1. Introduction
Emission regulation has played a significant role shaping automotive industry technology [
1]. Thanks to established regulations and stringent implementation, the automotive industry has made tremendous progress in emission control in the last century. This impact is not limited only to hardware technology but has also influenced fluid specifications.
Table 1 chronologically lists several critical emission regulation milestones that have driven fluid requirement changes [
2,
3]. Phosphorus and sulfur are the two important elements that have been regulated in both fuels and lubricants due to their harmful impact on emissions catalyst performance. The availability of low and even ultra-low sulfur fuel ensures the functionality and durability of the advanced emission control system. In response to these fluid specification changes, it is crucial to maintain engine durability and system compatibility.
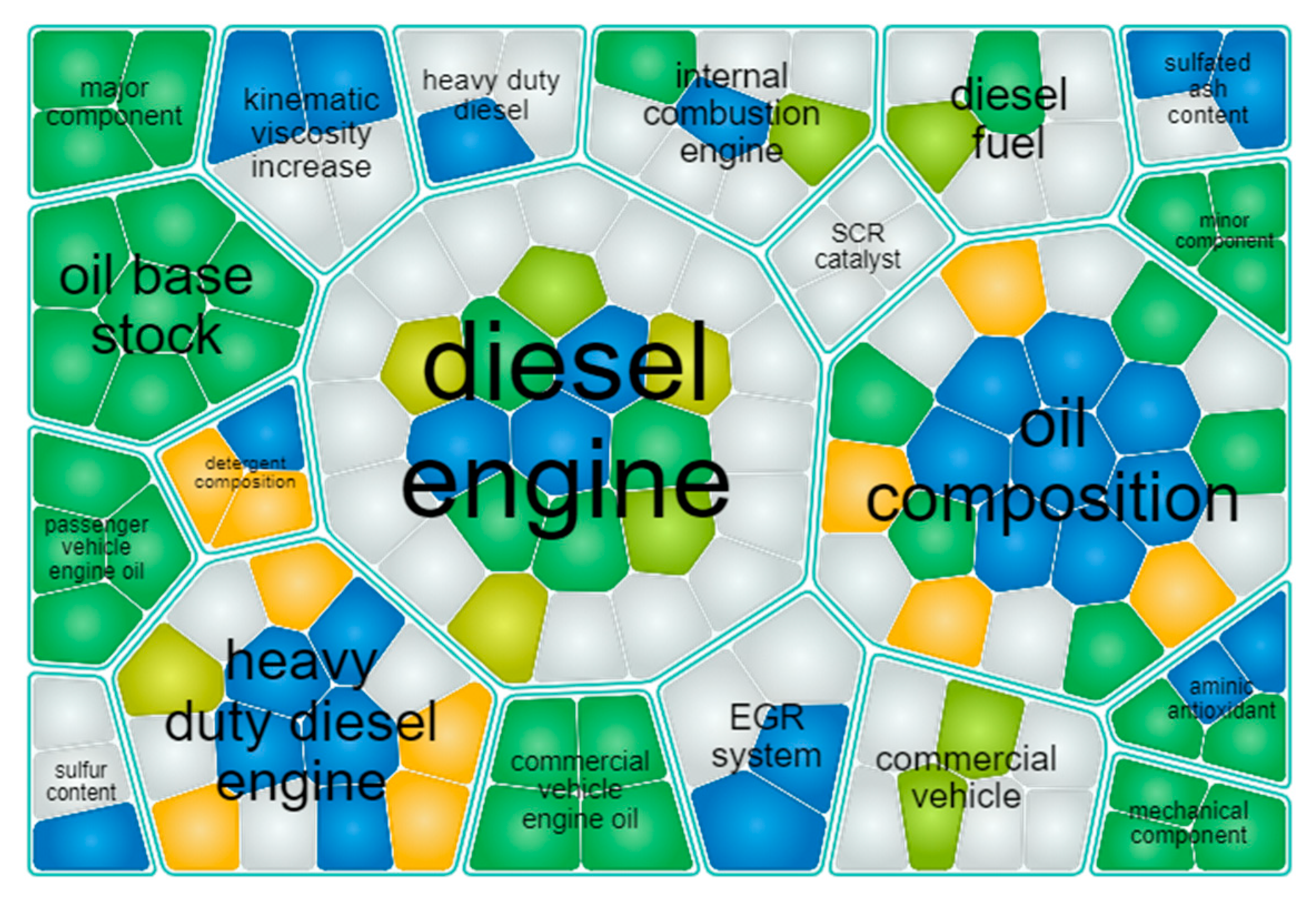
An analysis of intellectual property developed for commercial vehicle emission control and efficiency improvements illustrates the importance of both hardware and fluids in meeting emission system performance requirements (
Figure 1). Words in the cell diagram developed though patent analysis [
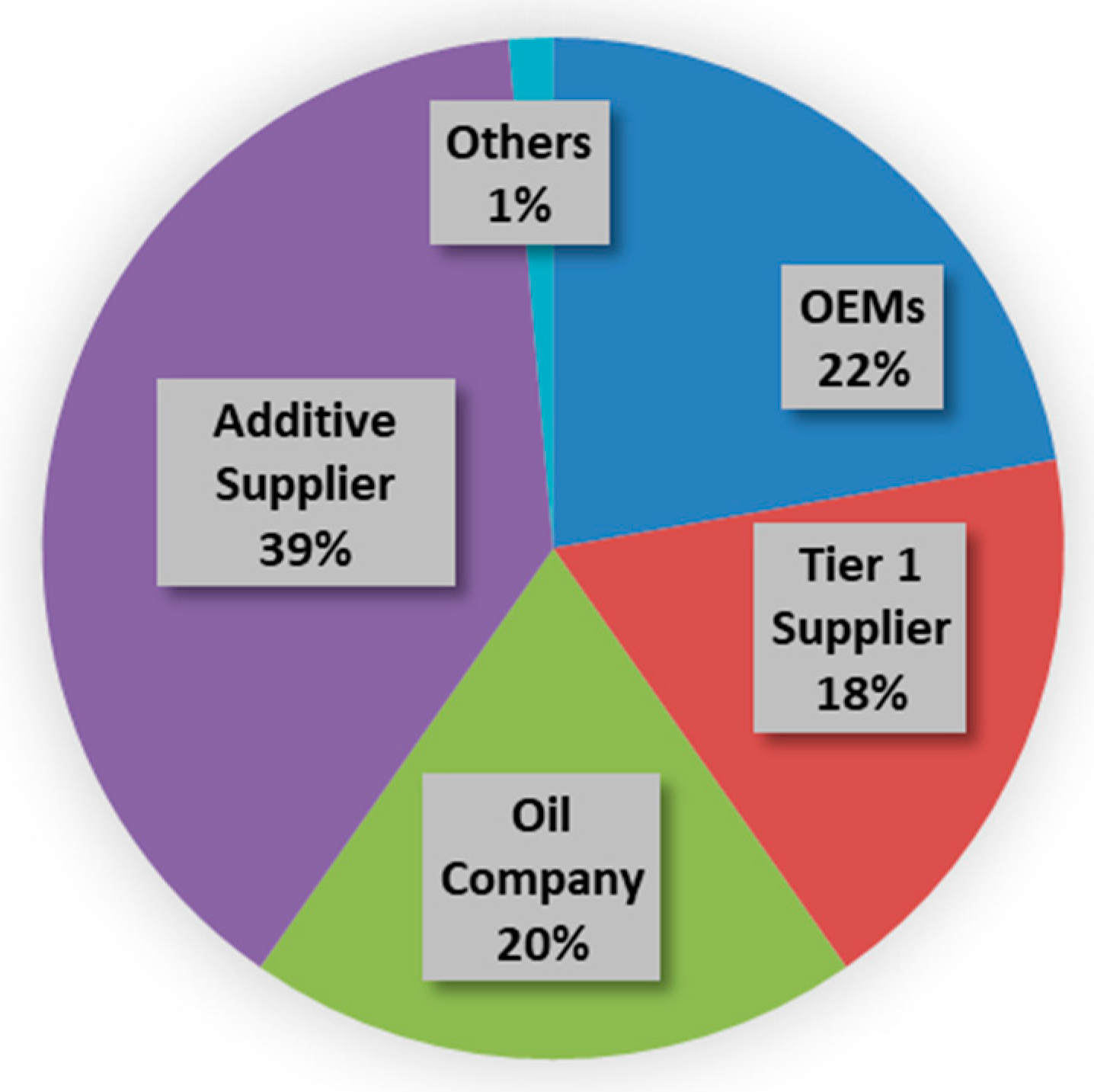
4] reflect the key patented technology areas. The number of cells proportionally represents the number of patents, and the color represents different assignees. Fuel and oil, as well as associated areas, are well represented in the diagram. The pie chart (
Figure 2) shows key industry players contributing to the intellectual property (IP) space. All the assignees are aggregated into industry groups, original equipment manufacturers (OEM), Tier 1 suppliers, oil companies, and additive suppliers. OEMs, Tier 1 suppliers, and oil companies have a similar share in this pie chart, about 20%, while additive companies take the lead with 39%. This intellectual property analysis provides strong evidence that fluid performance and capabilities are essential for efficiency improvement and emission control technology performance, and additive companies have made outstanding contributions to the technology advancement. A few selected patents are listed in the references [
5,
6,
7,
8].
