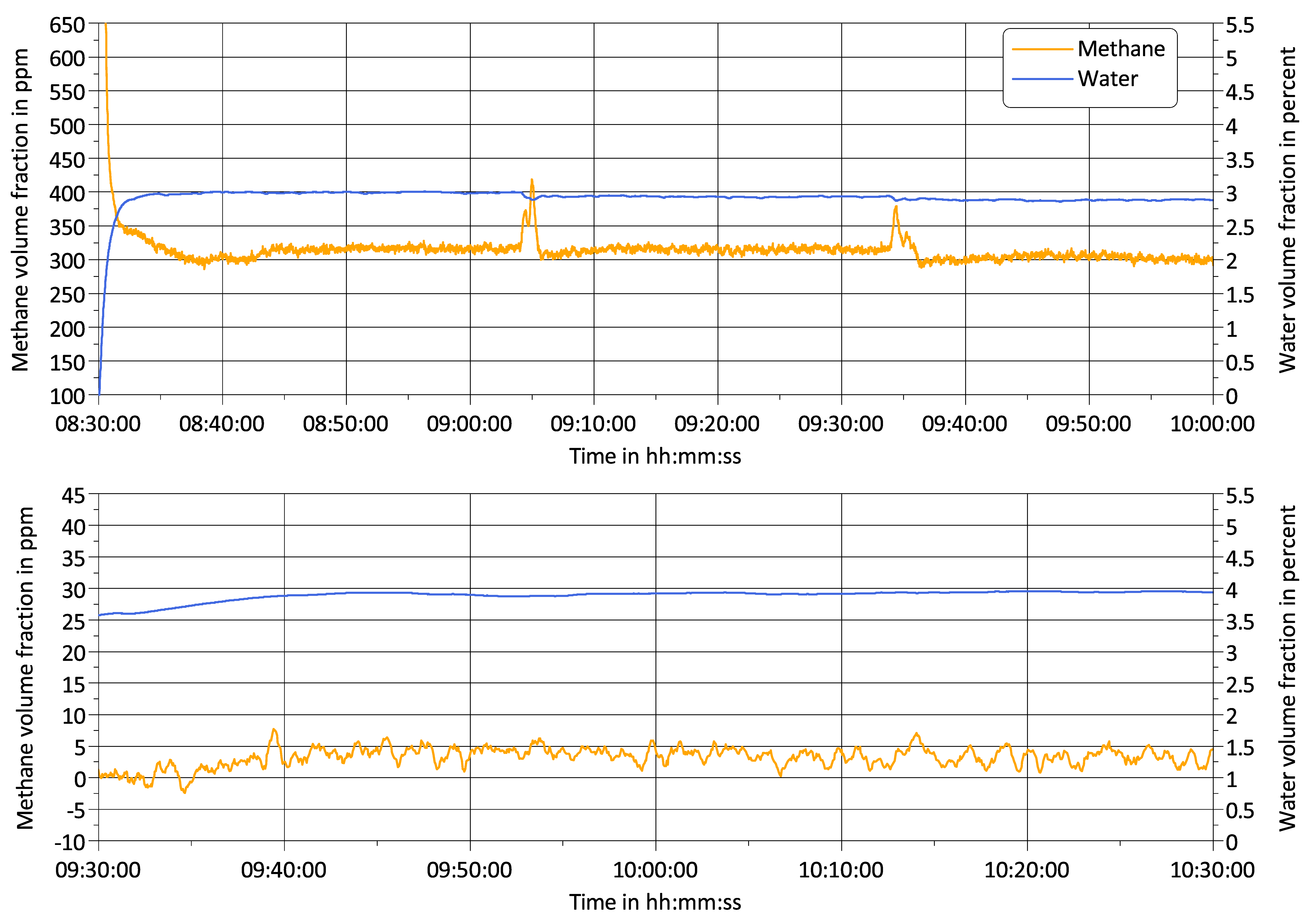
3.2. Challenge 2: Signal Bias by Unburned Methane
3.3. Challenge 3: Background Fluctuations
4. Summary and Conclusions
5. Patents
- Title: Verfahren zur Bestimmung von Isotopenverhältnissen (Method for determining isotope ratios)
- Application Number: A 51070/2019
- Granted: 15/11/2021
- State: Granted/Registered
- Applicant: LEC GmbH
- Representative: Hübscher & Partner Patent Attornys GmbH
- Inventor: Bernhard Rossegger, Michael Engelmayer
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| % (w/w) | Weight-percent |
| 1H | Protium |
| 2H | Deuterium |
| BSLOC | Brake specific lube oil consumption |
| CH4 | Methane |
| CRDS | Cavity ring-down spectroscopy |
| delta2H or δ2H | Hydrogen isotopic ratio |
| FTIR | Fourier transform infrared spectroscopy |
| IR | Infrared |
| IRMS | Isotope ratio mass spectroscopy |
| H2O | Dihydrogen oxide/water |
| LOC | Lubricating oil consumption |
| NMR | Nuclear magnetic resonance |
| PCE | Passenger car engine |
| Pd | Palladium |
| ppm | Parts per million |
| Pt | Platinum |
| Rh | Rhodium |
| SCE | Single-cylinder engine |
| SCR | Selective catalytic reaction |
| SO2 | Sulfur dioxide |
| TAN | Total acid number |
| TBN | Total base number |
| TBO | Time between overhaul |
References
- Schweizer, T.; Zöbinger, N.; Eder, M.; Schießl, R. Initial Pre-Ignition; FVV: Frankfurt, Germany, 2021. [Google Scholar]
- Rossegger, B.; Engelmayer, M. Verfahren zum Bestimmen des Ölverbrauchs von Maschinen. Austria Patent A 50410/2018, 15 May 2018. [Google Scholar]
- Rossegger, B.; Eder, M.; Vareka, M.; Engelmayer, M.; Wimmer, A. A novel method for lubrication oil consumption measurement for tribological assessments of internal combustion engines. Tribol. Int. 2021, 162, 107141. [Google Scholar] [CrossRef]
- Rossegger, B.; Schneider, M.; Leis, A.; Engelmayer, M.; Wimmer, A. New Approaches to Lube Oil Consumption Measurement Based on the Tracer Method; SAE Technical Paper: Graz, Austria, 2019. [Google Scholar] [CrossRef]
- Evans, R. A Tritium-Tracer Technique for the Measurement of Oil Consumption in Gasoline and Diesel Engines. Int. J. Appl. Radiat. Isot. 1973, 24, 19–27. [Google Scholar] [CrossRef]
- Robota, J.; Plückers, T.; Delvigne, O. Courtois, Oil Emission Measurement for a Holistic Estimation of Oil Consumption; ATZ extra; Springer Fachmedien Wiesbaden GmbH: Wiesbaden, Germany, 2015. [Google Scholar]
