Abstract
Osteoarthritis (OA) is one of the frequent conditions in the orthopaedic practice. The number of OA patients is increasing and the efficacy of the current treatment methods is relatively low in terms of slowing or even preventing of the disease progression. The current research suggests that the lubrication function of the cartilage depends on its articulating surfaces. These surfaces are characterized by extracellular matrices with a three-dimensional porous structure that ensures a proper lubrication regime to protect the surface against the wear. Viscosupplementation is one of the possible treatments to slow the OA progression. This therapeutic intervention is frequently used in the clinical practice for the knee osteoarthritis. Viscosupplementation can, to a certain extent, supplement the lubrication ability of the cartilage by doping the hyaluronic acid (HA) and thus delay the degradation. However, selection of a proper viscosupplement remains a challenge, both in terms of the correct evaluation of the HA properties and their interaction with different stages of the OA. The viscoupplements differ in their HA molecular weight that may influence the CoF development from both the short term and the long-term perspective. The aim of this study is to analyze the coefficient of friction (CoF) between the real surfaces of a bovine cartilage after applying viscosupplements. The experiments were conducted on a pin-on-plate tribometer with a real bovine cartilage to simulate the lubrication regimes of a human joint. The model joint was doped with 4 different commercially available viscosupplements with different molecular weights and cross-linking of the HA. The OA damage was simulated by using a model synovial fluid with a concentration that corresponds to an OA patient. A compression of the cartilage surface was observed during the experiment and the interstitial fluid drained away from the porous cartilage structure. This, in combination with a migrating contact area (MCA), led the synovial fluid (SF) to mix with the viscosupplement. Decrease in the CoF was observed after the application of the viscosupplements with an increasing molecular weight. This was observed under a functional boosted cartilage lubrication regime, what suggests that the viscosupplementation yields the benefits especially for the conditions where the cartilage is not substantially damaged by the OA.
1. Introduction
Osteoarthritis (OA) is one of the most challenging conditions in the orthopaedic practice. This statement is linked to both the incidence of a disease in older population as well as to relatively low efficacy of the current treatment methods in terms of slowing or even preventing of the disease progression. In fact, all intraarticular and surrounding periarticular structures including muscles are affected during OA development with cartilage degradation being the hallmark of the disease. Patients with an advanced OA located at the sites of lower limb have difficulties to walk requiring often total joint replacement (TJR) to relieve them from the symptoms and improve the functions. About 10% of men and 18% of women aged over 60 years undergo hip or knee TJR [1].
A more precise understanding of the pathophysiology of OA might open a way to more targeted and thus more effective treatment strategies. A joint cartilage is damaged during osteoarthritic process directly via the intraarticular injury, and indirectly as a result of chronic overload and chronic inflammation [2]. The latter mechanism is associated with a growing concentrations of inflammatory cytokines and enzymes observed in the affected joints that lead to the destruction of cartilage matrix [3]. Cartilage degradation in the OA has several stages with the initial pathological change linked with depletion of the hyaluronan-aggrecan network followed by degradation of the collagen fibrils. The last stage is associated with a severe cartilage disintegration and bone-on-bone contact of articulating joint surfaces [4,5].
Surface mechanisms are impacted by the cartilage deterioration as well. The porous cartilage surface structure leads to several dominant lubrication regimes: a boundary lubrication [6,7] and two cartilage-specific lubrication regimes: a weeping and a boosted lubrication regime. McCutchen et al. studied the weeping regime that was based on the porous structure and elasticity of the cartilage, where the pressurized synovial fluid flowed to separate the surfaces during movement [8]. Several following studies showed that the pressurized interstitial fluid was able to transfer most of the load during the first 100–200 s [9]. After this, the lubrication regime changed to the boosted lubrication regime as observed by Walker et al. They found that the joint cartilage behaved much like a filter that releases water and retains macromolecules—a process inverted to the weeping lubrication regime. This enabled creation of a viscous lubrication film containing mostly the hyaluronic acid as a lubricant [10,11,12].
In case of a prolonged static load of a joint, the fluid flows away from the contact area, what leads to a boundary lubrication regime, where the two lubricated surfaces come into physical contact. A damaged or an OA-degraded joint surface loses its properties and thus disables the boosted and wheeping regimes, what leads to a more rapid deterioration and pain symptoms.
A variety of methods have been tested to affect the clinical course of the disease, however, no one of them has a disease-modifying effect [13]. A viscosupplementation (VS) is still among the most frequent therapeutic interventions used in the clinical practice for knee osteoarthritis (KOA). The treatment was first described by Balazs et al. [14] in 1993 as an injection of the hyaluronic acid (HA) into affected joints to restore the physiologic viscoelasticity in the synovial fluid (SF). A number of following studies tested different molecular weights of the HA, ranging from 0.5 to 10 MDa [15] and analysed the impact of the HA cross-linking and different concentrations on the SF behaviour and biotribology [16,17].
The HA exhibits a unique viscoelastic behavior with regard to the joint functions. During an impact loading, the HA chains remain unchanged, and its elastic properties moderate the surface load. Vice versa, when the motion is slow, the HA chains unravel acting as a viscous lubricant [18,19]. The HA also participates in the biological processes in the joint. Therefore, an exogenous HA delivers both the biologic stimulation and an improvement of the physical and tribological features of the SF, i.e., a higher viscosity or a better force absorption and lubrication features of the SF [13]. As mentioned above, the viscosity of the SF varies significantly depending on the age, gender, body mass index, or the overall health conditions of a patient. Galandáková et al. [20] investigated the SF of patients with the end-stage of the OA, finding that the viscosity varied from 8 mPa/s up to 171 mPa/s.
Behavior of the lubricant in the contact is often characterized by the coefficient of friction between the contact surfaces. In most of the existing experiments, the cartilage samples articulate against a geometrically precise object. Cartilage samples can maintain their hydration and lubrication properties provided that particular kinematic conditions are met. One of most important conditions is that the contact area moves faster than is the time necessary for the fluid diffusion [21]. This is described as a stationary contact area (SCA). If the articulating surfaces are curved and the contact is not stationary, a migrating contact area (MCA) occurs. The MCA is closer to the real joint behaviour [9,22,23].