In this review article, we explore several aspects for understanding the role of powertrain fluids on particulate emissions and the environment. First, the need to assess the emissions control hardware and fluids as a complete system is considered with a focus on how the fluid can impact the overall performance. Second, an example is provided showing how fuel composition can impact emissions performance directly and indirectly. These provide insight into both the methodologies and context with which to assess lubricant additive effects Finally, the lubricant additive system is considered with examples highlighting how it can influence the emissions control system.
2. System Approach for Emission Control
Stringent emission regulation has become a significant driving force for technology advancement, and the regulatory targets can only be effectively and efficiently achieved through optimizing the emission control system including both hardware and fluid. Various emission control strategies are applied throughout a defined system that encompasses aspects ranging from precombustion to tailpipe emissions. In this system, the fluids (fuel and lubricants) and the hardware (engine and aftertreatment) all participate cooperatively to optimize for efficiency and durability. To evaluate this highly complex system, we must first understand individual components and their interactions. It is essential to include combustion to address emission control. Combustion gives the remanence of the fuel as well as the lubricant. For example, the lower sulfur content in fuel reduces the load on the emissions systems enabling a design for better exhaust flow and allowing improved overall vehicle operating efficiency. In addition, an appropriate testing procedure can assess the interaction of fuel and lubricant combustion products with the emission control system. Below, examples showing how the emission control systems interact are discussed.
Considering specifically the impact of the combustion process on the overall emission system, engine architecture, combustion cycle, and engine calibration all play a role. Brake thermal efficiency has significantly increased over the last several decades through compression ratio increase enabled through more complex control of valve timing. Combustion cycles such as the Miller and Atkinson cycle have been more widely adopted to achieve efficiency and emission reduction through early or late intake valve closing. Starting in the 1990s, the Miller cycle has been applied to diesel engines and some International Maritime Organization (IMO) Tier 1 marine engines for NO
x reduction [
4,
9]. The Atkinson cycle engine is known to be an efficient platform for hybrid electronic vehicle (HEV) to improve fuel economy. It has the potential to achieve more constant volume combustion while the combustion quality and stability are greatly improved [
10]. By applying the Miller cycle to a turbocharged diesel engine, a significant reduction of NO
x was achieved. However, particulate emissions greatly increased likely due to the higher burning temperature [
11]. Exhaust gas recirculation (EGR) has been used for emission control since the 1970s [
12], and further advancement was made by designing dual loop EGR [
13]. With different EGR strategies, it is expected that the efficiency can be improved further while emission is reduced. Another important technology adopted for heavy-duty low-load NOx emission control is cylinder deactivation, where cylinders are partially deactivated at low loads to increase the exhaust temperature of the firing cylinders [
14,
15].
Gasoline and diesel engines present different emission challenges. Accordingly, different aftertreatment devices have been utilized to meet regulation requirements. Conventional port fuel injection (PFI) gasoline-powered engines emit hydrocarbon (HC), carbon monoxide (CO), and nitrogen oxides (NO
x). An effective three-way catalyst (TWC) can meet most emission requirements [
16]. Additional improvement can be made by using a close-coupled catalyst to replace or supplement the under-floor one. Increasing efficiency gains led to significant penetration of gasoline direct injection (GDI) engines. Due to the combustion feature of direct injection, the GDI engine tends to emit high particulate emissions. Gasoline particulate filter (GPF) has become a cost-effective technology solution to reduce particulate emission from GDI vehicles [
17]. Diesel engine emission control has accomplished great success in the past decades, primarily in particulate emissions and NO
x emission control. The latest diesel emission control system includes diesel oxidation catalyst (DOC) [
18], diesel particulate filter (DPF) [
19], selective catalytic reduction (SCR) catalyst [
20,
21], and ammonium slip catalyst (ASC) [
16]. This system has been widely deployed to meet the current legislation requirement. Future heavy-duty emission regulation on NO
x will likely drive the adoption of close-coupled SCR technology to meet lower NO
x limits, cold start performance, low-load conversion efficiency, and durability requirements [
14]. This close-coupled SCR system is in conjunction with dual urea injection, whereby urea is dosed at two locations allowing achievement of low NO
x emission targets under a wide range of operations.
Fuel and lubricant additives have different roles within the engine system to improve performance. A critical function of fuel additives is to enable clean combustion, allowing emissions reduction directly or indirectly [
22,
23]. This more complete combustion process can reduce the load on the catalyst due to less formation of partial oxidation products [
24]. The primary function of a lubricant is to protect the engine, allowing it to deliver the desired efficiency, performance and durability with different engine designs dictating specific lubricant performance requirements. For example, engines employing the Atkinson or Miller cycles will likely require a high oil quality to provide sufficient oxidation protection for engines and bearings [
25]. With adoption of some of the more advanced EGR strategies, higher carbon deposits are observed on the engine parts, and higher wear appears on piston rings requiring the lubricant oil to mitigate deposit and wear [
26]. For cylinder deactivation technology, it may induce power loss or fuel penalty due to friction loss. The deactivated cylinders are expected to have lower contact temperature and increased viscous friction [
27]. Consequently, the lubricant oil desirably needs a high viscosity index to have minimal viscous impact in the deactivated cylinders.
Fluid technologies are required to ensure powertrain systems operate as designed, however, they can have additional impact beyond their primary function Fuel and lubricant additives either directly or indirectly find their way into the combustion chambers of engines and thus can have positive and negative impacts on engine controls and exhaust aftertreatment devices. Fluid technology can be an enabler for optimizing the system, and there are still significant opportunities to optimize fuels and lubricants to drive efficiency and durability in the emissions control system. This review work will elaborate the impact of fluids on combustion and emission control through specific examples.
3. Fuel Composition Impact on Emissions
Tailpipe emissions are derived primarily from fuel combustion. The design and proper emission system hardware operation has the greatest influence on emissions, but fuel properties can also have a meaningful impact, both direct and indirect, on tailpipe emissions. Furthermore, understanding mechanisms for emission formation and impacts of factors such as vehicle operation, fuel properties and hardware design allow appropriate additive use to enable robust performance across the entire system.
Governments utilize regulatory emission standards with the overall goal of protecting air quality. Fuel economy standards are directed toward controlling the final product of combustion, CO
2, with additional tailpipe emission standards focused on incomplete combustion products. In addition to limiting the gaseous emission components such as, carbon monoxide, hydrocarbons, and nitrogen oxides, in many countries current emission regulation includes tailpipe limits on particulate mass and particle number [
28]. Consequently, it is important to understand the role of lubricant additives on particle emission characteristics and have robust methodologies to evaluate particulate emissions.