- Zellbeck, H.; Roethig, J.; Seibold, J.; Zeuner, A. A method for measuring oil consumption by labelling with radioactive bromine. Tribotest 2006, 6, 251–265. [Google Scholar] [CrossRef]
- Lee, P.; Majkowksi, R.; Schreck, R. Method for Determining Fuel and Engine Oil Consumption Using Tunable Diode Laser Spectroscopy. U.S. Patent 4,990,780, 5 February 1991. [Google Scholar]
- Rossegger, C.; Schubert-Zallinger, A. Wimmer, Lube Oil Consumption Measurement on Internal Combustion Engines. In Proceedings of the STLE Annual Meeting, Atlanta, Georgia, 21–25 May 2017. [Google Scholar]
- Sellmeier, S.; Alonso, E.; Boesl, U. Dynamic Oil Consumption Measurement of Internal Combustion Engines Using Laser Spectroscopy; Analytical Chemistry: Garching, Germany, 2013. [Google Scholar]
- V&F Analyse. Messtechnik GmbH, IMR-MS—How It Works; Technical Description: Absam, Austria, 2013. [Google Scholar]
- Buttini, P.; Manni, M. Emissions of Deuterated Tracers from Oil in Light and Heavy Duty Diesel Engine. In SAE Transactions; Section 4: Journal of Fuels and Lubricants; SAE International: Warrendale, PA, USA, 2001; Volume 110, pp. 1748–1756. [Google Scholar]
- Atkinson, J.G.; Luke, M.O. Deuterated Lubricating Oils. U.S. Patent No. US3746634A, 17 July 1973. [Google Scholar]
- Satller, A. Hydrogen/Deuterium (H/D) Exchange Catalysis in Alkanes, Corporate Strategic Research, ExxonMobil Research & Engineering Company; ACS Catalysis: Annandale, NJ, USA, 2018; Volume 8, pp. 2296–2312. [Google Scholar]
- Gralher, B.; Herbstritt, B.; Weiler, M.; Wassenaar, L.I.; Stumpp, C. Correcting Laser-Based Water Stable Isotope Reading Biased by Carrier Gas Changes. Environ. Sci. Technol. 2016, 50, 7074–7081. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; Rella, C. Effects of Variation in Background Mixing Ratios of N2, O2, and Ar on the Measurement of D18-H2O and D2H-H2O Values by Cavity Ring-Down Spectroscopy; Department of Ecology and Evolutionary Biology, University of Arizone, Atmospheric Measurement Techniques: Tucson, AZ, USA, 2017. [Google Scholar]
- Hendry, M.; Richman, B.; Wassenaar, L. Correcting for Methane Interferences on δ2H and δ18O Measurements in Pore Water Using H2O(liquid)–H2O(vapor) Equilibration Laser Spectroscopy. Anal. Chem. 2011, 83, 5789–5796. [Google Scholar] [CrossRef] [PubMed]
- Boukha, Z.; de Rivas, B.; Gonzalez-Velasco, J.; Gutierrez-Ortiz, J.; Lopez-Fonseca, R. Comparative Study of the Efficiency of Different Noble Metals. Materials 2021, 14, 3612. [Google Scholar] [CrossRef] [PubMed]