Experimental studies designed for the cartilage on cartilage (CoC) contact pair configuration with the MCA are rare [19,24]. However, this type of experiments can bring the most relevant results for the lubrication analysis and describe the cartilage behaviour in the initial stages of the OA.
A degraded cartilage with damaged surface structures loses its lubrication abilities. Therefore, VS can, to a certain extent, supplement the lubrication ability of the cartilage by doping the HA and thus delay the degradation. However, the selection of the proper viscosupplement and the determination of the appropriate time intervals for administration remains a challenge. The characteristics of the applied HA may influence the coefficient of friction (CoF) as well as the biological behaviour of the viscosupplement. However, the long-term clinical effect of the treatment is controversial and often influenced by the individual conditions of the patients. Several studies concluded that VS had limited to negligible effects, often explained as placebo [25,26,27,28]. In fact, there is a discordance between the wide clinical usage of the VS and the results of the systematic reviews/meta-analysis. The solution may lie in further research in this field, including more experimental studies are necessary to describe the viscosupplement behaviour directly in the joint contacts and its tribological effects on the joint surfaces or on the lubrication mechanisms. The aim of this study is to analyze the CoF between the real surfaces of the bovine cartilage after applying viscosupplements with different molecular weights.
2. Materials and Methods
2.1. Simulator
A universal tribometer (UMT Bruker TriboLab, Billerica, MA, USA) in the pin-on-plate configuration was used for the CoF measurements. The device was equipped with a two-axis piezoelectric force sensor (DFH 5.0 G), with 5 N load and 2.5 mN resolution. The cartilage pin was secured by a clutching clamping and pushed against the cartilage plate. A significant inequality of the samples was compensated with a tilting table, dimensioned for a normal load of 10 kN. Heating was secured by two heating cartridges for 37 degrees. The speed motion of the pin and the normal load were maintained by a feedback regulation integrated in the experimental device (Figure 1).
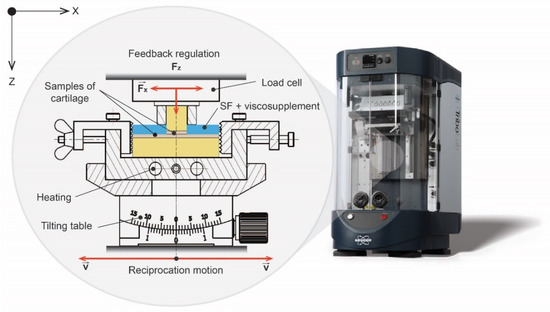
Figure 1.
Scheme of pin-on-plate tribometer.
2.2. Samples
The samples were harvested from calves that were approximately 24 months old. The time between the slaughter and the samples harvesting did not exceed 48 h. In the meantime, the tissue was kept at 8 °C. The femoral condyle was cut with an oscillating saw to harvest the plate sample. Subsequently, the cartilage pins of about 9.5 mm on average were pressed. The cartilage plates and pins were removed along with the subchondral bone and rinsed in the PBS. The samples were stored in a phosphate buffered saline (PBS) at −22 °C (Figure 2).
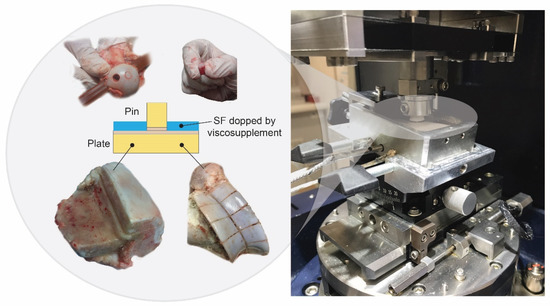
Figure 2.
Harvesting of the articular cartilage samples.
The model of synovial fluid was prepared according to the research findings of Galandova et al. to simulate the structure of the SF in patients suffering from the 2nd and 3th stage of osteoarthritis [20]. A PBS served as a base of the synovial fluid, with gradually mixed-in albumin (powder, ≥96%; A2153, Sigma-Aldrich, St. Louis, MO, USA), y-globulin (powder, ≥ 99%; G5009, Sigma-Aldrich, St. Louis, MO, USA), HA (molecular weight = 820–1020 kDa, Contipro, Dolní Dobrouč, Czech Republic) and phospholipids (powder, type XVI-E, lyophilized powder; ≥99%; vesicle form; P3556, Sigma-Aldrich, St-Louis, MO, USA). Mixing-in was conducted overnight for 12 h at the temperature of 37 °C.
Four commercial viscosupplementation agents were used (Table 1): Hyalgan® (Fidia Farmaceutici, Padova, Italy), Ostenil® (TRB Chemedica), Synvisc ONE® (Sanofi Genzyme, Ridgefield, NJ, USA) a Hyruan ONE® (LG Chemical Ltd., Pharmac.div., Yongjej-dong, Iksan). The viscosupplements were chosen based on their molecular weight and HA crosslinking. The agents were provided by local commercial suppliers and stored in the fridge at the temperature of 8 °C. The viscosupplements were diluted with the model SF at 1:1 ratio.

Table 1.
Specifications of viscosupplements.
2.3. Experimental Design
Viscosupplementation effects were observed using two types of experiments. The first experiment simulated the state where a viscosupplementation agent was applied to the patient. The friction pair was lubricated with the model SF for the first 600 s, then a viscosupplementation agent was added. After 1800 s, the contact was unloaded for 600 s to observe the impact of rehydration. The experiment then proceeded under the same conditions for another 1200 s. The measurements were repeated for all four viscosupplementation agents (Table 2).

Table 2.
Kinematic conditions.
The second experiment simulated the state, where the SF was fully mixed with the viscosupplementation agent after being applied to the joint. The measurements were first made only with the SF. Then the SF was drained from the cartilage using a vacuum pump for an hour. Next, the same measurements were repeated with a mixture of SF and the viscosupplementation agent. The measurements consisted of five loading phases (lasting 300 s each) and of four unloading phases (lasting 150 s each) to ensure the rehydration of the cartilage. The measurements were repeated for three contact pairs and for all the viscosupplementation agents. The actual load force and frictional forces were measured to calculate the CoF. The position of pin in load axes and the speed of the pin movement were also observed (Figure 3).