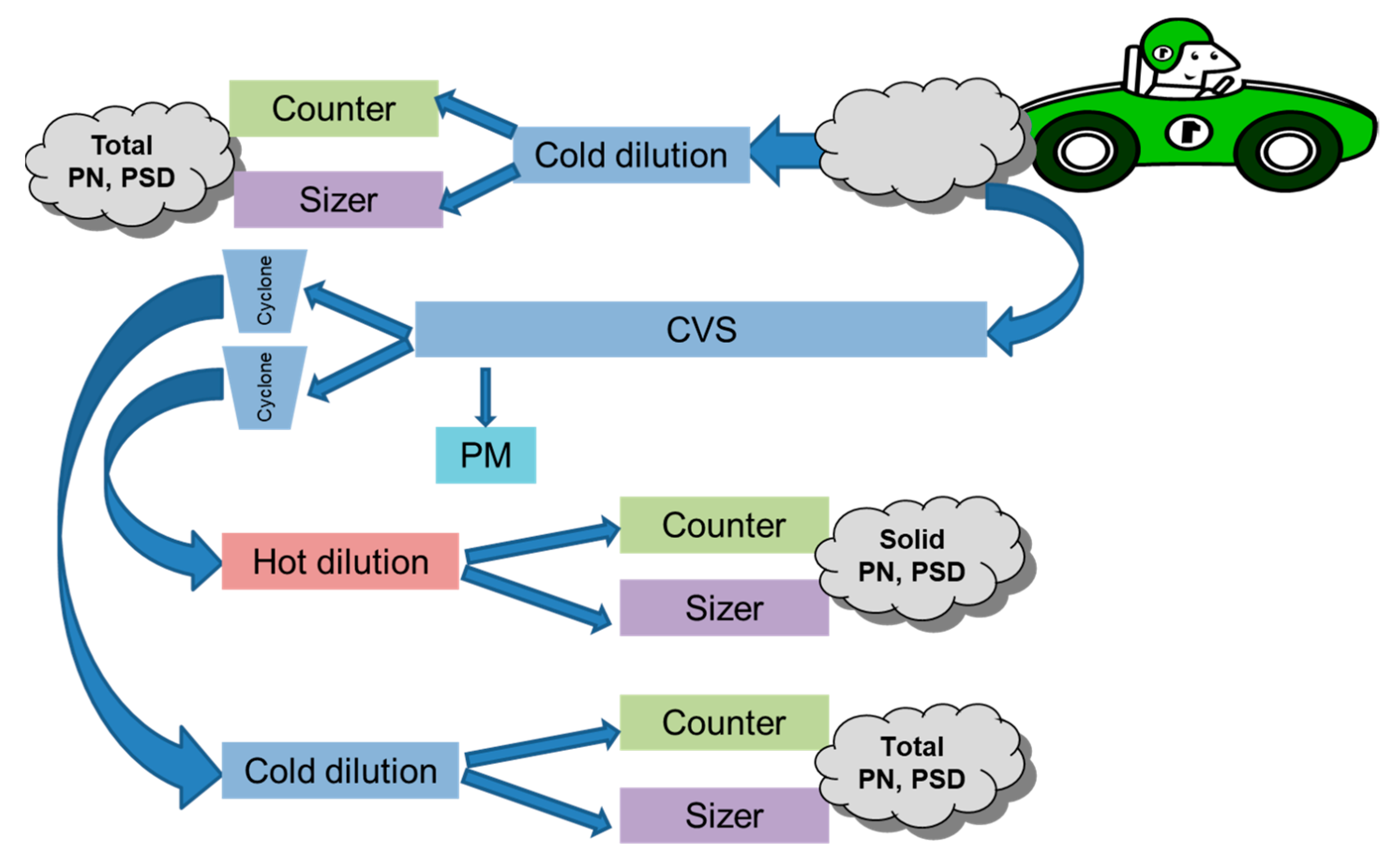
Figure 3 depicts a particulate emission measurement system enabling reliable measurements of particulate mass and number. Other approaches have been reported elsewhere [
29,
30]. There are three sampling locations that provide measurements meeting regulatory standards as well as measurements providing insight into the mechanisms driving particulate formation:
- (1)
The first sample location provides a direct measurement after the tailpipe in the presence of cold air dilution. This simulates real-world emission after the exhaust leaves the tailpipe and mixes with ambient air.
- (2)
The second location samples the exhaust downstream of the constant volume sampler (CVS) tunnel and cyclone separators, followed by continuous dilution with external heat. In this procedure, the evaporation tube is turned on to remove volatiles so that only solid particles are counted. This procedure is compliant with European Particle Measurement Programme (PMP) requirements.
- (3)
The third sample is similar to the second, except that the evaporation tube is turned off. Therefore, the volatiles are counted with the solid particles which together are considered total particles. The difference in the measurements between the second and third is the emission level of volatiles.
Below is a demonstration of particle emission assessment using realistic fuels in a model system. It illustrates the impact of fuel composition variance on vehicle particulate emissions as well as an assessment of the emissions potential impact on atmospheric particulates [
31].
Table 2 lists the properties of three test fuels used to evaluate fuel properties. F6 is a commercial fuel meeting China’s phase V requirement. F1 and F2 fuels were blended using selected components to reach the equivalent research octane number (RON). With those variations in the fuel components, some properties were altered accordingly. Those changes can potentially lead to an impact on combustion and emissions. In this work, F1 was blended with 80% F6 and 20% refinery catalytic stream to match RON and aromatics content with F6, but with a higher olefin level. For F2 fuel, 20% refinery reformate stream was blended with 80% of F6. The additional reformate stream significantly increases the aromatics content in the fuel to 36.7%.
A 1.4 L turbocharged GDI engine with a compression ratio of 10 was selected for engine testing evaluating fuel properties impact on emission characteristics. The engine was coupled to an electric eddy current dynamometer, and a TWC was connected in tandem. Exhaust emission was sampled both pre-and-post-TWC. Various engine testing conditions were chosen to characterize the emission characteristics. The test was equipped with an AVL CEB II emission gas analyzer and DMS 500 to measure gaseous and particle emissions. In addition, standard particulate matter, high efficiency (99+%) fiberglass and Teflon filters with the size of 47 mm were applied to collect particulates for mass measurement and further chemistry analysis. The information derived from the chemistry analysis allows the emitted particles’ toxicity assessment based on the polycyclic aromatic hydrocarbons (PAHs) species and level. EPA categorizes the 16 PAH compounds as “Priority Pollutant PAHs,” which are considered to pose significant potential health impacts and are preferentially monitored [
32,
33].