- Treschnitzer, K.; Lux, S.; Schulze, K. Bewertung von Reaktormodellen zur Mathematischen Beschreibung des Katalytischen Abbaus von Organischen Kohlenwasserstoffverbindungen in Industriellen Rauchgasreinigungsanlagen; Graz University of Technology, Chemische Thermische Prozesstechnik GmbH: Graz, Austria, 2014. [Google Scholar]
- Sherwood, S.; Schwietzke, V.; Arling, G. Etiope, Global Inventory of Gas Geochemistry Data from Fossil Fuel, Microbial, and Burning Sources, version 2017. Earth Syst. Sci. Data 2017, 9, 639–656. Available online: https://essd.copernicus.org/articles/9/639/2017 (accessed on 3 December 2020). [CrossRef] [Green Version]


| Tracer | Major Advantage | Major Downside | Source |
|---|---|---|---|
| Tritium (T; 3H) tracer | Unique substance in the system | Cost, effort, radioactive | [5] |
| Germanium (69Ge) | High sensitivity | Gamma emitter | [6] |
| Bromine (82Br) | Precisely detectable | Short half-life, radioactive | [7] |
| Halogens (X) | Precisely detectable | Corrosive | [8] |
| Alkaline Earth Metals (AEMs) | Present in oil/additives by nature | Not detectable online | [9] |
| Zinc (Zn) | Present in oil/additives by nature | Adsorption/memory effects | [10] |
| Sulfur (S or SO2) | Precisely detectable | Insufficient lower detection limit and selectivity | [11] |
| Pyrene (C16H10) | Oil-like physicochemical properties | Decomposes during combustion | [10] |
| Deuterated polyaromatic hydrocarbons | Detectable online | Cost, low accuracy | [12] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossegger, B.; Leis, A.; Vareka, M.; Engelmayer, M.; Wimmer, A. Lubricating Oil Consumption Measurement on Large Gas Engines. Lubricants 2022, 10, 40. https://doi.org/10.3390/lubricants10030040
Rossegger B, Leis A, Vareka M, Engelmayer M, Wimmer A. Lubricating Oil Consumption Measurement on Large Gas Engines. Lubricants. 2022; 10(3):40. https://doi.org/10.3390/lubricants10030040
Chicago/Turabian StyleRossegger, Bernhard, Albrecht Leis, Martin Vareka, Michael Engelmayer, and Andreas Wimmer. 2022. "Lubricating Oil Consumption Measurement on Large Gas Engines" Lubricants 10, no. 3: 40. https://doi.org/10.3390/lubricants10030040
APA StyleRossegger, B., Leis, A., Vareka, M., Engelmayer, M., & Wimmer, A. (2022). Lubricating Oil Consumption Measurement on Large Gas Engines. Lubricants, 10(3), 40. https://doi.org/10.3390/lubricants10030040