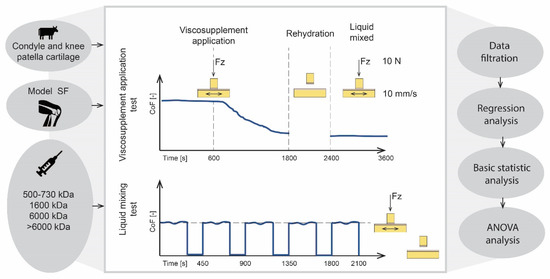
Figure 3.
Scheme of experimental design.
2.4. Data Processing
The raw data were filtered from the extreme speed and the load values to remove the data in the dead centre, where the boundary conditions of the experiment were not met. The filtration boundaries have been set for ± 5%. In the next step, we excluded the data distorted due to a large response to the feedback intervention. These data were filtered based on a sudden change of pin position. This could be the case of a large local curvature of surface where the boundary conditions were not met. For this purpose, a floating filter was developed to filter the data according to the standard deviation of pin position in the Z axes for each cycle of pin movement. In the next step, basic statistic values, such as quartile or arithmetic mean, were calculated. A regression analysis and two-sample t-tests with level of significance α = 0.05 were conducted. This approach was used for all the measurements. The tests simulating the application of the viscosupplementation agent were presented on a scatter chart, with time values on the x axes and ∆ CoF values on the y axes. The tests simulating the state after the mixing were presented on a boxplot.
3. Results and Discussion
Two sets of experiments were conducted to analyse the effects of commercial viscosupplements on CoF within the cartilage-on-cartilage contact as well as on the deformation of cartilage samples during the measurements. The first set of experiments, viscosupplementation application tests, was designed to simulate the state with a viscosupplement being applied to a patient. Therefore, a viscosupplement was added to the model SF during the measurement. The second set of measurements, the liquid mixing tests, simulated the state where the SF and the viscosupplement were fully mixed.
3.1. Viscosupplementation Application Tests
The viscosupplementation application tests, which lasted 3600 s, consisted of three phases: the run-in phase, the viscosupplementation phase, and the final phase after rehydration. The results of the time dependence of CoF for the four tested viscosupplements can be seen in Figure 4 and the evolution of cartilage deformation in the z-axis during measurements can be seen in Figure 5.
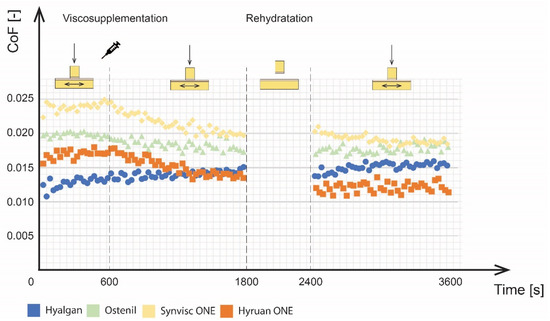
Figure 4.
Development of the CoF for various viscosupplements.
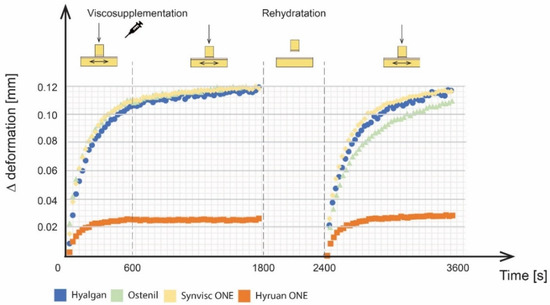
Figure 5.
Deformations of cartilage samples during the experiment.
During the run-in phase, the CoF values increased with time for all tested viscosupplements. This is mainly attributed to biphasic cartilage lubrication. Initial CoF values, ranging from 0.01 to 0.025, were observed for individual viscosupplements. At the end of the run-in phase, the CoF values of the individual viscosupplements were as follows: 0.024 for Synvisc ONE®, 0.020 for Ostenil®, 0.017 for Hyruan ONE® and 0.013 for Hyalgan®. However, it should be noted that the differences between individual viscosupplements could be significantly affected by the differences in the shape of cartilage sliding surfaces. Therefore, the overall efficacy was analysed by the percentage decrease of CoF between the run-in phase and the final phase after rehydration. A change in the pin position from 0 to 0.11 mm was also observed during the run-in phase for all viscosupplements except for Hyruan ONE®. This type of behaviour was attributed to the porous cartilage structure saturated with the lubricant together with the lubricant squeeze under the applied load. Hyruan ONE® showed the lowest grades of deformation, probably caused by the location of cartilage sample extraction on the surface of the bovine joint.
During the viscosupplementation phase, the viscosupplement was applied directly to the model SF between the articulating surfaces and the changes (increase/decrease) in cartilage-on-cartilage friction were analysed. The time of CoF stabilization was considered as the time when the liquids were ideally mixed. To determine the effects of the viscosupplementation agent on CoF, the phase ranging from 600 to 1200 s was interpolated by a linear regression line (Figure 6).
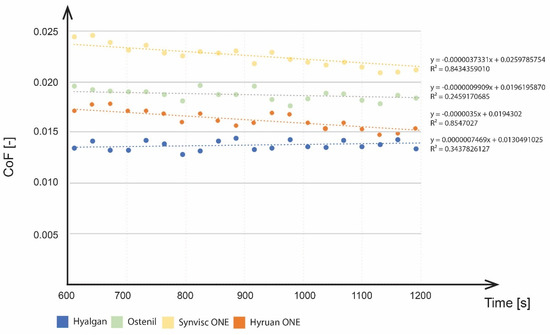
Figure 6.
CoF decrease during the viscosupplementation phase.