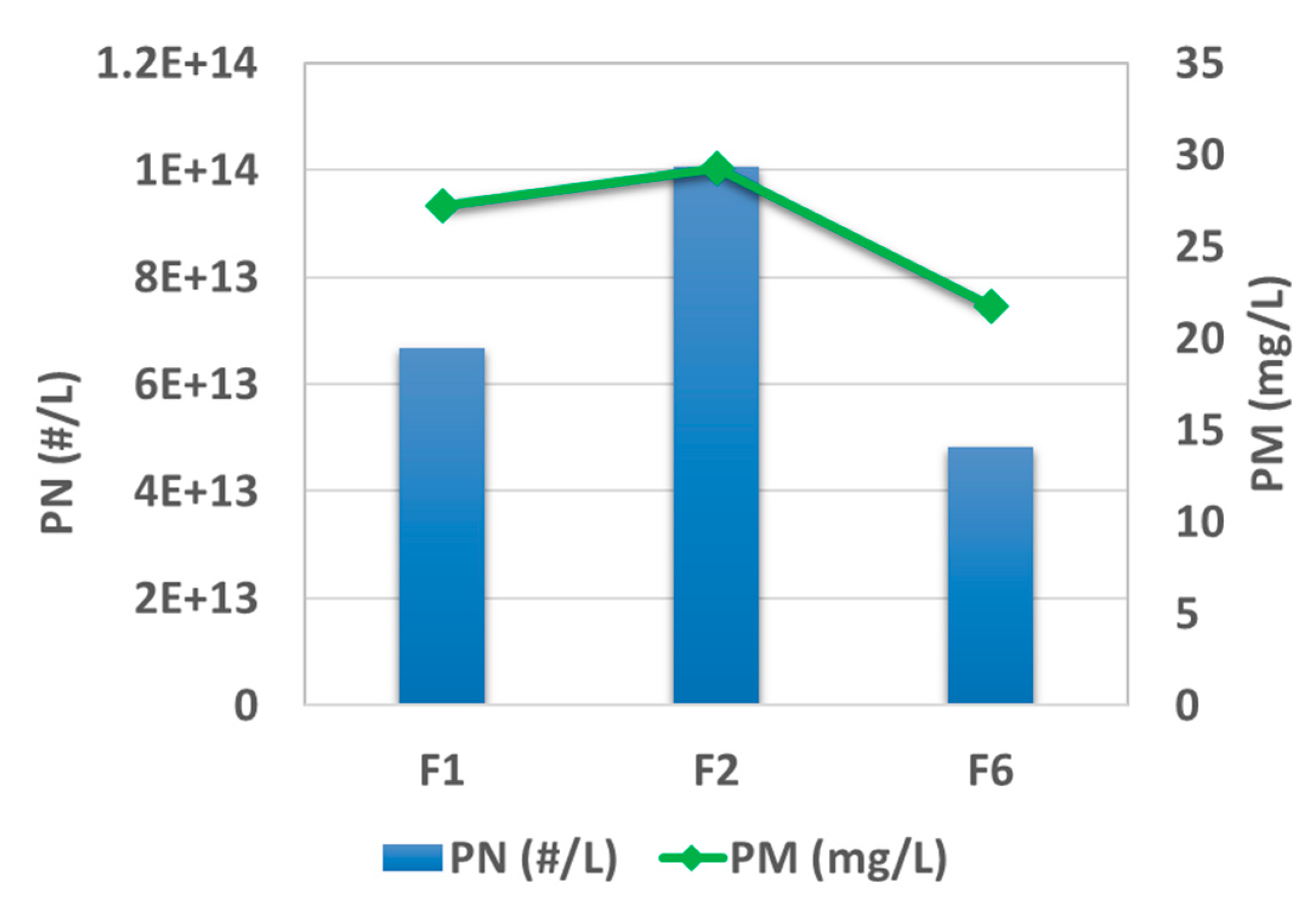
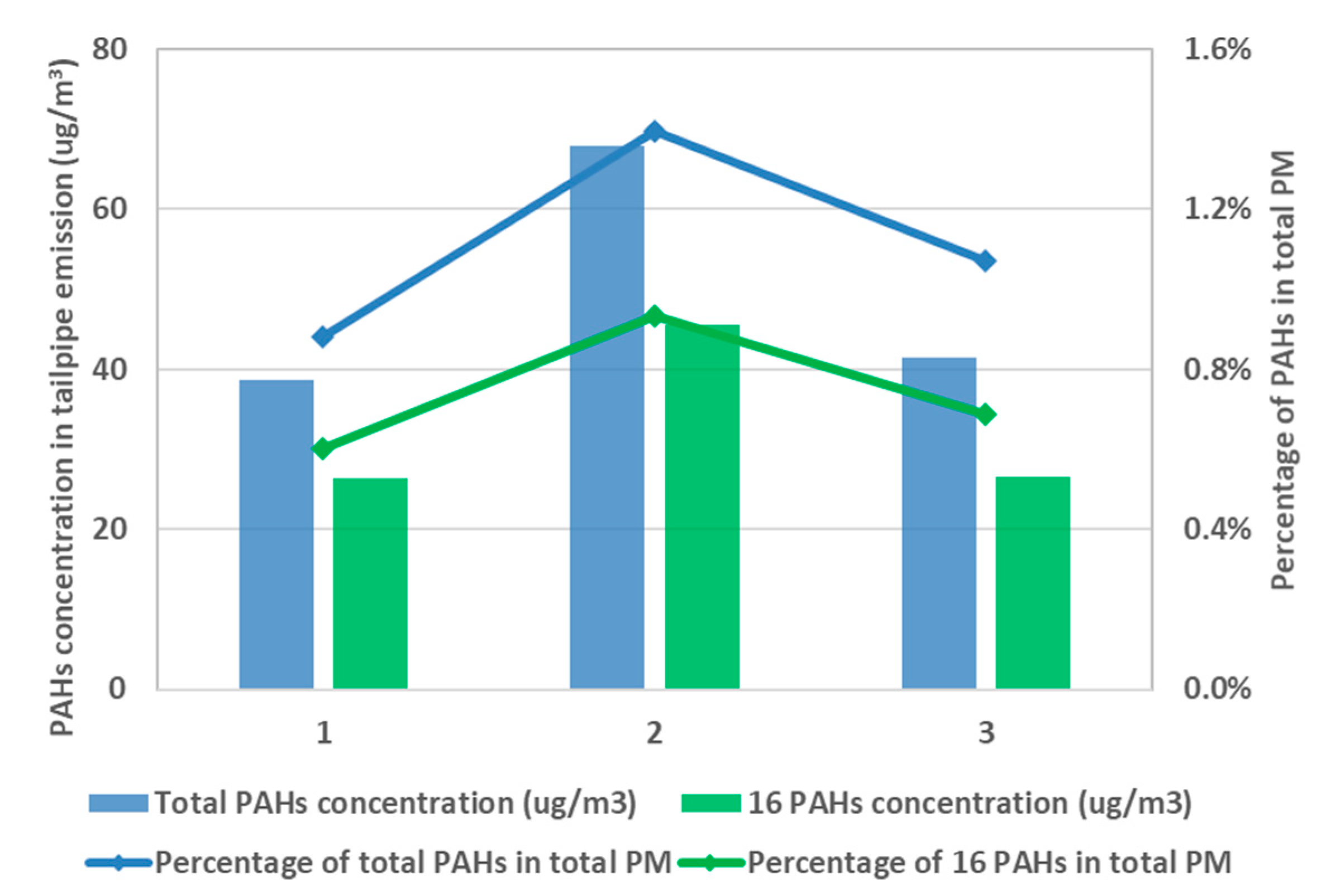
Figure 4 plots the results of post-TWC particle mass (PM) and particle number (PN) emission levels at the testing condition of 2000 rpm and 50% load. The rank of emission level for those three fuels is F2 > F1 > F6, for both mass and number. High aromatic fuel, F2, exhibits almost twice the PN emission (3.9 × 10
13 #/kw·h) as F6 under the same testing condition. This trend is consistently shown under other testing conditions, as presented elsewhere [
31]. In parallel, PAHs were characterized for the pre-TWC samples collected on the fiberglass filter, and the results are summarized in
Figure 5 for three test fuels. The results indicate that higher aromatic content in the fuel brings about an elevated level of PAH emission, which can potentially harm public health and the environment. High aromatic fuel, F2, leads to 70% more PAH emission than F6 given the same testing condition.
As exhaust leaves the tailpipe, it will enter the atmosphere. The constituents in the air will react with the exhaust, producing secondary organic aerosol (SOA). Various research mythologies have been developed to study SOA, covering the formation, transformation, and impact on public health and the environment [
34,
35]. However, the impact of fuel properties on SOA formation was not well studied or understood and is discussed below.
The work presented below used a smog chamber in conjunction with volatile organic compound (VOC) emission measurement equipment to investigate SOA formation. Other systems to assess this have been described elsewhere [
36,
37].
Figure 6 depicts the diagram of the smog chamber together with all the emission measurements. The test vehicle was a PFI vehicle, operated on the chassis dynamometer under a given driving cycle. The exhaust gas was first drawn into the CVS, in which the first stage of dilution took place. The total flow rate in CVS was kept at 5.5 m
3/min. Then, about 5.3 L/min of diluted exhaust gas was introduced to the 1.2 m
3 smog chamber, in which more fresh air would further dilute the exhaust gas as the smog chamber was exposed to the sunlight to promote the photochemistry reaction. The smog chamber was covered with two layers of anti-UV cloth to shield the chamber from sunlight prior to each test. Hydrogen peroxide was added to the chamber to provide an extra hydroxyl group (OH), assisting the formation of SOA. Details of the setup and measurement can be found in [
38].
Aromatics in the fuel can produce some aromatic VOCs after combustion. A chemical reaction occurs when exhaust containing aromatic VOC encounters the OH radical in the atmosphere. SOA will be generated from those reactions, and the fuel’s aromatic content will affect the formation potential. OH exposure is used to compare the impact of different fuels on SOA formation potential. and is determined based on the ratio between benzene and toluene [
39].
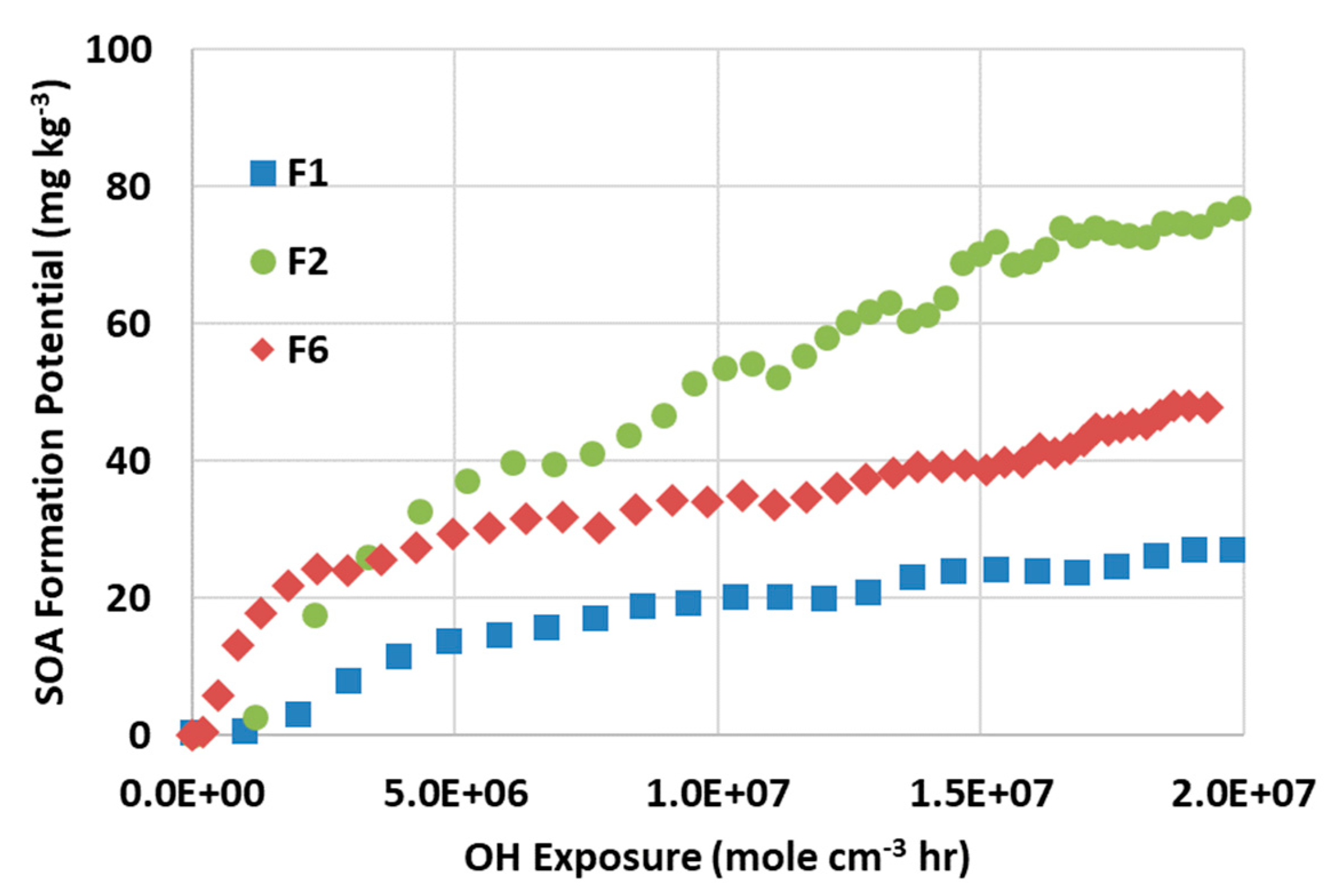
Figure 7 plots the SOA formation potential versus OH exposure for three fuels. Extended OH exposure promotes SOA generation, while the increasing rate slows down over time. F1 and F6 have similar SOA formation potential, while F2 has about a factor of three times higher. The elevated emission level with high aromatic fuel is well demonstrated for both primary particulates and SOA. These findings and other research work imply that emissions can be significantly reduced by improving fuel quality.
These studies illustrate the importance of rigorous research methodology and system approach. It provides a fundamental understanding of emission performance and technical guidance to evaluate the impact of fuel properties and particulate characteristics on emission at multiple levels, from vehicle tailpipe to ambient air quality. The results prove that the fuel properties impact the emission performance derived from combustion byproducts of different chemical components. This foundational work provides the critical base and relevant context for us to understand the system’s performance. This system understanding leads us to develop the testing to evaluate the role of lubricant additives in emission control.
4. Role of Lubricant Additive in Emission Control
4.1. Conventional Functionality of Lubricants (Engine Protection)
Lubricant additives became an important part of the lubricant package when petroleum-based lubricants became available in 1860 [
40]. With the advancement of engine technology, research and development of lubricant additives has been ongoing to meet the evolution in specification requirements and deliver performance value. Today, all lubricant contains additives to meet performance requirements.
The primary role of the engine oil is to protect the engine parts from deterioration due to friction, wear, corrosion, deposits, and oxidation, which led to the development of various additives. Anti-wear agents can protect metal surfaces from wear due to close contact. Antioxidant maintains oxidation stability and reduces the impact of oxidative decomposition. A friction modifier can reduce the friction coefficients improving fuel economy and help protect against wear. Detergent is vital in maintaining engine cleanliness from combustion contaminants and other impurities. Dispersants suspend and separate insoluble particles from fuel combustion or oil degradation. With a drive to lower viscosity oils for fuel economy benefits, viscosity modifiers provide more flexibility to meet those requirements. To provide satisfactory performance requirements at low temperatures, pour point depressant is often blended to improve the flow properties under cold operation. When the engine oil is contaminated with water, emulsifiers prevent phase separation for specific applications. All these functionalities mentioned above are essential and critical for lubricant performance, making the engine run smooth with desirable durability. Advanced engine technologies and more stringent performance requirements have brought more significant needs for lubricant additive improvement.
Although additives are utilized to enable the lubricant to meet performance requirements, some chemistry may directly or indirectly impact the emission level or the functionality of the emission control system. For example, zinc dialkyl dithiophosphate, known as ZDDP, has been used by the industry for over eighty years and is still the most cost-effective anti-wear agent and antioxidant. Meanwhile, the negative impact of phosphorous from ZDDP on the emission control catalyst has been studied and reported [
41,
42]. The controlled level of ZDDP can mitigate the known effect in the lubricant package. It was substantiated that a reasonable level of ZDDP will not harm the catalyst significantly, leading to the failure of emission performance compliance [
43,
44]. The primary role of detergents is to keep engines clean. Typical compounds are in the form of sulfonate, phenate, or salicylate. The most common metals are calcium and magnesium. These detergents can provide an efficient way to add basicity to the lubricant to neutralize combustion acids. During combustion, calcium and magnesium can form various compounds with phosphorous and sulfur, which are known to be harmful to emission control catalysts. The formation of specific compounds is known to provide a scavenging effect that can mitigate the negative impact of phosphorous and sulfur. The phosphorous level was set and controlled in industry specification ILSAC GF-3 onwards to 0.08% and successfully mitigated the earlier risks. In addition, Ca is known to be an oxidation catalyst, and its presence tends to assist oxidation of particulate in the exhaust [
45]. In this paper, the research work demonstrated: (1) the compatibility between lubricant formulation and emission control system (
Section 4.2), (2) the catalytic effect of lubricant chemistry on emission (
Section 4.3).