No significant efficacy was observed throughout the viscosupplementation phase for Hyalgan® and Ostenil® in terms of CoF changes. Synvisc ONE® and Hyruan ONE® showed more pronounced differences together with differences in the gradient of the CoF decrease. Taking into account the speed of the effect, Hyruan ONE® and Synvisc ONE® showed a significantly faster decrease in CoF values based on linear regression values. Ostenil® showed a slower decrease, while Hyalgan® even reported a slight increase in CoF values. The initial CoF value for Hyalgan® was 0.0134. This was followed by a slight increase to 0.0142. As mentioned above, the application of the other viscosupplements led to a decrease of the CoF. Ostentil showed the initial values of 0.0196, followed by a decrease to 0.0177. Synvisc ONE® showed the highest initial values of 0.0245, followed by a decrease to 0.0198. Hyruan ONE® showed initial values of 0.0172, followed by a decrease to 0.0136. The application of viscosupplementation agents had no effect on the progression of cartilage deformation. No local changes were observed after the application. In the last phase, all the fluids were considered mixed. Rehydration of cartilage did not have any further effects on the development of CoF for the three viscosupplements with a lower molecular weight (MW): Ostenil®, Hyalgan®, and Synvisc ONE®. Hyruan ONE® showed some effect of rehydration, with a decrease in the CoF value from 0.0136 to 0.012. When comparing the end CoF values after the first and third phases, we observed the following: Hyalgan® showed a CoF increase of 17.6%, Ostenil® 8% decrease, Synvisc ONE® 23% decrease, and Hyruan ONE® 28% decrease.
For cartilage deformation data in Figure 5, Hyruan ONE® reported an increase in the first 300 s. After that, it stabilized more or less at a value of 0.03. On the other hand, Hyalgan®, Ostenil® and Synvisc ONE® showed a similar logarithmic trend of the deformation development as during the run-in phase. Hyalgan® and Ostenil® showed the most significant increase in the first 500 s, when the cartilage deformation reached a value of 0.1 mm. During the same period, Ostenil® showed an increase of 0.08 mm. Compared to the first phase, this increase was lower and about 200 s longer. In the third phase, no flattening of cartilage deformation development was observed. The end values of the Z position were 0.113 mm for Hyalgan®, 0.109 mm for Ostenil®, and 0.116 mm for Synvisc ONE®.
3.2. Liquid Mixing Tests
Liquid mixing tests were performed on 12 pairs of bovine cartilage samples. First, a reference measurement with a model synovial fluid was made for each contact pair. Consequently, SF was drained from the cartilage structure and the new measurement was performed with a mixture of SF and viscosupplementation agent (mixed in a 1:1 ratio). Each of the viscosupplements was analyzed with three different cartilage samples. Based on the results, the average values and standard deviations of the CoF of the reference and comparative measurements were calculated (Figure 7).
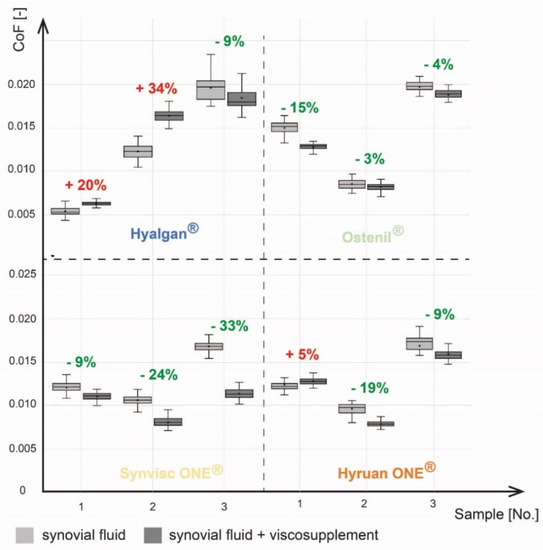
Figure 7.
Comparison of CoF after viscosuplementation.
The CoF values ranged from 0.0051 to 0.0163 for the reference measurements. All samples showed a significant change in CoF between the reference and comparative measurements. A significant decrease in CoF was observed for all measurements except for measurements 1a, 1b and 4a. An increase in CoF was observed for Hyalgan® in two cases. The first measurement showed an increase of 20% and the second 34%. In the last measurement, a decrease in CoF by 9% was observed. Ostenil® measurements showed a decrease in CoF in all cases. Similarly, a decrease in CoF was observed for Synvisc ONE® for all measurements. In the case of Hyruan ONE®, CoF decreased in two cases. A 5% increase in CoF was observed in the first measurement. A 19% and 9% decrease was observed for the second and third measurements. On the basis of these results, an average change in the CoF was calculated for each viscosupplementation agent. Hyalgan® showed an average Increase in CoF of 15%. There was an average decrease of 7% for Ostenil®. The highest average decrease in CoF of 21% was observed for Synvisc ONE®. The last product, Hyruan ONE®, showed an average decrease of 8%.
The results of cartilage deformation measurements during liquid mixing tests showed a logarithmic trend similar to that in the case of viscosupplementation application tests. Maximum values of deformation were always reached at the end of the individual loading phases, lasting 300 s. The average values and standard deviation of cartilage deformation at the end of the individual loading phases for clear SF and all viscosupplementation agents are shown in Figure 8. For clear SF, cartilage deformation values at the end of the loading phases were quite similar for all loading phases, between 0.067 and 0.072 mm. Hyalgan® reported higher values of cartilage deformations compared to pure SF. Furthermore, the cartilage deformation values increased from 0.078 mm to 0.089 mm during the measurement. Ostenil® also showed higher cartilage deformation values compared to pure SF. However, no trend in the results was observed while the deformation ranges between 0.069 and 0.078 mm. Hyruan ONE® reported a lower deformation than SF. Apart from the last loading phase, the results reported a downward trend. The deformation decreased from 0.065 mm to 0.057 mm. Except for the initial loading phase, the lowest cartilage deformation values between 0.05 and 0.053 mm were measured for Synvisc ONE®.
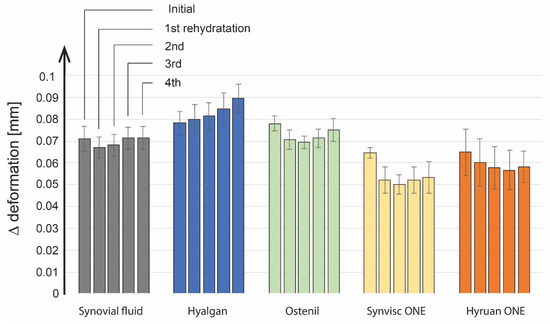
Figure 8.
Analysis of deformations for cartilage samples.