4.2. Compatibility Demonstration of Lubricant with Emission Control System
Gasoline particulate filters (GPF) have been adopted for gasoline-powered vehicles to reduce particulate emissions and concern was raised regarding lubricant compatibility. The combustion of lubricant produces ash, which will be accumulated in the filter. Unlike heavy-duty applications, no ash cleaning procedure is expected for light-duty gasoline applications. Therefore, the system must have enough capacity for all the ash produced in the useful life. Accordingly, lower ash levels can help reduce the risk of GPF operation with excessive ash accumulation in the filter. To investigate the compatibility between the GPF and lubricant, an accelerated aging protocol is needed to ensure the ash accumulation is representative of real-world full useful life (FUL) application. Meanwhile, thermal load, soot accumulation, and regeneration dynamics must be well captured in the aging protocol. The details of the aging protocol have been published previously [
46]. The final protocol consists of three stages of steady-state operation at different engine speed and load combinations. The cycle is repeated and applied to the system until the required aging target is reached.
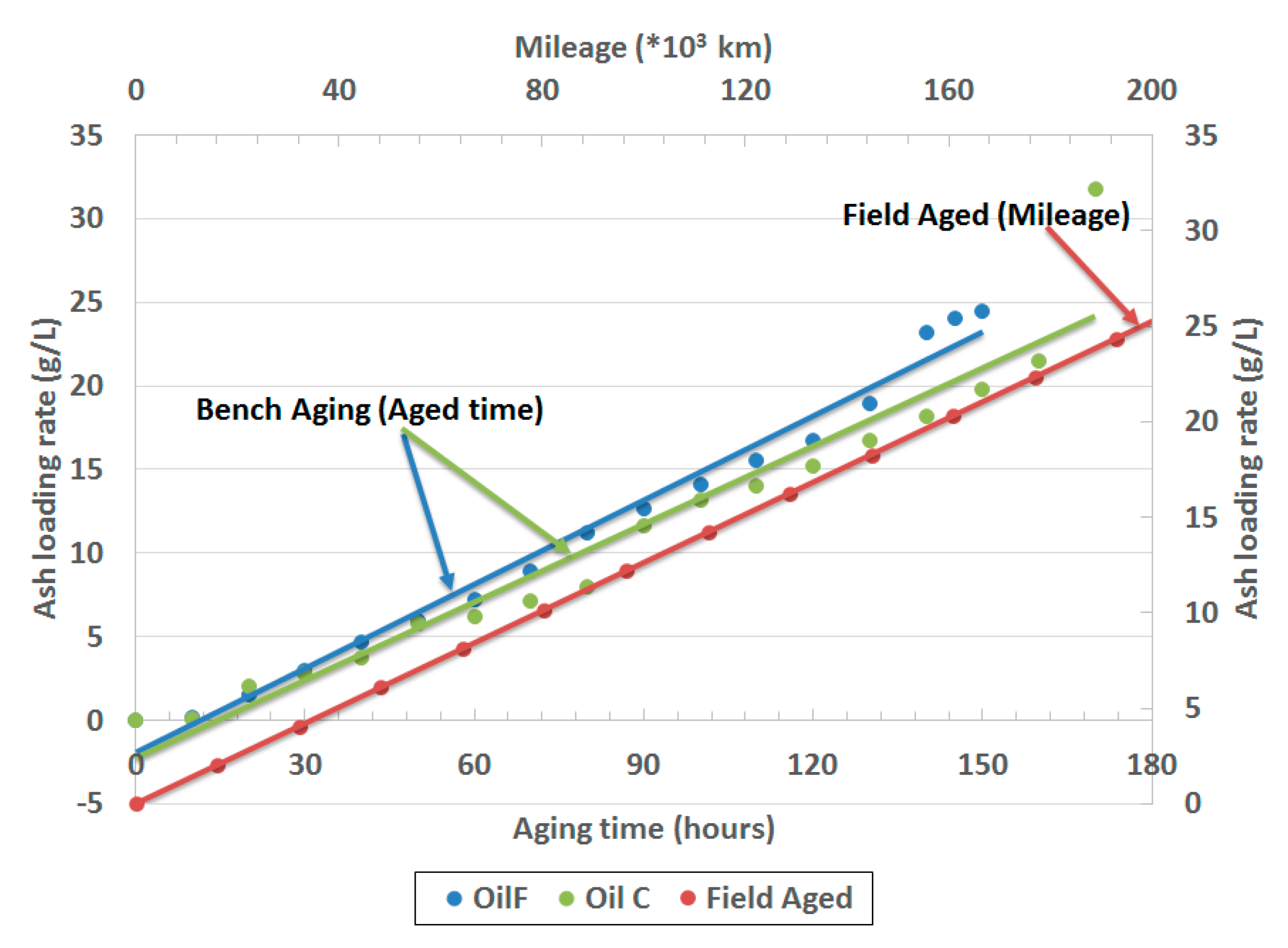
The FUL targeted in the cited work aimed to meet the China VI durability requirement, which is 200,000 km of driving. Two oils of different formulations were used for durability demonstration. They have the same SAPS (Sulfated Ash, Phosphorus, and Sulfur) level of 0.8% but use different detergent formulations. Oil F was Ca only detergent, while Oil C used mixed metal detergents including Ca and Mg. The results of ash loading are presented in
Figure 8. According to work done by Ford researchers, a reference of total ash loading was 25 g/L and was matched by the researchers [
47]. The durability demonstration adopts the proprietary aging protocol described elsewhere [
48]. During the aging testing, lubricant oil was doped at 1% (vol) to achieve accelerated ash loading while minimizing the impact on combustion stability and engine health. Ash accumulation based on the accelerated aging protocol successfully loaded an equivalent amount on the filter between 150 h and 170 h, respectively for the two oils described. Under the engine operating condition and resulting temperature profile, it would take 43 h for the catalytic converter to reach the amount of thermal load equivalent to 200,000 km driving exposure. Therefore, the total aging time for ash far exceeds what would be experienced from real-world operation over full useful life operation. The results suggest that the ash loading rates match the field aged profile (
Figure 8).
While the ash was loaded onto the GPF, periodic testing was conducted to evaluate the impact of aging, including ash loading, on vehicle emission performance. The vehicle was operated on a chassis dynamometer at those intervals over a Worldwide light-duty testing cycle (WLTC). Tailpipe exhaust was sampled for gaseous and particulate emission measurements.
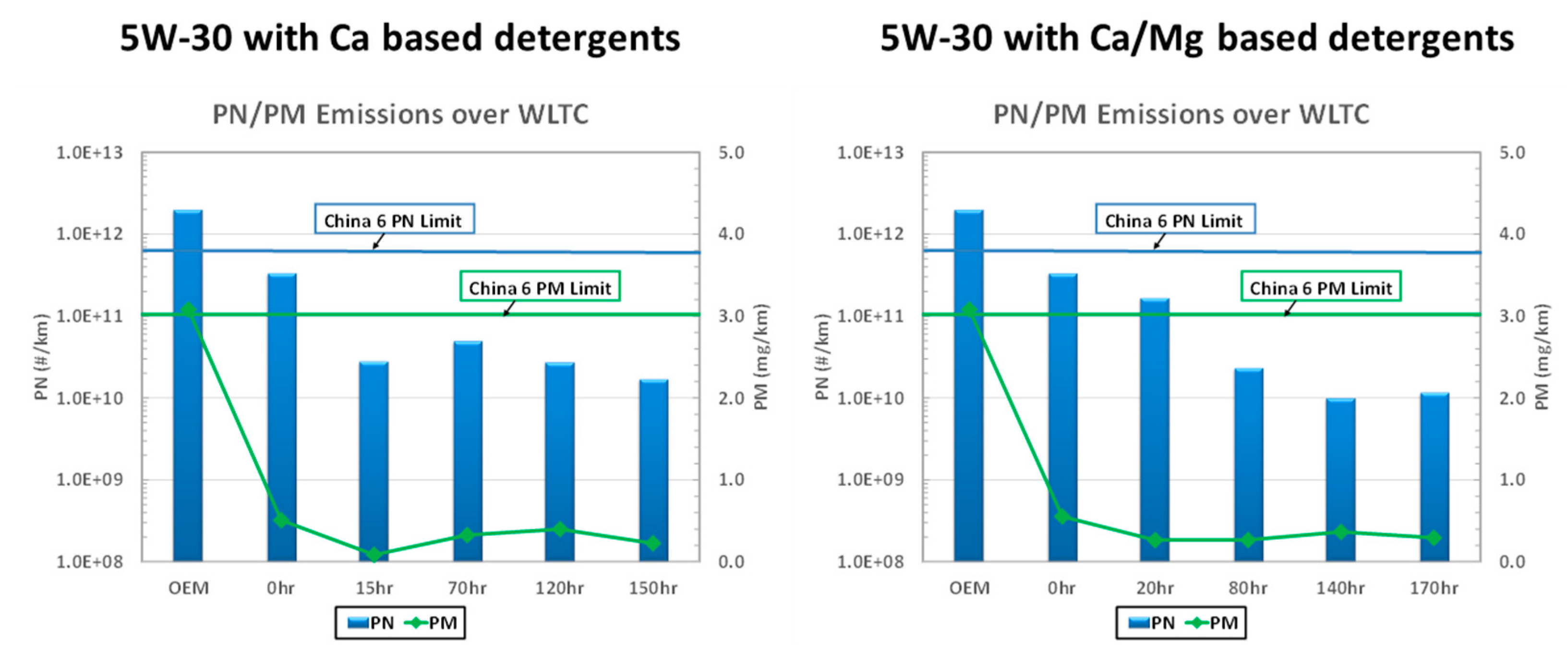
Figure 9 presents the results of particulate emission levels (both PN and PM) with the aged emission control system using two different lubricant oils (i.e., Oil F and Oil C). For both systems, the emission level drops initially after the de-greening and then reaches a steady level until the end of the testing. The initial de-greening and ash loading upon GPF help improve the filtration efficiency through the build-up of the filtration cake [
48]. Unlike diesel application, it takes longer to establish sufficient filtration cake on the GPF. The reason is that diesel engines emit much higher soot emissions than gasoline engines. After the efficiency reached the saturation level, no further reduction in particulate emission was observed until the completion of aging. In both cases, the vehicle emission performance was compliant with China VI emission requirement. The results manifest that lubricant oil at the SAPS level of 0.8% ash level is compatible with the durability requirement of GPF technology to meet legislation requirements.
4.3. Catalytic Effect Demonstration of Lubricant with Emission Control
The impact of lubricant on emission control systems is known as ash level and catalyst interaction. The ash accumulation on the filter can increase the backpressure, likely resulting in a fuel economy penalty. Specific chemistry in engine oil can lead to harmful effects on emission control catalysts through chemical and physical interaction. This can lead to catalyst poisoning and inefficiency. Both impacts may drive the need to lower the ash level or restrict the use of specific chemical components. However, the catalytic function of lubricant chemistry on particulate emission control, particularly soot oxidation, is not well known. Understanding the interaction between lubricant chemistry and particulates is vital for balanced engine oil formulation, through which optimized performance can be pursued.
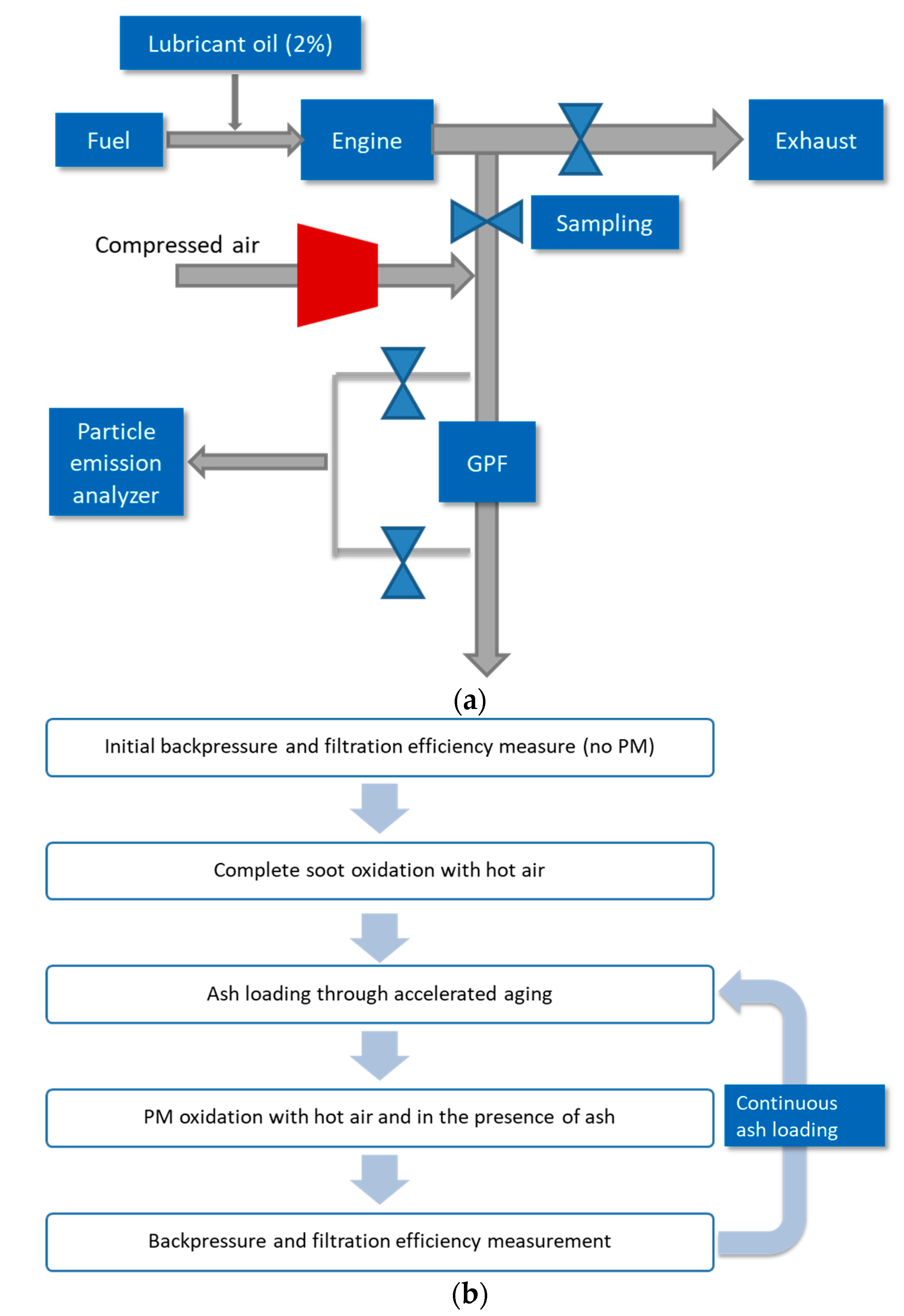
In the cited work, an engine bench test was set up to evaluate the impact of lubricant formulation chemistry on GPF performance and PM oxidation [
49].