Our study evaluated the effects of four commercially available viscosupplements for OA on friction between bovine cartilage samples, simulating a pre-osteoarthritic stage of joint lubricated by a model synovial fluid. The molecular weight of hyaluronic acid and the cross-linking of the HA were the key parameters for the selection of the viscosupplements. We analyzed two experimental setups. The viscosupplementation application tests simulated the behaviour of the viscosupplement with the solution applied directly to the osteoarthritic joint and the liquid mixing tests simulated a situation in which the SF and viscosupplement were already fully mixed. The results revealed a significant decrease in the CoF after the addition of the high-MW viscosupplement to the pure OA SF, as well as significant differences in results between the individual viscosupplements.
During all measurements, the reported values of CoF were not higher than 0.025. These values are relatively close to the real joint conditions with a migrating contact area [22,23]. Moreover, the CoF was constant during the measurements. The surface deformation increased, which corresponds to the assumed CoF development between two joint surfaces as described by Caligaris a Ateshian [29]. Due to a pressurized fluid and a compressed surface, we assumed a boosted lubrication regime as defined by Walker et al. [12]. The cartilage-on-cartilage configuration is a great benefit of this study compared to other studies in which various substitute materials such as glass, mica, or hydrogel have been often used to mitigate the cartilage structure [15,30,31]. This reimbursement leads to changes in the lubrication regimes within the contact. However, the use of two cartilage surfaces caused a problem for the CoF measurements due to a variation in the surface curvature of cartilage samples. This limited the repeatability of the data. Therefore, we mainly focused on the relative differences between the measurements to evaluate the effects of viscosupplements and to show their effects on cartilage friction. The individual viscosupplements were mutually compared using the ANOVA. The mutual comparison of the differences in the CoF decrease showed no statistical significance (p value < 0.05).
The absolute value of CoF can also be influenced by factors related to cartilage extraction, such as the location of the sampling [32], the cartilage structure, and the cartilage thickness that plays an important role in the cartilage lubrication [8,9,12]. It is also important to understand that the size of the contact area changes when two surfaces with various curvature radius articulate. This is accompanied by continuous compression during articulation and by changes in contact pressure with subsequent impact on CoF [33]. The compression of the cartilage structure is accompanied by a drain of the fluid from the cartilage structure. During the run-in phase of the viscosupplementation application tests, three of four cartilage samples showed a gradual increase in deformation with a maximum size of 0.11 mm. The deformation value of the last contact pair was only 0.03 mm. This was probably caused by a different thickness of the cartilage and the subchondral bone. In general, the fluid drain did not have any effect on the development of CoF. This was related to an interstitial lubrication regime, where the pressurized fluid flows through a porous cartilage structure to a high pressure area [10]. As the fluid flowed, the viscosupplement got mixed up with the SF.
During the viscosupplementation application tests, four commercially available HA-based viscosupplements showed different trends in CoF development (Figure 4). A CoF increase was observed for Hyalgan® after its application. The relative change in CoF in the final phase compared to the run-in phase was 0.0023. On the other hand, a decrease in CoF was observed for Ostenil®, with a gradient of −9.9 × 10−7 and a relative change in the CoF of 0.0023. Synvisc ONE® and Hyruan ONE® showed an effect three times faster compared to Ostenil®. The Synvisc ONE® gradient was −3.7 × 10−6 with an average decrease of 0.0041. The Hyruan ONE® gradient was −3.5 × 10−6 with an average decrease of 0.003. The results of the liquid mixing tests showed similar trends. Solutions with higher molecular weights showed a decrease in the CoF. However, in this case, it was Synvisc ONE® that showed the most substantial CoF decrease, by 22% on average. Hyruan ONE®, as the solution with the highest MW, also showed a decrease in CoF, with the exception of one sample with critical values of the surface curvature.
These results suggest a connection between the MW and the viscosupplement efficiency. Low-MW solutions, such as Hyalgan®, cannot interact with the cartilage surface and extracellular matrix to improve the lubrication characteristics. An approximately linear dependence between HA MW and CoF within the cartilage-on-cartilage contact was already published by Kwiecinski et al. [34]. The existing literature offers two possible explanations for this phenomenon. Viscosupplementation changes the rheological properties of synovial fluid. These changes are more significant for solutions containing high MW or cross-linked HA that cannot flow through the cartilage structure [18]. A highly viscous lubrication film is created on the cartilage surface, which is accompanied by a significant CoF decrease. Therefore, it is assumed that viscosupplementation changes the weeping lubrication regime to the boosted lubrication regime [12]. However, the human joint can operate at relatively high values of shear rate, up to 105 s−1 [35]. Therefore, due to the shear thinning behaviour of HA, improvement of SF rheology does not appear as the true viscosupplementation mechanism of action. Furthermore, some studies [15,36] have already published no direct dependency between the HA rheology and the cartilage-on-glass friction. Another explanation suggests that there are stronger adhesion forces between the high-MW HA and the cartilage surface [37]. Longer linear molecules of high-MW HA a make multiple contact with lubricin molecules within the cartilage superficial zone. The resulting robust gel-like layer is an essential foundation for an interaction with the phospholipids and the subsequent hydration lubrication between their phosphocholine headgroups [38].
Despite that we showed the contribution of different VS to both the decrease of CoF and cartilage deformation experimentally the clinical usefulness of VS is still difficult to prove in the randomized clinical studies and their meta-analysis. In fact, some meta-analysis find the clinically important effect maintaining for several weeks [39,40,41] while the others report very limited or no benefit from the intra-articular administration of VS especially when the long-term effects of this treatment was evaluated [42,43,44]. There was reported significant inter-trial heterogeneity related to trial size, particular product used, frequency of VS administration, blinded outcome assessment or publication status [43]. The contradictory findings of the research are consequently reflected in the guidelines of international medical associations such as the Osteoarthritis Research Society International (OARSI) [45].
On the other hand, at least one recent meta-analysis revealed the clinical effect for high molecular weight HA concluding that the high-molecular weight product had better outcomes for up to 6 months at the site of the hip [46]. In addition, a recent randomized-controlled trial reports better effect on patient’s performance, disability and activity of daily living for a combination of platelet-rich plasma (PRP) and high molecular weight HA [47]. Interestingly, some clinical data support also the intraarticular administration of high molecular weight HA with corticosteroids or other substances to affect simultaneously the inflammation accompanying OA progression [48]. Taken together, it seems that the HA product characteristics like molecular weight and cross-linking could affect its performance on the OA joint. However, Caligaris et al. [29] questioned the effects of viscosupplementation on joints with damaged cartilage. This corresponds to the theoretical assumptions on lubrication regimes, where cartilage plays a crucial role. In such cases, the improved viscoelastic and frictional properties of the synovial fluid cannot compensate for the degradation of the cartilage extracellular matrix. As a result, only earlier stages of OA are appropriate for VS interventions. Further high-quality investigations evaluating the effect of intraarticular administration of high-molecular weight HA in earlier stages of KOA in particular patients are therefore highly warranted to identify those who will show clinically meaningful response to HA injections as opposed to those who are unlikely to benefit from this intervention.