Figure 10a describes the engine bench diagram, and
Figure 10b describes the test procedure. A naturally aspirated 2.4 L 4-cylinder GDI engine was used. 2% lubricant oil was doped to fuel before being injected into the engine combustion chamber. The exhaust was partially drawn to the sampling line where a test prototype bench-scale GPF (2″ in diameter and 6′ in length) was placed. Through oil dosing, ash was expected to be loaded on the GPF at an accelerated rate. Flow control valves were applied to adjust the flow rates for the main exhaust and the partial one for GPF testing. Compressed air was mixed with the partial exhaust before passing through the GPF. For PM oxidation, the air must be preheated to ensure the exhaust temperature at the inlet of GPF reaches 600 °C. Only cold air is supplied to the system for backpressure and filtration efficiency measurement. As indicated in the diagram, particle emission analyzers were applied to measure the mass and number, respectively. The filtration efficiency was calculated based on the emissions difference between upstream and downstream GPF.
A fluid matrix was designed where detergent metal (calcium or magnesium) type and amount and ZDDP amount were varied. All other essential components, including, dispersant, antioxidant, friction modifier, and viscosity modifier, were treated at constant levels. All the lubricant oils used were fully formulated with the same SAE grade of 5W-20.
A comprehensive evaluation of the impact of oil properties on GPF performance has been reported elsewhere [
49].
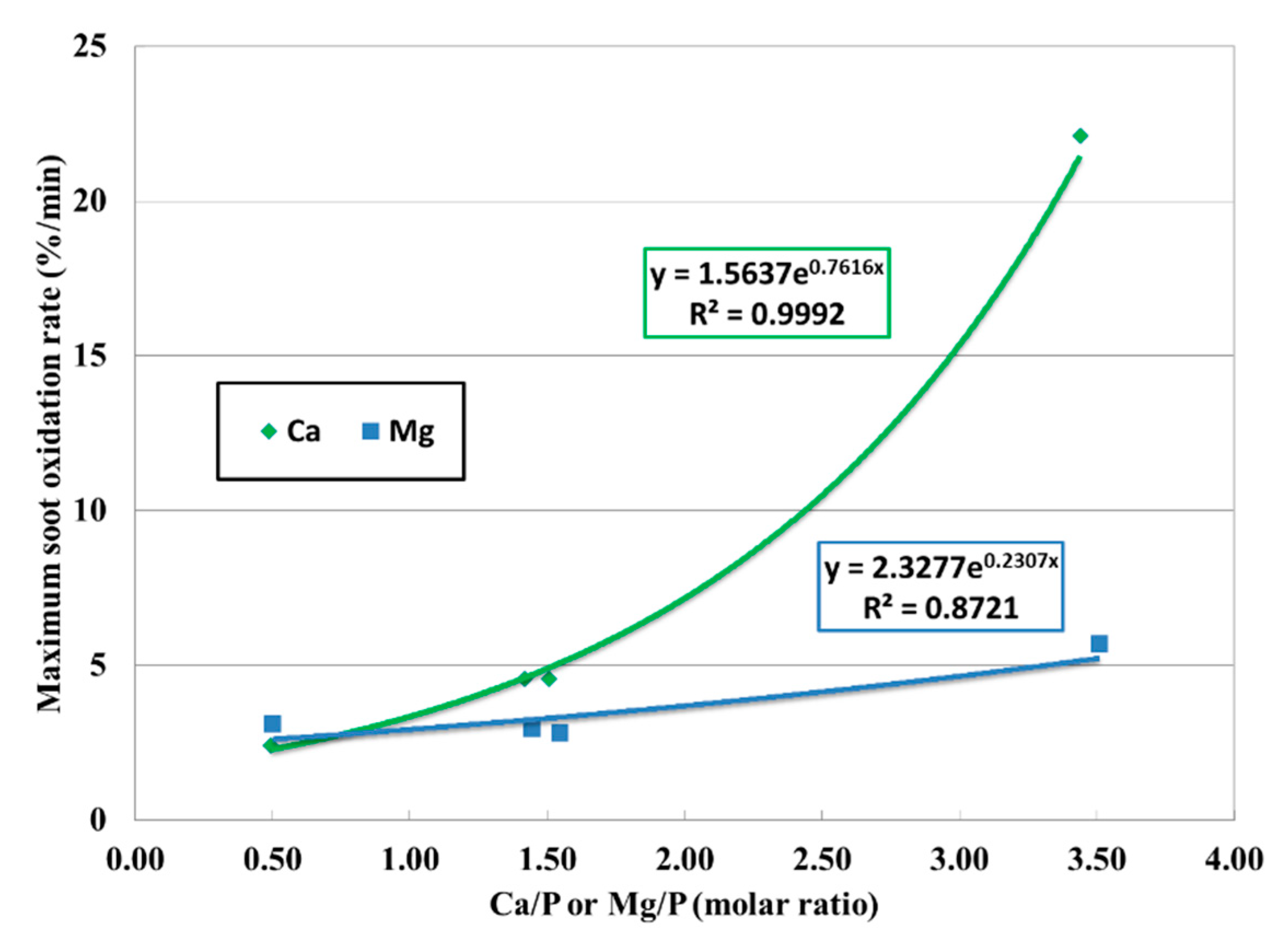
Figure 11 [
50] exhibited how PM oxidation was affected by the ratio between detergent metal and phosphorous. As Mg-based detergent was used in the oil (the blue line), little impact was observed regarding the PM oxidation rate. However, higher Ca/P increases the PM oxidation rate for calcium based detergent oil (the green line). This difference is attributed mainly to the detergent chemistry considering the other factors are maintained at the same level. As an oxidation catalyst, calcium can assist the PM combustion better than magnesium. The ratio between Ca and P will drive the dynamics of the oxidation and reduction process potentially through changing the catalytic activity, and the overall outcome is reflected in the PM oxidation rate.
The effect of lubricant additives on emission control systems is complex and dynamic. The findings in the literature successfully demonstrate the GPF performance with different oil formulations. A balanced SAPS level can be defined to maximize the filtration efficiency without sacrificing the durability requirements. Meanwhile, the choice of chemistry for lubricant additive can potentially impact PM oxidation with the assistance of certain elements that can act as an in situ catalyst. These comprehensive studies and insightful findings provide scientific guidance for lubricant formulation based on the system approach, leading to a holistic solution to system performance requirements.
5. Conclusions
Engine emissions technology is a complex system requiring multiple components to work together efficiently for optimal performance. The active development of intellectual property over the last 20 years illustrates the important role lubricants and fuels play in meeting vehicle emission performance requirements. Optimal performance of the emissions system requires the consideration of both the combustion and emission control processes. The lubricant and fuel additives can improve the overall emissions performance.
Particulate emissions are an area of particular focus for vehicle emissions control. Particulate mass and number emissions as well as particle characteristics and secondary organic aerosols are all to be considered as part of the complete system that influences emissions. Fuel composition can have impact on vehicle emissions performance both directly and indirectly. Fuel additives help improve the combustion properties improving emissions of the overall system. Limiting the aromatic content in fuel can noticeably reduce the particulate emission and contribute to reduced secondary organic aerosols.
Lubricant ash is an important consideration in vehicles equipped with GPF systems. With their widespread introduction, there was concern that high ash might prevent full useful life of the GPF system; however, research has shown that even at 0.8% SAPS the durability and performance of the GPF were retained. Furthermore, the accumulation of the ash in the filter was shown to have a positive effect on filtration performance. Finally, the detergent can have a positive effect on the reduction of soot accumulation in the GPF acting to catalyze its combustion potentially improving system performance.
Emission regulations are an increasingly important technology driver posing challenges to deliver higher efficiency, lower emission, and extended durability. Recognizing the system approach and applying technical rigor is critical to meeting those requirements and entails a comprehensive assessment of the fluid’s role in emission control. This work provides an overview and context for assessing the role of lubricant additives in the performance of the complete system. Understanding the full impact of the fluids, lubricant and fuel, and the powertrain hardware provides the foundation to design additives to deliver optimized performance for the vehicle with advanced emission control systems.