3.3. Limitations
The main challenge of our research was to obtain several cartilage surfaces with approximately the same geometry and mechanical properties. For example, Richard et al. [49] reported differences in Young’s modulus and the Poisson ratio between the cartilage samples extracted from various areas of the human femoral head, which can consequently influence the friction of the articular cartilage [32]. This surely affects the overall CoF values and the evaluation of the viscosupplementation effects. The short-term experiments were repeated on three different samples extracted from the same joint type on the basis of the joint surface curvature. For future research, it would be beneficial to repeat the measurements on more samples and to consider extracting the samples from an identical area of the joint. Our research covered four viscosupplements selected on the basis of their molecular weight. Because of the minimal effects of the viscosupplement containing low-MW HA on the CoF, it would be interesting to select a wider range of the MW values graded in greater detail. It will allow us to define the threshold value of the MW, which affects the development of the CoF.
In our study, a model synovial fluid, which corresponds to patients with the end stage of OA, was used. However, viscosupplementation is expected to be more effective in the early stages of osteoarthritis, during which the cartilage extracellular matrix is not completely worn out. It could also be interesting to study the interactions between the viscosupplement and the individual SF constituents in more detail.
4. Conclusions
The aim of this study was to observe the effects of the VS on the tribological behaviour of a cartilage to contribute to the discussion over the efficiency of the viscosupplementation in OA joints. The experiments were conducted on a pin-on-plate tribometer with a real bovine cartilage to simulate the lubrication regimes of a human joint. The OA damage was simulated using a model synovial fluid. Two distinctive states were selected for our evaluation: the state after the viscosupplementation application and the state when the SF was fully mixed with the viscosupplementation agent.
- A decrease in the CoF was observed after application of viscosupplements with a higher molecular weight to the SF. Various viscosupplements also differ significantly from each other based on their molecular weight. We observed a linear decrease of the CoF with an increasing molecular weight.
- The surface of the cartilage was compressed during the experiments and the interstitial fluid drained from its porous structure. This, together with the effects of the migrating contact area led to mixing of the SF with the viscosupplement.
- The viscosupplementation decreases the CoF under a functional boosted cartilage lubrication regime. This means that the viscosupplementation yields the most benefits for conditions where the cartilage is not substantially damaged by the OA.
Author Contributions
M.R. and M.O. conceived the idea and designed the experiments. M.R. and D.R. performed and analysed the experiments and wrote the original draft of the manuscript. J.G. and I.K. supervised the study. M.V. supervised and financed the study. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Czech Science Foundation: “An Investigation of Synovial Fluid Viscosupplementation and its Impact on Friction and Lubrication” (grant number 20-00483S) and supported by the Grant Agency of Ministry of Health of the Czech Republic (grant number NU20-06-00269).
Data Availability Statement
Not applicable.
Acknowledgments
This research was carried out under the project “An Investigation of Synovial Fluid Viscosupplementation and its Impact on Friction and Lubrication” (grant number 20-00483S) with financial support from the Czech Science Foundation and AZV MH CR NU20-06-00269.
Conflicts of Interest
The authors declare no conflict of interest.
References
- OECD. Hip and Knee Replacement. 2021. Available online: https://doi.org/10.1787/8b492d7a-en (accessed on 20 November 2022).
- Vincent, T.L.; Alliston, T.; Kapoor, M.; Loeser, R.F.; Troeberg, L.; Little, C.B. Osteoarthritis Pathophysiology: Therapeutic Target Discovery May Require a Multifaceted Approach. Clin. Geriatr. Med. 2022, 38, 193–219. [Google Scholar] [CrossRef] [PubMed]
- Shiozawa, J.; de Vega, S.; Yoshinaga, C.; Ji, X.; Negishi, Y.; Momoeda, M.; Nakamura, T.; Yoshida, H.; Kaneko, H.; Ishijima, M.; et al. Expression and regulation of recently discovered hyaluronidases, HYBID and TMEM2, in chondrocytes from knee osteoarthritic cartilage. Sci. Rep. 2022, 12, 17242. [Google Scholar] [CrossRef] [PubMed]
- Pritzker, K.P.H.; Gay, S.; Jimenez, S.A.; Ostergaard, K.; Pelletier, J.P.; Revell, P.A.; Salter, D.; van den Berg, W.B. Osteoarthritis cartilage histopathology: Grading and staging. Osteoarthr. Cartil. 2006, 14, 13–29. [Google Scholar] [CrossRef]
- Zhang, E.; Yan, X.; Zhang, M.; Chang, X.; Bai, Z.; He, Y.; Yuan, Z. Aggrecanases in the human synovial fluid at different stages of osteoarthritis. Clin. Rheumatol. 2013, 32, 797–803. [Google Scholar] [CrossRef]
- Schmidt, T.A.; Gastelum, N.S.; Nguyen, Q.T.; Schumacher, B.L.; Sah, R.L. Boundary lubrication of articular cartilage-Role of synovial fluid constituents. Arthritis Rheum. 2007, 56, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.S.; Holmes, M.H.; Lai, W.M.; Mow, V.C. Boundary Conditions at the Cartilage-Synovial Fluid Interface for Joint Lubrication and Theoretical Verifications. J. Biomech. Eng. 1989, 111, 78–87. [Google Scholar] [CrossRef]
- McCutchen, C.W. Paper 1: Physiological Lubrication. In Proceedings of the Institution of Mechanical Engineers, Conference Proceedings; SAGE Publications: London, UK, 1966; Volume 181, pp. 55–62. [Google Scholar] [CrossRef]
- Ateshian, G.A.; Hung, C.T. The natural synovial joint: Properties of cartilage. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2006, 220, 657–670. [Google Scholar] [CrossRef]
- Ermakov, S.; Beletskii, A.; Eismont, O.; Nikolaev, V. Modern Concepts of Friction, Wear and Lubrication of Joints. In Liquid Crystals in Biotribology: Synovial Joint Treatment; Ermakov, S., Beletskii, A., Eismont, O., Nikolaev, V., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 99–121. [Google Scholar]
- Jahn, S.; Seror, J.; Klein, J. Lubrication of Articular Cartilage. Annu. Rev. Biomed. Eng. 2016, 18, 235–258. [Google Scholar] [CrossRef] [PubMed]
- Walker, P.; Dowson, D.; Longfield, M.; Wright, V. Boosted lubrication in synvial joints by fluid entrapment and enrichment. Ann. Rheum. Dis. 1968, 27, 512–520. [Google Scholar] [CrossRef]
- Cooper, C.; Rannou, F.; Richette, P.; Bruyere, O.; Al-Daghri, N.; Altman, R.D.; Brandi, M.L.; Basset, S.C.; Herrero-Beaumont, G.; Migliore, A.; et al. Use of Intraarticular Hyaluronic Acid in the Management of Knee Osteoarthritis in Clinical Practice. Arthritis Care Res. 2017, 69, 1287–1296. [Google Scholar] [CrossRef]
- Balazs, E.A.; Denlinger, J.L. Viscosupplementation-A new concept in the treatment of osteoarthritis. J. Rheumatol. 1993, 20, 3–9. [Google Scholar]
- Rebenda, D.; Vrbka, M.; Nečas, D.; Toropitsyn, E.; Yarimitsu, S.; Čípek, P.; Pravda, M.; Hartl, M. Rheological and frictional analysis of viscosupplements towards improved lubrication of human joints. Tribol. Int. 2021, 160, 107030. [Google Scholar] [CrossRef]
- Xiao, J.; Hu, Y.; Huang, L.; Huang, Z.-F.; Jiang, W.-Z.; Luo, Y.-Q.; Jia, M.-Y.; Chen, D.; Shi, Z.-J. Injection route affects intra-articular hyaluronic acid distribution and clinical outcome in viscosupplementation treatment for knee osteoarthritis: A combined cadaver study and randomized clinical trial. Drug Deliv. Transl. Res. 2021, 11, 279–291. [Google Scholar] [CrossRef]
- Prekasan, D.; Saju, K.K. Tribological effectiveness of viscosupplements for osteoarthritis in knee joint. SN Appl. Sci. 2019, 1, 1018. [Google Scholar] [CrossRef]
- Rebenda, D.; Vrbka, M.; Čípek, P.; Toropitsyn, E.; Nečas, D.; Pravda, M.; Hartl, M. On the Dependence of Rheology of Hyaluronic Acid Solutions and Frictional Behavior of Articular Cartilage. Materials 2020, 13, 2659. [Google Scholar] [CrossRef] [PubMed]
- Čípek, P.; Rebenda, D.; Nečas, D.; Vrbka, M.; Krupka, I.; Hartl, M. Visualization of Lubrication Film in Model of Synovial Joint. Tribol. Ind. 2019, 41, 387–393. [Google Scholar] [CrossRef]
- Galandáková, A.; Ulrichová, J.; Langová, K.; Hanáková, A.; Vrbka, M.; Hartl, M.; Gallo, J. Characteristics of synovial fluid required for optimization of lubrication fluid for biotribological experiments. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1422–1431. [Google Scholar] [CrossRef]
- Moore, A.C.; Schrader, J.L.; Ulvila, J.J.; Burris, D.L. A review of methods to study hydration effects on cartilage friction. Tribol.-Mater. Surf. Interfaces 2017, 11, 202–214. [Google Scholar] [CrossRef]
- Boettcher, K.; Kienle, S.; Nachtsheim, J.; Burgkart, R.; Hugel, T.; Lieleg, O. The structure and mechanical properties of articular cartilage are highly resilient towards transient dehydration. Acta Biomater. 2016, 29, 180–187. [Google Scholar] [CrossRef]
- Klein, J. Hydration lubrication. Friction 2013, 1, 1–23. [Google Scholar] [CrossRef]
- Heiner, A.D.; Smith, A.D.; Goetz, J.E.; Goreham-Voss, C.M.; Judd, K.T.; McKinley, T.O.; Martin, J.A. Cartilage-on-cartilage versus metal-on-cartilage impact characteristics and responses. J. Orthop. Res. 2013, 31, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Vincent, P. Intra-articular hyaluronic acid in knee osteoarthritis: Clinical data for a product family (ARTHRUM), with comparative meta-analyses. Curr. Ther. Res. 2021, 95, 100637. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.V.; Jüni, P.; Saadat, P.; Xing, D.; Yao, L.; Bobos, P.; Agarwal, A.; Hincapié, C.A.; da Costa, B.R. Viscosupplementation for knee osteoarthritis: Systematic review and meta-analysis. BMJ 2022, 378, e069722. [Google Scholar] [CrossRef]
- Conrozier, T.; Raman, R.; Chevalier, X.; Henrotin, Y.; Monfort, J.; Diraçoglù, D.; Bard, H.; Baron, D.; Jerosch, J.; Richette, P.; et al. Viscosupplementation for the treatment of osteoarthritis. The contribution of EUROVISCO group. Ther. Adv. Musculoskelet. Dis. 2021, 13, 1759720X211018605. [Google Scholar] [CrossRef] [PubMed]
- Divine, J.G.; Zazulak, B.T.; Hewett, T.E. Viscosupplementation for Knee Osteoarthritis: A Systematic Review. Clin. Orthop. Relat. Res.® 2007, 455, 113–122. [Google Scholar] [CrossRef]
- Caligaris, M.; Ateshian, G.A. Effects of sustained interstitial fluid pressurization under migrating contact area, and boundary lubrication by synovial fluid, on cartilage friction. Osteoarthr. Cartil. 2008, 16, 1220–1227. [Google Scholar] [CrossRef]
- Murakami, T.; Yarimitsu, S.; Nakashima, K.; Sawae, Y.; Sakai, N. Influence of synovia constituents on tribological behaviors of articular cartilage. Friction 2013, 1, 150–162. [Google Scholar] [CrossRef]
- Hilšer, P.; Suchánková, A.; Mendová, K.; Filipič, K.E.; Daniel, M.; Vrbka, M. A new insight into more effective viscosupplementation based on the synergy of hyaluronic acid and phospholipids for cartilage friction reduction. Biotribology 2021, 25, 100166. [Google Scholar] [CrossRef]
- Chan, S.M.T.; Neu, C.P.; Komvopoulos, K.; Reddi, A.H. The role of lubricant entrapment at biological interfaces: Reduction of friction and adhesion in articular cartilage. J. Biomech. 2011, 44, 2015–2020. [Google Scholar] [CrossRef]
- Furmann, D.; Nečas, D.; Rebenda, D.; Čípek, P.; Vrbka, M.; Křupka, I.; Hartl, M. The Effect of Synovial Fluid Composition, Speed and Load on Frictional Behaviour of Articular Cartilage. Materials 2020, 13, 1334. [Google Scholar] [CrossRef]
- Kwiecinski, J.J.; Dorosz, S.G.; Ludwig, T.E.; Abubacker, S.; Cowman, M.K.; Schmidt, T.A. The effect of molecular weight on hyaluronan’s cartilage boundary lubricating ability–alone and in combination with proteoglycan 4. Osteoarthr. Cartil. 2011, 19, 1356–1362. [Google Scholar] [CrossRef] [PubMed]
- More, S.; Kotiya, A.; Kotia, A.; Ghosh, S.K.; Spyrou, L.A.; Sarris, I.E. Rheological properties of synovial fluid due to viscosupplements: A review for osteoarthritis remedy. Comput. Methods Programs Biomed. 2020, 196, 105644. [Google Scholar] [CrossRef] [PubMed]
- Bonnevie, E.D.; Galesso, D.; Secchieri, C.; Bonassar, L.J. Frictional characterization of injectable hyaluronic acids is more predictive of clinical outcomes than traditional rheological or viscoelastic characterization. PLoS ONE 2019, 14, e0216702. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lin, W.; Fan, Y.; Kampf, N.; Wang, Y.; Klein, J. Effects of Hyaluronan Molecular Weight on the Lubrication of Cartilage-Emulating Boundary Layers. Biomacromolecules 2020, 21, 4345–4354. [Google Scholar] [CrossRef]
- Seror, J.; Zhu, L.; Goldberg, R.; Day, A.J.; Klein, J. Supramolecular synergy in the boundary lubrication of synovial joints. Nat. Commun. 2015, 6, 6497. [Google Scholar] [CrossRef]
- De Lucia, O.; Jerosch, J.; Yoon, S.; Sayre, T.; Ngai, W.; Filippou, G. One-year efficacy and safety of single or one to three weekly injections of hylan G-F 20 for knee osteoarthritis: A systematic literature review and meta-analysis. Clin. Rheumatol. 2021, 40, 2133–2142. [Google Scholar] [CrossRef]
- Concoff, A.; Sancheti, P.; Niazi, F.; Shaw, P.; Rosen, J. The efficacy of multiple versus single hyaluronic acid injections: A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2017, 18, 542. [Google Scholar] [CrossRef]
- Campbell, K.A.; Erickson, B.J.; Saltzman, B.M.; Mascarenhas, R.; Bach, B.R., Jr.; Cole, B.J.; Verma, N.N. Is Local Viscosupplementation Injection Clinically Superior to Other Therapies in the Treatment of Osteoarthritis of the Knee: A Systematic Review of Overlapping Meta-analyses. Arthroscopy 2015, 31, 2036–2045. [Google Scholar] [CrossRef]
- Jevsevar, D.; Donnelly, P.; Brown, G.A.; Cummins, D.S. Viscosupplementation for Osteoarthritis of the Knee: A Systematic Review of the Evidence. JBJS 2015, 97, 2047–2060. [Google Scholar] [CrossRef]
- Rutjes, A.W.; Jüni, P.; da Costa, B.R.; Trelle, S.; Nüesch, E.; Reichenbach, S. Viscosupplementation for osteoarthritis of the knee: A systematic review and meta-analysis. Ann. Intern. Med. 2012, 157, 180–191. [Google Scholar] [CrossRef]
- Miller, L.E.; Fredericson, M.; Altman, R.D. Hyaluronic Acid Injections or Oral Nonsteroidal Anti-inflammatory Drugs for Knee Osteoarthritis: Systematic Review and Meta-analysis of Randomized Trials. Orthop. J. Sports Med. 2020, 8, 2325967119897909. [Google Scholar] [CrossRef] [PubMed]
- McAlindon, T.E.; Bannuru, R.R.; Sullivan, M.C.; Arden, N.K.; Berenbaum, F.; Bierma-Zeinstra, S.M.; Hawker, G.A.; Henrotin, Y.; Hunter, D.J.; Kawaguchi, H.; et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr. Cartil. 2014, 22, 363–388. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Z.; Huang, H.T.; Ho, C.J.; Shih, C.L.; Chen, C.H.; Cheng, T.L.; Wang, Y.C.; Lin, S.Y. Molecular Weight of Hyaluronic Acid Has Major Influence on Its Efficacy and Safety for Viscosupplementation in Hip Osteoarthritis: A Systematic Review and Meta-Analysis. Cartilage 2021, 13, 169S–184S. [Google Scholar] [CrossRef] [PubMed]
- Nouri, F.; Babaee, M.; Peydayesh, P.; Esmaily, H.; Raeissadat, S.A. Comparison between the effects of ultrasound guided intra-articular injections of platelet-rich plasma (PRP), high molecular weight hyaluronic acid, and their combination in hip osteoarthritis: A randomized clinical trial. BMC Musculoskelet. Disord. 2022, 23, 856. [Google Scholar] [CrossRef] [PubMed]
- Anil, U.; Markus, D.H.; Hurley, E.T.; Manjunath, A.K.; Alaia, M.J.; Campbell, K.A.; Jazrawi, L.M.; Strauss, E.J. The efficacy of intra-articular injections in the treatment of knee osteoarthritis: A network meta-analysis of randomized controlled trials. Knee 2021, 32, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Richard, F.; Villars, M.; Thibaud, S. Viscoelastic modeling and quantitative experimental characterization of normal and osteoarthritic human articular cartilage using indentation. J. Mech. Behav. Biomed. Mater. 2013, 24, 41–52. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).